Using Algal Indices to Assess the Ecological Condition of the Aras River, Northwestern Iran
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Selection of Sampling Stations
2.3. Laboratory Processes
2.4. Data Analysis and Interpretation
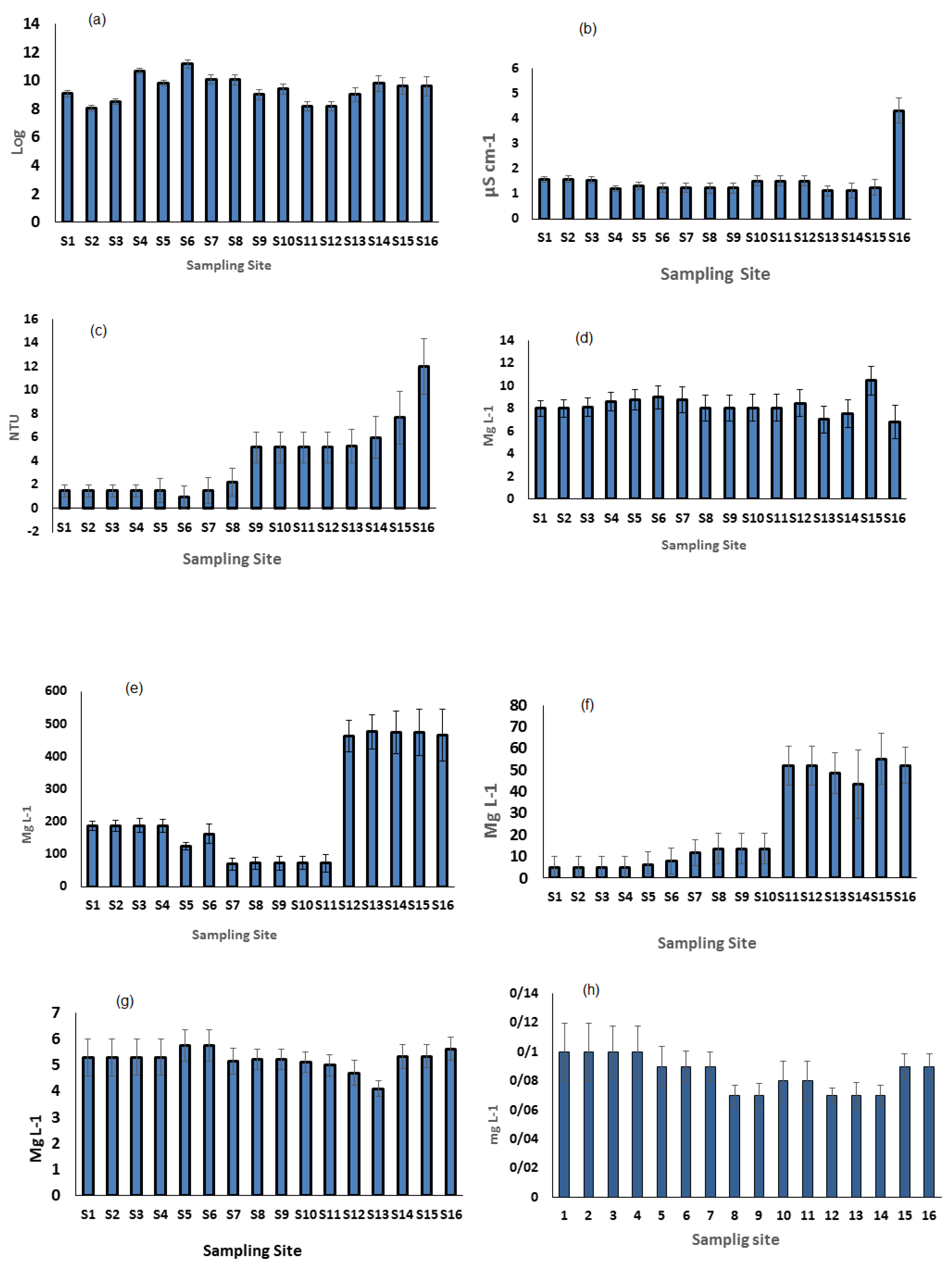
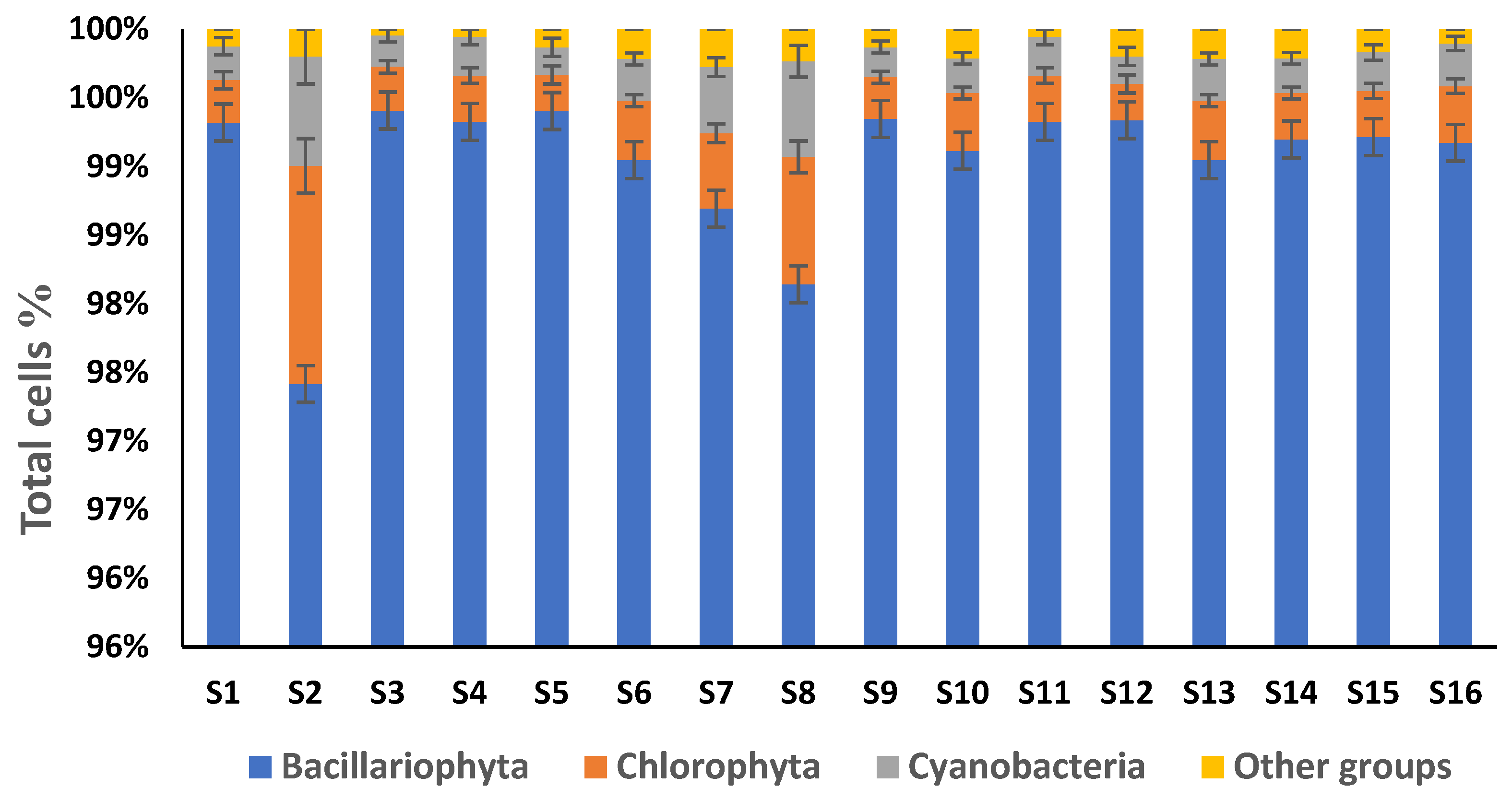
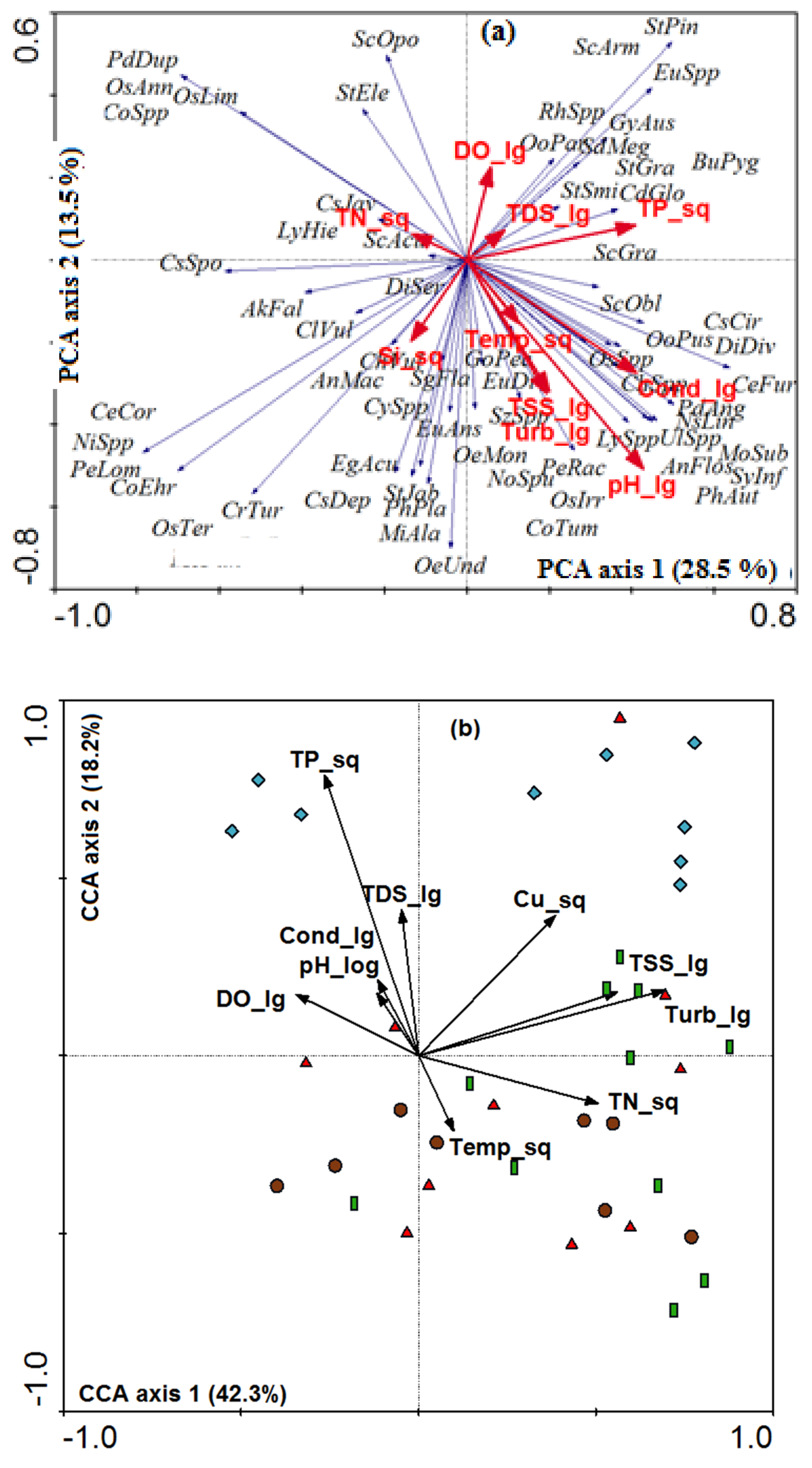
3. Results
Average Annual Water Chemistry in the Aras River
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atazadeh, E.; Gell, P.; Mills, K.; Newall, P. Community structure and ecological responses to hydrological changes in benthic algal assemblages in a regulated river: Application of algal metrics and multivariate techniques in river management. Environ. Sci. Pollut. Res. 2021, 28, 39805–39825. [Google Scholar] [CrossRef] [PubMed]
- Tornés, E.; Mor, J.-R.; Mandaric, L.; Sabater, S. Diatom responses to sewage inputs and hydrological alteration in Mediterranean streams. Environ. Pollut. 2018, 238, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M. Sewage Pollution of Ground Water. In Proceedings of the 35th Indian Engineering Congress, New Delhi, India, 18–20 December 2020; p. 4. [Google Scholar]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.; Malik, M.F.; Liaqat, S.; Aslam, A.; Mumtaz, K.; Ch, A.A.D.M.; Nisa, K.; Khurshid, F.; Arif, F.; Khalid, M.S.Z.; et al. Water pollution and industries. Pure Appl. Biol. 2020, 9, 2214–2224. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H. Water Pollution from Agriculture: A Global Review; FAO IWMI: Rome, Italy, 2017. [Google Scholar]
- Zhang, L.; Tan, X.; Chen, H.; Liu, Y.; Cui, Z. Effects of Agriculture and Animal Husbandry on Heavy Metal Contamination in the Aquatic Environment and Human Health in Huangshui River Basin. Water 2022, 14, 549. [Google Scholar] [CrossRef]
- Markiewicz, A.; Björklund, K.; Eriksson, E.; Kalmykova, Y.; Strömvall, A.M.; Siopi, A. Emissions of organic pollutants from traffic and roads: Priority pollutants selection and substance flow analysis. Sci. Total Environ. 2017, 580, 1162–1174. [Google Scholar] [CrossRef]
- Milla, O.V.; Huang, W.-J. Relationship between solid waste pollution and polluted drinking water in El Salvador. J. Int. Cooper 2012, 2, 37–60. [Google Scholar]
- Appannagari, D.R.R. Environmental pollution causes and consequences: A study. North Asian Int. Res. J. Soc. Sci. Humanit. 2017, 3, 151–161. [Google Scholar]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Haseena, M.; Malik, M.F.; Javed, A.P.; Arshad, S.; Asif, N.; Zulfiqar, S.; Hanif, J. Water pollution and human health. Environ. Risk Assess. Remediat. 2017, 1, 16–19. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of water pollution on human health and disease heterogeneity: A review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Falkenmark, M. The Massive water scarcity now threatening Africa: Why isn’t it being addressed? Ambio 1989, 18, 112–118. [Google Scholar]
- Falkenmark, M. Rapid Population Growth and Water Scarcity: The Predicament of Tomorrow’s Africa. Popul. Dev. Rev. 1990, 16, 81–94. [Google Scholar] [CrossRef]
- Falkenmark, M.; Widstrand, C. Population and water resources: A delicate balance. Popul. Bull. 1992, 47, 1–36. [Google Scholar] [PubMed]
- Falkenmark, M.; Kijne, J.W.; Taron, B.; Murdoch, G.; Sivakumar, M.V.K.; Craswell, E. Meeting Water Requirements of an Expanding World Population [and Discussion]. Philos. Trans. R. Soc. B 1997, 352, 929–936. [Google Scholar] [CrossRef]
- Clement, K. Regional policy and the environment—The case of Germany. Eur. Environ. 2001, 11, 103–111. [Google Scholar]
- Figuières, C.; Guyomard, H.; Rotillon, G. Sustainable development: Between moral injunctions and natural constraints. Sustainability 2010, 2, 3608–3622. [Google Scholar] [CrossRef]
- Molden, D.; Frenken, K.; Barker, R.; de Fraiture, C.; Mati, B.; Svendsen, M.; Sadoff, C.; Finlayson, C.M. Trends in water and agricultural development. In Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Molden, D., Ed.; Earthscan: London, UK; International Water Management Institute: Colombo, Sri Lanka, 2007; pp. 57–89. [Google Scholar]
- de Fraiture, C.; Wichelns, D. Satisfying future water demands for agriculture. Agric. Water Managem. 2010, 97, 502–511. [Google Scholar] [CrossRef]
- Atazadeh, E.; Barton, A.; Shirinpour, M.; Zarghami, M.; Rajabifard, A. River management and environmental water allocation in regulated ecosystems of arid and semi-arid regions–a review. Fundam. Appl. Limnol. 2020, 193, 327–345. [Google Scholar] [CrossRef]
- Dixit, S.S.; Smol, J.P.; Kingston, J.C.; Charles, D.F. Diatoms: Powerful indicators of environmental change. Envimn. Si. Technol. 1992, 26, 22–33. [Google Scholar] [CrossRef]
- Rimet, F. Recent views on river pollution and diatoms. Hydrobiologia 2012, 683, 1–24. [Google Scholar] [CrossRef]
- Pan, Y.; Stevenson, R.J.; Hill, B.H.; Herlihy, A.T.; Collins, G.B. Using diatoms as indicators of ecological conditions in lotic systems: A regional assessment. J. N. Amer. Benthol. Soc. 1996, 15, 481–495. [Google Scholar] [CrossRef]
- Desianti, N.; Potapova, M.; Enache, M.; Belton, T.J.; Velinsky, D.J.; Thomas, R.; Mead, J. Sediment diatoms as environmental indicators in New Jersey coastal lagoons. J. Coast. Res. 2017, 78, 127–140. [Google Scholar] [CrossRef]
- Vilmi, A.; Karjalainen, S.M.; Landeiro, V.L.; Heino, J. Freshwater diatoms as environmental indicators: Evaluating the effects of eutrophication using species morphology and biological indices. Environ. Monit. Assess. 2015, 187, 243. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.J.; Vos, P. Learning from the past: Diatoms as palaeoecological indicators of changes in marine environments. Neth. J. Aquat. Ecol. 1992, 26, 19–30. [Google Scholar] [CrossRef]
- Wolin, J.A.; Stone, J.R. Diatoms as Indicators of Water-Level Change in Freshwater Lakes. In The Diatoms Applications to the Environmental and Earth Sciences; Stoermer, E.F., Smol, J.P., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 174–185. [Google Scholar]
- Battarbee, R.; Charles, D.F.; Bigler, C.; Cumming, B.F.; Renberg, I. Diatoms as indicators of surface-water acidity. In The Diatoms Applications for the Environmental and Earth Sciences; Smol, J.P., Stoermer, E.F., Eds.; Cambridge University: Cambridge, UK, 2010; pp. 98–121. [Google Scholar]
- Brundtland, G.H. To meet human needs. World Health 1986, 12, 2–3. [Google Scholar]
- Gleick, P.H. Basic water requirements for human activities: Meeting basic needs. Water Int. 1996, 21, 83–92. [Google Scholar] [CrossRef]
- Parker, H.; Oates, N.E.M. How Do Healthy Rivers Benefit Society? A Review of the Evidence; PreventionWeb.nEt. ODI and WWF: London, UK, 2016. [Google Scholar]
- Wada, Y.; Bierkens, M.F.P.; de Roo, A.; Dirmeyer, P.A.; Famiglietti, J.S.; Hanasaki, N.; Konar, M.; Liu, J.; Müller Schmied, H.; Oki, T.; et al. Human–water interface in hydrological modelling: Current status and future directions. Hydrol. Earth Syst. Sci. 2017, 21, 4169–4193. [Google Scholar] [CrossRef]
- Gimelli, F.M.; Rogers, B.C.; Bos, J.J. Linking water services and human well-being through the fundamental human needs framework: The case of India. Water Altern. 2019, 12, 715–733. [Google Scholar]
- Gurnell, A.M.; Scott, S.J.; England, J.; Gurnell, D.; Jeffries, R.; Shuker, L.; Wharton, G. Assessing river condition: A multiscale approach designed for operational application in the context of biodiversity net gain. River Res. Appl. 2020, 36, 1559–1578. [Google Scholar] [CrossRef]
- HaRa, J.; Mamun, M.; An, K.-G. Ecological River Health Assessments Using Chemical Parameter Model and the Index of Biological Integrity Model. Water 2019, 11, 1729. [Google Scholar] [CrossRef]
- Karr, J.R. Defining and measuring river health. Freshw. Biol. 1999, 41, 221–234. [Google Scholar] [CrossRef]
- Barinova, S. On the classification of water quality from an ecological point of view. Int. J. Environ. Sci. Nat. Res. 2017, 2, 555581. [Google Scholar] [CrossRef]
- Collins, G.B.; Weber, C.I. Phycoperiphyton (algae) as indicators of water quality. Trans. Am. Microsc. Soc. 1978, 97, 36–43. [Google Scholar] [CrossRef]
- Gökçe, D. Algae as an indicator of water quality. In Algae-Organisms for Imminent Biotechnology; Thajuddin, N., Dhanasekaran, D., Eds.; IntechOpen Limited: London, UK, 2016; pp. 498–501. [Google Scholar]
- Quiñones, J.D.; Mopan, A.M. Identification of algae as water quality bioindicators in the water intakes of two municipalities of the department of Quindío. South Fla. J. Dev. 2021, 2, 5568–5578. [Google Scholar] [CrossRef]
- Stevenson, J. Ecological assessments with algae: A review and synthesis. J. Phycol. 2014, 50, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Lehman, J.T.; Sandgren, C.D. Species-specific rates of growth and grazing loss among freshwater algae. Limnol. Oceanogr. 1985, 30, 34–46. [Google Scholar] [CrossRef]
- Burns, A.; Ryder, D.S. Potential for biofilms as biological indicators in Australian riverine systems. Ecol. Manag. Restor. 2001, 2, 53–64. [Google Scholar] [CrossRef]
- Ivorra, N.; Hettelaar, J.; Tubbing, G.M.J.; Kraak, M.H.S.; Sabater, S.; Admiraal, W. Translocation of microbenthic algal assemblages used for in situ analysis of metal pollution in rivers. Arch. Environ. Contam. Toxicol. 1999, 37, 19–28. [Google Scholar] [CrossRef]
- Wilhm, J.; Cooper, J.; Burks, S. Species composition of algae and benthic macroinvertebrates in the Blue and Kiamichi Rivers. Proc. Okla. Acad. Sci. 1979, 59, 85–88. [Google Scholar]
- Smol, J.P.; Cumming, B.F. Tracking long-term changes in climate using algal indicators in lake sediments. J. Phycol. 2000, 36, 986–1011. [Google Scholar] [CrossRef]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchay, A.; Poulain, J.; Wincker, P.; Lucicone, D.; de Vargas, C.; Bittner, L.; et al. Insights into global distribution and diversity in the worlds ocean. Proc. Natl. Acad. Sci. USA 2016, 113, E1516–E1525. [Google Scholar] [CrossRef] [PubMed]
- Mariacristina, T.; Antonio, D. Biology monitoring of some Apennine Rivers using the Diatom-Based Eutrophication/Pollution index compare to other European Diatom indices. Diatom Res. 2006, 21, 159–174. [Google Scholar]
- Kelly, M.; Juggins, S.; Guthrie, R.; Pritchard, S.; Jamieson, J.; Rippey, B.; Hirst, H.; Yallop, M. Assessment of ecological status in UK rivers using diatoms. Freshw. Biol. 2008, 53, 403–422. [Google Scholar]
- Keck, F.; Rimet, F.; Franc, A. Phylogenetic signal in diatom ecology: Perspectives for aquatic ecosystems biomonitoring. Ecol. Appl. 2016, 26, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Valentin, V.; Frédéric, R.; Isabelle, D.; Olivier, M.; Yorick, R.; Agnès, B. Assessing pollution of aquatic environments with diatoms’ DNA metabarcoding: Experience and developments from France Water Framework Directive networks. Metabarcoding Metagenomics 2019, 3, e39646. [Google Scholar] [CrossRef]
- Kelly, M.; King, L.; Chatháin, B.N. The conceptual basis of ecological status assessments using diatoms. Biol. Environ. 2009, 109, 175–189. [Google Scholar] [CrossRef]
- Stenger-Kovács, C.; Buczkó, K.; Hajnal, É.; Padisák, J. Epiphytic, littoral diatoms as bioindicators of shallow lake trophic status: Trophic Diatom Index for Lakes (TDIL) developed in Hungary. Hydrobiologia 2007, 589, 141–154. [Google Scholar] [CrossRef]
- Sen, M.; Shan, H. A review of electrochemical macro-to micro-hole drilling processes. Int. J. Mach. Tools Manuf. 2005, 45, 137–152. [Google Scholar] [CrossRef]
- Kock, A.; Taylor, J.C.; Malherbe, W. Diatom community structure and relationship with water quality in Lake Sibaya, KwaZulu-Natal, South Africa. S. Afr. J. Bot. 2019, 123, 161–169. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Yang, S. Assessment of Anthropogenic Impact versus Climate Change on the Succession of the Diatom Community in Lugu Lake (Yunnan-Guizhou Plateau, China) Using the Sedimentary Record of Geochemical Elements. Water 2019, 11, 655. [Google Scholar] [CrossRef]
- Thacker, M.; Karthick, B. Response of Diatoms to the Changing Water Quality in the Myristica Swamps of the Western Ghats, India. Diversity 2022, 14, 202. [Google Scholar] [CrossRef]
- Fontana, L.; Sun, M.; Huang, X.; Xiang, L. The impact of climate change and human activity on the ecological status of Bosten Lake, NW China, revealed by a diatom record for the last 2000 years. Holocene 2019, 29, 1871–1884. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Burford, M.A.; Zhang, Q. The performance of diatom indices in assessing temporal changes in water quality in a large lowland river ecosystem. River Res. Appl. 2021, 37, 423–432. [Google Scholar] [CrossRef]
- Pfister, L.; McDonnell, J.J.; Wrede, S.; Hlúbiková, D.; Matgen, P.; Fenicia, F.; Ector, L.; Hoffmann, L. The rivers are alive: On the potential for diatoms as a tracer of water source and hydrological connectivity. Hydrol. Process. 2009, 23, 2841–2845. [Google Scholar] [CrossRef]
- Anderson, N.J. Miniview: Diatoms, temperature and climatic change. Eur. J. Phycol. 2000, 35, 307–314. [Google Scholar] [CrossRef]
- Tilstone, G.H.; Míguez, B.M.; Figueiras, F.G.; Fermín, E.G. Diatom dynamics in a coastal ecosystem affected by upwelling: Coupling between species succession, circulation and biogeochemical processes. Mar. Ecol. Prog. Ser. 2000, 205, 23–41. [Google Scholar] [CrossRef]
- Klaus, J.; Wetzel, C.E.; Martínez-Carreras, N.; Ector, L.; Pfister, L. A tracer to bridge the scales: On the value of diatoms for tracing fast flow path connectivity from headwaters to meso-scale catchments. Hydrol. Process. 2015, 29, 5275–5289. [Google Scholar] [CrossRef]
- Benoiston, A.-S.; Ibarbalz, F.M.; Bittner, L.; Guidi, L.; Jahn, O.; Dutkiewicz, S.; Bowler, C. The evolution of diatoms and their biogeochemical functions. Philos. Trans. R. Soc. B 2017, 372, 20160397. [Google Scholar] [CrossRef]
- da Silva, C.F.M.; Torgan, L.C.; Schneck, F. Temperature and surface runoff affect the community of periphytic diatoms and have distinct effects on functional groups: Evidence of a mesocosms experiment. Hydrobiologia 2019, 839, 37–50. [Google Scholar] [CrossRef]
- Johannessen, S.C.; Macdonald, R.W.; Wright, C.A. Rain, runoff, and diatoms: The effects of the North Pacific 2014–2015 warm anomaly on particle flux in a Canadian West Coast Fjord. Estuaries Coasts 2019, 42, 1052–1065. [Google Scholar] [CrossRef]
- Zaferani, S.; Biester, H. Biogeochemical processes accounting for the natural mercury variations in the Southern Ocean diatom ooze sediments. Ocean Sci. 2020, 16, 729–741. [Google Scholar] [CrossRef]
- Lavoie, I.; Campeau, S.; Darchambeau, F.; Cabana, G.; Dillon, P.J. Are diatoms good integrators of temporal variability in stream water quality? Freshw. Biol. 2008, 53, 827–841. [Google Scholar] [CrossRef]
- Ajani, P.A.; Kahlke, T.; Siboni, N.; Carney, R.; Murray, S.A.; Seymour, J.R. The microbiome of the cosmopolitan diatom leptocylindrus reveals significant spatial and temporal variability. Front. Microbiol. 2018, 9, 2758. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.L.; Muotka, T.; Karjalainen, S.M.; Laamanen, T.; Aroviita, J. Excess of nitrogen reduces temporal variability of stream diatom assemblages. Sci. Total Environ. 2020, 713, 136630. [Google Scholar] [CrossRef] [PubMed]
- Propst, D.L.; Gido, K.B. Responses of native and nonnative fishes to natural flow regime mimicry in the San Juan River. Trans. Am. Fish. Soc. 2004, 133, 922–931. [Google Scholar] [CrossRef]
- Stevenson, J.R.; Novoveska, L.; Rising, C.M.; Wiley, M.J. Comparing responses of diatom species composition to natural and anthropogenic factors in streams of glaciated ecoregions. Nova Hedwig. 2009, 135, 1–13. [Google Scholar]
- Çelekli, A.; Lekesiz, Ö. Eco-assessment of West Mediterranean basin’s rivers (Turkey) using diatom metrics and multivariate approaches. Environ. Sci. Pollut. Res. Int. 2020, 27, 27796–27806. [Google Scholar] [CrossRef]
- Lohr, M.; Wilhelm, C. Xanthophyll synthesis in diatoms: Quantification of putative intermediates and comparison of pigment conversion kinetics with rate constants derived from a model. Planta 2001, 212, 382–391. [Google Scholar] [CrossRef]
- Wu, X.; Gao, G.; Giordano, M.; Gao, K. Growth and photosynthesis of a diatom grown under elevated CO2 in the presence of solar UV radiation. Fundam. Appl. Limnol. 2012, 180, 279–290. [Google Scholar] [CrossRef]
- Wu, Y.; Yue, F.; Xu, J.; Beardall, J. Differential photosynthetic responses of marine planktonic and benthic diatoms to ultraviolet radiation under various temperature regimes. Biogeosciences 2017, 14, 5029–5037. [Google Scholar] [CrossRef]
- Lepetit, B.; Campbell, D.A.; Lavaud, J.; Büchel, C.; Goss, R.; Bailleul, B. Photosynthetic light reactions in diatoms. II. The dynamic regulation of the various light reactions. In The Molecular Life of Diatoms; Falciatore, A., Mock, T., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Depauw, F.A.; Rogato, A.; d’Alcalá, M.R.; Falciatore, A. Exploring the molecular basis of responses to light in marine diatoms. J. Exp. Bot. 2012, 63, 1575–1591. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; Thoms, S.; Bracher, A.; Liu, Y.; Völker, C. Modeling photoprotection at global scale: The relative role of nonphotosynthetic pigments, physiological state, and species composition. Glob. Biogeochem. Cycles 2019, 33, 904–926. [Google Scholar] [CrossRef]
- Smerilli, A.; Balzano, S.; Maselli, M.; Blasio, M.; Orefice, I.; Galasso, C.; Sansone, C.; Brunet, C. Antioxidant and Photoprotection Networking in the Coastal Diatom Skeletonema marinoi. Antioxidants 2019, 8, 154. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic Pigments in Diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- Korhola, A.; Weckström, J.; Nyman, M. Predicting the long-term acidification trends in small subarctic lakes using diatoms. J. Appl. Ecol. 1999, 36, 1021–1034. [Google Scholar] [CrossRef]
- Murtaugh, J.J.; Bunch, R.L. Acidic components of sewage effluents and river water. J. Water Pollut. Control Fed. 1965, 37, 410–415. [Google Scholar]
- Mattson, M.D. Acid lakes and rivers. In Environmental Geology. Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar] [CrossRef]
- Cattaneo, A.; Couillard, Y.; Wunsam, S.; Courcelles, M. Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Québec, Canada). J. Paleolimnol. 2004, 32, 163–175. [Google Scholar] [CrossRef]
- Pandey, L.K.; Kumar, D.; Yadav, A.; Rai, J.; Gaur, J.P. Morphological abnormalities in periphytic diatoms as a tool for biomonitoring of heavy metal pollution in a river. J. Ecol. Indic. 2014, 36, 272–279. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; van Dame, H. Inferring pH from diatoms: A comparison of old and new calibration methods. Hydrobiologia 1989, 178, 209–223. [Google Scholar] [CrossRef]
- Weckström, J.; Korhola, A.; Blom, T. Diatoms as quantitative indicators of pH and water temperature in subarctic Fennoscandian lakes. Hydrobiologia 1997, 347, 171–184. [Google Scholar] [CrossRef]
- Hervé, V.; Derr, J.; Douady, S.; Quinet, M.; Moisan, L.; Lopez, P.J. Multiparametric analyses reveal the pH-dependence of silicon biomineralization in diatoms. PLoS ONE 2012, 7, e46722. [Google Scholar] [CrossRef] [PubMed]
- Taraldsvik, M.; Myklestad, S. The effect of ph on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom skeletonema costatum. Eur. J. Phycol. 2000, 35, 189–194. [Google Scholar] [CrossRef]
- Pedersen, M.F.; Hansen, P.J. Effects of high pH on a natural marine planktonic community. Mar. Ecol. Prog. Ser. 2003, 260, 19–31. [Google Scholar] [CrossRef]
- Scholz, B. Effects of varying pH on the growth and physiology of five marine microphytobenthic diatoms isolated from the Solthörn tidal flat (southern North Sea, Germany). Phycologia 2014, 53, 252–264. [Google Scholar] [CrossRef]
- Gao, K.; Campbell, D.A. Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: A review. Funct. Plant Biol. 2014, 41, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.V.; Rajashekhar, M. Effect of pH, salinity and temperature on the growth of six species of marine phytoplankton. J. Algal Biomass Util. 2014, 5, 55–59. [Google Scholar]
- Lethier, H. World Heritage Thematic Study for Central Asia; Priority Sites for World Heritage Nomination under Criteria (ix) and (x); IUCN and IUCN ECARO: Gland, Switzerland; Belgrade, Serbia, 2020. [Google Scholar]
- Bruning, J.; Chapp, A.; Kaurala, G.A.; Wang, R.; Techtmann, S.; Chen, Q.-H. Gut microbiota and short chain fatty acids: Influence on the autonomic nervous system. Neurosci. Bull. 2020, 36, 91–95. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Sournia, A. Phytoplankton Manual; Sournia, A., Ed.; UNESCO: Paris, France, 1978. [Google Scholar]
- Williams, O.J.; Beckett, R.E.; Maxwell, D.L. Marine phytoplankton preservation with Lugol’s: A comparison of solutions. J. Appl. Phycol. 2016, 28, 1705–1712. [Google Scholar] [CrossRef]
- Woelkerling, W.J.; Kowal, R.R.; Gough, S.B. Sedgwick-rafter cell counts: A procedural analysis. Hydrobiologia 1976, 48, 95–107. [Google Scholar] [CrossRef]
- Battarbee, R.W. Diatom analysis. In Handbook of Holocene Palaeoecology and Palaeohydrology; Huntley, B., Ed.; Wiley-Interscience: Chichester, UK, 1986; pp. 527–570. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil. Naviculaceae. In Süßwasserflora von Mitteleuropa, Band 2/1; Gustav Fischer Verlag: Stuttgart, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil. Bacillariaceae, Epithemiaceae, Surirellaceae. In Süßwasserflora von Mitteleuropa, Band 2/2; Gustav Fischer Verlag: Stuttgart, Germany, 1988; p. 610. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil. Centrales, Fragilariaceae, Eunotiaceae. In Süßwasserflora von Mitteleuropa, Band 2/3; Gustav Fischer Verlag: Stuttgart, Germany, 1991; p. 598. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil. Achnanthaceae, kritsche erganzungen zu Navicula (Lineolatae) und Gomphonema Gesamliteraturverzeichnis. In Süßwasserflora von Mitteleuropa, Band 2/4; Gustav Fischer Verlag: Stuttgart, Germany, 1991; p. 468. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. (Eds.) The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Allott, T.E.H.; Harriman, R.; Battarbee, R.W. Reversibility of lake acidification at the Round Loch of Glenhead, Galloway, Scotland. Environ. Pollut. 1992, 77, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Rosyada, S.; Soeprobowati, T.R. Digestion diatom using Battarbee and Ruhland methods for Pengilon Lake, Dieng, Wonosobo, Central Java, Indonesia. J. Phys. Conf. Ser. 2021, 1943, 012063. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Bothwell, M.L.; Lowe, R.L. Algal Ecology: Freshwater Benthic Ecosystem; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Suren, A.M.; Biggs, B.J.F.; Kilroy, C.; Bergey, L. Benthic community dynamics during summer low-flows in two rivers of contrasting enrichment 1. Periphyton. N. Z. J. Mar. Freshw. Res. 2003, 37, 53–70. [Google Scholar] [CrossRef]
- Lavoie, I.; Vincent, W.F.; Pienitz, R.; Painchaud, J. Benthic algae as bioindicators of agricultural pollution in the streams and rivers of southern Québec (Canada). Aquat. Ecosyst. Health Manag. 2004, 7, 43–58. [Google Scholar] [CrossRef]
- Nusch, E. Comparison of different methods for chlorophyll and phaeopigment determination. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1980, 14, 14–36. [Google Scholar]
- Snow, G.C.; Bate, G.C.; Adams, J.B. The effects of a single freshwater release into the Kromme estuary. Part 2: Microalgal response. Water S.A. 2000, 26, 301–310. [Google Scholar]
- Hilmer, T. Factors Influencing the Estimation of Primary Production in Small Estuaries. Ph.D. Thesis, University of Port Elizabeth, Port Elizabeth, South Africa, 1990. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002; 500p. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Poole, R.W. An Introduction to Quantitative Ecology; McGraw-Hill: New York, NY, USA, 1974. [Google Scholar]
- Ryder, D.S.; Watts, R.J.; Nye, E.; Burns, A. Can flow velocity regulate epixylic biofilm structure in a regulated floodplain river? Mar. Freshw. Res. 2006, 57, 29–36. [Google Scholar] [CrossRef]
- Atazadeh, I. Biomass and Remote Sensing of Biomass; IntechOpen Limited: London, UK, 2011. [Google Scholar]
- Atazadeh, I.; Mills, K.; Barton, A.; Gell, P. Configuring consumptive water transfers for ecological benefit: An algal response model for water resource operations. In Proceedings of the 34th Hydrology and Water Resources Symposium, Sydney, Australia, 19–22 November 2012; Engineers Australia: Canberra, Australia. [Google Scholar]
- Chester, E.; Robson, B. Do recolonisation processes in intermittent streams have sustained effects on benthic algal density and assemblage composition? Mar. Freshw. Res. 2014, 65, 784–790. [Google Scholar] [CrossRef]
- Atazadeh, I.; Sharifi, M.; Kelly, M.G. Evaluation of the trophic diatom index for assessing water quality in River Gharasou, western Iran. Hydrobiologia 2007, 589, 165–173. [Google Scholar] [CrossRef]
- Medley, C.N.; Clements, W.H. Responses of diatom communities to heavy metals in streams: The influence of longitudinal variation. Ecol. Appl. 1998, 8, 631–644. [Google Scholar] [CrossRef]
- Jeffries, M.; Mills, D. Freshwater Ecology: Principles and Applications; Belhaven Press: London, UK, 1990. [Google Scholar]
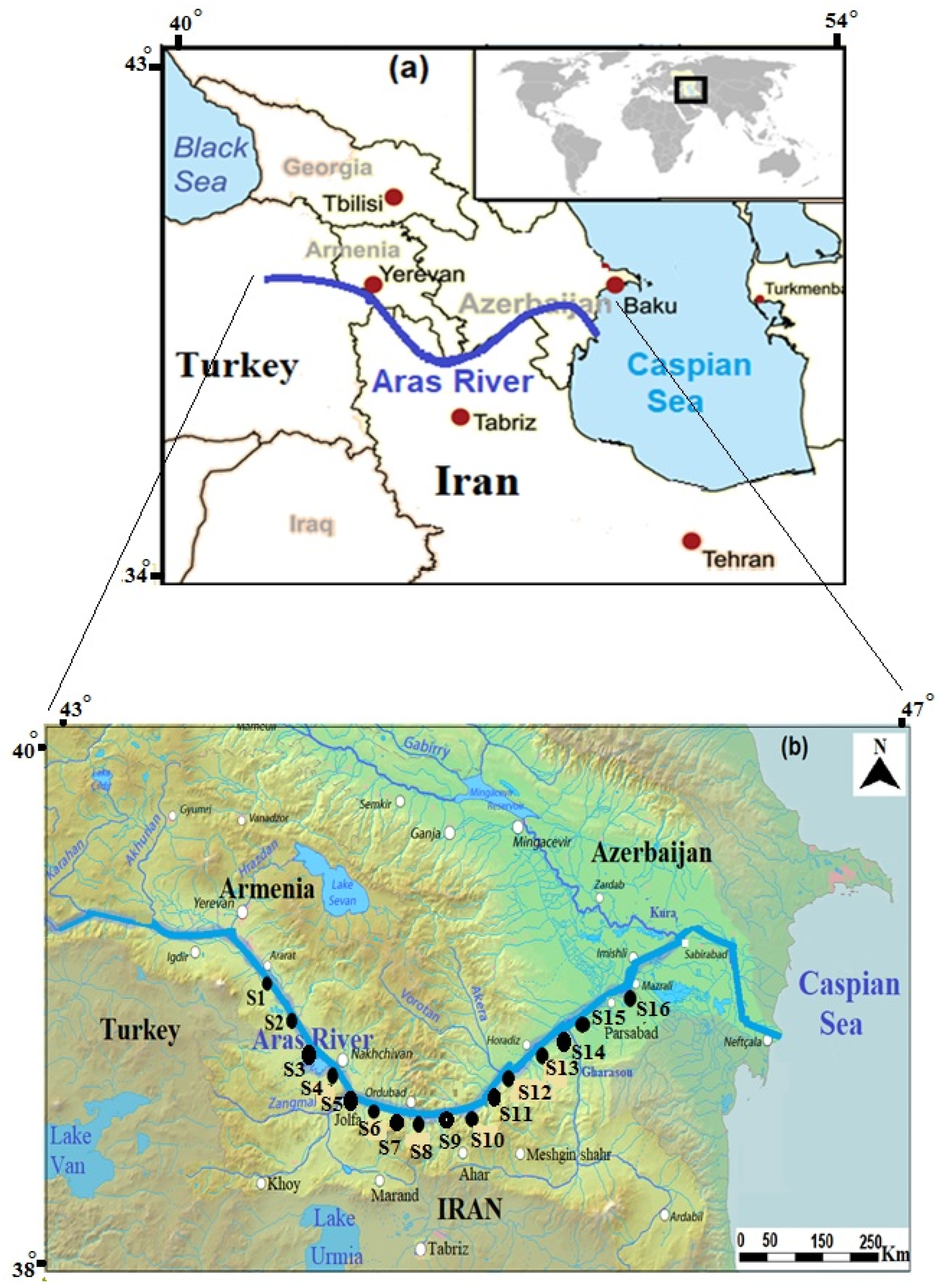



| Code | Site Name | Latitude | Longitude | Altitude (m) |
|---|---|---|---|---|
| S1 | Poldasht | 45.06581333 | 39.34696033 | 787 |
| S2 | Before Aras Dam | 45.22557378 | 39.19943588 | 770 |
| S3 | After Aras Dam | 45.35672307 | 39.13794955 | 764 |
| S4 | Jolfa | 45.62766533 | 38.93720633 | 707 |
| S5 | Jolfa to Siah Rud | 46.10739022 | 38.86254289 | 615 |
| S6 | 10 km to Siah Rud | 46.17854387 | 38.84061849 | 553 |
| S7 | Sfiah Rud | 46.00456867 | 38.86773967 | 648 |
| S8 | Nurdouz | 46.20829367 | 38.83874067 | 669 |
| S9 | Tatar Oliya | 46.77221433 | 39.04058333 | 360 |
| S10 | 10 km Tatar Oliya | 46.78872228 | 39.05588419 | 359 |
| S11 | Ving village | 46.83488333 | 39.01158833 | 339 |
| S12 | Before Khodafarin Dam | 47.35944405 | 39.41834632 | 142 |
| S13 | After Khodafarin Dam | 47.33519688 | 39.40097177 | 146 |
| S14 | Aslandooz | 47.41018800 | 39.44133100 | 157 |
| S15 | Oltan | 47.76384033 | 39.60737133 | 63 |
| S16 | Pars Abad | 47.91895151 | 39.64515549 | 45 |
| EPI-D | TI | TIT | DSIAR | TDI | IPS | |
|---|---|---|---|---|---|---|
| S1 | 0.75 | 0.88 | 1.08 | 80 | 35 | 4.1 |
| S2 | 0.78 | 0.94 | 1.15 | 74 | 42 | 4.26 |
| S3 | 0.91 | 1.1 | 1.21 | 75 | 41 | 4.50 |
| S4 | 0.89 | 0.98 | 1.08 | 65 | 46 | 3.27 |
| S5 | 0.88 | 1.1 | 1.99 | 55 | 55 | 3.55 |
| S6 | 1.9 | 2.5 | 3.84 | 51 | 58 | 4.07 |
| S7 | 1.5 | 2.56 | 2.66 | 52 | 59 | 3.28 |
| S8 | 1.8 | 2.8 | 2.74 | 53 | 65 | 2.50 |
| S9 | 1.5 | 2.6 | 3.01 | 42 | 72 | 2.48 |
| S10 | 1.3 | 2.1 | 2.64 | 48 | 75 | 3.22 |
| S11 | 1.6 | 1.21 | 2.65 | 48 | 76 | 3.66 |
| S12 | 1.5 | 1.23 | 2.51 | 46 | 78 | 4.00 |
| S13 | 1.5 | 1.54 | 2.48 | 45 | 81 | 3.19 |
| S14 | 1.5 | 2.01 | 2.61 | 38 | 85 | 2.68 |
| S15 | 1.8 | 1.47 | 2.19 | 25 | 89 | 3.08 |
| S16 | 1.4 | 1.08 | 2.10 | 20 | 86 | 3.47 |
| Unit | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 9.06 | 8.06 | 8.53 | 10.6 | 9.8 | 11.1 | 10.06 | 10.06 | 9.06 | 9.46 | 9.1 | 8.17 | 9 | 9 | 9.6 | 9.5 | |
| Temperature | °C | 8.1 | 8.1 | 8.1 | 8.1 | 8.9 | 9.1 | 9.74 | 8.97 | 8.7 | 8.7 | 8.7 | 8.7 | 8.3 | 8.9 | 9.6 | 9.4 |
| Conductivity | µS cm−1 | 1.56 | 1.58 | 1.55 | 1.2 | 1.3 | 1.2 | 1.23 | 1.22 | 1.54 | 1.55 | 1.55 | 1.08 | 1.12 | 1.1 | 1.2 | 4.3 |
| Turbidity | NTU | 1.47 | 1.47 | 1.47 | 1.47 | 1.5 | 0.9 | 1.4 | 2.2 | 5.1 | 5.1 | 5.1 | 5.1 | 5.2 | 6 | 7.6 | 12 |
| Dissolved oxygen | mg L−1 | 8 | 8 | 8 | 8 | 8.7 | 8.9 | 8.7 | 8 | 8 | 8 | 8 | 8 | 7.006 | 7.5 | 10.4 | 6.7 |
| Total dissolved solids | mg L−1 | 8 | 7.5 | 0.8 | 0.72 | 0.73 | 0.72 | 0.76 | 0.72 | 0.66 | 0.71 | 0.75 | 0.58 | 0.54 | 0.6 | 0.7 | 2.2 |
| Total suspended solids | mg L−1 | 187 | 187 | 187 | 187 | 124 | 162 | 69.45 | 71.55 | 463 | 463 | 463 | 463 | 475 | 474 | 475 | 465 |
| Mg2+ | mg L−1 | 1.2 | 1.2 | 1.7 | 1.7 | 2 | 2.2 | 2.2 | 2.1 | 2.5 | 2.6 | 2.6 | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 |
| Zn | mg L−1 | 5 | 5 | 5 | 5 | 6.3 | 8 | 11.6 | 13.6 | 51.8 | 51.8 | 51.8 | 51.8 | 48.4 | 43.5 | 55.1 | 52.1 |
| K+ | mg L−1 | 5.29 | 5.29 | 5.29 | 5.29 | 5.7 | 5.7 | 5.1 | 5.2 | 4.6 | 4.6 | 4.6 | 4.6 | 4.09 | 5.3 | 5.3 | 5.6 |
| CL− | mg L−1 | 22 | 25 | 23 | 25 | 31 | 31 | 32 | 32 | 33 | 28 | 28 | 29 | 29 | 28 | 28 | 28 |
| Ca2+ | mg L−1 | 1.6 | 1.6 | 1.6 | 1.8 | 1.8 | 2.2 | 2.2 | 3.3 | 3.2 | 3.3 | 3.1 | 3.2 | 3.4 | 3.4 | 3.6 | 3.6 |
| So42− | mg L−1 | 0.45 | 0.45 | 0.52 | 0.52 | 0.46 | 0.46 | 0.48 | 0.48 | 0.55 | 0.54 | 0.62 | 0.55 | 0.42 | 0.42 | 0.56 | 0.56 |
| NH3 | mg L−1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.09 | 0.09 | 0.09 | 0.07 | 0.07 | 0.08 | 0.08 | 0.08 | 0.07 | 0.07 | 0.09 | 0.09 |
| Total phosphorus | mg L−1 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.06 | 0.06 | 0.07 | 0.07 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Total oxidized nitrogen | mg L−1 | 0.06 | 0.08 | 0.07 | 0.08 | 0.08 | 0.08 | 0.05 | 0.05 | 0.07 | 0.06 | 0.06 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Total nitrogen | mg L−1 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 | 0.8 | 0.8 | 0.8 | 0.8 |
| Bacillariophyta | Taxa No. | Chlorophyta | Taxa No. | Cyanobacteria | Taxa No. | Other Groups | Taxa No. |
|---|---|---|---|---|---|---|---|
| Amphora | 15 | Chlorella | 1 | Anabaena | 2 | Chara | 1 |
| Aneumastus | 3 | Cladophora | 1 | Chroococcus | 1 | Ceratium | 2 |
| Asterionella | 2 | Chlorococcum | 1 | Microcystis | 2 | Cryptomonas | 1 |
| Anomoeoneis | 2 | Chlamydomonas | 1 | Nostoc | 1 | Euglena | 1 |
| Achnanthidium | 2 | Oedogonium | 1 | Oscillatoria | 1 | Gymnodinium | 1 |
| Aulacoseira | 1 | Scenedesmus | 5 | Phormidium | 1 | Peridinium | 1 |
| Adlafia | 1 | Schroederia | 1 | Spirulina | 1 | Staurastrum | 2 |
| Amphipleura | 1 | Stigeoclonium | 1 | Tolypothrix | 1 | Spirogyra | 1 |
| Brebissonia | 1 | Ulothrix | 1 | Staurodesmus | 1 | ||
| Brachysira | 3 | ||||||
| Brevisira | 1 | ||||||
| Bacillaria | 1 | ||||||
| Berkeleya | 1 | ||||||
| Brockmanniella | 1 | ||||||
| Ctenophora | 1 | ||||||
| Cymbopleura | 1 | ||||||
| Craticula | 1 | ||||||
| Chamaepinnularia | 1 | ||||||
| Campylodiscus | 1 | ||||||
| Cymbella | 20 | ||||||
| Caloneis | 9 | ||||||
| Cocconeis | 7 | ||||||
| Cymatopleura | 4 | ||||||
| Cyclotella | 3 | ||||||
| Cyclostephanos | 1 | ||||||
| Caloneis | 1 | ||||||
| Denticula | 7 | ||||||
| Diatoma | 6 | ||||||
| Didymosphenia | 3 | ||||||
| Diploneis | 2 | ||||||
| Discotella | 1 | ||||||
| Diploneis | 1 | ||||||
| Dimeregramma | 1 | ||||||
| Delicata | 1 | ||||||
| Eunotia | 8 | ||||||
| Entomoneis | 2 | ||||||
| Encyonopsis | 2 | ||||||
| Epithemia | 1 | ||||||
| Eucocconeis | 1 | ||||||
| Fragilaria | 9 | ||||||
| Surirella | 13 | ||||||
| Nitzschia | 35 | ||||||
| Pinnularia | 18 | ||||||
| Gomphonema | 25 | ||||||
| Geissleria | 4 | ||||||
| Stauroneis | 9 | ||||||
| Gyrosigma | 2 | ||||||
| Gomphosphenia | 2 | ||||||
| Halamphora | 8 | ||||||
| Total | 246 | 13 | 10 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parikhani, F.; Atazadeh, E.; Razeghi, J.; Mosaferi, M.; Kulikovskiy, M. Using Algal Indices to Assess the Ecological Condition of the Aras River, Northwestern Iran. J. Mar. Sci. Eng. 2023, 11, 1867. https://doi.org/10.3390/jmse11101867
Parikhani F, Atazadeh E, Razeghi J, Mosaferi M, Kulikovskiy M. Using Algal Indices to Assess the Ecological Condition of the Aras River, Northwestern Iran. Journal of Marine Science and Engineering. 2023; 11(10):1867. https://doi.org/10.3390/jmse11101867
Chicago/Turabian StyleParikhani, Fatemeh, Ehsan Atazadeh, Jafar Razeghi, Mohammad Mosaferi, and Maxim Kulikovskiy. 2023. "Using Algal Indices to Assess the Ecological Condition of the Aras River, Northwestern Iran" Journal of Marine Science and Engineering 11, no. 10: 1867. https://doi.org/10.3390/jmse11101867
APA StyleParikhani, F., Atazadeh, E., Razeghi, J., Mosaferi, M., & Kulikovskiy, M. (2023). Using Algal Indices to Assess the Ecological Condition of the Aras River, Northwestern Iran. Journal of Marine Science and Engineering, 11(10), 1867. https://doi.org/10.3390/jmse11101867






