Culture of Gracilaria gracilis and Chondracanthus teedei from Vegetative Fragments in the Field and Carpospores in Laboratory
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Species
2.2. Species Identification
2.3. Cultivation in the Natural Environment (In Situ)
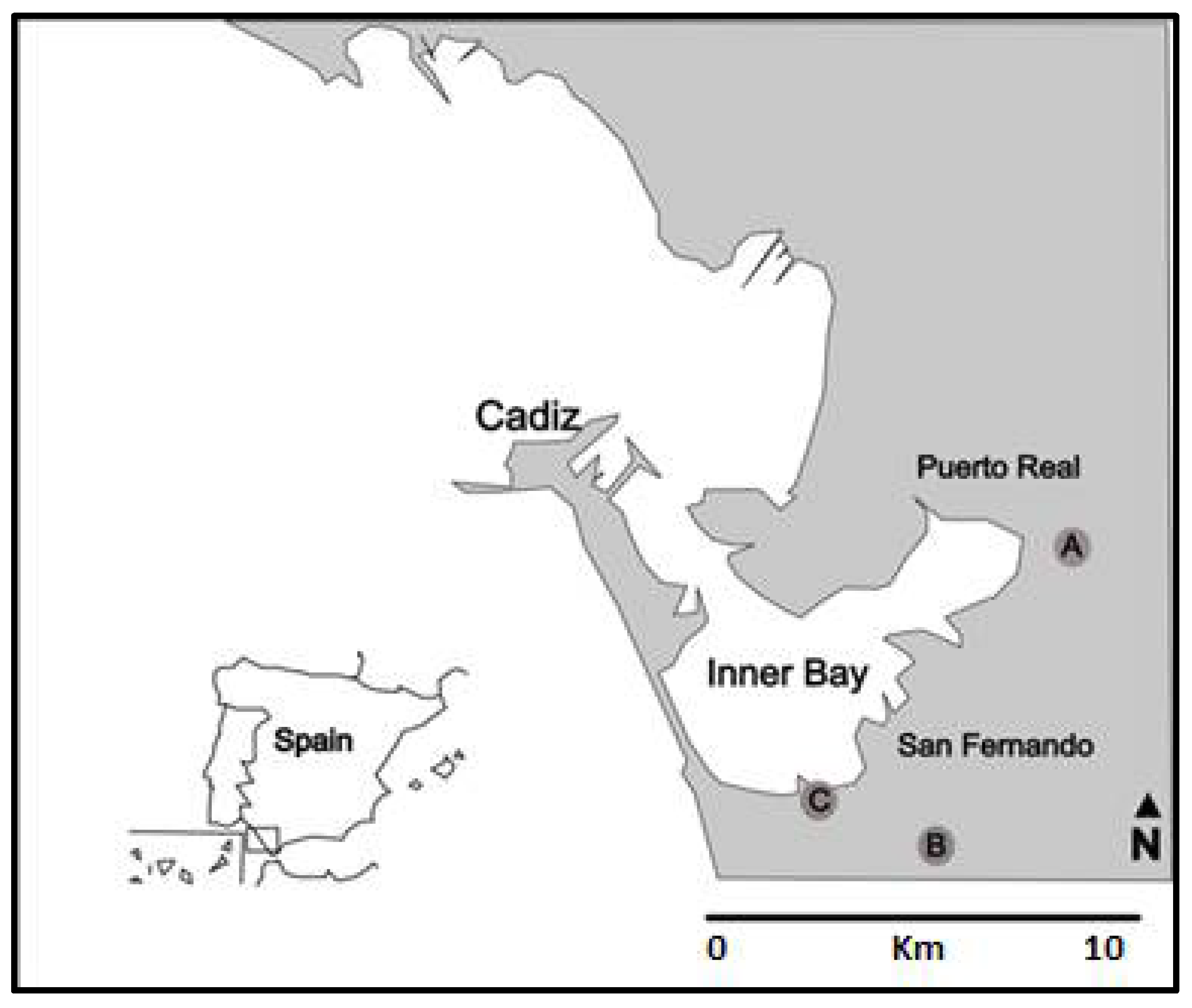
2.3.1. Characteristics of the Culture Site
2.3.2. Cultivation System and Growth Rate
2.4. Culture in the Laboratory
2.4.1. Collection Sites
2.4.2. Collection and Cleaning of Carpogonial Branches
2.4.3. Release of Carpospores
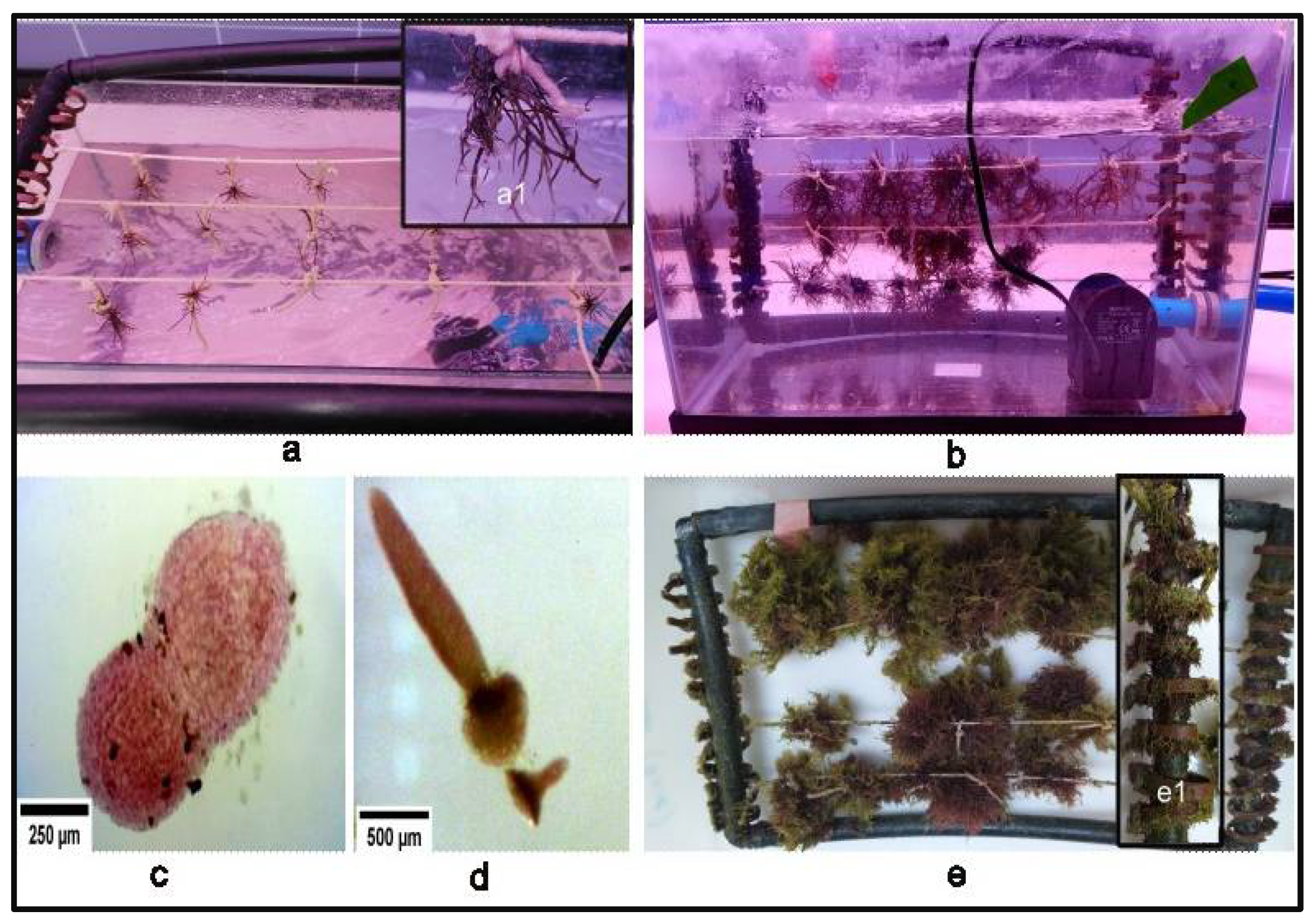
2.4.4. Cultivation of Thalli in the Aquarium and Growth Rate
2.5. Statistical Analysis
3. Results
3.1. Species Identification
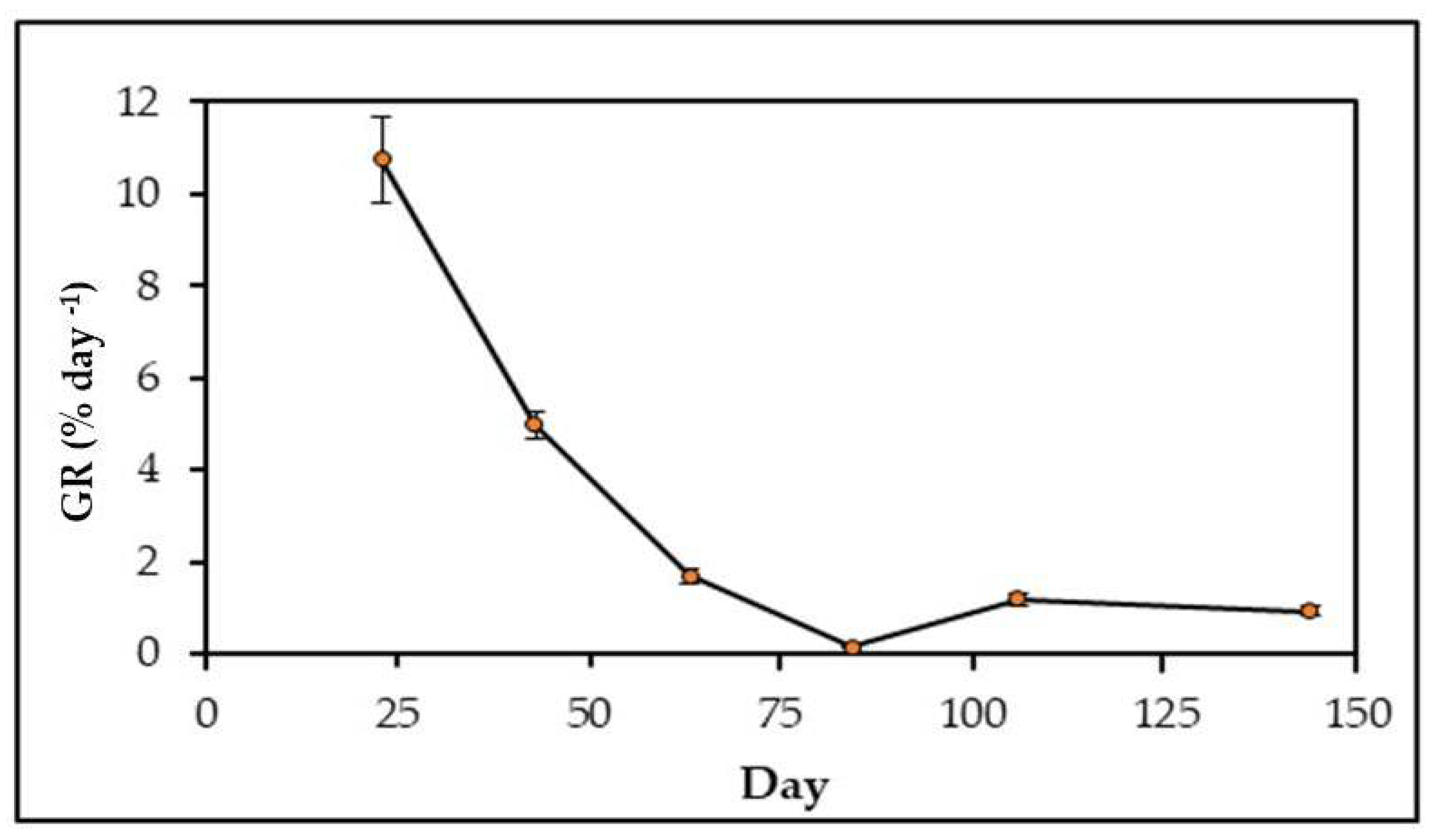
3.2. Field Growth Rates
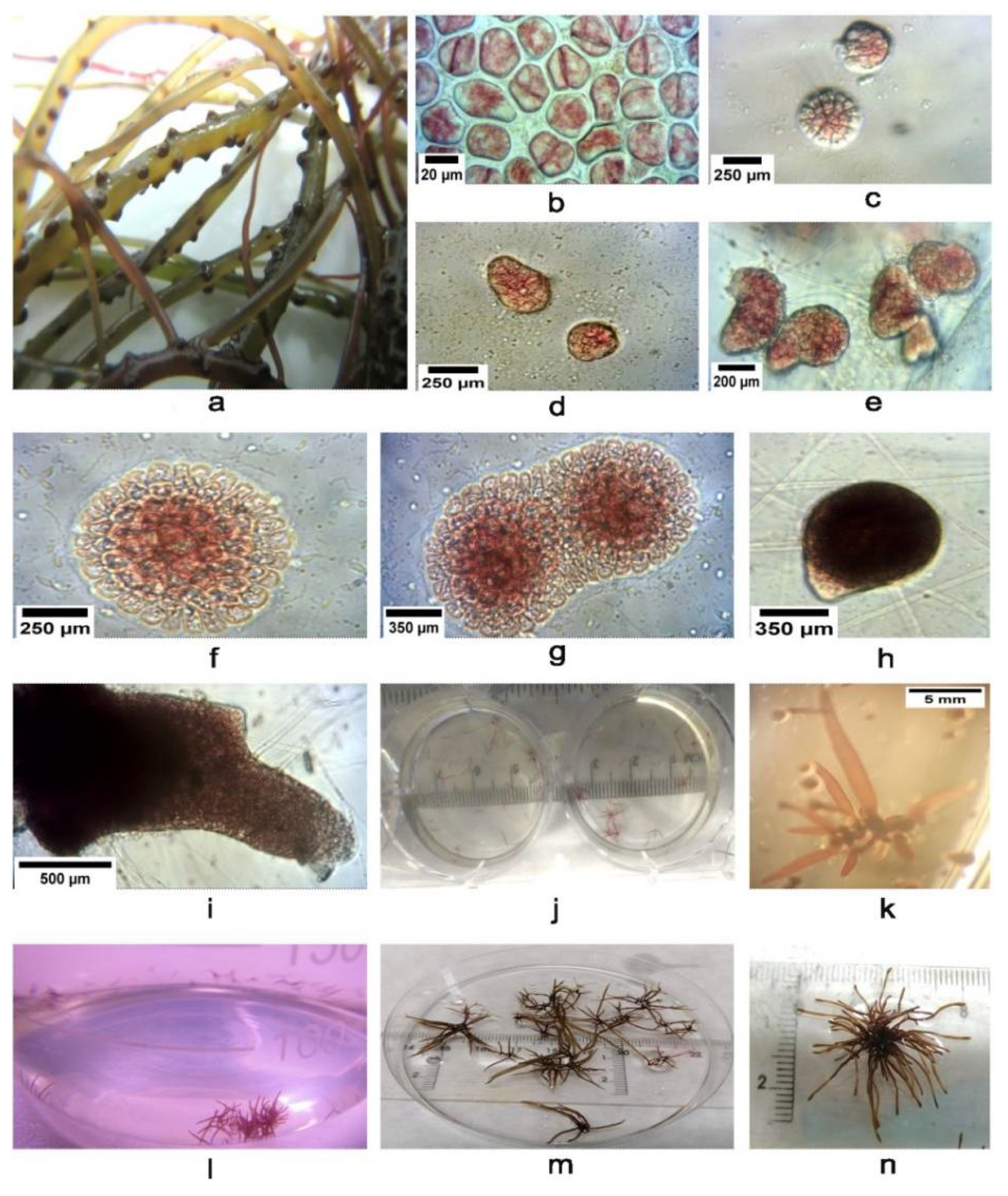
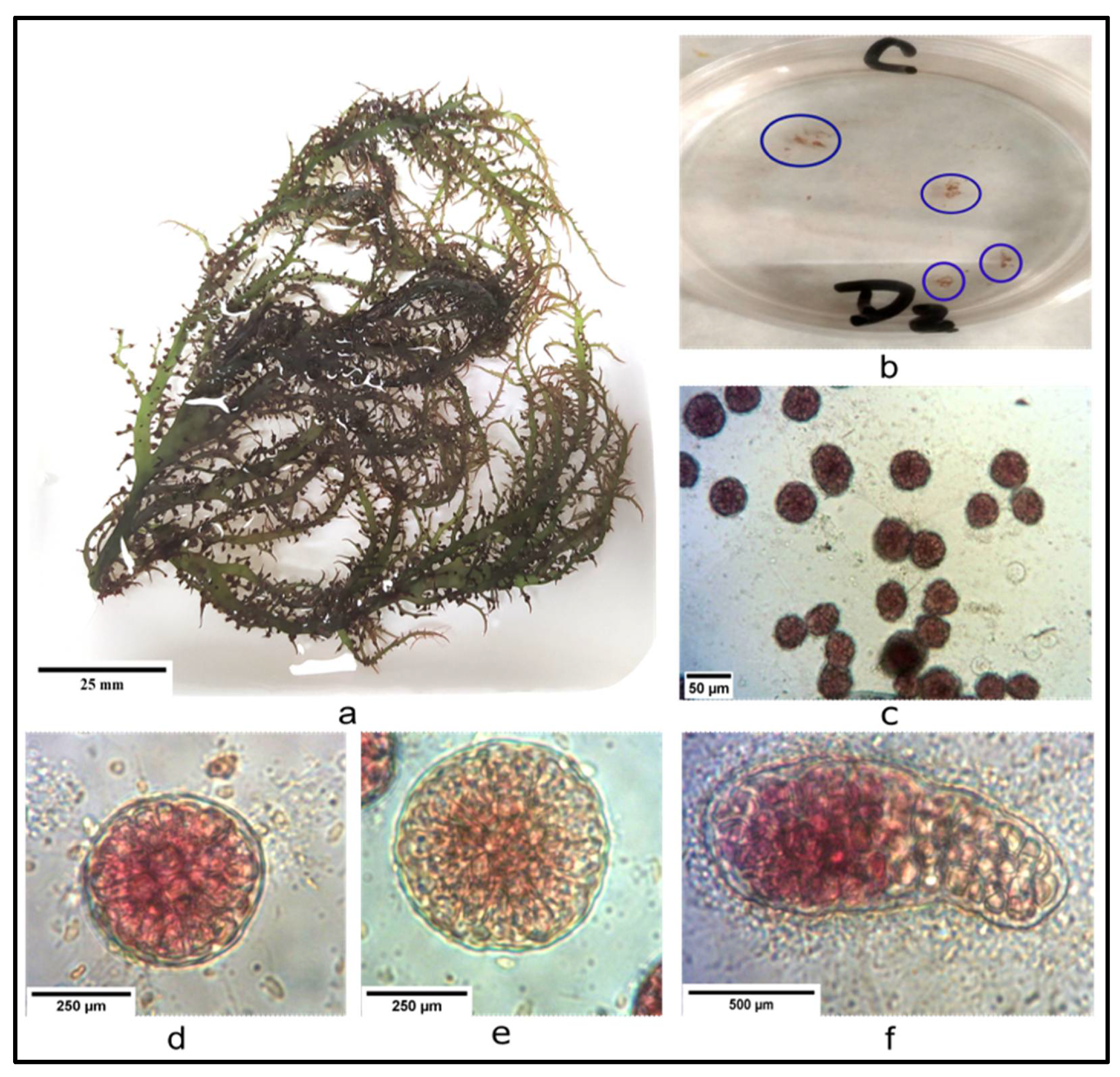
3.3. Harvesting, Cleaning and Sporulation
3.4. Spore Culture
4. Discussion
4.1. Species Identification
4.2. Cleaning and Collection
4.3. Growing from Spores of Gracilaria Gracilis
4.4. Growing from Spores of Chondracanthus Teedei
4.5. Growth Rates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Ferreira Barbosa, D.; Lucia Pereira Dias, T.; Faria Lopes, S.; Cristina de Souza Duarte, R.; Maria Duarte do Amaral, F. Community Structure and Functional Traits of Mollusks Associated with Coastal Reef Macroalgae in Northeastern Brazil. Mar. Ecol. 2019, 40, e12563. [Google Scholar] [CrossRef]
- Lynch, S.A.; Breslin, R.; Bookelaar, B.; Rudtanatip, T.; Wongprasert, K.; Culloty, S.C. Immunomodulatory and Antiviral Effects of Macroalgae Sulphated Polysaccharides: Case Studies Extend Knowledge on their Importance in Enhancing Shellfish Health, and the Control of a Global Viral Pathogen Ostreid Herpesvirus-1 MicroVar. Polysaccharides 2021, 2, 202–217. [Google Scholar] [CrossRef]
- Ismail, M.M.; Alotaibi, B.S.; EL-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, R.; Acquaviva, M.; Stabili, L.; Cecere, E.; Petrocelli, A.; Narracci, M. Antibacterial Activity of Marine Macroalgae against Fish Pathogenic Vibrio Species. Open Life Sci. 2013, 8, 646–653. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Čož-Rakovac, R.; Šubarić, D.; Jerković, I.; Ačkar, Đ.; Jokić, S. Macroalgae in the Food Industry—Opportunities and Challenges. Eng. Power 2020, 15, 14–19. [Google Scholar]
- Yang, L.-E.; Lu, Q.-Q.; Brodie, J. A Review of the Bladed Bangiales (Rhodophyta) in China: History, Culture and Taxonomy. Eur. J. Phycol. 2017, 52, 251–263. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Kim, J.K.; Yarish, C.; Pereira, R. Tolerances to Hypo-Osmotic and Temperature Stresses in Native and Invasive Species of Gracilaria (Rhodophyta). Phycologia 2016, 55, 257–264. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The Red Seaweed Gracilaria gracilis as a Multi Products Source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for Food and Industrial Applications. In Food Industry, 1st ed.; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2013; p. 760. [Google Scholar] [CrossRef]
- Neveux, N.; Bolton, J.J.; Bruhn, A.; Roberts, D.A.; Ras, M. The Bioremediation Potential of Seaweeds: Recycling Nitrogen, Phosphorus, and Other Waste Products. In Blue Biotechnology: Production and Use of Marine Molecules; La Barre, S., Bates, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 217–239. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- FAO. FAO Aquaculture News. No. 63 (May). Rome. 2021. Available online: http://www.fao.org/3/cb4850en/cb4850en.pdf (accessed on 10 April 2022).
- Callaway, E. Lab Staple Agar Runs Low: Dwindling Seaweed Harvest Imperils Reagent Essential for Culturing Microbes. Nature 2015, 7581, 171. Available online: Link.gale.com/apps/doc/A437223628/HRCA?u=anon~ac3ff0d9&sid=googleScholar&xid=b176955b (accessed on 30 January 2022). [CrossRef]
- Lauzon-Guay, J.-S.; Ugarte, R.A.; Morse, B.L.; Robertson, C.A. Biomass and Height of Ascophyllum Nodosum after Two Decades of Continuous Commercial Harvesting in Eastern Canada. J. Appl. Phycol. 2021, 33, 1695–1708. [Google Scholar] [CrossRef]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Bravo, A.; Buschmann, A.H.; Valenzuela, M.E.; Uribe, M.; Vergara, P.A.; Buitano, M.S. Evaluation of Artificial Intertidal Enclosures for Gracilaria Farming in Southern Chile. Aquac. Eng. 1992, 11, 203–216. [Google Scholar] [CrossRef]
- Friedlander, M. Gracilaria conferta and its Epiphytes: The effect of culture conditions on growth. Bot. Mar. 1992, 35, 423–428. [Google Scholar] [CrossRef]
- Hurtado-Ponce, A.Q. Vertical Rope Cultivation of Gracilaria (Rhodophyta) Using Vegetative Fragments. Bot. Mar. 1990, 33, 477–482. [Google Scholar] [CrossRef]
- de Oliveira Bastos, E.; Horta, P.A.; Hayashi, L. Strain Selection in Chondracanthus teedei (Gigartinaceae, Rhodophyta) Using Tetraspore and Carpospore Progeny: Growth Rates, Tolerance to Temperature and Carrageenan Yield. J. Appl. Phycol. 2021, 33, 2379–2390. [Google Scholar] [CrossRef]
- Santelices, B.; Doty, M.S. A Review of Gracilaria Farming. Aquaculture 1989, 78, 95–133. [Google Scholar] [CrossRef]
- Levy, I.; Beer, S.; Friedlander, M. Strain Selection in Gracilaria spp. 2. Selection for High and Low Temperature Resistance in G. Verrucosa Sporelings. J. Appl. Phycol. 1990, 2, 163–171. [Google Scholar] [CrossRef]
- Glenn, E.P.; Moore, D.; Fitzsimmons, K.; Azevedo, C. Spore Culture of the Edible Red Seaweed, Gracilaria parvispora (Rhodophyta). Aquaculture 1996, 142, 59–74. [Google Scholar] [CrossRef]
- Michetti, K.M.; Martín, L.A.; Leonardi, P.I. Carpospore Release and Sporeling Development in Gracilaria gracilis (Gracilariales, Rhodophyta) from the Southwestern Atlantic Coast (Chubut, Argentina). J. Appl. Phycol. 2013, 25, 1917–1924. [Google Scholar] [CrossRef]
- Freitas, M.V.; Mouga, T.; Correia, A.P.; Afonso, C.; Baptista, T. New Insights on the Sporulation, Germination, and Nutritional Profile of Gracilaria Gracilis (Rhodophyta) Grown under Controlled Conditions. J. Mar. Sci. Eng. 2021, 9, 562. [Google Scholar] [CrossRef]
- Pérez Massad, I.; Ávila, M.; Contreras-Porcia, L.; Bulboa Contador, C. Spores Re-Suspending Technology, a New System Improving Spore Seeding for Culture of Commercial Red Seaweeds. Aquaculture 2020, 526, 735374. [Google Scholar] [CrossRef]
- Hernández, I.; Cara, C.L.; Sánchez-García, J.; Macías, M.; Love, R.; Bermejo, R. Cultivos de Macroalgas en el Litoral Gaditano: Estado Actual y Perspectivas para su Desarrollo. Algas, Bol. Inf. Soc. Esp. Ficol. 2016, 52, 5–10. [Google Scholar]
- Bermejo, R.; Macías, M.; Cara, C.L.; Sánchez-García, J.; Hernández, I. Culture of Chondracanthus teedei and Gracilariopsis longissima in a Traditional Salina from Southern Spain. J. App. Phycol. 2019, 31, 561–573. [Google Scholar] [CrossRef]
- Bermejo, R.; Cara, C.L.; Macías, M.; Sánchez-García, J.; Hernández, I. Growth Rates of Gracilariopsis longissima, Gracilaria bursa-pastoris and Chondracanthus teedei (Rhodophyta) Cultured in Ropes: Implication for N Biomitigation in Cadiz Bay (Southern Spain). J. Appl. Phycol. 2020, 32, 1879–1891. [Google Scholar] [CrossRef]
- Bermejo, R.; Macías, M.; Sánchez-García, F.; Love, R.; Varela-Álvarez, E.; Hernández, I. Influence of Irradiance, Dissolved Nutrients and Salinity on the Colour and Nutritional Characteristics of Gracilariopsis longissima (Rhodophyta). Algal Res. 2020, 52, 102121. [Google Scholar] [CrossRef]
- Pereira, L.; Silva, P. A Concise Review of the Red Macroalgae Chondracanthus teedei (Mertens Ex Roth) Kützing and Chondracanthus teedei var. lusitanicus (J.E. De Mesquita Rodrigues) Bárbara & Cremades. J. Appl. Phycol. 2021, 33, 111–131. [Google Scholar] [CrossRef]
- Martín, L.A.; Boraso de Zaixso, A.L.; Leonardi, P.I. Biomass Variation and Reproductive Phenology of Gracilaria gracilis in a Patagonian Natural Bed (Chubut, Argentina). J. Appl. Phycol. 2011, 23, 643–654. [Google Scholar] [CrossRef]
- Kützing, F.T. Phycologia Generalis: Oder, Anatomie, Physiologie und Systemkunde der Tange; Brockhaus: Leipzig, Berlin, 1843. [Google Scholar] [CrossRef]
- Cabioc’h, J.; Floc’h, J.Y.; Le Toquin, A.; Boudouresque, C.F.; Meinesz, A.; Verlaque, M. Guide to the Algae of the Seas of Europe: Atlantic and Mediterranean; Omega: Jackson, MI, USA, 1995. [Google Scholar]
- Schmidt, É.C.; Pereira, B.; Pontes, C.L.M.; dos Santos, R.; Scherner, F.; Horta, P.A.; de Paula Martins, R.; Latini, A.; Maraschin, M.; Bouzon, Z.L. Alterations in Architecture and Metabolism Induced by Ultraviolet Radiation-B in the Carragenophyte Chondracanthus teedei (Rhodophyta, Gigartinales). Protoplasma 2012, 249, 353–367. [Google Scholar] [CrossRef]
- Zinoun, M.; Cosson, J.; Deslandes, E. Influence of Culture Conditions on Growth and Physicochemical Properties of Carrageenans in Gigartina teedii (Rhodophyceae-Gigartinales). Bot. Mar. 1993, 36, 131–136. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L.; Hernández, I.; Vergara, J.J.; Brun, F.G.; León, Á. Those Curious and Delicius Seaweeds a Fascinating Voyage from Biology to Gastronomy; University of Cadiz: Cadiz, Spain, 2018. [Google Scholar]
- Steentoft, M.; Irvine, L.M.; Farnham, W.F. Two Terete Species of Gracilaria and Gracilariopsis (Gracilariales, Rhodophyta) in Britain. Phycologia 1995, 34, 113–127. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Fredericq, S.; Butler, B.S.; Hommersand, M.H.; Chase, M.W. A Gene Phylogeny of the Red Algae (Rhodophyta) Based on Plastid RbcL. Proc. Natl. Acad. Sci. USA 1994, 91, 7281–7285. [Google Scholar] [CrossRef] [PubMed]
- Freshwater, D.W.; Rueness, J. Phylogenetic Relationships of Some European Gelidium (Gelidiales, Rhodophyta) Species, Based on RbcL Nucleotide Sequence Analysis. Phycologia 1994, 33, 187–194. [Google Scholar] [CrossRef]
- Ministry of Transport, Mobility and Urban Agenda, State Ports. Available online: http://www.puertos.es/es-es/oceanografia/ (accessed on 10 March 2022).
- Yarish, C.; Redmond, S.; Kim, J.K. Gracilaria Culture Handbook for New England; Wrack Lines: Groton, CT, USA, 2012; Volume 72, Available online: https://opencommons.uconn.edu/wracklines/72 (accessed on 1 July 2022).
- Provasoli, L. Media and Prospects for the Cultivation of Marine Algae. In Proceedings of the US-Japan Conference, Hakone, Japan, 12–15 September 1966; pp. 63–75. [Google Scholar]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Recipes for Freshwater and Seawater Media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Phycological Society; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 429–532. [Google Scholar] [CrossRef]
- Allen, E.J.; Nelson, E.W. On the Artificial Culture of Marine Plankton Organisms. J. Mar. Biol. Assoc. 1910, 8, 421–474. [Google Scholar] [CrossRef]
- Provasoli, L.; McLaughlin, J.J.A.; Droop, M.R. The Development of Artificial Media for Marine Algae. Archiv. Mikrobiol. 1957, 25, 392–428. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.; Ryther, J.H. Studies of Marine Planktonic Diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Angriani, E.; Made, S.; Tahang, H. Seaweed Cultivation Business Development Strategy (Gracillaria sp.) through Spores Culture in Takalar Regency. Int. J. Environ. Agric. Biotech. 2021, 6, 120–126. [Google Scholar] [CrossRef]
- Pérez-Vas, R.; Puime Guillén, F.; Enríquez-Díaz, J. Valuation of a Company Producing and Trading Seaweed for Human Consumption: Classical Methods vs. Real Options. Int. J. Environ. Res. Public. Health 2021, 18, 5262. [Google Scholar] [CrossRef]
- Barbier, M.; Charrier, B.; Araujo, R.; Holdt, S.; Jacquemin, B.; Rebours, C. Phycomorph European Guidelines for a Sustainable Aquaculture of Seaweeds. In PEGASUS; Barbier, M., Charrier, B., Eds.; COST Action FA1406. M.: Roscoff, France, 2019; p. 194. [Google Scholar] [CrossRef]
- Hayashi, L.; de Cantarino, J.; Critchley, A.T. Challenges to the Future Domestication of Seaweeds as Cultivated Species: Understanding Their Physiological Processes for Large-Scale Production. Adv. Bot. Res. 2020, 95, 57–83. [Google Scholar] [CrossRef]
- Hu, Z.; Guiry, M.D.; Duan, D. Using the Ribosomal Internal Transcribed Spacer (ITS) as a complement Marker for Species Identification of Red Macroalgae. Hydrobiologia 2009, 635, 279–287. [Google Scholar] [CrossRef]
- Yang, M.Y.; Geraldino, P.J.L.; Kim, M.S. DNA Barcode Assessment of Gracilaria Salicornia (Gracilariaceae, Rhodophyta) from Southeast Asia. Bot. Stud. 2013, 54, 27. [Google Scholar] [CrossRef]
- Montes, M.; Rico, J.M.; García-Vázquez, E.; Borrell, Y.J. Morphological and Molecular Methods Reveal the Asian Alga Grateloupia imbricata (Halymeniaceae) Occurs on Cantabrian Sea Shores (Bay of Biscay). Phycologia 2016, 55, 365–370. [Google Scholar] [CrossRef]
- Saunders, G.W. Applying DNA Barcoding to Red Macroalgae: A Preliminary Appraisal Holds Promise for Future Applications. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 1879–1888. [Google Scholar] [CrossRef]
- Hassan, R.; Othman, M.N.A.; Harith, M.N.; Md Sah, A.S.R. Morphological Diversity of Gracilaria Blodgettii Harvey 1853 (Gracilariaceae, Rhodophyta) from Sarawak, Malaysian Borneo. Scientifica 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Gurgel, C.F.D.; Fredericq, S. Systematics of the Gracilariaceae (Gracilariales, Rhodophyta): A Critical Assessment Based on Rbcl Sequence Analyses1: Systematics of the Gracilariaceae. J. Phycol. 2004, 40, 138–159. [Google Scholar] [CrossRef]
- Goff, L.J.; Moon, D.A.; Coleman, A.W. Molecular Delineation of Species and Species Relationships in the Red Algal Agarophytes Gracilariopsis and Gracilaria (Gracilariales). J. Phycol. 1994, 30, 521–537. [Google Scholar] [CrossRef]
- Kawai, H.; Motomura, T.; Okuda, K. Isolation and Purification Techniques for Macroalgae. In Algal Culturing Techniques; Andersen, R.A., Ed.; Phycological Society; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 133–143. [Google Scholar] [CrossRef]
- Le, H.N.; Hughes, A.D.; Kerrison, P.D. Early Development and Substrate Twine Selection for the Cultivation of Sargassum muticum (Yendo) Fensholt under Laboratory Conditions. J. Appl. Phycol. 2018, 30, 2475–2483. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Marian, F.D.; Rodrigo, M.; Robaina, R.R. Sporulation and Sterilization Method for Axenic Culture of Gelidium canariensis. Prog. Ind. Microbiol. 1999, 35, 227–229. [Google Scholar] [CrossRef]
- Bulboa Contador, C.; Massad, I.P.; Contreras-Porcia, L.; Zapata, J.; Castañeda, F.; Ramírez, M.E.; Gil-Kodaka, P. Concise Review of Genus Chondracanthus (Rhodophyta: Gigartinales). J. Appl. Phycol. 2020, 32, 773–785. [Google Scholar] [CrossRef]
- Kain, J.M.; Destombe, C. A Review of the Life History, Reproduction and Phenology of Gracilaria. J. Appl. Phycol. 1995, 7, 269. [Google Scholar] [CrossRef]
- Mantri, V.A.; Thakur, M.C.; Kumar, M.; Reddy, C.R.K.; Jha, B. The Carpospore Culture of Industrially Important Red Alga Gracilaria dura (Gracilariales, Rhodophyta). Aquaculture 2009, 297, 85–90. [Google Scholar] [CrossRef]
- Lefebvre, C.A.; Destombe, C.; Godin, J. Le fonctionnement du carposporophyte de Gracilaria verrucosa et ses repercussions sur la strategie de reproduction. Cryptogam. Algol. 1987, 8, 113–126. [Google Scholar]
- Guzmán-Urióstegui, A.; Robledo, D. Factors Affecting Sporulation of Gracilaria cornea (Gracilariales, Rhodophyta) Carposporophytes from Yucatan, Mexico. In Sixteenth International Seaweed Symposium; Kain, J.M., Brown, M.T., Lahaye, M., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 285–290. [Google Scholar] [CrossRef]
- Garza-Sánchez, F.; Zertuche-González, J.A.; Chapman, D.J. Effect of Temperature and Irradiance on the Release, Attachment and Survival of Spores of Gracilaria pacifica Abbott (Rhodophyta). Bot. Mar. 2000, 43, 205–212. [Google Scholar] [CrossRef]
- Bonomi, B.J.; Oliveira, E.C.; Plastino, E.M.; Oliveira, M.C. Life History, Morphological Variability and Growth Rates of the Life Phases of Gracilaria tenuistipitata (Rhodophyta: Gracilariales) in Vitro. Sci. Mar. 2010, 74, 297–303. [Google Scholar] [CrossRef]
- Guimarães, M.; Plastino, E.M.; Oliveira, E.C. Life History, Reproduction and Growth of Gracilaria domingensis (Gracilariales, Rhodophyta) from Brazil. Bot. Mar. 1999, 42, 481–486. [Google Scholar] [CrossRef]
- Barbosa, I.M. Efeitos da Qualidade de Radiação na Fisiologia de Chondracanthus Teedei (Gigartinales, Rhodophyta). Graduação em Oceanografia; Universidade Federal de Santa Catarina: Florianopolis, Brazil, 2019; p. 51. Available online: https://repositorio.ufsc.br/handle/123456789/197545 (accessed on 3 March 2022).
- Guiry, M.D. Structure, Life History and Hybridization of Atlantic Gigartina teedii (Rhodophyta) in Culture. Br. Phycol. J. 1984, 19, 37–55. [Google Scholar] [CrossRef]
- do Rosärio, M.; de Braga, A. Reproductive Characteristics of Gigartina teedii (Roth) Lamouroux (Rhodophyta, Gigartinales), a Turf-forming Species: Field and Laboratory Culture Studies. Bot. Mar. 1990, 33, 401–410. [Google Scholar] [CrossRef]
- Wilson, A.J.; Critchley, A.T. Studies on Gracilaria gracilis (Stackhouse) Steentoft, Farnham and Irvine and Gracilaria aculeata (Hering) Papenfuss from Southern Africa. I. The Influence of Temperature, Irradiance, Salinity and Nitrogen-Nutrition on Growth. S. Afr. J. Bot. 1997, 63, 465–473. [Google Scholar] [CrossRef][Green Version]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Lo Giudice, A.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spanò, N.; et al. New Insights into the Culture Method and Antibacterial Potential of Gracilaria gracilis. Mar. Drugs 2018, 16, 492. [Google Scholar] [CrossRef]
- Mensi, F.; Nasraoui, S.; Bouguerra, S.; Ben Ghedifa, A.; Chalghaf, M. Effect of Lagoon and Sea Water Depth on Gracilaria gracilis Growth and Biochemical Composition in the Northeast of Tunisia. Sci. Rep. 2020, 10, 10014. [Google Scholar] [CrossRef]
- Martínez-Aragón, J.F.; Hernández, I.; Pérez-Lloréns, J.L.; Vázquez, R.; Vergara, J.J. Biofiltering Efficiency in Removal of Dissolved Nutrients by Three Species of Estuarine Macroalgae Cultivated with Sea Bass (Dicentrarchus labrax) Waste Waters 1. Phosphate. J. Appl. Phycol. 2002, 14, 365–374. [Google Scholar] [CrossRef]
- Hernández, I.; Pérez-Pastor, A.; Vergara, J.J.; Martínez-Aragón, J.F.; Fernández-Engo, M.Á.; Pérez-Lloréns, J.L. Studies on the Biofiltration Capacity of Gracilariopsis longissima: From Microscale to Macroscale. Aquaculture 2006, 252, 43–53. [Google Scholar] [CrossRef]

| Parameter | Conditions |
|---|---|
| Irradiance | 30 µmol m−2 s−1 |
| Temperature | ±18 °C |
| Light: dark cycle | 12:12 |
| Salinity | 34‰ |
| Culture medium | Changed once a week |
| Species (Initial Identification) | Genbank (% Similarity) |
|---|---|
| Chondracanthus teedei (Gametophyte) | Chondracanthus teedei (99.21) |
| Gracilariopsis longissima (Sporophyte) | Gracilaria gracilis (99.72) |
| Gracilariopsis longissima (Gametophyte) | Gracilaria gracilis (99.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Campos, M.; Pérez-Lloréns, J.L.; Barrena, F.; Pérez-González, C.M.; Hernández, I. Culture of Gracilaria gracilis and Chondracanthus teedei from Vegetative Fragments in the Field and Carpospores in Laboratory. J. Mar. Sci. Eng. 2022, 10, 1041. https://doi.org/10.3390/jmse10081041
López-Campos M, Pérez-Lloréns JL, Barrena F, Pérez-González CM, Hernández I. Culture of Gracilaria gracilis and Chondracanthus teedei from Vegetative Fragments in the Field and Carpospores in Laboratory. Journal of Marine Science and Engineering. 2022; 10(8):1041. https://doi.org/10.3390/jmse10081041
Chicago/Turabian StyleLópez-Campos, Malurisbel, José Lucas Pérez-Lloréns, Felipe Barrena, Claudia M. Pérez-González, and Ignacio Hernández. 2022. "Culture of Gracilaria gracilis and Chondracanthus teedei from Vegetative Fragments in the Field and Carpospores in Laboratory" Journal of Marine Science and Engineering 10, no. 8: 1041. https://doi.org/10.3390/jmse10081041
APA StyleLópez-Campos, M., Pérez-Lloréns, J. L., Barrena, F., Pérez-González, C. M., & Hernández, I. (2022). Culture of Gracilaria gracilis and Chondracanthus teedei from Vegetative Fragments in the Field and Carpospores in Laboratory. Journal of Marine Science and Engineering, 10(8), 1041. https://doi.org/10.3390/jmse10081041







