Life History Parameters to Inform Pattern of Prenatal Investment in Marine Mammals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Algorithm of Self-Organizing Map
2.3. Statistical Analysis
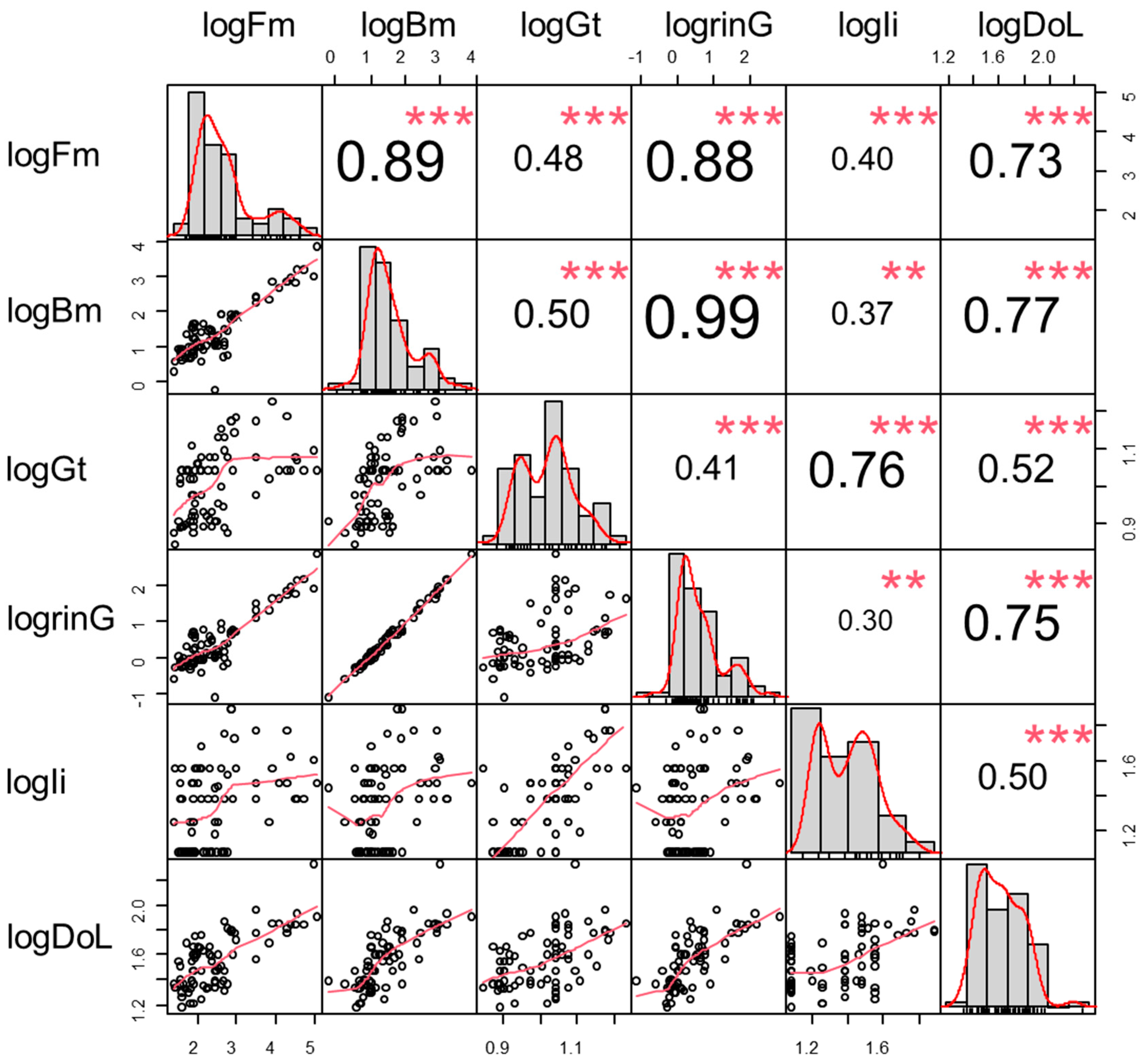
3. Results
3.1. Life History Traits
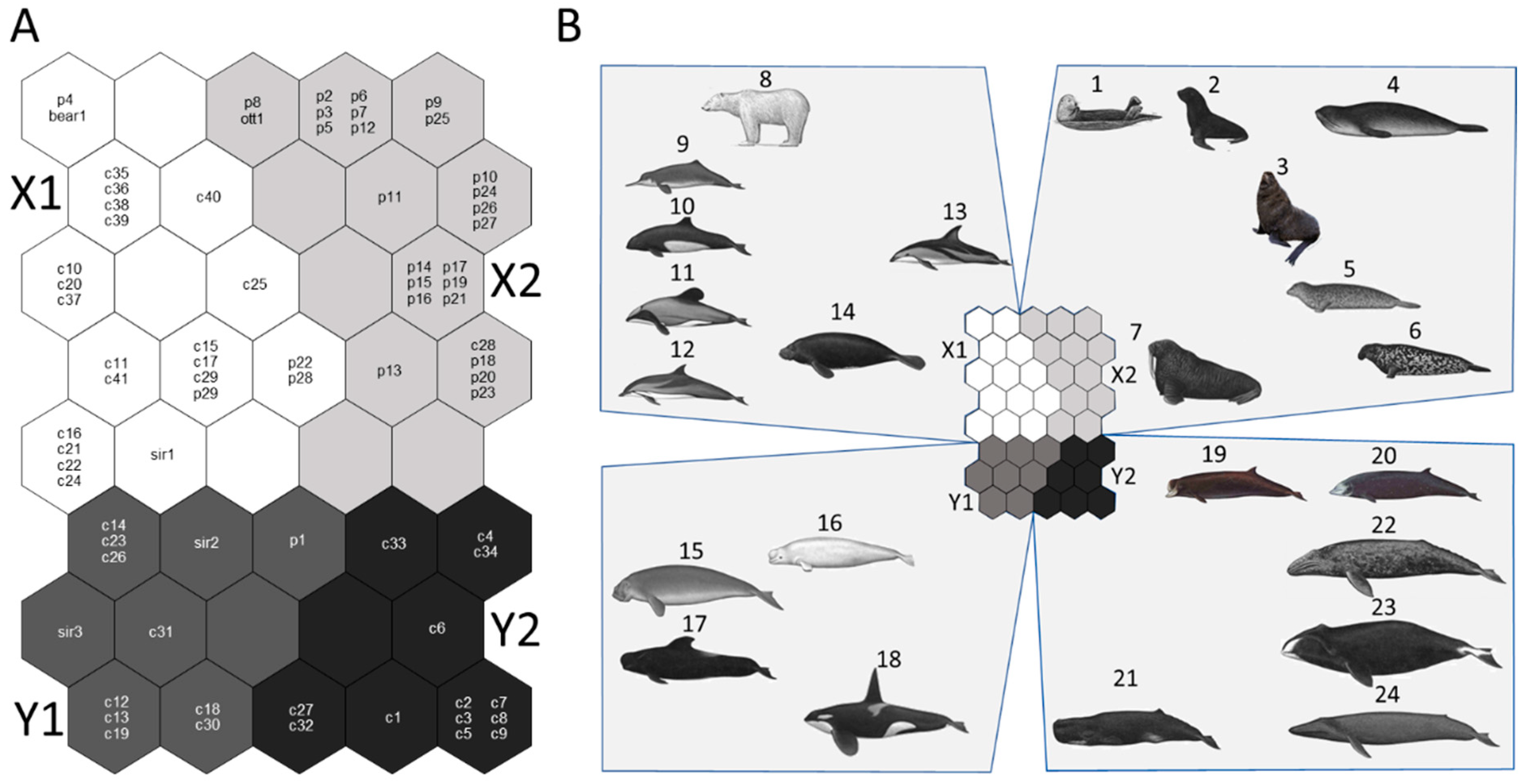
3.2. Optimal Map Size and Species Clustering
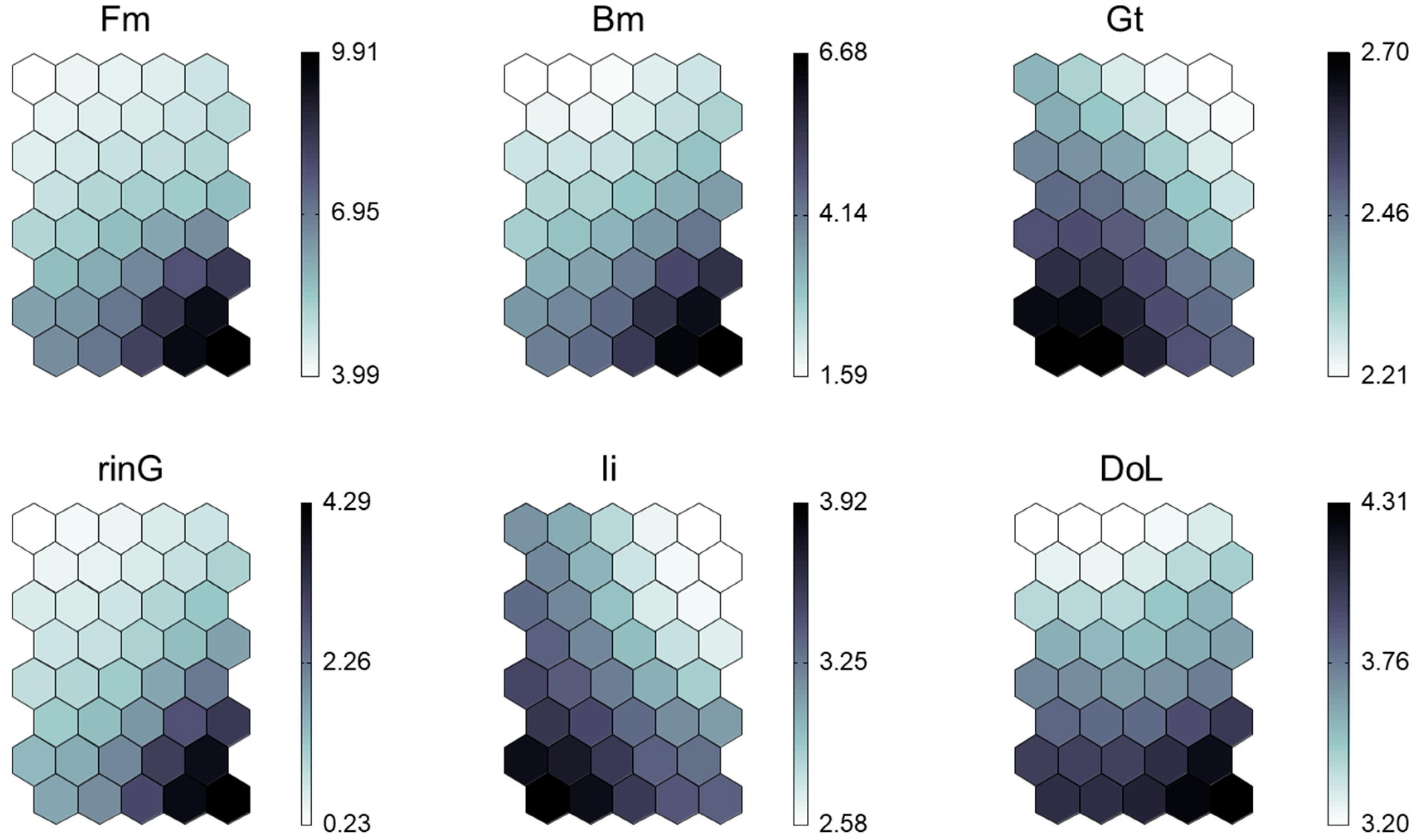
3.3. Trait Characteristics of Clusters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivers, R. Parental Investment and Sexual Selection; Biological Laboratories, Harvard University: Cambridge, MA, USA, 1972; pp. 136–179. [Google Scholar]
- Clutton-Brock, T.H. Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems; University of Chicago Press: Chicago, IL, USA, 1988; pp. 1–533. [Google Scholar]
- Mcnamara, J.M.; Szekely, T.; Webb, J.N.; Houston, A.I. A dynamic game-theoretic model of parental care. J. Theor. Biol. 2000, 205, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Kokko, H.; Jennions, M.D. Parental investment, sexual selection and sex ratios. J. Evol. Biol. 2008, 21, 919–948. [Google Scholar] [CrossRef] [PubMed]
- Tallamy, D.W.; Wood, T.K. Convergence patterns in subsocial insects. Annu. Rev. Entomol. 1986, 31, 369–390. [Google Scholar] [CrossRef]
- Wilson, E.O. Sociobiology: The New Synthesis; Harvard University Press: Cambridge, MA, USA, 2000; pp. 1–720. [Google Scholar]
- Bonsall, M.B.; Klug, H. The evolution of parental care in stochastic environments. J. Evol. Biol. 2011, 24, 645–655. [Google Scholar] [CrossRef]
- Ferrière, R.; Dieckmann, U.; Couvet, D. Evolutionary Conservation Biology; Cambridge University Press: Cambridge, UK, 2004; pp. 1–428. [Google Scholar]
- Bowen, W.D. Role of marine mammals in aquatic ecosystems. Mar. Ecol. Prog. Ser. 1997, 158, 267–274. [Google Scholar] [CrossRef]
- Moore, S.E. Marine mammals as ecosystem sentinels. J. Mammal. 2008, 89, 534–540. [Google Scholar] [CrossRef]
- Jefferson, T.A.; Webber, M.A.; Pitman, R.L. Marine Mammals of the World; Academic Press: London, UK, 2015; pp. 1–608. [Google Scholar]
- Chivers, S.J. Cetacean Life History. In Encyclopedia of Marine Mammals; Academic Press: San Diego, CA, USA, 2009; pp. 215–220. [Google Scholar]
- Stephens, P.A.; Houston, A.I.; Harding, K.C.; Boyd, I.L.; McNamara, J.M. Capital and income breeding: The role of food supply. Ecology 2014, 95, 882–896. [Google Scholar] [CrossRef]
- McCann, T.S.; Fedak, M.A.; Harwood, J. Parental investment in southern elephant seals. Mirounga Leonina. Behav. Ecol. Sociobiol. 1989, 25, 81–87. [Google Scholar] [CrossRef]
- Boness, D.J.; Bowen, W.D. The evolution of maternal care in pinnipeds. BioScience 1996, 46, 645–654. [Google Scholar] [CrossRef]
- Oftedal, O.T. Use of maternal reserves as a lactation strategy in large mammals. Proc. Nutr. Soc. 2000, 59, 99–106. [Google Scholar] [CrossRef]
- Mann, J. Maternal Care and Offspring Development in Odontocetes. In Ethology and Behavioral Ecology of Odontocetes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 95–116. [Google Scholar]
- Read, A.J. Trends in the maternal investment of harbour porpoises are uncoupled from the dynamics of their primary prey. Proc. R. Soc. Lond. B 2001, 268, 573–577. [Google Scholar] [CrossRef]
- Georges, J.Y.; Guinet, C. Prenatal investment in the subantarctic fur seal, Arctocephalus tropicalis. Can. J. Zool. 2001, 79, 601–609. [Google Scholar] [CrossRef]
- Huang, S.; Chou, L.; Shih, N.; Ni, I. Implication of life history strategies for prenatal investment in cetaceans. Mar. Mamm. Sci. 2010, 27, 182–194. [Google Scholar] [CrossRef]
- Schulz, T.M.; Bowen, W.D. Pinniped lactation strategies: Evaluation of data on maternal and offspring life history traits. Mar. Mamm. Sci. 2004, 20, 86–114. [Google Scholar] [CrossRef]
- Ferguson, S.H. The influences of environment, mating habitat, and predation on evolution of pinniped lactation strategies. J. Mamm. Evol. 2006, 13, 63–82. [Google Scholar] [CrossRef]
- Klein, C. Cetacean Maternal Investment: Importance in Conservation Across Species and Drivers for Interspecific Altruism. Master’s Thesis, University of South Florida, FL, USA, 2021; pp. 7–28. [Google Scholar]
- Schulz, T.M.; Bowen, W.D. The evolution of lactation strategies in pinnipeds: A phylogenetic analysis. Ecol. Monogr. 2005, 75, 159–177. [Google Scholar] [CrossRef]
- Fu, L. The discriminate analysis and dimension reduction methods of high dimension. Open. J. Soc. Sci. 2015, 3, 7–13. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, C.; Xiang, W.; Tian, D.; Deng, X.; Zhao, M. Application of artificial neural networks in global climate change and ecological research: An overview. Chin. Sci. Bull. 2010, 55, 3853–3863. [Google Scholar] [CrossRef]
- Zarra, T.; Galang, M.G.; Ballesteros, F.; Belgiorno, V.; Naddeo, V. Environmental odour management by artificial neural network—A review. Environ. Int. 2019, 133, 105189. [Google Scholar] [CrossRef]
- Kang, H.; Jin Jeon, D.; Kim, S.; Jung, K. Estimation of fish assessment index based on ensemble artificial neural network for aquatic ecosystem in South Korea. Ecol. Indic. 2022, 136, 108708. [Google Scholar] [CrossRef]
- Lin, M.; Liu, M.; Dong, L.; Caruso, F.; Li, S. Modeling intraspecific variation in habitat utilization of the Indo-Pacific humpback dolphin using self-organizing map. Ecol. Indic. 2022, 144, 109466. [Google Scholar] [CrossRef]
- Park, Y.; Verdonschot, P.F.M.; Chon, T.; Lek, S. Patterning and predicting aquatic macroinvertebrate diversities using artificial neural network. Water Res. 2003, 37, 1749–1758. [Google Scholar] [CrossRef]
- Lin, M.; Lek, S.; Ren, P.; Li, S.; Li, W.; Du, X.; Guo, C.; Gozlan, R.E.; Li, Z. Predicting impacts of South-to-North Water Diversion Project on fish assemblages in Hongze Lake, China. J. Appl. Ichthyol. 2017, 33, 395–402. [Google Scholar] [CrossRef]
- Allen, J.A.; Murray, A.; Noad, M.J.; Dunlop, R.A.; Garland, E.C. Using self-organizing maps to classify humpback whale song units and quantify their similarity. J. Acoust. Soc. Am. 2017, 142, 1943–1952. [Google Scholar] [CrossRef]
- Usman, A.M.; Ogundile, O.O.; Versfeld, D.J.J. Review of automatic detection and classification techniques for cetacean vocalization. IEEE Access 2020, 8, 105181–105206. [Google Scholar] [CrossRef]
- Mann, J.; Connor, R.C.; Tyack, P.L.; Whitehead, H. Cetacean Societies: Field Studies of Dolphins and Whales; The University of Chicago Press: Chicago, IL, USA, 2000; pp. 1–448. [Google Scholar]
- NOAA Fisheries. NOAA Fisheries. 2000. Available online: https://www.fisheries.noaa.gov/ (accessed on 5 June 2023).
- Pomeroy, P. Reproductive cycles of marine mammals. Anim. Reprod. Sci. 2011, 124, 184–193. [Google Scholar] [CrossRef]
- Berta, A.; Kovacs, K.M.; Sumich, J.L. Marine Mammals: Evolutionary Biology; Academic Press: London, UK, 2015; pp. 1–738. [Google Scholar]
- Würsig, B.G.; Thewissen, J.G.M.; Kovacs, K.M. Encyclopedia of Marine Mammals; Academic Press: San Diego, CA, USA, 2018; pp. 1–1190. [Google Scholar]
- Cardillo, M.; Mace, G.M.; Gittleman, J.L.; Jones, K.E.; Bielby, J.; Purvis, A. The predictability of extinction: Biological and external correlates of decline in mammals. Proc. R. Soc. B 2008, 275, 1441–1448. [Google Scholar] [CrossRef]
- Trites, A.W.; Pauly, D. Estimating mean body masses of marine mammals from maximum body lengths. Can. J. Zool. 1998, 76, 886–896. [Google Scholar] [CrossRef]
- Blueweiss, L.; Fox, H.; Kudzma, V.; Nakashima, D.; Peters, R.; Sams, S. Relationships between body size and some life history parameters. Oecologia 1978, 37, 257–272. [Google Scholar] [CrossRef]
- Charnov, E.L. Evolution of life history variation among female mammals. Proc. Natl. Acad. Sci. USA 1991, 88, 1134–1137. [Google Scholar] [CrossRef]
- Pontier, D.; Gaillard, J.M.; Allainé, D.; Allaine, D. Maternal investment per offspring and demographic tactics in placental mammals. Oikos 1993, 66, 424. [Google Scholar] [CrossRef]
- Kohonen, T.; Honkela, T. Kohonen network. Scholarpedia 2007, 2, 1568. [Google Scholar] [CrossRef]
- Kohonen, T. Self-organized formation of topologically correct feature maps. Biol. Cybern. 1982, 43, 59–69. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Vesanto, J.; Himberg, J.; Alhoniemi, E.; Parhankangas, J. SOM toolbox for Matlab 5; Helsinki University of Technology Finland: Helsinki, Finland, 2000; p. 57. [Google Scholar]
- Kiviluoto, K. Topology Preservation in Self-organizing Maps. In Proceedings of the International Conference on Neural Networks (ICNN’96), Washington, DC, USA, 3–6 June 1996; pp. 294–299. [Google Scholar]
- Park, Y.S.; Chon, T.S.; Bae, M.J.; Kim, D.H.; Lek, S. Multivariate Data Analysis by Means of Self-organizing Maps. In Ecological Informatics; Springer: Berlin/Heidelberg, Germany, 2018; pp. 251–272. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, Netherlands, 1998; pp. 1–870. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2020. Available online: https://www.r-project.org/ (accessed on 25 July 2023).
- Kovacs, K.M.; Lavigne, D.M. Maternal investment in otariid seals and walruses. Can. J. Zool. 1992, 70, 1953–1964. [Google Scholar] [CrossRef]
- Lockyer, C. All creatures great and smaller: A study in cetacean life history energetics. J. Mar. Biol. Assoc. UK 2007, 87, 1035–1045. [Google Scholar] [CrossRef]
- Roff, D.A. Contributions of genomics to life-history theory. Nat. Rev. Genet. 2007, 8, 116–125. [Google Scholar] [CrossRef]
- Huang, S.; Chou, L.; Ni, I. Comparable length at weaning in cetaceans. Mar. Mamm. Sci. 2009, 25, 875–887. [Google Scholar] [CrossRef]
- Churchill, M.; Clementz, M.T.; Kohno, N. Predictive equations for the estimation of body size in seals and sea lions (Carnivora: Pinnipedia). J. Anat. 2014, 225, 232–245. [Google Scholar] [CrossRef]
- Adams, D.C.; Collyer, M.L. Phylogenetic comparative methods and the evolution of multivariate phenotypes. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 405–425. [Google Scholar] [CrossRef]
- Tuomi, J. Mammalian reproductive strategies: A generalized relation of litter size to body size. Oecologia 1980, 45, 39–44. [Google Scholar] [CrossRef]
- Geffen, E.; Gompper, M.E.; Gittleman, J.L.; Luh, H.K.; MacDonald, D.W.; Wayne, R.K. Size, life-history traits, and social organization in the Canidae: A reevaluation. Am. Nat. 1996, 147, 140–160. [Google Scholar] [CrossRef]
- Houston, A.I.; Stephens, P.A.; Boyd, I.L.; Harding, K.C.; McNamara, J.M. Capital or income breeding? A theoretical model of female reproductive strategies. Behav. Ecol. 2006, 18, 241–250. [Google Scholar] [CrossRef]
- Jonsson, K.I. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 1997, 78, 57–66. [Google Scholar] [CrossRef]
- Ejsmond, M.J.; Varpe, Ø.; Czarnoleski, M.; Kozłowski, J. Seasonality in offspring value and trade-offs with growth explain capital breeding. Am. Nat. 2015, 186, 111–125. [Google Scholar] [CrossRef]
- Boyd, I.L. Time and energy constraints in pinniped lactation. Am. Nat. 1998, 152, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Horning, M.; Mellish, J.A.E. Predation on an upper trophic marine predator, the Steller sea lion: Evaluating high juvenile mortality in a density dependent conceptual framework. PLoS ONE 2012, 7, e30173. [Google Scholar] [CrossRef] [PubMed]
- Boyd, I.L. Environmental and physiological factors controlling the reproductive cycles of pinnipeds. Can. J. Zool. 1991, 69, 1135–1148. [Google Scholar] [CrossRef]
- New, L.F.; Moretti, D.J.; Hooker, S.K.; Costa, D.P.; Simmons, S.E. Using energetic models to investigate the survival and reproduction of beaked whales (family Ziphiidae). PLoS ONE 2013, 8, e68725. [Google Scholar] [CrossRef]
- Senigaglia, V.; Christiansen, F.; Sprogis, K.R. Food-provisioning negatively affects calf survival and female reproductive success in bottlenose dolphins. Sci. Rep. 2019, 9, 8981. [Google Scholar] [CrossRef]
- Whitehead, H.; Shin, M. Current global population size, post-whaling trend and historical trajectory of sperm whales. Sci. Rep. 2022, 12, 19468. [Google Scholar] [CrossRef] [PubMed]
- Swartz, S.L.; Taylor, B.L.; Rugh, D.J. Gray whale Eschrichtius robustus population and stock identity. Mamm. Rev. 2006, 36, 66–84. [Google Scholar] [CrossRef]

| Abbr | Variables | Range | Mean ± SD |
|---|---|---|---|
| Fm | Adult female mass (kg) | 24.00–105,000.00 | 5643.80 ± 17,641.19 |
| Bm | Birth mass (kg) | 0.60–7250.00 | 236.54 ± 880.65 |
| Gt | Gestation duration (m) | 7.00–17.00 | 10.50 ± 2.32 |
| rinG | Prenatal growth (kg/m) | 0.08–659.09 | 20.54 ± 79.49 |
| Ii | Interbirth interval (m) | 12.00–78.00 | 25.83 ± 15.25 |
| DoL | Duration of Life (y) | 15.00–211.00 | 41.87 ± 27.41 |
| Variable | Cluster X1 | Cluster X2 | Cluster Y1 | Cluster Y2 | p |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| logFm | 2.01 ± 0.35 a | 2.03 ± 0.39 a | 2.78 ± 0.35 b | 4.26 ± 0.50 b | *** |
| logBm | 0.96 ± 0.36 a | 1.09 ± 0.41 a | 1.65 ± 0.37 b | 2.88 ± 0.40 b | *** |
| logGt | 1.02 ± 0.06 a | 0.91 ± 0.04 b | 1.13 ± 0.05 c | 1.07 ± 0.07 ac | *** |
| logrinG | −0.07 ± 0.33 a | 0.17 ± 0.40 ab | 0.52 ± 0.34 bc | 1.80 ± 0.40 c | *** |
| logIi | 1.40 ± 0.14 a | 1.09 ± 0.05 b | 1.65 ± 0.17 a | 1.48 ± 0.14 a | *** |
| logDoL | 1.43 ± 0.13 a | 1.46 ± 0.16 a | 1.74 ± 0.12 b | 1.85 ± 0.17 b | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Liu, M.; Turvey, S.T.; Lin, M.; Li, S. Life History Parameters to Inform Pattern of Prenatal Investment in Marine Mammals. J. Mar. Sci. Eng. 2023, 11, 2086. https://doi.org/10.3390/jmse11112086
Huang X, Liu M, Turvey ST, Lin M, Li S. Life History Parameters to Inform Pattern of Prenatal Investment in Marine Mammals. Journal of Marine Science and Engineering. 2023; 11(11):2086. https://doi.org/10.3390/jmse11112086
Chicago/Turabian StyleHuang, Xiaoyu, Mingming Liu, Samuel T. Turvey, Mingli Lin, and Songhai Li. 2023. "Life History Parameters to Inform Pattern of Prenatal Investment in Marine Mammals" Journal of Marine Science and Engineering 11, no. 11: 2086. https://doi.org/10.3390/jmse11112086
APA StyleHuang, X., Liu, M., Turvey, S. T., Lin, M., & Li, S. (2023). Life History Parameters to Inform Pattern of Prenatal Investment in Marine Mammals. Journal of Marine Science and Engineering, 11(11), 2086. https://doi.org/10.3390/jmse11112086








