Abstract
Environmental diagnostics are commonly used to identify anthropogenic influences in various environmental settings. However, the use of a single survey technique is limiting and leads to an incomplete and often inaccurate picture of reality. In this study, three real cases analyzing impacts on marine ecosystems have been presented to prove how the integration of different diagnostic techniques can be very beneficial to better understand the phenomena that occur as well as the impacts and associated damages. Studies combining classical diagnostics based on the determination of contaminant levels by chemical analysis, ecotoxicological tests and stress biomarkers with diagnostics by Field Emission Scanning Electron Microscopy-FESEM and X-ray diffraction-XRD microscopy are reported. Findings suggest that the embedding of chemical analyses, ecotoxicology and microchemical FESEM and XRD analyses allow us to hit the mark and give precise and effective responses in environmental management.
1. Introduction
Environmental pollution is one of the biggest issues that humanity will have to face to ensure a healthy environment for the next generation [1]. Environmental impact assessment requires accurate measurements of both environmental quality and the nature, magnitude, and location of human-induced impacts to better define strategies to manage, mitigate, and monitor adverse environmental impacts [2]. A proper assessment of impacts is still not possible due to their long-term evolution [1]. Several sources can affect environmental quality and protection [3]. Numerous definitions of environmental pollution can be found in the literature; nevertheless, it is accepted by almost all scientists that this term can be defined as human-mediated phenomena that cause environmental quality degradation [4].
Many types of chemicals can contribute to environmental pollution, including macronutrients, inorganic compounds (i.e., trace and rare earth elements), organic molecules (i.e., polycyclic aromatic hydrocarbon), and xenobiotics (i.e., PCBs, phthalates, pesticides, pharmaceuticals, etc., i.e., [5]). Recently, materials such as microplastics, nanoplastics, and nanoparticles (i.e., TiO2, ZnO) have been shown to be particularly harmful to species in terrestrial [6] and aquatic environments [7]. In addition, the great interest of industry in finding viable substitutes for commercial molecules developed by the chemical industry is growing and has led to the development of a new approach to chemistry called green chemistry [8]. The goal is to synthesize chemicals from petroleum-free products or purified bioactive molecules used for pharmaceutical purposes from biological sources [8]. However, the safety of these new commercial products, which are also of natural origin, must be carefully evaluated to verify their efficacy and effectiveness. This is because, as is well known in ecotoxicology, “Omnia venenum sunt: nec sine veneno quicquam existit. Dosis sola facit, ut venenum non fit” [9] applies, and natural chemicals are by no means safe, but can be poisoning, ecotoxic or even carcinogenic. However, pollution is not only caused by chemicals, but there are also many biological sources that can affect pollution and environmental safety, such as biological and microbiological pollution. Microbes introduced by human activities, such as effluent from municipal wastewater treatment plants or bioremediation of chemical spills, can affect ecosystem maintenance [10].
The widespread use of plastic in human activity has raised environmental concerns. After plastic enters (directly and/or indirectly) aquatic ecosystems through various sources (i.e., residential, domestic activities, tourism, etc.), it undergoes degradation (i.e., biological, mechanical, photooxidation by ultraviolet light) that alters the particle size and density [11]. Most plastics are highly resistant to (bio)chemical degradation, however, which is why they persist in the environment. Plastics physically break up into smaller and smaller fractions, termed microplastics (MPs, 1 µm–5 mm) [12]. Microplastics are classified into primary and secondary MPs. Primary MPs are manufactured plastic particles that go into a variety of products (i.e., cosmetics), while secondary MPs are formed during the use and disposal of plastic products (i.e., degradation of plastic bottles) or in the decomposition of macroplastics into MPs [6]. A variety of harmful effects of (micro)plastic and associated chemicals has been shown. For example, plastic debris can act as a vector for alien species and diseases. On this path it was observed that both birds and fishes ingest plastic marine debris [13], and a study found fishes that contain human pathogenic Vibrio spp. strains [14]. Since plastic marine debris persists longer than natural substrates (i.e., feathers, wood, and macroalgae), it can traverse significant distances, and it has been shown to transport invasive alien species. Furthermore, microplastic contamination is endangering animal life and thus also the food chain and public health [15].
Finally, but not least, we live in a world that is changing rapidly due to global changes. The effects of pollution on species have been shown to differ significantly when physicochemical environmental conditions change, such as the ecotoxicological responses of plants exposed to microplastics in the presence of acid rain compared to controls [16,17], and of marine animals exposed to chemicals, materials, and nanoparticles when pH and salinity change [18,19].
In ecology, phenomena occur at spatial and temporal scales that can include many confounding factors and produce highly variable signals that are often difficult to detect and interpret using a single approach. To assess this diversity, several approaches have been proposed in the literature over time. The first approach to pollution detection was based on chemical analyses of environmental matrices and biota [20]. This approach started in the 1970s when society demanded more environmental safety after the publication of her masterpiece “Silent Spring” [17]. Other impressive cases on the consequences of pollution such as Minamata (methylmercury; [21]) and Itai-Itai (cadmium, [22]).
The chemical approach proved to be limited and inadequate from the beginning. Although it is very accurate, precise, and reproducible, it is based on a defined list of chemicals and does not allow evaluation of the environmental impact of the measured levels. Ecotoxicology is a recently applied approach to determine the effects caused by exposure of target species at different trophic levels, using in vitro, in vivo, and in site approaches [23]. In ecotoxicological studies, a variety of biological indicators (i.e., microorganisms, plants, and animals) have been developed in the literature to detect changes in the ecosystem at both lethal and sublethal levels, and each indicator is used to identify individual contaminants or a category of contaminants [4]. Recently, micro- and nanochemical diagnostic techniques have improved the diagnostic performance of previous microscopy. The integration of a multidisciplinary and interdisciplinary approach is crucial to obtain a complete picture of environmental problems that will help optimize management strategies [24]. However, in the near future, the complexity of environmental issues will require a deeper integration of diagnostic techniques and human capabilities to improve the realism and effectiveness of diagnosis.
The increasing impact of pollution on the environment, inefficient use of resources, improper waste management, climate change, degradation of ecosystems and loss of biodiversity make necessary an integrated and multidisciplinary approach. In this study, the H0 hypothesis tested was to verify if the use of multiple approaches and techniques could solve issues related to environmental pollution and management. To do this, three real-world cases were selected and analyzed using the expertise of our research team. In particular, chemical, morphological/microchemical, ecotoxicological and biochemical approaches/techniques were applied.
2. Materials and Methods
2.1. Case Study I—TiO2 in Mussels
The main aim of this case study was to determine whether exposure of natural mussels (Mytilus galloprovincialis) to nano-TiO2 can induce a detectable stress signal using sublethal biomarkers. To do this, chemical analyses on mussel tissues, classical ecotoxicological endpoints (i.e., mortality during the exposure period), biomarkers of oxidative stress (superoxide dismutase, glutathione peroxidase, glutathione S-transferase and malondialdehyde levels) and microchemical—structural (FESEM, XRD) diagnostics were brought together. This research was performed by applying an in vivo approach following the laboratory exposure of tested animals.
Selected pollutants. Metal oxide nanoparticles (NPs) have been widely used in various fields due to their unique physical and chemical properties [25]. Among these, titanium dioxide nanoparticles (n-TiO2) are used for applications such as additives in pharmaceuticals, food colorants, paints, surface coatings, sunscreens, medical devices, plastics, boat paints, and toothpastes [26,27,28,29,30]. Nano-TiO2 particles are generally used in self-cleaning paints and self-sterilizing surfaces [31,32]. Cements and asphalts that are used for buildings, roads, pavement, and tunnel structures also contain n-TiO2 [33]. It can almost stabilize ingredients, enhance the penetration of vitamins and antioxidants into the skin, and improve the effectiveness of UV filters when present in cosmetics and sunscreens. Titanium gives material thermal stability, making it simple to clean surfaces, leading to anti-skid, anti-fogging, and anti-fouling characteristics. It has perfect thermal and electrical conductivity, perfect retention of gloss and other mechanical characteristics such as scratch resistance, anti-reflective in nature, chromate and lead free, good adherence to various types of materials [34]. Therefore, TiO2 is one of the most popular commercially available nano-sized materials that has found application in a variety of fields due to its wide availability, low cost, and high chemical stability [35]. However, it has been demonstrated by the literature that n-TiO2 is ecotoxic for aquatic organisms i.e., [7,36,37].
Selection of tested species. Mussels represented a very used species in ecotoxicology. Their biology, physiology and ecotoxicological responses are well known as it was used for the monitoring program known worldwide as “mussel watch” [38]. The organism used for the study was M. galloprovincialis Lamarck 1819, an aquatic plant-feeding marine bivalve. The organisms were collected from the Ligurian Sea (La Spezia, Liguria, Italy) in November 2021. The test organisms were measured with a caliper before the test was performed and belong to the size class 5.1 (±0.4) cm. Organisms underwent a preliminary analyzed to verify the health status of the tested animals and to exclude the presence of significant natural stressors (i.e., metals, hydrocarbons, pesticides) affecting selected populations of the original community (control analysis at time zero, T0). Analyses were performed on three pools, each of them containing three organisms. The abundance of bivalves in near-shore environments, their sedentary lifestyle, and predilection for suspension feeding should make these animals more susceptible to NPs entering coastal waters [39]. Previous studies have shown that several different types of NPs, including TiO2, can be captured and ingested by bivalves [40,41,42,43], however, the efficiency and rate of this capture has not been determined.
Experimental design. M. galloprovincialis was exposed to two different concentrations of titanium dioxide (TiO2; CAS n. 13463-67-7, Caelo, powder, particle size <21 nm, surface area 35–65 m2/g) for 60 days: 100 μg/L, and 10 μg/L. Exposure concentrations were determined after a preliminary range-finding test. Each experimental condition was performed in duplicate, and a single specimen was placed in each vessel of each replicate (n = 2). Samples were exposed to 800 mL of unfiltered seawater obtained from a location without environmental exposure. The required TiO2 concentration was added to each replicate, starting from a stock solution of 0.1 g/L prepared in distilled water (Figure 1). The suspension was placed in an ultrasound water bath for 30 min before being diluted to different exposure concentrations [44]. A negative control (seawater and M. galloprovincialis without TiO2) was also prepared in two replicates and treated as the other samples. The physicochemical water parameters (pH, dissolved oxygen, salinity) were measured daily and maintained at the natural level without significant variations.

Figure 1.
Field Emission Scanning Electron Microscopy-FESEM micrograph of TiO2 nanoparticles used in this study.
Experimental exposure. For the experimental exposure, unfiltered natural water was used, originating from an uncontaminated natural marine area (Talamone, Tuscany, Italy). The high standard of the water quality was confirmed with ecotoxicological tests performed on seawater collected from the Talamone site. The tests were conducted with a battery of three species from different trophic levels: bacteria (Aliivibrio fischeri), primary producer (Phaeodactylum tricornutum) and primary consumer (Paracentrotus lividus), which always gave values within the recorded effects (<5%). The salinity of the water used was standardized at 30.0 ± 0.2 g/L and pH 8.1 ± 0.2 according to the literature [45] The water was changed every three days. At each water change, the number of surviving mussels was determined and rinsed thoroughly. After each water change, the required concentration of TiO2 was added from the stock solution 0.1 g/L. Simultaneously with the water changes, the organisms were fed with freeze-dried fish food (30 mg/L). Each jar was kept under constant oxygenation for the duration of the experiment. Organisms were maintained at a controlled temperature (18 ± 2 °C) and alternated day and night in a 16:8-h ratio. At the end of the experiment, each specimen was filtered using a 0.45 µm nitrate cellulose fiber filter. After filtration, samples were collected for tissue analyzes (chemical and biochemical), and the shells were analyzed with microchemical and crystallographic methods (i.e., Field Emission Scanning Electron Microscopy-FESEM, X-ray diffraction-XRD). For biochemical analyzes, live animals were sacrificed, digestive glands and gills removed, weighed, and frozen with liquid nitrogen. Samples were then stored at −80 °C until analyzes were performed to assess induced oxidative stress using biomarkers. Biomarkers were selected based on previous in vivo exposure studies with this species (i.e., [46]). In addition, survival rates were calculated to evaluate the classical ecotoxicological endpoint (mortality).
Biomarkers analyses. Analyses were performed with protein fraction S9. Methods and expression of data were referred to previous research on mussels [47]. A buffer phosphate 50 mM + EDTA 2 mM was added to a test tube containing the tissues of the organisms at a ratio of 1:4 (w/v) for the digestive gland and a ratio of 1:2 (w/v) for the gills. Tissues added to the buffer were homogenized using an Ultraturrax and then centrifuged (12,000× g; 12 min; 4 °C) to extract the protein fraction. The supernatant was collected, placed in 2-mL Eppendorf tubes, and stored at −80 °C until analysis. Prior to analysis and to normalize the data, the protein content in each tissue of each sample must be quantified. Protein content was quantified by spectrophometry at 750 nm using the Lowry colorimetric method [48]. Protein fraction S9 was placed in a test tube containing NaOH 0.5 M, Folin-Ciocalteau reactive and a mixture of reactive (CuSO4·5 H2O, Rochelle salt and Na2CO3) and then analyzed. Each sample was subjected to a series of biomarker analyzes such as: lipid peroxidation (LPO), quantification of the enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione S-transferase (GST). Lipid peroxidation was tested using 10% of the complex protein [49]. The protein fraction was contacted with 1% phosphoric acid (v/v) and 0.6% thiobarbituric acid (w/v). After heating (96 °C for 25 min) and centrifugation (4000 rpm at room temperature for 5 min) with 1-buthanol, malondialdehyde (MDA) was quantified spectrophotometrically at a wavelength between 535 and 520 nm. The delta was calculated using the delta of absorbance at the beginning and at the end of measurement. The results were expressed as U/mg of protein. To quantify SOD [50], the S9 protein fraction was mixed with Tris-EDTA buffer (pH 8.2) and pyrogallol. This method quantifies SOD indirectly and is based on the ability of the enzyme to inhibit the autoxidation of pyrogallol. SOD enzyme was quantified with spectrophometry at 420 nm after a reaction time of 3 min. Results were expressed in U/mL. GPx analyzes [51] were performed with a fixed concentration of 0.5 mg/mL protein. The reaction of the S9 fraction was performed with a mixture of GSH (reduced glutathione) 10 mM, GSSG reductase 2.4 U/mL, and NADPH 1.5 mM. After heating at 37 °C for 5 min in the presence of hydrogen peroxide, GPx was quantified at 340 nm for 2 min. Results were determined by calculating a delta and were expressed in nmol/(mg.min). GST analysis [52] was performed with a protein fraction exposed to a reaction mixture of GSH (reduced glutathione) 10 mM and CDNB (1-chloro-2,4-dinitrobenzene) 60 mM. After mixing, GST was quantified at 340 nm for 5 min. The delta was calculated, and the results were expressed in μmol/(μg.min).
Data analyses. The content of MDA, SOD, GPx and GST were quantified on Mytilus galloprovincialis. The results were calculated considering the amount of proteins present in the studied organism. For each replicate, two organs were analyzed, and two analytical replicates were performed. A mean value was calculated from the two analytical replicates (±standard deviation). Then, all replicates of each analytical condition were also averaged (±standard deviation). Data normality and homoscedasticity were tested with the Shapiro-Wilk and the Levene test, respectively. A one-way ANOVA analysis was performed with XLSTAT software (2021) using Tukey’s test and Dunnett’s test to detect significant differences between the control and the treatments.
2.2. Case Study II—Recovery of Bioclast in Circular Economy
A complex mixture of shell powder was obtained by mechanically grinding mollusk shells recovered from natural sediments close to an aquaculture plant (Figure 2). Powder was used to evaluate the applicability of shell dusts as a soil conditioner in agriculture. This is of relevance for the circular economy because mollusk shells are considered a waste to be managed when discharged from aquaculture or derived from dredged sediments.

Figure 2.
Bioclast tested for evaluate their possible applications in circular economy.
The aim of this study was to determine the physico-chemical properties of such materials and to evaluate the effects on plant growth when added to natural soils.
Physicochemical analyses. Finely ground bioclasts were analyzed to determine the principal physicochemical composition of the shells. For these analyses, the methods applied, measurement units, and limit of quantifications are reported in Table 1.

Table 1.
Physicochemical analyses performed on powdered shells. In this table, methods, limit of quantification (LOQ) and measurement units are reported for each parameter.
Ecotoxicological assays. Powdered shells were added to natural soil and tested for 72 h to evaluate seed germination rates and the growth of roots in Lepidium sativum, Sinapsis alba, and Sorghum saccharatum, the most often used higher plants in the ecotoxicological studies for the evaluation of the potential impacts of pollutants on primary producers. Tests were performed following the UNI 11357:2010, using as negative control, natural soil, positive control (K2Cr2O7). Tested soil was produced by the addition of 5%, 10%, and 15% d.w. of powdered shell to natural soil used as negative control. After exposure of 72 h, the percentage of germination and elongation of the root system were determined to evaluate ecotoxicological effects. Negative control results were L. sativum, S. alba, and S. saccharatum (>90% germination seeds; >60 mm mean root length). Positive controls results were L. sativum (EC50 = 1.5 mg/L, range: 13.3–34.8 mg/L), S. alba (EC50 = 24.9 mg/L, range: 15.9–39.0 mg/L), S. saccharatum (EC50 = 20.4 mg/L, range: 9.3–41.4 mg/L).
2.3. Case Study III—An Environmental “Crime Scene”
In this case study, the environmental research was undertaken because of the need to evaluate the potential impact caused by an accidental spill in marine water of a yellow-colored effluent containing suspended solid particles of unknown origin. In this case, starting from a highly pigmented liquid discharge, a series of determinations were carried out aimed primarily at classifying the risk for the environment and secondly at understanding the origin of the pollutant. Ecotoxicological tests were performed on the species Daphnia magna (APAT CNR IRSA 8020 B Man 29, 2003; three replicates) to evaluate ecotoxicity for the aquatic environment. Negative (standard freshwater) and positive (K2Cr2O7) controls were performed to evaluate the general quality of the ecotoxicological assay. After 24 h of exposure, negative controls showed an absence of effects on tested animals while EC50 on positive controls results conform to the standard of the followed method (0.82 mg/L; range: 0.77–1.02 mg/L).
Principal physicochemical parameters were measured following methods reported in Table 2 on collected unknown effluent. Furthermore, 0.45 μm filtered solid materials were collected and analyzed using a FESEM microscope to empower the general understanding on recovered pollutants.

Table 2.
Physicochemical analyses performed on powdered shells. In this table, method, limit of quantification (LOQ) and measurement units are reported for analyzed variables.
2.4. Common Microchemical Analyses Performed in Tested Case-Studies
FESEM detection. Electron scanning microscopy was applied with two principal different aims: define the microstructure of tested materials and perform microchemical analyses on target particles that could be useful to better understand specific features. Microchemical analyses were performed using a high-ultra resolution scanning electron microscope (FESEM, Merlin, Zeiss) combined with EDS—WDS spectrometry (resolution better than 10 eV). FESEM was equipped with two pairs of secondary—backscattered electrons detectors located in sample chamber and in lens, respectively. The in-Lens detectors were required to achieve sub-nanometric resolution. In addition, there was also a charge compensator installed that allowed analysis of non-conductive and unprepared samples.
XRD diffractometry. Automated multipurpose X-ray Powder diffractometer (XRD, RIGAKU, Smartlab, Type XE high resolution analysis) is an analytical technique that provides phases identification, texture, and quantitative analysis of crystalline materials.
3. Results
3.1. Case Study I—TiO2 in Mussels
The mean size of the exposed animals was 52.7 ± 1.41 mm (range of variation: 49.9–54.2 mm). The population of mussel used for this research were shown to be unpolluted by classical chemicals. Metals, hydrocarbons, pesticides, and other tested xenobiotics results were included within natural levels for the species. Moreover, titanium levels in tissues at T0 were under the detection limits. Classical ecotoxicological tests do not indicate a significant mortality compared to negative controls during the exposure of mussels at the tested doses. At the end of the exposure, mortality was not relevant and almost all animals survived at all the exposure doses.
Biochemical analyses, on the contrary, showed significant effects following the exposure to TiO2 nanoparticles. Levels of biomarkers (mean and standard deviation) are shown in the Table 3.

Table 3.
Levels of biomarkers of oxidative stress in digestive gland (DG) and gills (G) of M. galloprovincialis exposed to TiO2 nanoparticles for 60 days. SD = Standard Deviation (n = 4 replicates); NC = negative control; T0 = biomarkers measured at the beginning of the study. SOD = superoxide dismutase; GPx = glutathione peroxidase; GST = glutathione S-transferase; MDA = malondialdehyde.
Results of one-way ANOVA are reported in Table 4. Levels of GPx in the gills were significantly higher compared to the negative control (T0) both for the treatment of 10 μg/L and 100 μg/L of TiO2 (Dunnett’s test; p < 0.05). Levels of GPx in the digestive gland were significantly higher compared to the negative control only for the treatment of 100 μg/L (Dunnett’s test; p < 0.05). The same trend was observed for GST in the gill. Levels of GST in the digestive gland were significantly higher compared to the negative control with all treatments (Dunnett’s test; p < 0.05). Finally, MDA levels were significantly higher only for the higher TiO2 concentration (100 μg/L) in the gills (Dunnett’s test; p < 0.05).

Table 4.
Results of one-way ANOVA and Dunnett’s post-hoc test on GPx (glutathione peroxidase), GST (glutathione S-transferase) and SOD (superoxide dismutase) levels. NG = negative control, gills; G1 = Gills tested at 100 μg/L; G2 = Gills tested at 10 μg/L; ND = negative controls (T0), digestive glands; DG1 = digestive glands tested at 100 μg/L; DG2 = digestive glands tested at 10 μg/L. Only significant comparisons were reported; critical value = 2.683.
The size distribution (94.2 ± 31.1 nm; range: 59.2–159.5 nm) and chemical composition of TiO2 nanoparticles were investigated through FESEM imaging and microanalysis, respectively. Nevertheless, FESEM analysis was not able to disclose the crystalline structure of TiO2, that was revealed by means of X-ray diffraction (Anatase) (Figure 3b).

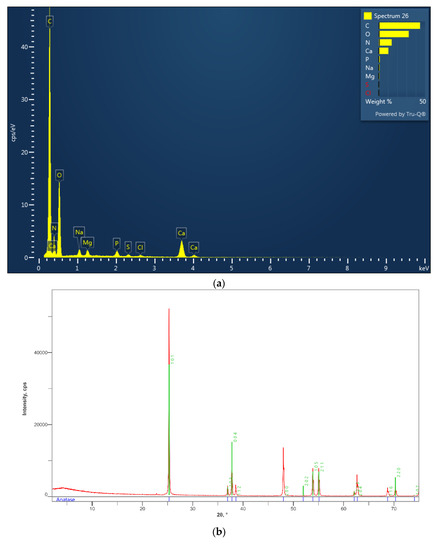
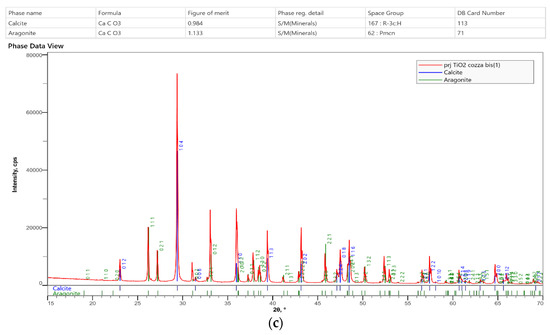
Figure 3.
The first figure is the detection area of tested shell. (a) Energy Dispersion Spectra showing the relative abundance of the elements composing the matrix as function of the peak’s intensity. (b) XRD diffractogram of the nanoparticles previously identified using microanalysis (TiO2). (c) XRD diffractogram of mussel shells. The phases composition is consistent with calcite-aragonite and there is no evidence of TiO2.
XRD phase analyses (Figure 3c) were consistent with both Aragonite and Calcite compounding the mussel shell umbo and did not reveal the presence of Anatase. Moreover, EDS analyses (Figure 3a) also indicated the presence of N and P in bulk composition, but no N- and P- bearing mineral phases were identify in X-ray diffractogram; thus indicating N and P were included in elemental form into the structure of the carbonate minerals.
3.2. Case Study II—Bioclasts in Circular Economy
The test matrix consists of pulverized carbonate-based marine bivalves from aquaculture with a principal grain-size composition (98.9%) in the size class included within the range 0.063–2 mm. The analyses showed values of As, Cd, Cr, Hg, Ni, Pb, and V below the LOQ. However, some other elements were recorded: Al (20.1 mg/kg), Fe (245 mg/kg), Cu (4.3 mg/kg), and Zn (12.5 mg/kg).
Ecotoxicological tests performed on plants (addition of shell powdered to natural soils at different percentages; 72 h of exposure) highlighted no significant effects on seed germination (>90% for all tested concentrations). Moreover, root growth did not show any significant difference between negative controls (natural soil without additions) and exposure concentrations (t-test, p > 0.05).
FESEM microscopy allowed detection of a high percentage of Ca and Fe. The X-Ray diffraction revealed that Ca was present in the form of aragonite (Figure 4).
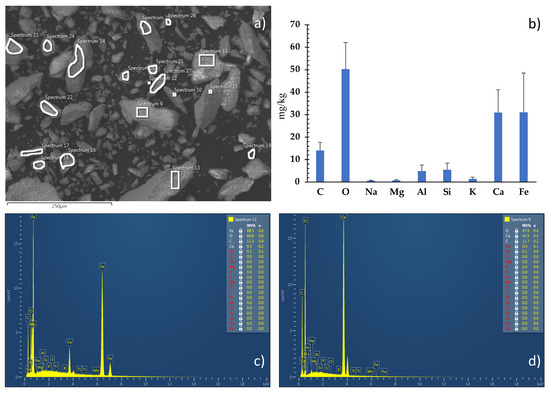
Figure 4.
(a) FESEM micrograph of the pulverized carbonate matrix, with highlighted the areas selected for chemical analyses. (b) Weight percentage diagram of the elements identified using chemical microanalyses. (c,d) Energy Dispersion Spectra showing the relative abundance of the elements composing the matrix as a function of the peak’s intensity.
3.3. Case Study III—An Environmental “Crime Scene”
Physicochemical analyses highlighted pH values close to 8.3, and very high levels of TSS (1325 mg/L). Most of the trace elements showed values below the LOQ, except As (0.009 mg/L), Cu (0.07 mg/L), and Zn (0.04 mg/L). Nutrients were high, in particular, NH4+ (4.9 mg/L), and TP (1.0 mg/L). On the contrary, nitrites and nitrates results were close to 0.5 and 0.3 mg/L, respectively. Both chemical (67 mg/L) and biological oxygen demand (20 mg/L) results were measurable. Any other chemicals of ecological concern had results below the LOQ. From a chemical point of view, the effluent was considered safe to be discharge in municipal wastewater treatment plants as pertaining to the Italian Law (L.D. 152/2006, All. 5, part III, Table 3). Ecotoxicological tests performed on D. magna also suggested the absence of toxicity. Moreover, it was observed the absence of immobilized animals (24 h of exposure) and EC50 was not calculable at the tested concentrations. Nevertheless, the effluent showed significant pigmentation and to better understand its origin, FESEM microchemical analyses was performed (Figure 5). The microscope analyses detected the presence of large quantities of biological materials (pollen). The mean dimension of the pollen particles was 13.0 μm length × 9.0 μm width. The dense stratum of pollen included round particles of cordierite albeit (about 6 μm diameter) in smaller percentages. Chemical analyses on the recorded particles highlighted the presence of Mg, Fe, and Al silicates.
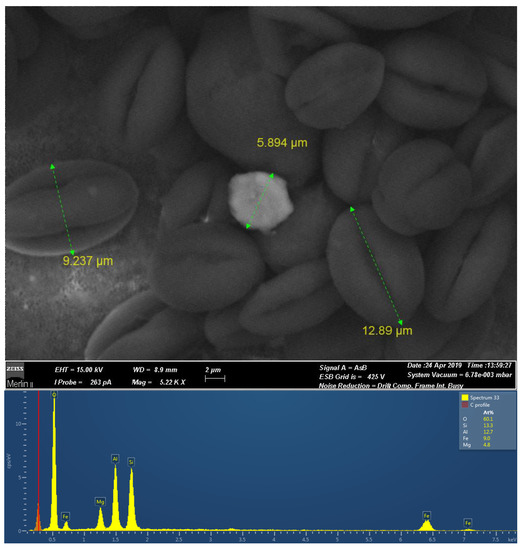
Figure 5.
FESEM micrographs in compositional contrast (Backscattered electrons) of the pigment, including a cordierite crystal (the brighter area) in a high-density pollen matrix.
4. Discussion
4.1. Case Study I—TiO2 in Mussels
In the environment, n-TiO2 could be released during manufacturing, transport, and disposal, including air, soil, and water [60]. Man-made sources of n-TiO2 are found in river water because of the release of synthetic n-TiO2 from urban applications into the aquatic environment [26]. Nano-TiO2 can enter an organism through the respiratory system, skin, and digestive systems. The differences in the physicochemical properties of n-TiO2 lead to different interactions with organisms and induce different toxicity [61]. The toxic effects induced in bacteria, algae, cells, yeasts, plants, invertebrates, and vertebrates are demonstrated by the literature [7,36,62,63,64,65,66]. The levels of reactive oxygen species-ROS (i.e., superoxide anions, hydroxyl radicals, and nonradical hydrogen peroxide) are increased when oxidative stress is activated inside cells. Then, the consequential damage includes damage to the phospholipid membrane, mitochondrial structure, and mitochondrial membrane depolarization. Subsequently, lipid peroxidation and cell membrane damage, changes in mitochondrial membrane permeability, cell death, and apoptosis. In algal species, it is reported to adsorb onto algal cell surfaces and cause membrane lipid peroxidation [67]. As one of the widely accepted toxic mechanisms of nanomaterials, the production of ROS is shown after exposure to n-TiO2 [68]. Most studies assessing the effects of NPs in aquatic invertebrates have focused on freshwater species, invertebrates (mainly crustaceans, Daphnia spp. in particular), and vertebrates (fish) [30,37,69,70]. Several studies have already revealed the impacts of n-TiO2 in bivalve species, such as the scallop Chlamys farreri, the clam Ruditapes philippinarum, and the oyster Crassostrea virginica. Moreover, alterations of the immune system, oxidative status, and metabolism have been reported [71,72,73,74,75,76,77,78,79]. For example, ZnO nanoparticles have strong impacts on immune-related functions and the gene expression profile in the hemocytes of the marine bivalves Mytilus edulis [80]. Less information is available from suspension-feeding organisms in estuarine and marine environments, which represent a key target group for nanoparticle toxicology [81]. Furthermore, some studies have suggested that nanoparticles can adsorb and concentrate different types of pollutants and can enhance the uptake of co-existing contaminants in organisms [82,83,84,85,86,87]. In this study, mortality of mussels was not relevant and almost all animals survived at all the exposure doses of titanium dioxide. However, biochemical biomarkers revealed sublethal effects, highlighting the need to assess more than one endpoint during in vivo tests. Findings are consistent with the literature. Indeed, Canesi et al. [88] reported that lower concentrations affect the physiology of mussels when exposed for a longer time. GST is one of the most useful biomarkers to use when it is suspected that organisms have encountered toxicants that may have caused oxidative stress [88,89]. Among the biochemical biomarkers, lipid peroxidation shows a less evident response compared to the others. The same thing was observed by Monteiro et al. [90] who stated that despite organisms being unable to increase their metabolic activity, they were able to activate their antioxidant defenses (SOD, GST and GPx enzymes), although not enough to prevent the occurrence of cellular damage (LPO levels), with induction of oxidative stress. The activation of antioxidant enzymes increased to eliminate the excess of reactive oxygen species produced after contamination with n-TiO2.
Previous studies have shown that many types of nanoparticles, including n-TiO2, can be captured, and ingested by filter-feeding organisms such as bivalves (i.e., [90]), although the modality and especially the speed and quantity with which they capture the nanoparticles is not clear. According to Doyle et al. [74], after exposure to n-TiO2, oxidative stress is present, but there is no evidence of the accumulation of nanoparticles in the tissues of both gills and digestive gland, which could confirm the fact that the organism manages to expel the particles. Titanium is the ninth most abundant element in the Earth’s crust, being present in rocks, soils, and sediments [91]. Because this element is poorly soluble in water [92,93], dissolved Ti is usually present in riverine, estuarine, and coastal waters, with concentrations between 0.01 and 5.5 mg/L [94,95]. Despite that, animals exposed to n-TiO2 do not show TiO2 or Ti in their recent or older deposited conchiolin. In fact, results obtained with microchemical analyses do not highlight the presence of titanium in the umbo structure. The large presence of Ti could have determined the development of detoxification mechanisms for this element by aquatic species that have a larger exposure to its effects, such mussels. Particle size is a critical determining factor in the capture and ingestion of particulate matter by suspension-feeding bivalves [96]. For example, bivalves can capture particles greater than 5 mm with an efficiency of approximately 100%. Capture efficiency, however, decreases as particles become smaller [97,98]. Many studies allude to the ingestion of aggregated NPs by suspension feeding bivalves, but do not include ecologically relevant experiments addressing particle capture and ingestion efficiencies [39]. To better clarify this aspect, further investigations can be performed on the histology and bioaccumulation of titanium regarding bivalve mussels and, specifically, M. galloprovincialis.
4.2. Case Study II—Bioclasts in the Circular Economy
Dredging of sediments in marine areas, harbors and river estuaries represent a key strategy that needs to be performed to maintain bottom depth and the human use of the natural resource [99]. This is also important in highly human-exploited ecosystem such as coastal lagoon and aquaculture plants. In these areas numerous mussel shells are sedimented and represent an important quantity to be managed. The disposal of collected shells represent a critical aspect for stakeholders as well as, in Italy, collected bioclasts are considered to be a waste when they are removed from the water. A circular economy has developed in the last decade to reduce waste production and improve recycling [100]. Based on this point of view, the chance to reuse bioclast produced in the environment as a resource in human production represents a target of particular interest. Some geographical areas are affected by a large production of bivalves such Orbetello, Lesina and Varano lagoons, and the Po River delta [101,102]. For example, the Varano and Lesina channels have intense mussel production that has led to the accumulation of a dense layers of bivalve shells in sediments. The use of shell dusts in agriculture as amending compound is outlined in the literature [103]. As reported in the previous Section 4.1, the presence of metals such as TiO2, can lead to their accumulation in mollusk shells. The results of our study have allowed us to exclude the presence of pollutants in bioclast dust. On the contrary, ecotoxicological tests performed on plants of agricultural interest at different doses of shell dust added to natural soils can allow us to evaluate the safeness of the application of this amendment to soils even if they are washed before being used. Finally, microchemical analyses are important in this phase to determine the structural and ultrastructural mineralization of carbonate. In this study, for example, the aragonite and orthorhombic phase of calcium carbonate, CaCO3, has been reported. During the formation of the shells, calcium carbonate binds to proteins forming two different types of crystals: calcite and aragonite; aragonite is more easily soluble than calcite. The calcium carbonate deposit may contain impurities and trace elements that can be released into the environment with the dissolution of the aragonite itself.
4.3. Case Study III—An Environmental “Crime Scene”
In this study, a classical analysis was performed concerning the chemical composition of effluent and ecotoxicological tests on D. magna species. It classified the effluent as acceptable to be discharged and not ecotoxic for the aquatic environment. Information was obtained on the real nature of the material that caused the color of the effluent and the high content of TSS. Microchemical analyses using electronic microscopy was a key technique to determine the solution of the case. It showed the presence of cordierite within a pollen matrix. It is important to point out that the impact of wastewater discharges to inland and marine water bodies is gaining attention. Drought conditions frequently exacerbate the effects, as low flows reduce the dilution of discharges. In other cases, the flow from some wastewater discharges can contribute significantly to waterway health if they are of high environmental quality and well managed. These concerns are driving the need for a more detailed assessment of the impact of wastewater discharges using new diagnostic methods to aid decision-making. A risk assessment process will yield more targeted and defensible information than a less formal process. These data are especially useful for decision makers and managers who need to evaluate alternatives, compare, or prioritize risks, determine the most cost-effective actions to maximize environmental gains, or determine the extent to which stressors must be reduced to achieve a given outcome.
5. Conclusions
In the Era of human technology and increasing sensitiveness toward environmental pollution, a single diagnostic approach to environmental study is considered weak and insufficient to completely understand phenomena that occur, and to give an exhaustive response to stakeholders. Integrating chemical analyses, ecotoxicology, biochemical biomarker responses, and microchemical FESEM and XRD analyses hit the mark and give precise and effective responses for environmental management. The possibility of having these early and non-destructive diagnostic techniques opens interesting scenarios for environmental monitoring techniques on marine organisms, including those of ecological importance. Finally, animal welfare is an increasing need and the development of not invasive and not-destructive diagnostic methods must be improved. Analyses performed using an FESEM and XRD approach on mussels and bioclasts (case studies I and II) did not require large quantities of samples, and shell splinter can be collected from the umbo zone (recent grown structure) of the animals without sacrificing them. This technique allows for a great added value as the animal that has not been killed can be exposed again and it is therefore possible to monitor the conchiolin deposition of the contaminant over time.
Author Contributions
Conceptualization, M.R. and A.C.; methodology, S.A., F.P., A.C. and A.S.; validation, A.C. and S.A.; formal analysis, M.R., P.P. and A.S.; investigation, S.A., F.P. and A.C.; resources, M.R.; data curation, S.A. and P.P.; writing—original draft preparation, M.R. and P.P.; writing—review and editing, M.R. and P.P.; supervision, project administration, funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ASSING SpA, grant number RG05100022021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Original data are available on request.
Acknowledgments
Authors are grateful to the administrative staff of BsRC supporting all researchers’ requests and to CERTEMA for supporting scientific collaborations among researchers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. 21—Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Morgan, K.R. Environmental impact assessment: The state of the art. Impact Assess. Proj. Apprais. 2012, 30, 5–14. [Google Scholar] [CrossRef]
- Zaghloul, A.; Saber, M.; Gadow, S.; Awad, F. Biological indicators for pollution detection in terrestrial and aquatic ecosystems. Bull. Natl. Res. Cent. 2020, 44, 127. [Google Scholar] [CrossRef]
- Russell, V.S. Pollution: Concept and definition. Biol. Conserv. 1974, 6, 157–161. [Google Scholar] [CrossRef]
- Pastorino, P.; Brizio, P.; Abete, M.C.; Bertoli, M.; Noser AG, O.; Piazza, G.; Prearo, M.; Elia, A.C.; Pizzul, E.; Squadrone, S. Macrobenthic invertebrates as tracers of rare earth elements in freshwater watercourses. Sci. Total Environ. 2020, 698, 134282. [Google Scholar] [CrossRef]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Anselmi, S.; Cavallo, A.; Ferrari, V.; Prevedelli, D.; Pastorino, P.; Renzi, M. Ecotoxicological effects of new generation pollutants (nanoparticles, amoxicillin and white musk) on freshwater and marine phytoplankton species. Chemosphere 2021, 279, 130623. [Google Scholar] [CrossRef]
- Xie, W.; Li, T.; Tiraferri, A.; Drioli, E.; Figoli, A.; Crittenden, J.C.; Liu, B. Toward the next generation of sustainable membranes from green chemistry principles. ACS Sustain. Chem. Eng. 2020, 9, 50–75. [Google Scholar] [CrossRef]
- Paracelso, P.T.B.a.H. Opera Omnia; Sumptibus Joan. Antonii, & Samuelis De Tournes: Geneva, Switzerland, 1658. [Google Scholar]
- Sicuro, B.; Castelar, B.; Mugetti, D.; Pastorino, P.; Chiarandon, A.; Menconi, V.; Galloni, M.; Prearo, M. Bioremediation with freshwater bivalves: A sustainable approach to reducing the environmental impact of inland trout farms. J. Environ. Manag. 2020, 276, 111327. [Google Scholar] [CrossRef]
- Bertoli, M.; Pastorino, P.; Lesa, D.; Renzi, M.; Anselmi, S.; Prearo, M.; Pizzul, E. Microplastics accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: Novel insights in a riverine ecosystem. Sci. Total Environ. 2022, 804, 150207. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Di Blasio, A.; Barcelò, D.; Anselmi, S.; Colussi, S.; Alberti, S.; Tedde, G.; Dondo, A.; Ottino, M.; et al. Microplastics Occurrence in the European Common Frog (Rana temporaria) from Cottian Alps (Northwest Italy). Diversity 2022, 14, 66. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Senderovich, Y.; Izhaki, I.; Halpern, M. Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE 2010, 5, e8607. [Google Scholar] [CrossRef] [PubMed]
- Cverenkárová, K.; Valachovičová, M.; Mackul’ak, T.; Žemlička, L.; Bírošová, L. Microplastics in the food chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef]
- Pignattelli, S.; Broccoli, A.; Piccardo, M.; Felline, S.; Terlizzi, A.; Renzi, M. Short-term physiological and biometrical responses of Lepidium sativum seedlings exposed to PET-made microplastics and acid rain. Ecotoxicol. Environ. Saf. 2021, 208, 111718. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Piccardo, M.; Terlizzi, A.; Renzi, M. Effects of polyethylene terephthalate (PET) microplastics and acid rain on physiology and growth of Lepidium sativum. Environ. Pollut. 2021, 282, 116997. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, M.; Provenza, F.; Grazioli, E.; Anselmi, S.; Terlizzi, A.; Renzi, M. Impacts of Plastic-Made Packaging on Marine Key Species: Effects Following Water Acidification and Ecological Implications. J. Mar. Sci. Eng. 2021, 9, 432. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/nanoplastic toxicity in plants: An imminent concern. Environ. Monit. Assess. 2023, 195, 27. [Google Scholar] [CrossRef]
- Carson, R.L. Primavera Silenziosa; Universale Economica Feltrinelli Editore: Milan, Italiy, 1968; p. 336. (In Italian) [Google Scholar]
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef]
- Kasuya, M.; Teranishi, H.; Aoshima, K.; Katoh, T.; Horiguchi, H.; Morikawa, Y.; Nishijo MIwata, K. Water Pollution by Cadmium and the Onset of Itai-itai Disease. Water Sci. Technol. 1992, 25, 149–156. [Google Scholar] [CrossRef]
- Bacci, E. Ecotoxicology of Organic Contaminants; CRC Press: Boca Raton, FL, USA, 1993; p. 176. [Google Scholar]
- Renzi, M.; Guerranti, C.; Anselmi, S.; Provenza, F.; Leone, M.; La Rocca, G.; Cavallo, A. A multidisciplinary approach to Posidonia oceanica detritus management (Port of Sperlonga, Italy): A story of turning a problem into a resource. Water 2022, 14, 2856. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef]
- Kaegi, R.; Ulrich, A.; Sinnet, B.; Vonbank, R.; Wichser, A.; Zuleeg, S.; Simmler, H.; Brunner, S.; Vonmont, H.; Burkhardt, M.; et al. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ. Pollut. 2008, 156, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ. Sci Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef] [PubMed]
- Wahie, S.; Lloyd, J.J.; Farr, P.M. Sunscreen ingredients and labelling: A survey of products available in the UK. Clin. Exp. Dermatol. Clin. Dermatol. 2007, 32, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Brennan, A.; Diamond, S.A. Phototoxicity of TiO2 nanoparticles under solar radiation to two aquatic species: Daphnia magna and Japanese medaka. Environ. Toxicol. Chem. 2012, 31, 1621–1629. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.L.; Jonsson, C.M.; Fraceto, L.F. Ecotoxicology of nano-TiO2–an evaluation of its toxicity to organisms of aquatic ecosystems. Int. J. Environ. Res. 2012, 6, 33–50. [Google Scholar]
- Chen, J.; Poon, C.S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussain, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Gnanasekar, K.; Rambabu, B. Nanostructure Semiconductor Oxide Powders and Thin Films for Gas Sensor. Surf. Sci. 2002, 200, 780. [Google Scholar]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Renzi, M.; Blašković, A. Ecotoxicity of nano-metal oxides: A case study on Daphnia magna. Ecotoxicology 2019, 28, 878–889. [Google Scholar] [CrossRef]
- Goldberg, E.; Bowen, V.; Farrington, J.; Harvey, G.; Martin, J.; Parker, P.; Risebrough, R.W.; Robertson, R.W.; Schneider, E.; Gamble, E. The Mussel Watch. Environ. Conserv. 1978, 5, 101–125. [Google Scholar] [CrossRef]
- Gagné, F.; Auclair, J.; Turcotte, P.; Fournier, M.; Gagnon, C.; Sauvé, S.; Blaise, C. Ecotoxicity of CdTe quantum dots to freshwater mussels: Impacts on immune system, oxidative stress and genotoxicity. Aquat. Toxicol. 2008, 86, 333–340. [Google Scholar] [CrossRef]
- Koehler, A.; Marx, U.; Broeg, K.; Bahns, S.; Bressling, J. Effects of nanoparticles in Mytilus edulis gills and hepatopancreas—A new threat to marine life? Mar. Environ. Res. 2008, 66, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Doyle, H.; Blasco, J.; Redmond, G.; Sheehan, D. Exposure of the blue mussel, Mytilus edulis, to gold nanoparticles and the pro-oxidant menadione. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 167–174. [Google Scholar] [CrossRef]
- Hanna, S.K.; Miller, R.J.; Muller, E.B.; Nisbet, R.M.; Lenihan, H.S. Impact of engineered zinc oxide nanoparticles on the individual performance of Mytilus galloprovincialis. PLoS ONE 2013, 8, e61800. [Google Scholar] [CrossRef]
- Canesi, L.; Frenzilli, G.; Balbi, T.; Bernardeschi, M.; Ciacci, C.; Corsolini, S.; Della Torre, C.; Fabbri, R.; Faleri, C.; Focardi, S.; et al. Interactive effects of n-TiO2 and 2, 3, 7, 8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2014, 153, 53–65. [Google Scholar] [CrossRef]
- Tang, Y.; Xin, H.; Yang, S.; Guo, M.; Malkoske, T.; Yin, D.; Xia, S. Environmental risks of ZnO nanoparticle exposure on Microcystis aeruginosa: Toxic effects and environmental feedback. Aquat. Toxicol. 2018, 204, 19–26. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Pinheiro, J.P.; Cancio, I.; Bebianno, M.J. Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquat. Toxicol. 2012, 118, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Chora, S.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J. Proteomic response of mussels Mytilus galloprovincialis exposed to CuO NPs and Cu2+: An exploratory biomarker discovery. Aquat. Toxicol. 2014, 155, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Provenza, F.; Anselmi, S.; Specchiulli, A.; Piccardo, M.; Barceló, D.; Prearo, M.; Pastorino, P.; Renzi, M. Sparkling plastic: Effects of exposure to glitter on the Mediterranean mussel Mytilus galloprovincialis. Environ. Toxicol. Pharmacol. 2022, 96, 103994. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Badary, O.A.; Abdel-Maksoud, S.; Ahmed, W.A.; Owieda, G.H. Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci. 2005, 76, 2125–2135. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Cicero, A.M.; Di Girolamo, I. Metodologie Analitiche di Riferimento del Programma di Monitoraggio per il Controllo dell’Ambiente Marino Costiero (Triennio 2001–2003); Ministero dell’Ambiente e della Tutela del Territorio, ICRAM: Rome, Italy, 2001. [Google Scholar]
- IRSA/CNR. Analytical Methods for Water; Polygraphic Institute of the Italian Government: Rome, Italy, 1994; Volume 100. [Google Scholar]
- US EPA. Method 3051a: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils; Revision 1; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- US EPA. Method 6020B: Inductively Coupled Plasma—Mass Spectrometry, Part of Test Methods for Evaluating Solid Waste, Physical/Chemical Methods; Revision 2; US EPA: Washington, DC, USA, 2014. [Google Scholar]
- APAT. Agenzia per la protezione dell’ambiente e per i servizi tecnici. Manuali e Linee Guida 29/2003: Metodi analitici per le acque. 2003. Vol I. Available online: https://www.irsa.cnr.it/wp/wp-content/uploads/2022/04/Vol2_Sez_4000_InorganiciNonMetallici.pdf. (accessed on 27 December 2022).
- Ottaviani, M.; Bonadonna, L. (Eds.) Rapporti ISTISAN 2007/31. Metodi Analitici di Riferimento per le Acque Destinate al Consumo Umano ai Sensi del D.L. 31/2001; Metodi Chimici. Met ISS BCA 023; Istituto Superiore Di Sanita’: Rome, Italy, 2007; p. 328. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2277_allegato.pdf (accessed on 30 November 2020).
- EC 1-2008 UNI EN ISO 15586:2004. Qualità dell’acqua—Determinazione di elementi in traccia mediante spettrometria di assorbimento atomico con fornetto di grafite. Available online: https://store.uni.com/p/UNINI1558600C1/ec-1-2008-uni-en-iso-15586-2004/UNINI1558600C1. (accessed on 27 December 2022).
- Lopez-Serrano Oliver, A.; Munoz-Olivas, R.; Sanz Landaluze, J.; Rainieri, S.; Cámara, C. Bioaccumulation of ionic titanium and titanium dioxide nanoparticles in zebrafish eleutheroembryos. Nanotoxicology 2015, 9, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Seitz, F.; Rosenfeldt, R.R.; Schulz, R. Effects of nanoparticles in fresh waters: Risks, mechanisms and interactions. Freshw. Biol. 2016, 61, 2185–2196. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Bradley, T.; Moore, J.T.; Kuykindall, T.; Minella, L. Acute and chronic toxicity of nano-scale TiO2 particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity. Nanotoxicology 2009, 3, 91–97. [Google Scholar] [CrossRef]
- Kulacki, K.J.; Cardinale, B.J.; Keller, A.A.; Bier, R.; Dickson, H. How do stream organisms respond to, and influence, the concentration of titanium dioxide nanoparticles? A mesocosm study with algae and herbivores. Environ. Toxicol. Chem. 2012, 31, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Binh CT, T.; Adams, E.; Vigen, E.; Tong, T.; Alsina, M.A.; Gaillard, J.F.; Gray, K.A.; Peterson, C.G.; Kelly, J.J. Chronic addition of a common engineered nanomaterial alters biomass, activity and composition of stream biofilm communities. Environ. Sci. Nano 2016, 3, 619–630. [Google Scholar] [CrossRef]
- Pastorino, P.; Broccoli, A.; Bagolin, E.; Anselmi, S.; Cavallo, A.; Prearo, M.; Renzi, M. A Multidisciplinary Approach to Evaluate the Effects of Contaminants of Emerging Concern on Natural Freshwater and Brackish Water Phytoplankton Communities. Biology 2021, 10, 1039. [Google Scholar] [CrossRef]
- Metzler, D.M.; Li, M.; Erdem, A.; Huang, C.P. Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chem. Eng. J. 2011, 170, 538–546. [Google Scholar] [CrossRef]
- González, I.D.; Navarro, R.M.; Alvarez-Galvan, M.C.; Rosa, F.; Fierro, J.L.G. Performance enhancement in the water–gas shift reaction of platinum deposited over a cerium-modified TiO2 support. Catal. Commun. 2008, 9, 1759–1765. [Google Scholar] [CrossRef]
- Handy, R.D.; Henry, T.B.; Scown, T.M.; Johnston, B.D.; Tyler, C.R. Manufactured nanoparticles: Their uptake and effects on fish—A mechanistic analysis. Ecotoxicology 2008, 17, 396–409. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. Int. J. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Barmo, C.; Ciacci, C.; Canonico, B.; Fabbri, R.; Cortese, K.; Balbi, T.; Marcomini, A.; Pojana, G.; Gallo, G.; Canesi, L. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2013, 132, 9–18. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, R.; Gallo, G.; Vallotto, D.; Marcomini, A.; Pojana, G.J.A. T. Biomarkers in Mytilus galloprovincialis exposed to suspensions of selected nanoparticles (Nano carbon black, C60 fullerene, Nano-TiO2, Nano-SiO2). Aquat. Toxicol. 2010, 100, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Balbi, T.; Grassi, G.; Frenzilli, G.; Bernardeschi, M.; Smerilli, A.; Guidi, P.; Canesi, L.; Nigro, M.; Monaci, F.; et al. Titanium dioxide nanoparticles modulate the toxicological response to cadmium in the gills of Mytilus galloprovincialis. J. Hazard. Mater. 2015, 297, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Ward, J.E.; Mason, R. An examination of the ingestion, bioaccumulation, and depuration of titanium dioxide nanoparticles by the blue mussel (Mytilus edulis) and the eastern oyster (Crassostrea virginica). Mar. Environ. Res. 2015, 110, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, D.; Ning, K.; Sui, Y.; Hu, M.; Lu, W.; Wang, Y. Hemocyte responses of the thick shell mussel Mytilus coruscus exposed to nano-TiO2 and seawater acidification. Aquat. Toxicol. 2016, 180, 1–10. [Google Scholar] [CrossRef]
- Johnson, B.D.; Gilbert, S.L.; Khan, B.; Carroll, D.L.; Ringwood, A.H. Cellular responses of eastern oysters, Crassostrea virginica, to titanium dioxide nanoparticles. Mar. Environ. Res. 2015, 111, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Marisa, I.; Marin, M.G.; Caicci, F.; Franceschinis, E.; Martucci, A.; Matozzo, V. In vitro exposure of haemocytes of the clam Ruditapes philippinarum to titanium dioxide (TiO2) nanoparticles: Nanoparticle characterisation, effects on phagocytic activity and internalisation of nanoparticles into haemocytes. Mar. Environ. Res. 2015, 103, 11–17. [Google Scholar] [CrossRef]
- Shi, W.; Han, Y.; Guo, C.; Zhao, X.; Liu, S.; Su, W.; Zha, S.; Wang, Y.; Liu, G. Immunotoxicity of nanoparticle nTiO2 to a commercial marine bivalve species, Tegillarca granosa. Fish Shellfish. Immunol. 2017, 66, 300–306. [Google Scholar] [CrossRef]
- Wu, F.; Falfushynska, H.; Dellwig, O.; Piontkivska, H.; Sokolova, I.M. Interactive effects of salinity variation and exposure to ZnO nanoparticles on the innate immune system of a sentinel marine bivalve, Mytilus edulis. Sci. Total Environ. 2020, 712, 136473. [Google Scholar] [CrossRef]
- Xia, B.; Zhu, L.; Han, Q.; Sun, X.; Chen, B.; Qu, K. Effects of TiO2 nanoparticles at predicted environmental relevant concentration on the marine scallop Chlamys farreri: An integrated biomarker approach. Environ. Toxicol. Pharmacol. 2017, 50, 128–135. [Google Scholar] [CrossRef]
- Baun, A.; Hartmann, N.B.; Grieger, K.; Kusk, K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: A brief review and recommendations for future toxicity testing. Ecotoxicology 2008, 17, 387–395. [Google Scholar] [CrossRef]
- Caballero-Diaz, E.; Guzman-Ruiz, R.; Malagon, M.M.; Simonet, B.M.; Valcarcel, M. Effects of the interaction of single-walled carbon nanotubes with 4-nonylphenol on their in vitro toxicity. J. Hazard. Mater. 2014, 275, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Zhu, B.; Xiao, X.; Li, Y.; Dirbaba, N.B.; Zhou, B.; Wu, H. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat. Toxicol. 2015, 161, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, Y.; Song, C.; Zhu, X.; Xing, B. Titanium dioxide nanoparticles as carrier facilitate bioaccumulation of phenanthrene in marine bivalve, ark shell (Scapharca subcrenata). Environ. Pollut. 2014, 192, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Shi, X.; Zhang, L.; Wang, Q.; Wang, X.; Guo, Y.; Zhou, B. Effect of titanium dioxide nanoparticles on the bioavailability, metabolism, and toxicity of pentachlorophenol in zebrafish larvae. J. Hazard. Mater. 2015, 283, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Shi, Q.; Guo, Y.; Hua, J.; Wang, X.; Zhou, B. Enhanced bioconcentration of bisphenol A in the presence of nano-TiO2 can lead to adverse reproductive outcomes in zebrafish. Environ. Sci. Technol. 2016, 50, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, R.; Liu, J.; Wei, Z.; Wang, L.; Yang, S.; Huang, Q.; Wang, Z. Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity. Environ. Pollut. 2016, 208, 732–738. [Google Scholar] [CrossRef]
- Canesi, L.; Borghi, C.; Ciacci, C.; Fabbri, R.; Vergani, L.; Gallo, G. Bisphenol-A alters gene expression and functional parameters in molluscan hepatopancreas. Mol. Cell. Endocrinol. 2007, 276, 36–44. [Google Scholar] [CrossRef]
- Jemec, A.; Tišler, T.; Drobne, D.; Sepčić, K.; Jamnik, P.; Roš, M. Biochemical biomarkers in chronically metal-stressed daphnids. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 61–68. [Google Scholar] [CrossRef]
- Monteiro, R.; Costa, S.; Coppola, F.; Freitas, R.; Vale, C.; Pereira, E. Toxicity beyond accumulation of Titanium after exposure of Mytilus galloprovincialis to spiked seawater. Environ. Pollut. 2019, 244, 845–854. [Google Scholar] [CrossRef]
- Skrabal, S.A. Distributions of dissolved titanium in Chesapeake Bay and the Amazon River Estuary. Geochim. Et Cosmochim. Acta 1995, 59, 2449–2458. [Google Scholar] [CrossRef]
- Knauss, K.G.; Dibley, M.J.; Bourcier, W.L.; Shaw, H.F. Ti (IV) hydrolysis constants derived from rutile solubility measurements made from 100 to 300 °C. Appl. Geochem. 2001, 16, 1115–1128. [Google Scholar] [CrossRef]
- Schmidt, J.; Vogelsberger, W. Aqueous long-term solubility of titania nanoparticles and titanium (IV) hydrolysis in a sodium chloride system studied by adsorptive stripping voltammetry. J. Solut. Chem. 2009, 38, 1267–1282. [Google Scholar] [CrossRef]
- Yan, L.; Stallard, R.F.; Key, R.M.; Crerar, D.A. Trace metals and dissolved organic carbon in estuaries and offshore waters of New Jersey, USA. Geochim. Cosmochim. Acta 1991, 55, 3647–3656. [Google Scholar] [CrossRef]
- Yokoi, K.; van den Berg, C.M. Determination of titanium in sea water using catalytic cathodic stripping voltammetry. Anal. Chim. Acta 1991, 245, 167–176. [Google Scholar] [CrossRef]
- Ward, J.E.; Shumway, S.E. Separating the grain from the chaff: Particle selection in suspension-and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol. 2004, 300, 83–130. [Google Scholar] [CrossRef]
- Møhlenberg, F.; Riisgård, H.U. Efficiency of particle retention in 13 species of suspension feeding bivalves. Ophelia 1978, 17, 239–246. [Google Scholar] [CrossRef]
- Riisgård, H.U. Efficiency of particle retention and filtration rate in 6 species of Northeast American bivalves. Mar. Ecol. Prog. Ser. Oldendorf 1988, 45, 217–223. [Google Scholar] [CrossRef]
- Calliari, L.J.; Machado, A.A.; Marroig, P.; Vinzon, S.; Gianuca, N. Mud deposits at Cassino beach: Role of dredging. Geo-Mar. Lett. 2020, 40, 1031–1043. [Google Scholar] [CrossRef]
- Scialla, S.; Carella, F.; Dapporto, M.; Sprio, S.; Piancastelli, A.; Palazzo, B.; Adamiano, A.; Degli Esposti, L.; Iafisco, M.; Piccirillo, C. Mussel Shell-Derived Macroporous 3D Scaffold: Characterization and Optimization Study of a Bioceramic from the Circular Economy. Mar. Drugs 2020, 18, 309. [Google Scholar] [CrossRef]
- Martino, S. Fisheries and Aquaculture in the Lagoon of Orbetello. In Proceedings of the European Association of Agricultural Economists 95th Seminar, Civitavecchia, Italy, 9–10 December 2005; pp. 363–378. [Google Scholar]
- Tamburini, E.; Turolla, E.; Fano, E.A.; Castaldelli, G. Sustainability of mussel (Mytilus galloprovincialis) farming in the Po river delta, northern Italy, based on a life cycle assessment approach. Sustainability 2020, 12, 3814. [Google Scholar] [CrossRef]
- Wang, W.; Wei, W.; Gao, S.; Chen, G.; Yuan, J.; Li, Y. Agricultural and aquaculture wastes as concrete components: A Review. Front. Mater. 2021, 8, 762568. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).