Assessing the Relationship between Sea Turtle Strandings and Anthropogenic Impacts in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
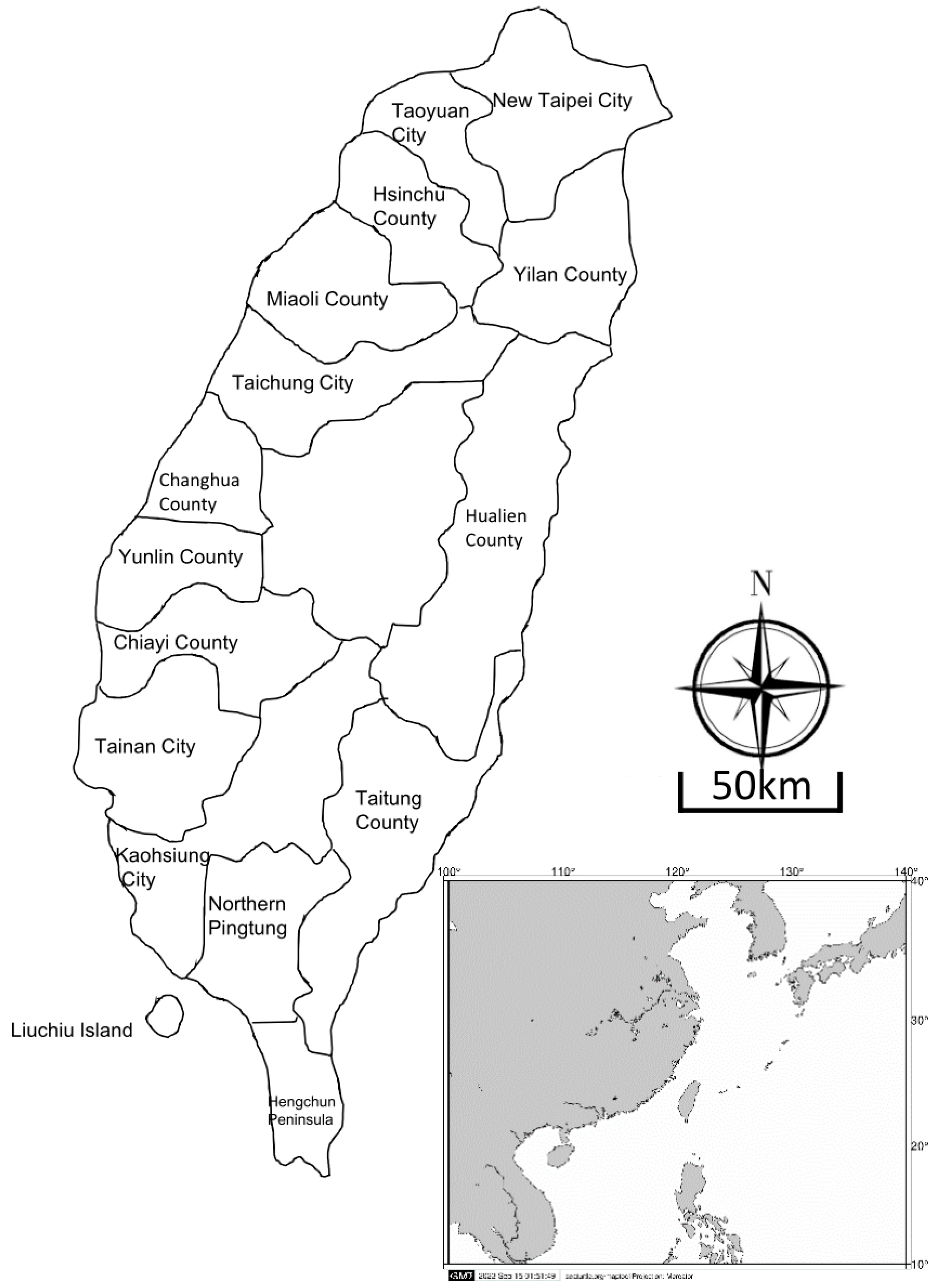
2.2. Study Area
2.3. Data Analysis
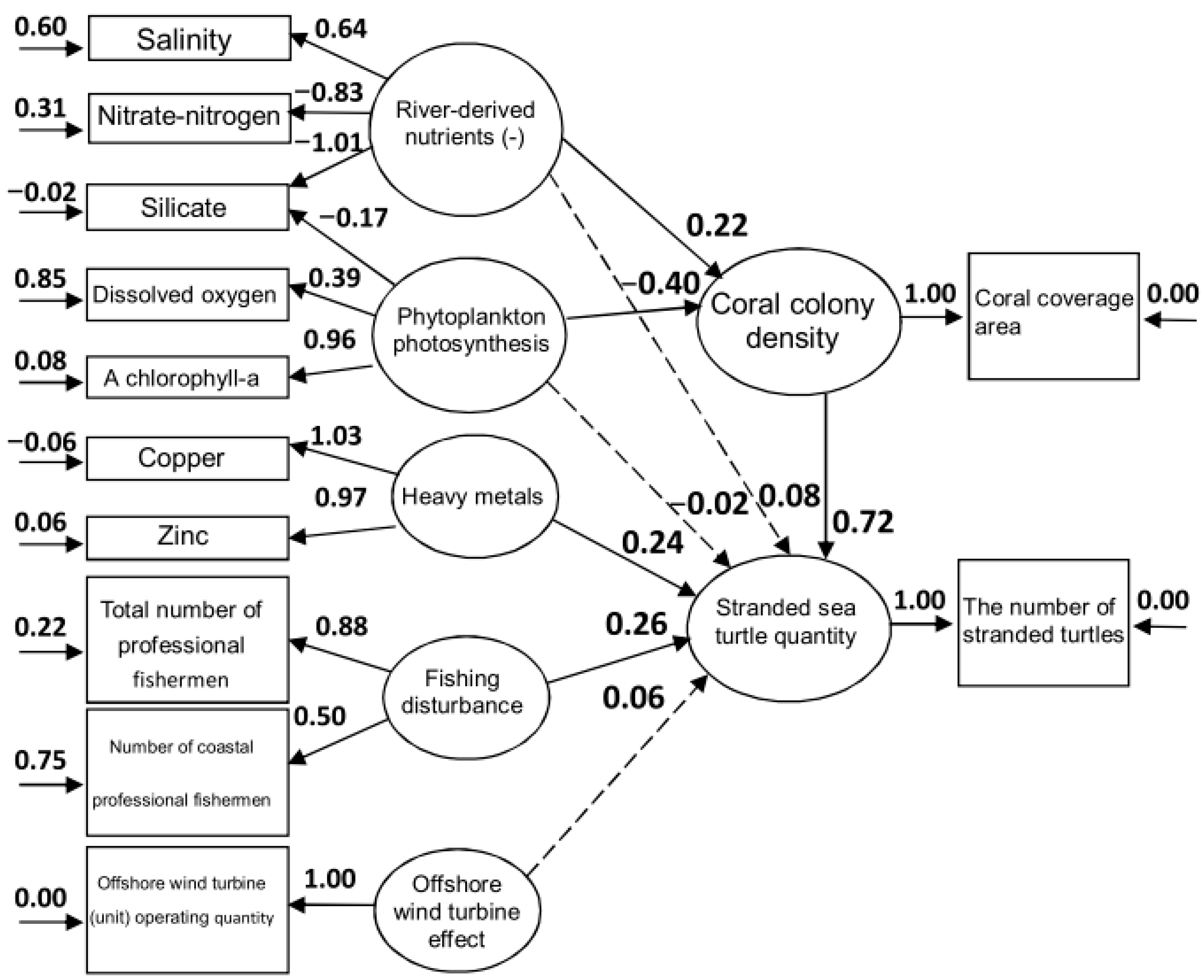
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguirre, A.A.; Lutz, P.L. Marine turtles as sentinels of ecosystem health: Is fibropapillomatosis an indicator? EcoHealth 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Tsai, M.A.; Chang, C.C.; Li, T.H. Antimicrobial-resistance profiles of gram-negative bacteria isolated from green turtles (Chelonia mydas) in Taiwan. Environ. Pollut. 2021, 277, 116870. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.W.; Fan, T.Y.; Ng, C.K.Y.; Cai, Y.; Balazs, G.H.; Li, T.H. Tale of the unlucky tags: The story of a rescued, rehabilitated, and released green sea turtle (Chelonia mydas) in southern Taiwan. Bull. Mar. Sci. 2017, 93, 689–690. [Google Scholar] [CrossRef]
- Hamann, M.; Godfrey, M.H.; Seminoff, J.A.; Arthur, K.; Barata, P.C.R.; Bjorndal, K.A.; Bolten, A.B.; Broderick, A.C.; Campbell, L.M.; Carreras, C.; et al. Global research priorities for sea turtles: Informing management and conservation in the 21st century. Endanger. Species Res. 2010, 11, 245–269. [Google Scholar] [CrossRef]
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of fibropapillomatosis in green turtles (Chelonia mydas). Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef]
- Li, T.H.; Hsu, W.L.; Lan, Y.C.; Balazs, G.H.; Work, T.M.; Tseng, C.T.; Chang, C.C. Identification of Chelonid herpesvirus 5 (ChHV5) in endangered green turtles (Chelonia mydas) with fibropapillomatosis in Asia. Bull. Mar. Sci. 2017, 93, 1011–1022. [Google Scholar] [CrossRef]
- Perrault, J.R.; Stacy, N.I.; Lehner, A.F.; Mott, C.R.; Hirsch, S.; Gorham, J.C.; Buchweitz, J.P.; Bresette, M.J.; Walsh, C.J. Potential effects of brevetoxins and toxic elements on various health variables in Kemp’s ridley (Lepidochelys kempii) and green (Chelonia mydas) sea turtles after a red tide bloom event. Sci. Total Environ. 2017, 605–606, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.A.; Stockin, K.A. Anthropogenic impacts on green turtles Chelonia mydas in New Zealand. Endanger. Species Res. 2018, 37, 1–9. [Google Scholar] [CrossRef]
- Ng, C.K.Y.; Lam, J.C.W.; Zhang, X.H.; Gu, H.X.; Li, T.H.; Ye, M.B.; Xia, Z.R.; Zhang, F.Y.; Duan, J.X.; Wang, W.X.; et al. Levels of trace elements, methylmercury and polybrominated diphenyl ethers in foraging green turtles in the South China region and their conservation implications. Environ. Pollut. 2018, 234, 735–742. [Google Scholar] [CrossRef]
- Li, T.H.; Cai, Y.R.; Wu, P.Y.; Ng, C.K.Y.; Balazs, G.H. Lesson to learn from an endangered green turtle (Chelonia mydas): Marine debris ingestion, rehabilitation and satellite tracking. Indian J. Anim. Res. 2020, 1, 5. [Google Scholar] [CrossRef]
- Manes, C.; Herren, R.M.; Page, A.; Dunlap, F.D.; Skibicki, C.A.; Rollinson Ramia, D.R.; Farrell, J.A.; Capua, I.; Carthy, R.R.; Duffy, D.J. Green Turtle Fibropapillomatosis: Tumor Morphology and Growth Rate in a Rehabilitation Setting. Vet. Sci. 2023, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Yetsko, K.; Farrell, J.A.; Blackburn, N.B.; Whitmore, L.; Stammnitz, M.R.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Krstic, A.; et al. Molecular characterization of a marine turtle tumour epizootic, profiling external, internal and postsurgical regrowth tumours. Commun. Biol. 2021, 4, 152. [Google Scholar] [CrossRef]
- Lloret, J.; Turiel, A.; Solé, J.; Berdalet, E.; Sabatés, A.; Olivares, A.; Gili, J.M.; Vila-Subirós, J.; Sardá, R. Unravelling the ecological impacts of large-scale Offshore wind farms in the Mediterranean Sea. Sci. Total Environ. 2022, 824, 153803. [Google Scholar] [CrossRef] [PubMed]
- EEA. Europe’s Onshore and offshore Wind Energy Potential: An Assessment of Environmental and Economic Constraints; EEA Technical Report; No 6; EEA: Copenhagen, Denmark, 2009. [Google Scholar]
- Dannheim, J.; Bergström, L.; Birchenough, S.N.; Brzana, R.; Boon, A.R.; Coolen, J.W.; Dauvin, J.C.; De Mesel, I.; Derweduwen, J.; Gill, A.B.; et al. Benthic effects of offshore renewables: Identification of knowledge gaps and urgently needed research. ICES J. Mar. Sci. 2020, 77, 1092–1108. [Google Scholar] [CrossRef]
- Bennun, L.; van Bochove, J.; Ng, C.; Fletcher, C.; Wilson, D.; Phair, N.; Carbone, G. Mitigating Biodiversity Impacts Associated with Solar and Wind Energy Development: Guidelines for Project Developers; The Biodiversity Consultancy: Gland, Switzerland; IUCN: Cambridge, UK, 2021. [Google Scholar]
- ICES. Workshop on socio-economic implications of offshore wind on fishing communities (WKSEIOWFC). ICES Sci. Rep. 2021, 3, 33. [Google Scholar]
- Foley, A.M.; Stacy, B.A.; Hardy, R.F.; Shea, C.P.; Minch, K.E.; Schroeder, B.A. Characterizing watercraft-related mortality of sea turtles in Florida. J. Wildl. Manag. 2019, 83, 1057–1072. [Google Scholar] [CrossRef]
- Lavender, A.L.; Bartol, S.M.; Bartol, I.K. A two-method approach for investigating the hearing capabilities of loggerhead sea turtles (Caretta caretta). In Proceedings of the 31st Annual Symposium on Sea Turtle Biology and Conservation, San Diego, CA, USA, 10–16 April 2011. [Google Scholar]
- Nelms, S.E.; Piniak, W.E.D.; Weir, C.R.; Godley, B.J. Seismic surveys and marine turtles: An underestimated global threat? Biol. Conserv. 2016, 193, 49–65. [Google Scholar] [CrossRef]
- Irwin, W.P.; Lohmann, K.J. Disruption of magnetic orientation in hatchling loggerhead sea turtles by pulsed magnetic fields. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 475–480. [Google Scholar] [CrossRef]
- Lohmann, K.J.P.; Luschi, P.; Hays, G.C. Goal navigation and island-finding in sea turtles. J. Exp. Mar. Biol. Ecol. 2008, 356, 83–95. [Google Scholar] [CrossRef]
- Renaud, M.L.; Carpenter, J.A. Movements and submergence patterns of loggerhead turtles (Caretta caretta) in the Gulf of Mexico determined through satellite telemetry. Bull. Mar. Sci. 1994, 55, 1–15. [Google Scholar]
- Hochscheid, S.; Bentivegna, F.; Hamza, A.; Hays, G.C. When surfacers do not dive: Multiple significance of extended surface times in marine turtles. J. Exp. Biol. 2010, 213, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Iverson, A.R.; Fujisaki, I.; Lamont, M.M.; Hart, K.M. Loggerhead sea turtle (Caretta caretta) diving changes with productivity, behavioral mode, and sea surface temperature. PLoS ONE 2019, 14, e0220372. [Google Scholar] [CrossRef] [PubMed]
- Clukey, K.E.; Lepczyk, C.A.; Balazs, G.H.; Work, T.M.; Lynch, J.M. Investigation of plastic debris ingestion by four species of sea turtles collected as bycatch in pelagic Pacific longline fisheries. Mar. Pollut. Bull. 2017, 120, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.Y.; Gu, H.X.; Li, T.H.; Ye, M.B.; Xia, Z.R.; Zhang, F.Y.; Duan, J.X.; Hsu, C.K.; Balazs, G.H.; Murphy, M.B. Insights into identifying habitat hot spots and migratory corridors of green turtles in the South China region. Aquatic. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1181–1191. [Google Scholar] [CrossRef]
- Shigenaka, G. Oils and Sea Turtle: Biology, Planning, and Response; Office of Response and Restoration, NOAA Ocean Service: Washington, DC, USA, 2010.
- Li, T.H.; Wu, P.Y.; Chen, I.C. Lethal Lesions Caused by Propeller Cuts on the Endangered Green Turtle Chelonia mydas on Liuchiu Island, Taiwan. Indian J. Anim. 2022, 1, 3. [Google Scholar] [CrossRef]
- Tomás, J.; Gozalbes, P.; Raga, J.A.; Godley, B.J. Bycatch of loggerhead sea turtles: Insights from 14 years of stranding data. Endanger. Species Res. 2008, 5, 161–169. [Google Scholar] [CrossRef]
- Sönmez, B. Sixteen year (2002–2017) record of sea turtle strandings on Samandağ Beach, the eastern Mediterranean coast of Turkey. Zool. Stud. 2018, 57, 53. [Google Scholar]
- Guimarães, S.M.; de Almeida, L.G.; Nunes, L.A.; Lacerda, P.D.; de Amorim, C.E.S.; Burato, M.; Baldassin, P.; Werneck, M.R. Distribution and potential causes of sea turtles stranding in the state of Rio de Janeiro, southern Brazil. Herpetol. Conserv. Biol. 2021, 16, 225–237. [Google Scholar]
- Work, T.M.; Balazs, G.H.; Summers, T.M.; Hapdei, J.R.; Tagarino, A.P. Causes of mortality in green turtles from Hawaii and the insular Pacific exclusive of fibropapillomatosis. Dis. Aquat. Organ. 2015, 115, 103–110. [Google Scholar] [CrossRef]
- Cheng, I.J.; Wang, H.Y.; Hsieh, W.Y.; Chan, Y.T. Twenty-three years of sea turtle stranding/bycatch research in Taiwan. Zool. Stud. 2019, 58, 44. [Google Scholar]
- Chen, Z.L. 108 Annual Coral Reef Ecosystem Survey Project; Ocean Conservation Division, Ocean Affairs Council: Kaohsiung City, Taiwan, 2019.
- Chou, W.R.; Hsieh, H.Y.; Hong, G.K.; Ko, F.C.; Meng, P.J.; Tew, K.S. Verification of an Environmental Impact Assessment Using a Multivariate Statistical Model. J. Mar. Sci. Eng. 2022, 10, 1023. [Google Scholar] [CrossRef]
- Burstyn, I. Principal component analysis is a powerful instrument in occupational hygiene inquiries. Ann. Occup. Hyg. 2004, 48, 655–661. [Google Scholar] [PubMed]
- Chou, W.R.; Fang, L.S.; Wang, W.H.; Tew, K.S. Environmental influence on coastal phytoplankton and zooplankton diversity: A multivariate statistical model analysis. Environ. Monit. Assess. 2012, 184, 5679–5688. [Google Scholar] [CrossRef]
- Malaeb, Z.A.; Summers, J.K.; Pugesek, B.H. Using structural equation modeling to investigate relationships among ecological variables, Environ. Ecol. Stat. 2000, 7, 93–111. [Google Scholar] [CrossRef]
- Shipley, B. Cause and Correlation in Biology: A User’s Guide to Path Analysis, Structural Equations and Causal Inference; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Fan, Y.; Chen, J.; Shirkey, G.; John, R.; Wu, S.R.; Park, H.; Shao, C. Applications of structural equation modeling (SEM) in ecological studies: An updated review. Ecol. Process. 2016, 5, 19. [Google Scholar] [CrossRef]
- Bjorndal, K.A. Foraging ecology and nutrition of sea turtles. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; p. 34. [Google Scholar]
- Li, T.H.; Chang, C.C. The impact of fibropapillomatosis on clinical characteristics, blood gas, plasma biochemistry, and hematological profiles in juvenile green turtles (Chelonia mydas). Bull. Mar. Sci. 2020, 96, 723–734. [Google Scholar] [CrossRef]
- Ng, C.K.Y.; Matsuzawa, Y. Sea Turtles in the East Asia Region; MTSG Annual Regional Report; The Marine Turtle Specialist Group: Ross, CA, USA, 2020. [Google Scholar]
- Chen, H.; Kuo, R.J.; Chang, T.C.; Hus, C.K.; Bray, R.A.; Cheng, I.J. Fluke (Spirorchiidae) infections in sea turtles stranded on Taiwan: Prevalence and pathology. J. Parasitol. 2012, 98, 437–439. [Google Scholar] [CrossRef]
- Liu, T.K.; Chen, P.; and Chen, H.Y. Comprehensive assessment of coastal eutrophication in Taiwan and its implications for management strategy. Bull. Mar. Sci. 2015, 97, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Van Houtan, K.S.; Hargrove, S.K.; Balazs, G.H. Land use, macroalgae, and a tumour-forming disease in marine turtles. PLoS ONE 2010, 5, e12900. [Google Scholar] [CrossRef]
- Van Houtan, K.S.; Smith, C.M.; Dailer, M.L.; Kawachi, M. Eutrophication and the dietary promotion of sea turtle tumours. PeerJ. 2014, 2, e602. [Google Scholar] [CrossRef] [PubMed]
- Dujon, A.M.; Schofield, G.; Venegas, R.M.; Thomas, F.; Ujvari, B. Turtles in the cancer risk landscape: A global meta-analysis of fibropapillomatosis prevalence and associated risk factors. Sea Pathogens. 2021, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Lei, I.I.; Byadgi, O.V.; Chen, I.C.; Tsai, M.A. Evidence of chelonid herpesvirus 5 infection in green turtle (Chelonia mydas) indicated a possible tumorigenesis activation by transcriptome analysis. Front. Mar. Sci. 2023, 10, 1185111. [Google Scholar] [CrossRef]
- Thayer, G.W.; Bjorndal, K.A.; Ogden, J.C.; Williams, S.L.; Zieman, J.C. Role of larger herbivores in seagrass communities. Estuaries 1984, 7, 351–376. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Hoey, A.S.; Bellwood, D.R. The Role of Turtles as Coral Reef Macroherbivores. PLoS ONE 2012, 7, e39979. [Google Scholar] [CrossRef]
- Day, R.D.; Segars, A.L.; Arendt, M.D.; Lee, A.M.; Peden-Adams, M.M. Relationship of blood mercury levels to health parameters in the loggerhead sea turtle (Caretta caretta). Environ. Health Perspect. 2007, 115, 1421–1428. [Google Scholar] [CrossRef]
- Day, R.D.; Keller, J.M.; Harms, C.A.; Segars, A.L.; Cluse, W.M.; Godfrey, M.H.; Lee, A.M.; Peden-Adams, M.; Thorvalson, K.; Dodd, M.; et al. Comparison of mercury burdens in chronically debilitated and healthy loggerhead sea turtles (Caretta caretta). J. Wildl. Dis. 2010, 46, 111–117. [Google Scholar] [CrossRef]
- Camacho, M.; Orós, J.; Boada, L.D.; Zaccaroni, A.; Silvi, M.; Formigaro, C.; López, P.; Zumbado, M.; Luzardo, O.P. Potential adverse effects of inorganic pollutants on clinical parameters of loggerhead sea turtles (Caretta caretta): Results from a nesting colony from Cape Verde, West Africa. Mar. Environ. Res. 2013, 92, 15–22. [Google Scholar] [CrossRef]
- Rees, A.F.; Alfaro-Shigueto, J.; Barata, P.C.R.; Bjorndal, K.A.; Bolten, A.B.; Bourjea, J.; Broderick, A.C.; Campbell, L.M.; Cardona, L.; Carreras, C.; et al. Are we working towards global research priorities for management and conservation of sea turtles? Endanger. Species Res. 2016, 31, 337–382. [Google Scholar] [CrossRef]
- Formia, A.; Deem, S.; Billes, A.; Ngouessono, S.; Parnell, R.; Collins, T.; Sounguet, G.-P.; Gibudi, A.; Villarubia, V.A.; Balazs, G.H.; et al. Fibropapillomatosis confirmed in Chelonia mydas in the gulf of Guinea, West Africa. Mar. Turtle Newslett. 2007, 116, 20. [Google Scholar]
- Manes, C.; Pinton, D.; Canestrelli, A.; Capua, I. Occurrence of fibropapillomatosis in green turtles (Chelonia mydas) in relation to environmental changes in coastal ecosystems in Texas and Florida: A retrospective study. Animals 2022, 12, 1236. [Google Scholar] [CrossRef]
- Klimley, A.P.; Putman, N.F.; Keller, B.A.; Noakes, D. A call to assess the impacts of electromagnetic fields from subsea cables on the movement ecology of marine migrants. Conserv. Sci. Pract. 2021, 3, e436. [Google Scholar] [CrossRef]
- Yalçin-Özdilek, S.; Yalçin, S. Wind Energy Plants and Possible Effects on Samandag Sea Turtles. Mar. Turtle Newsletter. 2012, 133, 7–9. [Google Scholar]
- Catry, P.; Senhoury, C.; Sidina, E.; Bar, N.E.; Bilal, A.S.; Ventura, E.; Godley, B.J.; Pires, A.J.; Regalla, A.; Patricio, A.R. Satellite tracking and field assessment highlight major foraging site for green turtles in the Banc d’Arguin, Mauritania. Biol. Conserv. 2023, 277, 109823. [Google Scholar] [CrossRef]
- Farrell, J.A.; Whitmore, L.; Mashkour, N.; Rollinson Ramia, D.R.; Thomas, R.S.; Eastman, C.B.; Burkhalter, B.; Yetsko, K.; Mott, C.; Wood, L.; et al. Detection and population genomics of sea turtle species via noninvasive environmental DNA analysis of nesting beach sand tracks and oceanic water. Mol. Ecol. Resour. 2022, 22, 2471–2493. [Google Scholar] [CrossRef]
| Species | n | % | Curved Carapace Length (cm) | Oceanic Juvenile (%) | ||
|---|---|---|---|---|---|---|
| Range | Mean | SD | ||||
| Green turtle | 692 | 85.4 | 6.50–130.00 | 53.95 | 17.21 | 5 (0.7%) |
| Hawksbill turtle | 48 | 5.9 | 12.00–90.00 | 43.50 | 15.47 | 6 (12.5%) |
| Olive ridley turtle | 37 | 4.5 | 22.00–124.50 | 58.59 | 18.17 | 1 (2.7%) |
| Loggerhead turtle | 30 | 3.7 | 11.50–120.00 | 78.86 | 21.62 | 2 (6.6%) |
| Leatherback turtle | 3 | 0.3 | 140.00–150.00 | 144.50 | 5.07 | - |
| Total | 810 | 100 | 14 (1.7%) | |||
| Life Stage | n | % |
|---|---|---|
| Juvenile (CCL< 67 cm) | 567 | 81.9 |
| Sub-adult (CCL 67–84 cm) | 70 | 10.1 |
| Adult (CCL > 84 cm) | 55 | 7.9 |
| Total | 692 | 100 |
| Variable | Range | Mean | SD |
|---|---|---|---|
| Temperature (°C) | 15.8 (16.8–32.6) | 26.193 | 3.9008 |
| Salinity (psu) | 11.6 (23.4–35.0) | 32.956 | 1.9412 |
| Dissolved oxygen (mg/L) | 4.4 (4.5–8.9) | 6.631 | 0.7615 |
| Dissolved oxygen saturation (%) | 87.2 (54.2–141.4) | 95.927 | 12.2051 |
| A chlorophyll-a (μg/L) | 22.4 (0.1–22.4) | 1.389 | 2.4330 |
| Ammonium (mg/L) | 0.46 (0.01–0.46) | 0.0642 | 0.08872 |
| Nitrate-nitrogen (mg/L) | 0.415 (0.005–0.420) | 0.07183 | 0.079210 |
| Phosphate (mg/L) | 0.17 (0.00–0.18) | 0.0318 | 0.03600 |
| Nitrite-nitrogen (mg/L) | 0.130 (0.001–0.130) | 0.01262 | 0.021747 |
| Silicate (mg/L) | 4.0010 (0.0090–4.0100) | 0.425836 | 0.6160038 |
| Copper (mg/L) | 0.0349 (0.0001–0.0350) | 0.001175 | 0.0035777 |
| Zinc (mg/L) | 0.0429 (0.0001–0.0430) | 0.005887 | 0.0054381 |
| Lead (mg/L) | 0.00195 (0.00005–0.00200) | 0.0001832 | 0.00031616 |
| Variables | Two-Way ANOVA | Multiple Comparison * | |
|---|---|---|---|
| F | p-Value | ||
| Station | 42.65 | <0.001 | A1aA12bA14bA15bA16bA13bcA2cdA3cdA4cdA9cdA10cdA11cdA5dA6dA7dA8d |
| Season | 16.37 | <0.001 | S1aS2bS3bS4b |
| Station × Season ** | 5.712 | <0.001 | S1 A1aA12bA14bA15bA16bA2cA3cA4cA5cA6cA7cA8cA9cA10cA11cA13c |
| S2 A1aA14aA15aA16aA2abA3abA4abA7abA8abA9abA10abA11ab A12abA13abA5bA6b | |||
| S3 A1aA12bA2bcA3bcA4bcA5bcA6bcA9bcA10bcA11bcA13bcA14bcA15bcA16bcA7cA8c | |||
| S4 A1aA14bA15bA16bA9bcA10bcA11bcA12bcA13bcA2cA3cA4cA5cA6cA7cA8c | |||
| Area | The Number of Professional Fishermen | The Number of Coastal Professional Fishermen |
|---|---|---|
| A1 | 41,909 | 1225 |
| A16 | 16,135 | 3254 |
| A15 | 3020 | 1216 |
| A14 | 3555 | 914 |
| A12 | 4597 | 2045 |
| A13 | 7355 | 1789 |
| A11 | 18,693 | 4686 |
| A10 | 48,540 | 15,221 |
| A9 | 17,646 | 1991 |
| A8 | 7442 | 684 |
| A7 | 18,889 | 2162 |
| A6 | 6989 | 453 |
| A5 | 4304 | 0 |
| A4 | 8926 | 3613 |
| A3 | 1902 | 1535 |
| A2 | 623 | 623 |
| Area | % |
|---|---|
| A1 | 27.56 |
| A16 | 23.02 |
| A15 | 26.88 |
| A14 | 38.21 |
| A12 | 44.69 |
| A13 | 15.70 |
| A11 | 0 |
| A10 | 0 |
| A9 | 0 |
| A8 | 0 |
| A7 | 0 |
| A6 | 0 |
| A5 | 0 |
| A4 | 0 |
| A3 | 0 |
| A2 | 0 |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Temperature | −0.10 | −0.12 | 0.00 | −0.87 |
| Salinity | −0.71 | 0.03 | 0.02 | 0.51 |
| Dissolved oxygen | −0.04 | 0.83 | 0.02 | 0.39 |
| Dissolved oxygen saturation | −0.20 | 0.89 | 0.03 | −0.00 |
| A chlorophyll-a | 0.13 | 0.71 | −0.07 | −0.07 |
| Ammonium | 0.51 | 0.05 | −0.06 | 0.04 |
| Nitrate-nitrogen | 0.89 | −0.08 | 0.02 | 0.19 |
| Phosphate | 0.58 | −0.01 | −0.06 | 0.52 |
| Nitrite-nitrogen | 0.69 | 0.08 | 0.06 | −0.02 |
| Silicate | 0.87 | −0.21 | 0.00 | 0.07 |
| Copper | −0.08 | −0.04 | 0.89 | 0.03 |
| Zinc | −0.04 | −0.05 | 0.77 | −0.23 |
| Lead | 0.10 | 0.05 | 0.80 | 0.16 |
| Eigenvalues | 3.22 | 2.06 | 2.04 | 1.57 |
| Total variance (%) | 20.73 | 40.60 | 56.32 | 68.42 |
| (A) PC1 | |||
| Variables | Two-Way ANOVA | Multiple Comparison* | |
| F | p-Value | ||
| Station | 6.562 | <0.001 | A11a A7ab A8ab A5bc A6bc A13bc A2c A3c A4c A1cd A9cd A10cd A12cd A14d A15d A16d |
| Season | 2.491 | 0.065 | |
| Station × Season ** | 1.383 | 0.136 | |
| (B) PC2 | |||
| Variables | Two-way ANOVA | Multiple Comparison * | |
| F | p-value | ||
| Station | 2.208 | 0.033 | A11a A13ab A2abc A3abc A4abc A5abc A6abc A7abc A8abc A9abc A10abc A14bc A15bc A16bc A12c |
| Season | 2.352 | 0.077 | |
| Station × Season ** | 0.9 | 0.601 | |
| (C) PC3 | |||
| Variables | Two-way ANOVA | Multiple Comparison | |
| F | p-value | ||
| Station | 0.631 | 0.75 | |
| Season | 0.773 | 0.512 | |
| Station × Season ** | 0.451 | 0.986 | |
| (D) PC4 | |||
| Variables | Two-way ANOVA | Multiple Comparison * | |
| F | p-value | ||
| Station | 7.062 | <0.001 | S4aS1bS3bS2c |
| Season | 65.28 | <0.001 | A1aA5aA6aA2abA3abA4abA14bcA15bcA16bcA12bcd A9cdA10cdA13cdA7dA8dA11d |
| Station × Season ** | 1.917 | 0.014 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, W.-R.; Wu, P.-Y.; Li, T.-H. Assessing the Relationship between Sea Turtle Strandings and Anthropogenic Impacts in Taiwan. J. Mar. Sci. Eng. 2023, 11, 1962. https://doi.org/10.3390/jmse11101962
Chou W-R, Wu P-Y, Li T-H. Assessing the Relationship between Sea Turtle Strandings and Anthropogenic Impacts in Taiwan. Journal of Marine Science and Engineering. 2023; 11(10):1962. https://doi.org/10.3390/jmse11101962
Chicago/Turabian StyleChou, Wei-Rung, Po-Yu Wu, and Tsung-Hsien Li. 2023. "Assessing the Relationship between Sea Turtle Strandings and Anthropogenic Impacts in Taiwan" Journal of Marine Science and Engineering 11, no. 10: 1962. https://doi.org/10.3390/jmse11101962
APA StyleChou, W.-R., Wu, P.-Y., & Li, T.-H. (2023). Assessing the Relationship between Sea Turtle Strandings and Anthropogenic Impacts in Taiwan. Journal of Marine Science and Engineering, 11(10), 1962. https://doi.org/10.3390/jmse11101962





