Abstract
The common bottlenose dolphin (Tursiops truncatus) is a cosmopolitan delphinid, regularly present in the Mediterranean Sea. According to previous studies, this dolphin tends to form resident geographical units scattered on the continental shelf. We investigated how the physiographic characteristics of the area of residence, with special reference to the size and shape of the continental shelf, affect the home range and the group size of the local units. We analysed and compared data collected between 2004–2016 by 15 research groups operating in different study areas of the Mediterranean Sea: the Alboran Sea, in the South-Western Mediterranean, the Gulf of Lion and the Pelagos Sanctuary for the marine mammals, in the North-Western Mediterranean, and the Gulf of Ambracia, in the North-Central Mediterranean Sea. We have found that in areas characterised by a wide continental platform, dolphins have wider home ranges and aggregate into larger groups. In areas characterized by a narrow continental platform, dolphins show much smaller home ranges and aggregate into smaller groups. The results obtained from this collective research effort highlight the importance of data sharing to improve our scientific knowledge in the field of cetaceans and beyond.
1. Introduction
The common bottlenose dolphin—Tursiops truncatus Montagu, 1821—hereafter referred to as bottlenose dolphin, is a cosmopolitan Delphinidae that can be found worldwide in all the oceans. Its distribution is usually confined between 45\1\2 parallels, in tropical and temperate waters of both hemispheres, except for the North Atlantic, where it can reach the 65\1\2 parallel [1,2,3,4,5].
This wide distribution of the species is associated with a certain level of morphometric differentiation among the different areas and latitudes, which seems consistent with the presence of a globally distributed offshore ecotype and several inshore ecotypes, scattered along the coasts [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20].
The inshore bottlenose dolphin communities show a high level of site fidelity, with home range sizes that can vary from tens of km2 [21] to hundreds of km2 [22]. According to a global synthesis provided by Wells and Scott [3], the home range of the inshore bottlenose dolphins generally includes areas of about 100–150 km2, even if wider home ranges have been reported for local geographical units [23]. It has been hypothesized that the home range sizes and movement patterns of this species depend on habitat differences [24], food resources [25] and reproductive resources [26].
In the context of the Mediterranean Sea, the bottlenose dolphin is considered a regularly present species and the second most sighted cetacean after the striped dolphin, Stenella coeruleoalba [27,28,29,30,31].
According to Notarbartolo di Sciara and Demma [29], the Mediterranean bottlenose dolphin would be more closely related to the Atlantic inshore ecotype, while Cañadas et al. [32,33], who studied this species in the context of the Alboran Sea, suggested a closer link with the offshore Atlantic ecotype. This apparent contradiction could in fact derive from the different ecological habits of the dolphins that inhabit the Alboran Sea (as also emerges from the present study). Gaspari et al. [34] found that samples from deeper parts of the Mediterranean (e.g., Ionian Sea) were genetically more similar to samples from the Atlantic offshore ecotype and suggested a potential differentiation between offshore and inshore ecotypes also in the Mediterranean context. However, the results from TursioMed, a networking project compiling census data at the Mediterranean level and involving 29 institutes from eight Mediterranean countries, covering 13 years of survey effort from 2004 to 2016 [35], showed that most sightings of the species occurred within the 200 m isobath marking the border of the continental shelf, while sightings outside this limit have been reported to be quite rare despite the sampling effort. This pattern of distribution seems quite consistent in all sampled study areas, with the already mentioned exception of the Alboran Sea, possibly confirming the inshore habit of the Mediterranean bottlenose dolphin, as originally stated by Notarbartolo Sciara and Demma [29]. Therefore, it is unclear to what extent the genetic differences found by Gaspari and co-authors, who examined mainly stranded animals, should be correlated to ecological habits (see also [14]).
According to Moura et al. [36], the Mediterranean and Black Sea (inshore) populations derive from a globally distributed offshore population, which would explain the presence of an “offshore” genetic inheritance in Mediterranean bottlenose dolphins, regardless of their current ecological habits.
Most studies based on photo identification data are consistent in describing the Mediterranean bottlenose dolphin as a primarily resident species. According to Gnone et al. [37], who studied the movement patterns of this species in the Pelagos Sanctuary (a specially protected area in the northwest of the Mediterranean basin, see below), the bottlenose dolphins move around their usual area of residence within a maximum distance of 50 kms. The resident behaviour and a local specialization, especially in feeding techniques, seem to produce a segregation between neighbouring dolphins and a clustering of the (meta) population in geographical and demographical units [37]. According to Carnabuci et al. [38], the connectivity (or disconnectivity) through the bottlenose dolphin units living in the Pelagos Sanctuary retraces the landscape features and its habitat breakages (see also [39]). The dolphins living in the Tuscany Archipelagos, for example, and those inhabiting the waters off the northwest coast of Corsica, are separated by a remarkable habitat breakage, delimited by the Corsica’s ‘‘finger’’ (Cape Corso). The east side (Tuscany Archipelagos) is characterized by shallow waters and sandy/muddy ecosystems, while the west side (northwest coast of Corsica) presents a typical narrow rocky platform and a steep slope. The “ecological distance” seems to overcome the geographical distance, producing a clear separation of the two units despite the geographical proximity.
The specialization on the residence habitat and the isolation between geographical units could be responsible for the genetic structure described by Natoli et al. [40] and, on a finer scale, by Gaspari et al. [34] and Brotons et al. [41].
As bottlenose dolphins form resident units scattered over the continental shelf, it is crucial to understand how the physiographic characteristics of the area of residence, which can be very different even in neighbouring areas, can affect the geographical units, shaping their structure.
For this purpose, we analysed six geographical units of bottlenose dolphins living in different areas of the Mediterranean Sea, comparing their home ranges (both as individuals and as a whole unit), their aggregation behaviour (group size), their abundance and density.
2. Material and Methods
2.1. Study Areas
The data were collected by different research groups in four areas of the Mediterranean basin, recognized as Important Marine Mammal Areas (IMMA) or Specially Protected Area of Mediterranean Importance (SPAMI), and in the neighbouring waters (Figure 1).
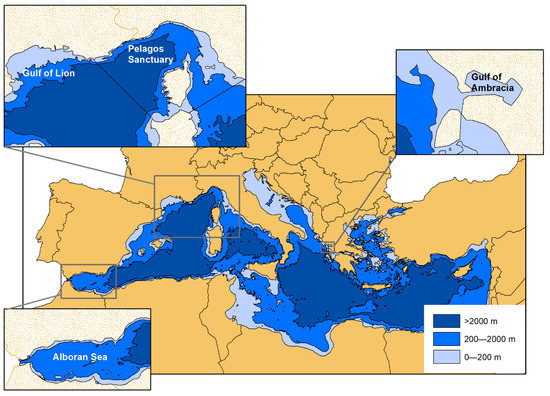
Figure 1.
The geographical location of the study areas within the Mediterranean basin: Alboran Sea, Gulf of Lione, Pelagos Sanctuary, Gulf of Ambracia.
2.1.1. Alboran Sea
The Alboran Sea is the westernmost area of the Mediterranean. Located at the entrance of the basin, it is connected to the Atlantic Ocean through the Strait of Gibraltar and represents a transition area from the ocean to the semi-enclosed Mediterranean Sea. It covers a water area of approximately 56,000 km2, between Spain and Morocco, with an average depth of 445 m and a maximum depth of 1500 m in its eastern portion. The seabed shows a complex profile and is crossed, in its central part, by an important chain of seamounts, the Alboran Ridge. The area has been recognized as IMMA, where the bottlenose dolphin is reported as a qualifying species [42]. Most of the data analysed in the present study come from the northern side of the Alboran Sea.
2.1.2. Gulf of Lion
The Gulf of Lion is a wide embayment, located in the easternmost portion of the French Mediterranean coast, up to the border with Spain. It covers an area of approximately 13,000 km2 over the continental shelf, with an average depth of about 250 m and no relevant habitat breakages. The Gulf of Lion is also included in the IMMAs list for the bottlenose dolphin identified as a qualifying species [43].
2.1.3. Pelagos Sanctuary
The Pelagos Sanctuary for Mediterranean Marine Mammals is the largest Mediterranean Marine Protected Area and it is listed as the biggest SPAMI within the framework of the Barcelona Convention. It is located in the north-western portion of the basin, and it covers an area of about 87,500 km2, extending over waters belonging to the territorial waters of France, Italy and the Principality of Monaco (and the adjacent international waters), including Corsican and north Sardinian coasts [44]. It comprises pelagic areas, deep water zones and the continental shelf, with remarkable oceanographic and physiographic differences. The western portion is characterized by typical rocky coasts, with a narrow continental shelf, furrowed by several submarine canyons, and a wide bathyal plain (2500–2700 m) [45]; the eastern area, on the contrary, has a much wider continental shelf, with typical sandy/muddy ecosystems. Compared to other Mediterranean regions, this area is characterized by very high levels of offshore primary productivity, induced by the interaction between geomorphologic, oceanographic and climatic factors [46]. All cetaceans regularly observed in the Mediterranean Sea can be found in this region, including the common bottlenose dolphin [37,39,47,48,49,50,51,52]. The Sanctuary area partially overlaps with the “North Western Mediterranean Sea, Slope and Canyon System IMMA”, which includes the bottlenose dolphin as (secondary) qualifying species [53].
2.1.4. Gulf of Ambracia
The Gulf of Ambracia (or Gulf of Amvrakikos) is a semi-enclosed gulf in north-western Greece. It has a total surface of about 400 km2, and it is connected to the Ionian Sea through a 700 m wide canal. The average depth is only 30 m, the maximum depth is 63 m, and its bottom mostly consists of mud or sand. Its waters are rich in nutrients and characterized by abundant wildlife. The dolphins of the Gulf of Ambracia are genetically differentiated from the surrounding Mediterranean metapopulation [54]. The area is recognized as IMMA, where the bottlenose dolphin is the only qualifying species [55].
2.2. Data Collection
We analysed the data collected between 2004–2016 by 15 different research groups operating in the above described Mediterranean regions (see Table 1) and shared on the Intercet platform (www.intercet.it, accessed on 10 May 2022) within the TursioMed project [35].

Table 1.
Summary of information on the research groups involved in the study.
Dedicated surveys were carried out by expert researchers between March–October, in favourable weather conditions (sea state < 4 in the Douglas scale), following random tracks within predefined sampling areas. Each research team used a GPS device to record the effort tracks (the routes travelled by the research vessel during the sampling activity) and the sighting points of the target species. Researchers counted the number of individuals observed in each group and collected photographic data for individual photo-identification [56].
2.3. Data Analysis
2.3.1. Distribution
The GIS system ArcGIS 9.3 was used to analyse the georeferenced data (the effort tracks and the sighting points of the target species) collected by the research groups involved in the study.
2.3.2. Photographic Data and Matching Process
The research groups selected their photographic data separately, but following common rules: (a) the quality of the image; and (b) the distinctiveness of the animals [35].
(a) Photographic quality was based on focus, lighting (the sun should be behind the camera for proper dorsal fin illumination), angle of the fin to the photographer (the fin should be in an orthogonal position with respect to the camera, to avoid deformation of the profile), contrast between dorsal fin and background and visibility of the fin [57,58,59].
(b) Identification value was based on the presence of notches, deformities, unusual fin shapes, scars and discoloration [60]. Notches, deformities and unusual fin shapes were considered to be permanent marks [58] and were used as primary distinctive elements for the photo-identification of the animals. Scars and discolorations were only used in association with primary distinctive elements.
The catalogues curated by the single research groups were then pooled to be matched. The matching between catalogues was performed with aid of FinfindR (https://github.com/haimeh/finFindR, accessed on 10 May 2022), a software for computer-assisted recognition and identification of the bottlenose dolphin dorsal fins [61]. Each recapture had to be confirmed visually by an experienced researcher, and if in doubt, a second person was asked to confirm the match.
We used the Simple Ratio Index (SRI) [62] to measure the degree of sharing between catalogues, calculated according to the following formula:
SRI = X/(a + b − X)
(a = total number of individuals in catalogue a; b = total number of individuals in catalogue b; and X = number of individuals shared between a and b).
The SRI can vary from 0 (no individuals shared between the catalogues matched) to 1 (all the individuals in the catalogue a are present in the catalogue b and vice versa), depending on the reciprocal size of the two catalogues and the number of individuals shared.
2.3.3. Connectivity Analysis and Cluster Identification
Following the matching, a network outline was drawn using the Spring embedding visualization [63,64] in NetDraw [65]. This was performed selecting those individuals sighted and recognised through photographic identification on at least 4 occasions (including the first identification event), 4 being the average number of times each individual was sighted, as in Carnabuci et al. [38].
A more severe selection could have provided more info about the “intimate” structure within each cluster, but we were actually looking to the network “macro-structure” on a Mediterranean scale. Furthermore, we could verify that the size of the minimum convex polygon (see below) did not increase with the increase in the number of captures >4, reassuring us this was an appropriate choice.
The connectivity analysis was then performed on a selection of 704 individuals of the 2604 photo-identified (about 27%). Two animals were assumed to be associated in the network if sighted in the same group on at least one occasion, following a 0–1 criterion (0, never sighted together; 1, sighted together at least once).
To identify the clusters within the network, we carried out a Girvan–Newman analysis, based on edge betweenness measurements [66,67,68,69]. The best division for the network was identified using a modularity index Q, where the highest Q value indicates the best division [69,70]. This index varies between 0 (community structure no better than in a random network) and 1 (strong community structure) and it can be considered meaningful if it falls in the range 0.3–0.7 [71].
Each identified cluster was assigned a different colour, which was maintained in all the figures to facilitate the comprehension of the analytical process.
2.3.4. Mapping the Different Geographical Units
Following the connectivity analysis and cluster identification, all the sightings of the individuals selected (all the individuals with at least 4 captures, see above) were then plotted on the map using the same cluster colours, in order to identify the areas of occupancy of the bottlenose dolphins belonging to each cluster.
2.3.5. Movement Analysis through Minimum Convex Polygon (MCP)
For each of the selected individuals, we have drawn the minimum convex polygon [72] as a simplified representation of its home range. Each (convex) polygon includes all the sighting points of the same individual and describes its maximum recorded movements. Since the distribution of sightings clearly shows that the bottlenose dolphins are confined within the border of the continental shelf area (except for the bottlenose dolphins inhabiting the Alboran Sea), we cropped the MCPs following the 200 m isobath profile. This crop has been implemented for all the individual MCPs, with the exception of the dolphins inhabiting the Alboran Sea (see above) and the ones residing in the Gulf of Ambracia (which is entirely included within the continental shelf).
Cropping the MCP along the 200 m isobath is a needed correction to avoid serious bias when drawing the MCPs. The bias derives from the curvature of the coast profile and is proportional to its degree. Since the shelf usually follows the coastal contour, an MCP drawn across a concave coastline will result in the inclusion of pelagic waters outside the 200 isobath that were actually free of sightings and thus will result in an overestimated area.
A boxplot was used for a descriptive analysis of MCP area values and to identify outliers within each cluster. We then compared the average size of the individual MCPs belonging to each cluster, excluding the outliers identified through the boxplot analysis. The statistical significance of the differences in the average size of the cluster MCPs was assessed with a Kruskal–Wallis test for independent samples (a non-parametric test was chosen in consideration of the Kolmogorov–Smirnov normality test results). For the pairwise comparison of the clusters, the significance was adjusted by the Bonferroni correction for multiple tests.
The average MCP of the different clusters were then compared to the corresponding MP and to the average width of the continental platform in the area of occupancy, to test for possible correlation through a nonlinear regression analysis.
To identify the home range of the different clusters/geographical units, we overlapped the MCPs of the single individuals belonging to the same cluster, so as to obtain a complex multi-polygon (MP).
The home range of a geographical unit can be underestimated if the sampling effort was concentrated in an area too small to describe the movements of the dolphins in their full extent. We then calculated, for each cluster, the ratio between the average MCP (the average of the individual MCPs) and the MP of the cluster, to obtain an “overlap index”. The overlap index can be used as a measure of the extent of effort relative to the size of the average MCP. For example, if the overlap index approaches 1, it means that the total sampled area approximates the average MCP, suggesting a possible bias given by effort coverage (since the MCP cannot be larger than the same sampled area). Therefore, the overlap index can provide information on the reliability of the results obtained.
2.3.6. Group Size
For each cluster/geographical unit, we calculated the average group size, considering all the sightings where at least one of the selected individuals was present. We then compared the mean group sizes across clusters/geographical units, testing the statistical significance of the differences through a Kruskal–Wallis test for independent samples (also in this case a non-parametric test was chosen in consideration of the Kolmogorov–Smirnov normality test results). For the pairwise comparison of the clusters, the significance was adjusted by the Bonferroni correction for multiple tests.
We used a nonlinear regression analysis to test the relationship between: the individual’s MCP and the average size of the groups in which the same individual was sighted, defined as the “individual group size” (IGS), and the average MCP and the average group size in the different clusters The statistical significance of the correlations found was verified with the ANOVA Regression test.
2.3.7. Abundance Estimates and Density
We estimated the abundance of each cluster/geographical unit using a closed population mark-recapture model [73], performed with Capture [74,75], within the Mark program [76]. Through this analysis, we produced single year abundance estimates of the animals identified by means of permanent natural markings, considering all the sightings where at least one individual of the above-described selection (all the individuals with at least 4 captures) was identified. This model, applied to the annual dataset, was considered the most appropriate approach according to the above home range analysis and literature consultation [37,58,59,77].
Mark–recapture models for closed populations rely on a series of assumptions: (a) the mark (or the recognition system) should be reliable during the study period; (b) the capture of an animal should not modify the probability of the same individual being captured again; (c) the population should be considered closed, meaning that emigration/immigration and birth/death events should not affect the size of the population during the study period, neither should the portion of marked and unmarked animals change within the population; (d) all of the individuals of the population should have the same probability of being captured at each sampling event. Strong violations of these assumptions may lead to bias in the abundance estimate and, therefore, a proper validation of the data collected should be done in advance [78]
In our study, assumption (a) is respected, as we considered permanent marks for individual photo-identification. Although these may change over time (new marks may be added), the use of multiple elements reduces the risk for false negative or positive matches, especially if the estimate is performed over a single year period. Assumption (b) is also respected, as the photographic capture technique does not significantly affect the behavioural response of the individual to the next capture event. Regarding the assumption (c), the bottlenose dolphins are long living and slow breeding animals; according to Rossi et al. [79], the annual fertility rate in the Mediterranean context is approximately 0.29–0.41 and is compensated by a similar death rate. In relation to immigration and emigration events, we based the assumption of closure on the previous cluster analysis. In fact, the connections between clusters are few over the entire research period (13 years) and are minimal (if any are present) within the years selected for the abundance estimates. This is consistent with the resident behaviour exhibited by the dolphins in the study areas, in agreement with Gnone et al. [37]. Assumption (d) is usually the most difficult to satisfy [58,80] and its violation may lead to an underestimation of the population size. Within this study, capture probabilities are unlikely to be constant, owing to the multiple research groups conducting the data collection. For this reason, a time dependent and/or heterogeneity model, which allows a certain tolerance of assumptions (d) should be used. We chose the time dependent variant (Chao Mt) of the Chao model [81] (Chao et al., 1992), based on Wilson et al. [58], Chilvers and Corkeron [60] and Gnone et al. [37].
For each cluster, we chose the annual estimate with the maximum number of individuals captured (photo-identified), considering it as the most reliable (best annual estimate). Finally, the population density was calculated as the ratio between the abundance estimate and the size of the cluster MP.
3. Results
3.1. Distribution
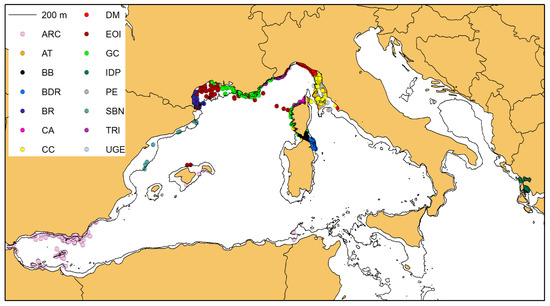
A total of 306,388 kms of sampling effort was performed by the research groups in their respective study areas (Table 2, Figure 2), and 3927 sightings of bottlenose dolphins were recorded (Table 2, Figure 3). In the surveyed areas, the distribution of bottlenose dolphins was clearly confined within the 200 m isobath that marks the boundary of the continental shelf, except for the Alboran Sea, where dolphins have been sighted even in deeper waters, confirming previous findings from Cañadas et al. [32].

Table 2.
The sampling effort implemented by each research group (Effort (km), the resulting sightings of bottlenose dolphins (Sightings Tt), the sightings during which photographic data for photo-identification of the individuals were collected (Sight. Photo-ID) and the total of individuals photo-identified (Ind. Photo-ID).
3.2. Photographic Data and Matching Process
In 2007 sightings (out of a total of 3927), the researchers collected photographic data for the photo-identification of individuals, and a gross total of 3513 dolphins were photo-identified by the different groups (Table 2, Figure 4). The comparison between catalogues resulted in 909 positive matches, lowering the net total to 2604 distinctively identified individuals (Table 3). Only in 13 cases (highlighted orange in Table 3) did the Simple Ratio Index (SRI) exceed 0.1, always between research groups sampling in the same area or in neighbouring areas, confirming the strong site-fidelity of the bottlenose dolphins.
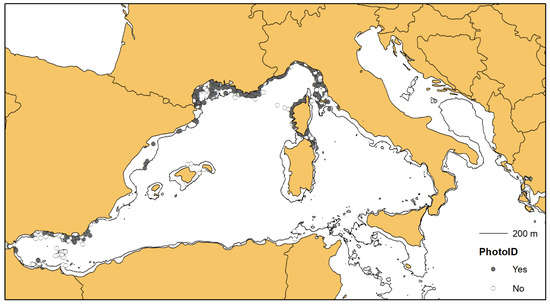
Figure 4.
Of the 3927 sightings of bottlenose dolphins, 2007 produced photo-identification data (see the dark points).

Table 3.
The result of the matching between photo-ID catalogues (the number of individuals shared across the different catalogues) and relative SRI between catalogues (in parentheses). The SRI can vary from 0 (no individuals shared between catalogues) to 1 (all the individuals in the first catalogue are present in the second one and vice versa). In green the cells with SRI > 0, in orange the cells with SRI > 0.1.
3.3. Connectivity Analysis and Cluster Identification
The connectivity analysis was performed on the photo-identified dolphins with at least 4 “captures”, for a total of 703 individuals. The Spring embedding visualization shows five major aggregations around the points of densest connection (Figure 5) and the Girvan–Newman analysis identifies six clusters within the same network (best division for the network was identified with the value of Qmax = 0.656), based on the betweenness of the bonds (Figure 5): (A) composed by 95 individuals; (B) composed by 132 individuals; (C) composed by 261 individuals; (D) composed by 66 individuals; (E) composed by 36 individuals; and (F) composed by 113 individuals.
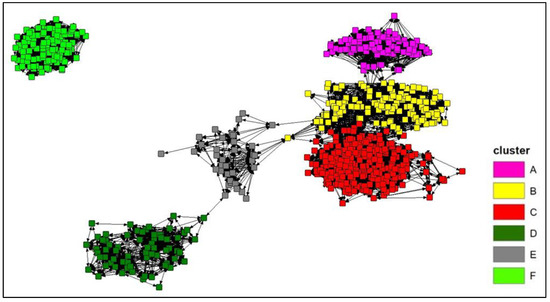
Figure 5.
The connectivity network of the photo-identified bottlenose dolphins with at least four captures (703 individuals) according to the Spring embedding lay out. A Girvan–Newman analysis was performed to identify the clusters within the network according to the betweenness of the bonds (see the different colours).
3.4. Mapping the Different Geographical Units
Following the connectivity analysis and cluster identification, all the sightings of the selected individuals were plotted on the map, using the same cluster colours coding as in Figure 4. The clusters clearly correspond to geographical units with their own area of distribution (Figure 6).
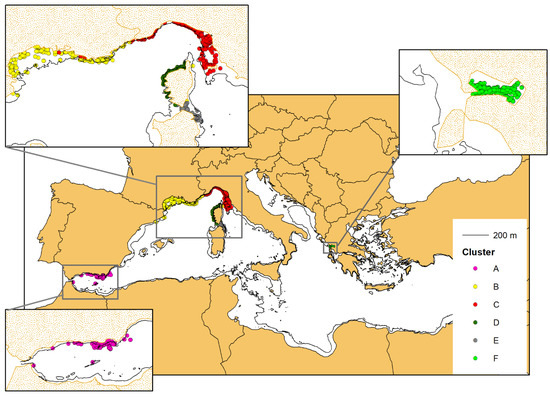
Figure 6.
The sighting positions of the photo-identified bottlenose dolphins with at least four captures (703 individuals). The colours correspond to the belonging cluster (see Figure 5).
3.5. Movement Analysis through Minimum Convex Polygon (MCP)
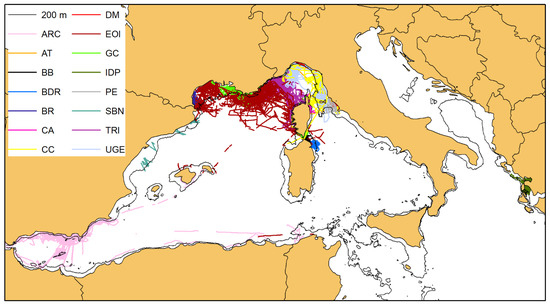
For each of the 703 individuals selected, we have drawn the respective Minimum Convex Polygon (MCP), as shown in Figure 7. When calculating the surface area (in km2) of the individual MCPs, the portion lying outside the 200 m isobath, delimiting the boundaries of the bottlenose dolphin habitat, was cropped (cropping has not been applied to the MCPs of the dolphins inhabiting the Alboran Sea, whose habitat does not appear to be so sharply confined to the continental shelf, see above).
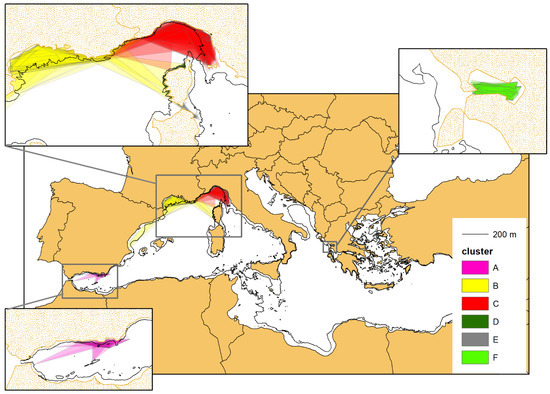
Figure 7.
The minimum convex polygons (MCPs) of the photo-identified bottlenose dolphins with at least four captures (703 individuals). The colours correspond to the belonging cluster (see Figure 4). When calculating the surface area (in km2) of the individual MCPs, the portion lying outside the 200 m isobath, delimiting the boundaries of the bottlenose dolphin habitat, was cropped, except for cluster A (purple colour), residing in the Alboran Sea (see the main text for further details).
The boxplot analysis identified a total of 19 outliers among the clusters (Figure 8). These individuals, representing a minority within each cluster (19 individuals out of 703), seem to travel for much longer distances and probably correspond to the so-called “long travellers”, already described by Gnone et al. [37] and Carnabuci et al. [38]. All the outliers identified were excluded from subsequent analyses.
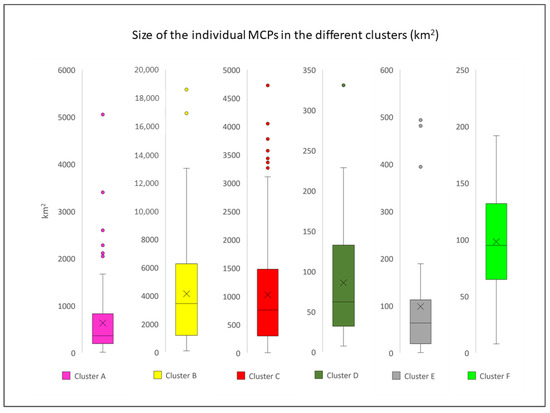
Figure 8.
Boxplot analysis of the MCPs surface areas (km2) for each cluster (each boxplot has a different scale).
For each cluster, the single MCPs were overlapped to obtain the complex multi-polygons (MPs), which represent an approximation of the area of occupancy of the different geographical units (Figure 9). The MPs tend to occupy all of the continental platform (CP), with the clear exception of cluster E, whose MP takes only a small portion of the available CP. This is consistent with the limited (nearshore) sampling effort carried out in the corresponding study area (see also the Discussion section).
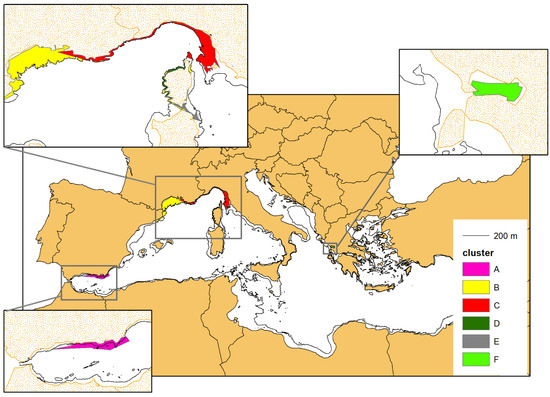
Figure 9.
The multi-polygons resulting from the overlap of the single MCPs of the individuals belonging to the same cluster (see Figure 7). The MCPs were cropped following the 200 m isobaths marking the border of the continental shelf, except for cluster A (purple colour), residing in the Alboran Sea (see the main text for further details).
The difference in the average size of the MCPs across the clusters (Table 4) was found statistically significant (p-value < 0.01) based on the Kruskal–Wallis test implemented. In the pairwise comparison of the clusters, the medians were found significantly different (p-value < 0.01) in all possible combinations except for clusters A–C, D–E, D–F, and E–F (p-value > 0.05).

Table 4.
The average area (km2) of the individual MCP, the area (km2) of the complex MP and the average width of the continental platform (CP) in the area of occupancy of the related cluster (see also Figure 9). We could not calculate the average CP width for the occupancy area of cluster F, as the Gulf of Ambracia is fully included in the continental platform.
The average MCP and the complex MP of each cluster are positively correlated (p-value < 0.01) and seem to grow proportionally to the average width of the continental platform in the corresponding area of occupancy (Table 4): as the continental platform gets wider also the average MCP increases, following an exponential curve (Figure 10).
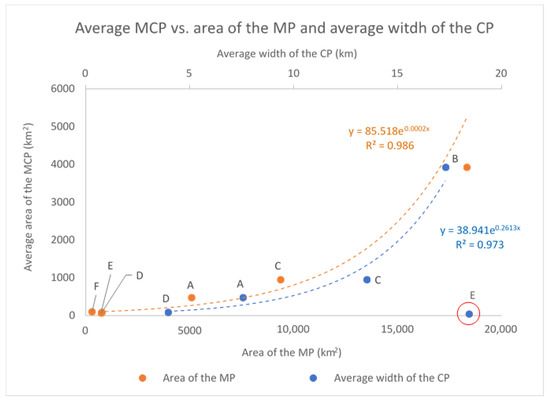
Figure 10.
The average MCP and the complex MP of each cluster are positively correlated (p-value < 0.01) and seem to grow proportionally to the average width of the continental platform in the corresponding area of occupancy, following an exponential curve. Cluster E (whose value was not included in the regression analysis) seems to escape this general pattern, since its average MCP is quite small compared to the available platform. This is due to the limited (inshore) effort carried out in the corresponding study area (see the text for discussion on this point).
As already mentioned, cluster E seems to escape this general pattern, since both its average MCP and MP are quite small considering the available platform, bit this is consistent with the nearshore sampling effort carried out in the area. In fact, most of the sightings of cluster E (565 over a total of 701, around 80%) were recorded inside the bay named “Golfo Aranci”, where dolphins are used to feed around an aquaculture facility, located a few hundred meters from the coast. The rest of the data (136 sightings, around 20%) come from the waters immediately adjacent to the gulf and from the waters between the islands of the Maddalena archipelago and the Strait of Bonifacio. Virtually no (photo-ID) data were collected in open waters, as it is clear from Figure 6, Figure 7 and Figure 9. We will further discuss this point in the Discussion section.
The average size of the individual MCPs and the size of the MP for each cluster are listed in Table 5. The ratio between these two values was calculated as the “overlap index”. This tends to be quite homogeneous across clusters, with the exception of cluster B (which has an overlap index about double that of clusters A, C, D, and E) and F (which has an overlap index more than triple that of clusters A, C, D, and E).

Table 5.
The average area (km2) of the individual MCPs and the area (km2) of the multi-polygons (MPs) of each cluster. In the last column, the ratio between these two values was used to calculate the “overlap index”.
3.6. Group Size
The average group size (the total number of individuals per sighting) of the clusters (Table 6) was found to be significantly different (p-value < 0.01) according to the Kruskal–Wallis analysis implemented. In the pairwise comparisons, the medians were found to be significantly different (p-value < 0.01) in all possible combinations, except for clusters D–E (p-value > 0.05).

Table 6.
The average group size (total number of individuals per sighting) in the different clusters/geographical units. In the third column the sample size (number of sightings) on which the average was calculated (n sight.).
Since cluster C occupies an area characterized by a large “body” on the east side, where the continental platform is quite wide, and by a long “tail” extending to the west, where the continental platform is rather narrow (see Figure 9), we also compared the average group size between these two areas. In the large portion (from La Spezia to the east end) the average group size is 15.52 (15.52 ± 0.68 SE, n = 479), while in the “tail” (from La Spezia to the west end) the average groups size is 13.05 (13.05 ± 0.87 SE, n = 136). The difference was found to be statistically significant (p-value < 0.05). Furthermore, the group size in the narrow portion ranges from 1 to 46 individuals, while in the large portion the group size ranges from 1 to 100 individuals (all the large aggregations > 46 individuals were observed in the wide platform portion).
The “individual group sizes” (IGS, the average size of the groups in which the same individual was sighted), displayed against the individual MCPs in a scatterplot, show an interesting pattern of distribution (Figure 11), with the points well distributed along a logarithmic curve. Through the colour of the points, the data show that dolphins living in wider areas have a wider range of both the MCP and the IGS, with the clear exception of the dolphins belonging to cluster A (in the Alboran Sea), which, although having small and narrow-ranged MCP, demonstrate a high and very wide-ranging IGS. We then tested the logarithmic relationship between MCP and IGS, excluding from the analysis all the dolphins belonging to cluster A (Figure 12). The relationship was found to be statistically significant according to the ANOVA Regression test (F = 279; p-value < 0.01).
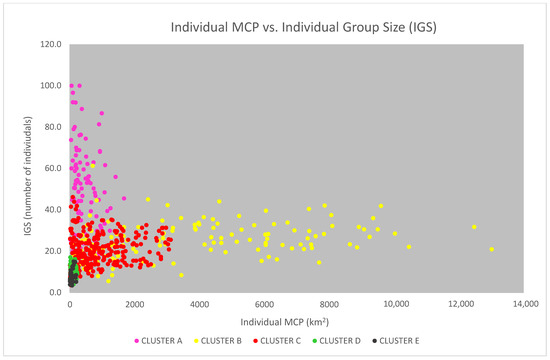
Figure 11.
The individual MCPs are displayed in the scatterplot against the individual group size (IGS). The colour of the points indicates the cluster to which they belong (the data from the cluster F—Gulf of Ambracia—were not available for this analysis).
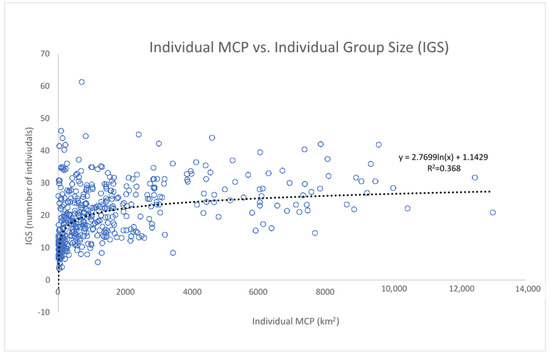
Figure 12.
The correlation between the individual group size (IGS) and the individual MCP is statistically significant according to an ANOVA test (F = 279; p-value < 0.01). Cluster A was excluded from the analysis (see above).
We should notice that the individuals belonging to cluster E (in the geographical area Corsica S–Sardinia NE) seem to behave like dolphins from cluster D (in Corsica W), despite the inhabit an area where the continental platform is much wider. As already mentioned, this is consistent with the peculiar sampling effort carried out in the area (see also the Discussion section).
Additionally, the (logarithmic) relationship between the average group size and the average MCP across the clusters was found to be statistically significant (p-value < 0.01), even if only four points were displayed in the graph (see Figure 13).
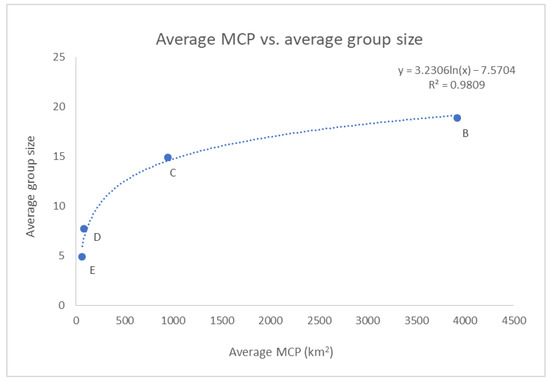
Figure 13.
The correlation between the average MCP and the average group size across the clusters resulted statistically significant (p-value < 0.01). Cluster A was excluded by the analysis as in Figure 12.
3.7. Abundance Estimates and Density
The abundance estimates (only marked individuals) of the clusters/geographical units, assessed with the mark–recapture technique, are described in Table 7. In the following Table 8, the abundance estimates of the geographical units are displayed together with the size of the cluster multi-polygons (MPs), considered as an approximation of the occupancy area of the same geographical units, and the density is calculated as the ratio between these two values. As a general pattern, the size of the geographical units seems to increase with the size of the MP; however, the density shows quite different values and has its maximum value in the Gulf of Ambracia (Table 8).

Table 7.
The abundance (best annual estimate, see Section 2.3.6.) of the clusters/geographical units, estimated with the mark–recapture technique.

Table 8.
The abundance estimates of each geographical unit are compared with the size of the relative MPs. The density is calculated as the ratio between these two values, being the MPs considered as an approximation of the area of occupancy of the same units.
4. Discussion and Conclusions
The analyses performed in this research confirm previous findings on the distribution of the bottlenose dolphin in the context of the Mediterranean Sea: the dolphins are clustered over the continental shelf in discrete geographical units, in agreement with Gnone et al. [37] and Carnabuci et al. [38].
The physiographic characteristics of the area of residence, and specifically the size and shape of the continental shelf, seem to affect the home range of the dolphins and, consequently, the size and structure of the geographical units.
Home range and group size
The dolphins living in the Gulf of Lion, an area characterised by a wide continental shelf, are observed to have a much wider individual home range (calculated as the average MCP) compared to the units living in areas where the continental platform is very narrow, like the west coast of Corsica (see Table 4, Figure 9). This phenomenon can in part be considered a simple “geometric” consequence of the shape and extension of the continental shelf, as it might be difficult (or impossible) for the dolphins to get a wide home range in a geographical context where the habitat boundaries are very narrow (such as the western coast of Corsica or the Gulf of Ambracia).
A direct consequence of this physical constraint seems to be the average size of the groups, as the dolphins living in those areas characterised by a narrow platform, such as the west coast of Corsica, not only show a smaller MCP, but they are also sighted in smaller groups (see Table 6, Figure 11, Figure 12 and Figure 13). This seems quite reasonable, since a bigger group needs to exploit a bigger territory to survive [82] and home range size and group size tend to be correlated [83,84].
The average group size is small (4.9 ± 0.12 SE) also in cluster E, which inhabits a stretch of coast (Corsica S–Sardinia NE) where the continental shelf is quite large, if compared, for example, with the west of Corsica (see Table 4). However, this is not surprising considering the local sampling context. As already mentioned, the dolphins were mainly sighted (80% of all sightings) while feeding around the aquaculture fish cages of “Golfo Aranci”, a peculiar context in which the dolphins are known to form small groups. Diaz Lopez [85], who studied the bottlenose dolphins of “Golfo Aranci” during their foraging activity around the fish cages, reported an average group size of 3.5 (3.51 ± 0.1 SE). The few other sightings were recorded in the inshore waters between the islands of the Maddalena archipelago and the Strait of Bonifacio. Virtually no effort was carried out in the open waters of the continental platform, so the data available for this unit are representative of both a limited geographical area and a peculiar behavioural context (the opportunistic feeding around the aquaculture fish cages). These results support the idea that bottlenose dolphins could “fuse” into smaller groups when they enter inshore areas and/or engage in specific feeding activities, according to the “fission-fusion” mechanism described by Connor and co-authors [86].
A correlation between the group size and the “openness” of the habitat has already been reported in cetaceans and is usually considered a social strategy against predators, assuming that a larger group can better face the predation risk [87]. Our results suggest that other variables should also be taken into consideration.
In the Alboran Sea, the bottlenose dolphins form larger groups (composed by 36.2 individuals on average), but they have relatively small MCPs, according to the analysis implemented with the available data. The average MCP could have been underestimated if the research effort had been invested in an area too small to cover the movements of the dolphins in their complete extension. However, in the geographical unit of the Alboran Sea, the overlap index (see Table 5) is relatively low (0.9), meaning that the total area sampled is much wider than the average individual MCP, a result that should reassure on the reliability of the results obtained.
In this regard, it should be noted that the bottlenose dolphins living in the Alboran Sea present quite different characteristics compared to the dolphins of the other geographical units analysed. Their distribution is not confined within the continental shelf and, according to Cañadas et al. [32], these dolphins should be considered more closely related to the Atlantic offshore ecotype, which would also account for the larger size of the groups [24,26,88]. The results are not conclusive, since the distribution of the dolphins in this area could also be affected by the peculiar bathymetric profile of the Alboran Sea, characterised by a gentle slope and wide offshore shallow water shoals (such as the Alboran Ridge), which could attract the bottlenose dolphins in the open sea. However, if it were proven that the dolphins inhabiting the Alboran Sea belong to a different (offshore) ecotype, we could assume that these dolphins are able to exploit food resources in deeper bathymetric habitats, where larger groups may be more successful in their feeding activity [87,89].
It should be mentioned that the Alboran Sea presents peculiar oceanographic characteristics if compared to the inside Mediterranean. The primary production is usually much higher, thanks to the inflow of Atlantic waters through the Strait of Gibraltar, which generates permanent anticyclonic gyres in the westernmost portion of the basin [90]. As so, some authors believe the Alboran Sea should be considered more properly as a portion of the Atlantic Ocean (at least from an oceanographic point of view), while the Mediterranean border should be moved east to the so-called Almeria-Oran front [90,91]. Additionally, as regards the cetacean fauna, the Alboran Sea shows quite a unique picture compared to the rest of the Mediterranean basin; the diversity of species is higher in this area, and the short-beaked common dolphin (Delphinus delphis), which is usually quite rare inside the Mediterranean, is the most sighted species in the shallow waters <400 m [32,33]. The presence of the common dolphin could possibly affect the behavioural ecology of the bottlenose dolphin in this sea area, shifting its preferential habitat offshore.
Size and density
In relation to the size of the geographical units identified, this seems to be correlated to the size of the area of occupancy (the complex multi-polygon of the cluster as a whole) which, in turn, is correlated to the size of the continental platform (Figure 10, Table 8). The bottlenose dolphin unit of the Gulf of Lion, for example, inhabiting a wide territory with no geographical or habitat breakages, was estimated to be larger than the others (see Table 7). However, when considering the density of the dolphins in their respective areas of occupancy, we found significant differences. In this regard, it should be noted that the density was estimated considering the cluster multi-polygon as an approximation of the area of residence of the related geographical unit. If the effort implemented over space was not sufficient to cover the movements of the dolphins in their complete extension, the density could be overestimated, so we should use some caution when comparing different geographical units. However, we got the maximum density (0.372, see Table 8) in the geographical units residing in the Gulf of Ambracia. Due to the peculiar physiography of this semi-closed gulf and the resident behaviour of the dolphins, this result should be considered quite reliable. In fact, the bottlenose dolphins living in the Gulf of Ambracia are considered a subpopulation, spending their entire life in the waters of the gulf [54].
It seems as though the different aggregation in bigger or smaller groups, adopted by the dolphins in the different geographical contexts, could be functional to exploit the area of residence (with its habitat constraint) to get the maximum density according to the local resources. Given this general pattern, the productivity and food resources available in the different areas of residence can obviously contribute to the density of dolphins in the same areas, as already described in both marine and land mammals [92,93].
While the size and shape of the continental shelf are stable physiographic features that can be easily measured and used as predictive parameters, the productivity and exploitability of the area of residence might change over time, according to local ecological variables, including anthropic pressures (such as the fishing effort and levy), but also according to the capability of the resident bottlenose dolphins to exploit the resources.
Behavioural plasticity is a key feature of the bottlenose dolphin and is probably transmitted from one generation to the next one as a local cultural tradition, allowing the dolphins to improve their ability to exploit the residency area and to adapt to changes. As part of this plastic behaviour, bottlenose dolphins can learn to obtain food from trawlers, gillnets and aquaculture fish cages, and these opportunistic strategies can become an integral part of their feeding habits [30,54,77,94,95,96,97,98,99,100,101,102,103,104,105,106].
Bottlenose dolphins can take advantage of the human activities either directly, for example, taking the fish entangled in gillnets or farmed fish [107,108,109], but also indirectly in the form of modified habitats that could be favourable for feeding (e.g., increase input of nutrients from aquaculture activities [99,110]). We know that human activities can have negative consequences on dolphin conservation (overfishing, bycatch, habitat degradation, noise pollution, etc.), but it can be difficult to measure the balance between negative and “positive” effects from the point of view of the bottlenose dolphins. Furthermore, the ability of bottlenose dolphins to exploit resources can change over space and time, depending on the human activities carried out locally and the behavioural adaptation of the resident dolphins, so it may be challenging to compare the productivity of the different areas in terms of food resources actually available to dolphins.
Conclusive remarks
The results obtained in this collective research effort highlight the importance of data sharing to better understand the ecology of cetaceans in different contexts, to grasp local peculiarities, but also to understand general patterns at a higher level.
The Mediterranean bottlenose dolphin population has been recently reassessed as Least Concern by the International Union for Conservation of Nature [111], with the exception of the subpopulation inhabiting the Gulf of Ambracia, Greece, which has been assessed as Critically Endangered [112].
The Mediterranean bottlenose dolphin population is also listed in the Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals (CMS), in the Appendix II of the Washington Convention on International Trade in Endangered Species (CITES), in the Appendix II of the Convention on the Conservation of European Wildlife and Natural Habitats (Bern Convention), in Appendix II of the Protocol to the Barcelona Convention on Specially Protected Areas of Mediterranean Importance (SPAMI). Finally, the bottlenose dolphin is included in the Annexes II and IV of the EU Habitats Directive (Council Directive 92/43/EEC) as a species of “community interest whose conservation requires both designation of special areas and strict protection” [113].
Our findings can help to better characterise the different geographical and management units that constitute the Mediterranean bottlenose dolphin metapopulation in relation to the physiographic characteristics of their area of residence. This can contribute to develop proper management and conservation measures for the bottlenose dolphin in the context of the Mediterranean Sea and beyond.
At the same time, further insights are needed to complete the picture. For example, we should consider that most of the data analysed were collected in daylight time and in the favourable conditions (i.e., the calm sea and long daylight time) given by the “good season” (late spring, summertime, early autumn). We cannot exclude that the behavioural patterns of the bottlenose dolphins, in relation to the continental platform, could change during nigh time or in winter. Further studies, possibly including other techniques such as passive acoustic, could help to monitor the behaviour of the dolphins in these other contexts.
Furthermore, the role of so-called “long travellers” needs to be better clarified. These dolphins, despite being a clear minority, could represent a means of continuity between the different geographical units, and their role in this regard could be more important than is understood.
Author Contributions
Conceptualization, G.G. and M.B.; methodology, G.G. and M.B.; formal analysis, M.B., G.G., A.A., Y.M.; data curation, G.G., M.B., Y.M., F.D., H.L., B.D.L., L.D., N.D.M., G.A., C.A., S.A., C.L., J.G., V.D.S., S.N., C.Á.C., M.G., A.M., J.A., A.A., N.T., M.-C.S., C.M., A.M.C.; writing-original draft preparation, G.G.; writing-review and editing, G.G., B.D.L., C.L., V.D.S., A.A., L.D., A.M.C., C.Á.C., M.B.; supervision, G.G. and M.B.; project administration, G.G. and M.F.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
Blue Planet Virginia Böger Stiftung X.X.
Institutional Review Board Statement
Ethical review and approval were waived for this study since the data collection activity, based on sighting and photo-identification, does not involve any direct contact with the animals and no significant impact.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used for this research are displayed on the Intercet platform (www.intercet.it).
Acknowledgments
We thank the Blue Planet Virginia Böger Stiftung X.X. foundation for supporting the TursioMed project, giving a fundamental impetus to the long-term networking program that made this scientific research possible. We would also like to thank Cybelle Planète, SCS and Participe Futur for their contribution to the data collection, in partnership with EcoOcean Institut. The research activity of Tethys Research Institute in the Gulf of Ambracia was carried out with the support of Ocean Care. We thank all the partners of GIONHA (Governance and Integrated Observation of Marine Natural Habitat) sharing their data on the Intercet Platform: Regione Liguria, Regione Toscana, Regione Sardegna, Provincia di Livorno, Cooperativa Pelagos, Area Marina Protetta Tavolara-Punta Coda Cavallo. Thanks to Alice Nebuloni for her support in the editing process. We thank the two anonymous reviewers whose remarks helped improve the quality of the paper during the review process. Special thanks to Daniele Zanzi for his interest and friendship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shane, S.H.; Wells, R.S.; Würsig, B. Ecology, behavior and social organization of the bottlenose dolphin: A review. Mar. Mammal Sci. 1986, 2, 34–63. [Google Scholar] [CrossRef]
- Rice, D.W. Marine Mammals of the World: Systematics and Distribution; The Society for Marine Mammology: San Diego, CA, USA, 1998. [Google Scholar]
- Wells, R.S.; Scott, M.D. Bottlenose dolphin Tursiops truncatus (Montagu, 1821). In Handbook of Marine Mammals, Volume VI.; The Second Book of Dolphins and Porpoises; Ridgway, S.H., Harrison, R., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 137–182. [Google Scholar]
- Wells, R.S.; Scott, M.D. Common Bottlenose Dolphin: Tursiops truncatus. In Encyclopedia of Marine Mammals; Academic Press: San Diego, CA, USA, 2009; pp. 249–255. [Google Scholar]
- Wells, R.S.; Scott, M.D. Bottlenose dolphin, Tursiops truncatus, common bottlenose dolphin. In Encyclopedia of Marine Mammals; Academic Press: San Diego, CA, USA, 2018; pp. 118–125. [Google Scholar]
- Walker, W.A. Geographic variation in morphology and biology of bottlenose dolphins (Tursiops truncatus) in the eastern North Pacific. NOAA/NMFS Southwest Fisheries Science Centre, Administrative Report No. LJ-81-3C. Available online: https://repository.library.noaa.gov/view/noaa/22873 (accessed on 10 May 2022).
- Van Waerebeek, K.; Reyes, J.C.; Read, A.J.; McKinnon, J. Preliminary observation of bottlenose dolphins from the Pacific coast of South America. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 143–154. [Google Scholar]
- Hoelzel, A.R.; Potter, C.W.; Best, P.B. Genetic differentiation between parapatric ‘nearshore’ and ‘offshore’ populations of the bottlenose dolphin. Proc. R Soc. B 1998, 265, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.G.; Rosel, P.E.; D’Agrosa, C.; Read, A.J. Improving management of overlapping bottlenose dolphin ecotypes through spatial analysis and genetics. Mar. Mammal Sci. 2003, 19, 502–514. [Google Scholar] [CrossRef]
- Parsons, K.M.; Durban, J.W.; Claridge, D.E.; Herzing, D.L.; Balcomb, K.C.; Noble, L.R. Population genetic structure of coastal bottlenose dolphins (Tursiops truncatus) in the Northern Bahamas. Mar. Mammal Sci. 2006, 22, 276–298. [Google Scholar] [CrossRef]
- Tezanos-Pinto, G.; Baker, C.S.; Russell, K.; Martien, K.; Baird, R.W.; Hutt, A.; Stone, G.; Mignucci-Giannoni, A.A.; Caballero, S.; Endo, T.; et al. A worldwide perspective on the population structure and genetic diversity of bottlenose dolphins (Tursiops truncatus) in New Zealand. J. Hered. 2008, 100, 11–24. [Google Scholar] [CrossRef]
- Mirimin, L.; Miller, R.; Dillane, E.; Berrow, S.D.; Ingram, S.; Cross, T.F.; Rogan, E. Fine-scale population genetic structuring of bottlenose dolphins in Irish coastal waters. Anim. Conserv. 2011, 14, 342–353. [Google Scholar] [CrossRef]
- Perrin, W.F.; Thieleking, J.L.; Walker, W.A.; Archer, F.I.; Robertson, K.M. Common bottlenose dolphins (Tursiops truncatus) in California waters: Cranial differentiation of coastal and offshore ecotypes. Mar. Mammal Sci. 2011, 27, 769–792. [Google Scholar] [CrossRef]
- Louis, M.; Viricel, A.; Lucas, T.; Peltier, H.; Alfonsi, E.; Berrow, S.; Brownlow, A.; Covelo, P.; Dabin, W.; Deaville, R.; et al. Habitat-driven population structure of bottlenose dolphins, Tursiops truncatus, in the North-East Atlantic. Mol. Ecol. 2014, 23, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.; Galimberti, M.; Archer, F.; Berrow, S.; Brownlow, A.; Fallon, R.; Nykänen, M.; O’brien, J.; Robertson, K.M.; Rosel, P.E.; et al. selection on ancestral genetic variation fuels repeated ecotype formation in bottlenose dolphins. Sci. Adv. 2021, 7, eabg1245. [Google Scholar] [CrossRef] [PubMed]
- Oudejans, M.G.; Visser, F.; Englund, A.; Rogan, E.; Ingram, S.N. Evidence for Distinct Coastal and Offshore Communities of Bottlenose Dolphins in the North East Atlantic. PLoS ONE 2015, 10, e0122668. [Google Scholar] [CrossRef]
- Chen, I.; Nishida, S.; Yang, W.-C.; Isobe, T.; Tajima, Y.; Rus Hoelzel, A. Genetic diversity of bottlenose dolphin (Tursiops sp.) populations in the western North Pacific and the conservation implications. Mar. Biol. 2017, 164, 202. [Google Scholar] [CrossRef] [PubMed]
- Fruet, P.F.; Secchi, E.R.; Di Tullio, J.C.; Simões-Lopes, P.C.; Daura-Jorge, F.; Costa, A.P.; Vermeulen, E.; Flores, P.A.C.; Genoves, R.C.; Laporta, P.; et al. Genetic divergence between two phenotypically distinct bottlenose dolphin ecotypes suggests separate evolutionary trajectories. Ecol. Evol. 2017, 7, 9131–9143. [Google Scholar] [CrossRef] [PubMed]
- de los Bayas-Rea, R.Á.; Félix, F.; Montufar, R. Genetic divergence and fine scale population structure of the common bottlenose dolphin (Tursiops truncatus) found in the Gulf of Guayaquil, Ecuador. PeerJ 2018, 6, e4589. [Google Scholar] [CrossRef]
- Segura-García, I.; Rojo-Arreola, L.; Rocha-Olivares, A.; Heckel, G.; Gallo-Reynoso, J.P.; Hoelzel, R. Eco-Evolutionary Processes Generating Diversity Among Bottlenose Dolphin, Tursiops truncatus, Populations off Baja California, Mexico. Evol. Biol. 2018, 45, 223–236. [Google Scholar] [CrossRef]
- Louis, M.; Buanic, M.; Lefeuvre, C.; Le Nilliot, P.; Ridoux, V.; Spitz, J. Strong bonds and small home range in a resident bottlenose dolphin community in a Marine Protected Area (Britanny, France, Northeast Atlantic). Mar. Mammal Sci. 2017, 33, 1194–1203. [Google Scholar] [CrossRef]
- Rako-Gospić, N.; Radulović, M.; Vučur, T.; Pleslić, G.; Holcer, D.; Mackelworth, P. Factor associated variations in the home range of a resident Adriatic common bottlenose dolphin population. Mar. Pollut. Bull. 2017, 124, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Holcer, D. Ecology of the Common Bottlenose Dolphin, Tursiops truncatus (Montagu, 1821) in the Central Adriatic Sea. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2012. [Google Scholar]
- Defran, R.H.; Weller, D.W.; Kelly, D.L.; Espinosa, M.A. Range characteristics of Pacific coast bottlenose dolphins (Tursiops truncatus) in the Southern California bight. Mar. Mammal Sci. 1999, 15, 381–393. [Google Scholar] [CrossRef]
- Ballance, L.T. Habitat use patterns and ranges of the bottlenose dolphin in the Gulf of California, Mexico. Mar. Mammal Sci. 1992, 8, 262–274. [Google Scholar] [CrossRef]
- Scott, M.D.; Wells, R.S.; Irvine, A.B. A long-term study of bottlenose dolphins on the west coast of florida. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic press: San Diego, CA, USA, 1990; pp. 235–244. [Google Scholar]
- Pilleri, G.; Gihr, M. Uber adriatische Tursiops truncatus (Montagu, 1821) und vergleichende Untersuchungen über mediterrane und atlantische Tümmler. Investig. Cetaceans 1969, 1, 66–73. [Google Scholar]
- Cagnolaro, L.; Di Natale, A.; Notarbartolo di Sciara, G.C. Guide per il Riconoscimento Delle Specie Animali Delle Acque Lagunari e Costiere Italiane. AQ/1/224, 9; Consiglio Nazionale delle Ricerche: Genova, Italy, 1983. [Google Scholar]
- Notarbartolo di Sciara, G.; Demma, M. Guida dei Mammiferi Marini del Mediterraneo; Franco Muzzio Editore: Padova, Italy, 1994. [Google Scholar]
- Bearzi, G.; Fortuna, C.; Reeves, R.R. Ecology and conservation of common bottlenose dolphins Tursiops truncatus in the Mediterranean Sea. Mammal Rev. 2009, 39, 92–123. [Google Scholar] [CrossRef]
- Notarbartolo di Sciara, G. Marine Mammals in the Mediterranean Sea: An Overview. Adv. Mar. Biol. 2016, 75, 1–36. [Google Scholar] [PubMed]
- Cañadas, A.; Sagarminaga, R.; Garcia-Tiscar, S. Cetacean distribution related with depth and slope in the Mediterranean waters off southern Spain. Deep. Sea Res. 2002, 49, 2053–2073. [Google Scholar] [CrossRef]
- Cañadas, A.; Sagarminaga, R.; De Stephanis, R.; Urquiola, E.; Hammond, P.S. Habitat preference modelling as a conservation tool: Proposals for marine protected areas for cetaceans in southern Spanish waters. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 495–521. [Google Scholar] [CrossRef]
- Gaspari, S.; Scheinin, A.; Holcer, D.; Fortuna, C.; Natali, C.; Genov, T.; Frantzis, A.; Chelazzi, G.; Moura, A.E. Drivers of Population Structure of the Bottlenose Dolphin (Tursiops truncatus) in the Eastern Mediterranean Sea. Evol. Biol. 2015, 42, 177–190. [Google Scholar] [CrossRef]
- Gnone, G.; Bellingeri, M.; Paraboschi, M.; Campana, I.; Alessi, J.; Nuti, S.; Salvioli, F.; Tepsich, P.; Rosso, M.; Moulins, A.; et al. TursioMed: Final Scientific Report. The final scientific report of the TursioMed project, funded by Blue Planet Virginia Böger Stiftung X.X. 2021; Unpublished work. [Google Scholar]
- Moura, A.E.; Shreves, K.; Pilot, M.; Andrews, K.R.; Moore, D.M.; Kishida, T.; Möller, L.; Natoli, A.; Gaspari, S.; McGowen, M.; et al. Phylogenomics of the genus Tursiops and closely related Delphininae reveals extensive reticulation among lineages and provides inference about eco-evolutionary drivers. Mol. Phylogenet. Evol. 2020, 146, 106756. [Google Scholar] [CrossRef]
- Gnone, G.; Bellingeri, M.; Dhermain, F.; Dupraz, F.; Nuti, S.; Bedocchi, D.; Moulins, A.; Rosso, M.; Alessi, J.; McCrea, R.S.; et al. Distribution, abundance, and movements of the bottlenose dolphin (Tursiops truncatus) in the Pelagos Sanctuary MPA (north-west Mediterranean Sea). Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 372–388. [Google Scholar] [CrossRef]
- Carnabuci, M.; Schiavon, G.; Bellingeri, M.; Fossa, F.; Paoli, C.; Vassallo, P.; Gnone, G. Connectivity in the network macrostructure of Tursiops truncatus in the Pelagos Sanctuary (NW Mediterranean Sea): Does landscape matter? Popul. Ecol. 2016, 58, 249–264. [Google Scholar] [CrossRef]
- Vassallo, P.; Marini, C.; Paoli, C.; Bellingeri, M.; Dhermain, F.; Nuti, S.; Airoldi, S.; Bonelli, P.; Laran, S.; Santoni, M.-C.; et al. Species-specific distribution model may be not enough: The case study of bottlenose dolphin (Tursiops truncatus) habitat distribution in Pelagos Sanctuary. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1689–1701. [Google Scholar] [CrossRef]
- Natoli, A.; Birkun, A.; Aguilar, A.; Lopez, A.; Hoelzel, A.R. Habitat structure and the dispersal of male and female bottlenose dolphins (Tursiops truncatus). Proc. R. Soc. B Biol. Sci. 2005, 272, 1217–1226. [Google Scholar] [CrossRef]
- Brotons, J.M.; Islas-Villanueva, V.; Alomar, C.; Tor, A.; Ruth, F.; Salud, D. Genetics and stable isotopes reveal non-obvious population structure of bottlenose dolphins (Tursiops truncatus) around the Balearic Islands. Hydrobiologia 2019, 842, 233–247. [Google Scholar] [CrossRef]
- IUCN-MMPATF. Alborán Sea IMMA. Full Accounts of Mediterranean IMMA Factsheet. IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force. 2017. Available online: https://www.marinemammalhabitat.org/wp-content/uploads/imma-factsheets/Mediterranean/Alboran-Sea-Mediterranean.pdf (accessed on 10 May 2022).
- IUCN-MMPATF. Shelf of the Gulf of Lyon IMMA Factsheet. IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force. 2017. Available online: https://www.marinemammalhabitat.org/portfolio-item/shelf-gulf-lion/ (accessed on 10 May 2022).
- Notarbartolo di Sciara, G.; Agardy, T.; Hyrenbach, D.; Scovazzi, T.; van Klaveren, P. The Pelagos Sanctuary for Mediterranean marine mammals. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 367–391. [Google Scholar] [CrossRef]
- Migeon, S.; Cattaneo, A.; Hassoun, V.; Larroque, C.; Corradi, N.; Fanucci, F.; Dano, A.; Mercier de Lepinay, B.; Sage, F.; Gorini, C. Morphology, distribution and origin of recent submarine landslides of the Ligurian Margin (North-western Mediterranean): Some insights into geohazard assessment. Mar. Geophys. Res. 2011, 32, 225–243. [Google Scholar] [CrossRef]
- Goffart, A.; Hecq, J.H.; Prieur, L. Contrôle du phytoplancton du bassin Ligure par le front liguro-provençal (secteur Corse). Oceanol. Acta 1995, 18, 329–342. [Google Scholar]
- Azzellino, A.; Panigada, S.; Lanfredi, C.; Zanardelli, M.; Airoldi, S.; Notarbartolo di Sciara, G. Predictive habitat models for managing marine areas: Spatial and temporal distribution of marine mammals within the Pelage’s Sanctuary (Northwestern Mediterranean Sea). Ocean Coast. Manag. 2012, 67, 63–74. [Google Scholar] [CrossRef]
- Lauriano, G.; Pierantonio, N.; Donovan, G.; Panigada, S. Abundance and distribution of Tursiops truncatus in the Western Mediterranean Sea: An assessment towards the Marine Strategy Framework Directive requirements. Mar. Environ. Res. 2014, 100, 86–93. [Google Scholar] [CrossRef]
- Marini, C.; Fossa, F.; Paoli, C.; Bellingeri, M.; Gnone, G.; Vassallo, P. Predicting bottlenose dolphin distribution along Liguria coast (northwestern Mediterranean Sea) through different modeling techniques and indirect predictors. J. Environ. Manag. 2015, 150, 9–20. [Google Scholar] [CrossRef]
- Laran, S.; Pettex, E.; Authier, M.; Blanck, A.; David, L.; Dorémus, G.; Falchetto, H.; Monestiez, P.; Pettex, E.; Stephan, E.; et al. Seasonal distribution and abundance of cetaceans within French waters-Part I: The North-Western Mediterranean, including the Pelagos sanctuary. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 20–30. [Google Scholar] [CrossRef]
- Pennino, M.G.; Mérigot, B.; Fonseca, V.P.; Monni, V.; Rotta, A. Habitat modeling for cetacean management: Spatial distribution in the southern Pelagos Sanctuary (Mediterranean Sea). Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 203–211. [Google Scholar] [CrossRef]
- Labach, H.; Azzinari, C.; Barbier, M.; Cesarini, C.; Daniel, B.; David, L.; Dhermain, F.; Di-Méglio, N.; Guichard, B.; Jourdan, J.; et al. Distribution and abundance of common bottlenose dolphin (Tursiops truncatus) over the French Mediterranean continental shelf. Mar. Mammal Sci. 2022, 38, 212–222. [Google Scholar] [CrossRef]
- IUCN-MMPATF. North West Mediterranean Sea, Slope and Canyon system IMMA Factsheet. IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force. 2017. Available online: https://www.marinemammalhabitat.org/portfolio-item/north-western-mediterranean-sea-slope-canyon-system/ (accessed on 10 May 2022).
- Gonzalvo, J.; Lauriano, G.; Hammond, P.S.; Viaud-Martinez, K.A.; Fossi, M.C.; Natoli, A.; Marsili, L. The Gulf of Ambracia’s Common Bottlenose Dolphins, Tursiops truncatus: A Highly Dense and yet Threatened Population. Adv. Mar. Biol. 2016, 75, 259–296. [Google Scholar]
- IUCN-MMPATF. Gulf of Ambracia IMMA Factsheet. IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force. 2017. Available online: https://www.marinemammalhabitat.org/portfolio-item/gulf-of-ambracia/ (accessed on 10 May 2022).
- Wursig, B.; Jefferson, T.A. Methods of photo-identificationfor small cetaceans. Rep. Int. Whal. Comm. 1990, 12, 43–52. [Google Scholar]
- Whitehead, H.; Gowans, S.; Faucher, A.; Mccarrey, S.W. Population analysis of northern bottlenose whales in the Gully, Nova Scotia. Mar. Mammal Sci. 1997, 13, 173–185. [Google Scholar] [CrossRef]
- Wilson, B.; Hammond, P.S.; Thompson, P.M. Estimating size and assessing trends in a coastal bottlenose dolphin population. Ecol. Appl. 1999, 9, 288–300. [Google Scholar] [CrossRef]
- Read, A.J.; Urian, K.W.; Wilson, B.; Waples, D.M. Abundance of bottlenose dolphins in the bays, sounds, and estuaries of North Carolina. Mar. Mammal Sci. 2003, 19, 59–73. [Google Scholar] [CrossRef]
- Chilvers, B.L.; Corkeron, P.J. Abundance of indo-pacific bottlenose dolphins, Tursiops aduncus, off point lookout, Queensland, Australia. Mar. Mammal Sci. 2003, 19, 85–95. [Google Scholar] [CrossRef]
- Thompson, J.W.; Zero, V.H.; Schwacke, L.H.; Speakman, T.R.; Quigley, B.M.; Morey, J.S.; McDonald, T.L. finFindR: Automated recognition and identification of marine mammal dorsal fins using residual convolutional neural networks. Mar. Mammal Sci. 2022, 38, 139–150. [Google Scholar] [CrossRef]
- Ginsberg, J.R.; Young, T.P. Measuring association between individuals or groups in behavioral studies. Anim. Behav. 1992, 44, 377–379. [Google Scholar] [CrossRef]
- Eades, P. A heuristic for graph drawing. Congr. Numer. 1982, 42, 149–160. [Google Scholar]
- Fruchterman, T.M.J.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Borgatti, S.P. NetDraw Software for Network Visualization; Analytic Technologies: Lexington, KT, USA, 2002. [Google Scholar]
- Freeman, L.C. A set of measures of centrality based upon betweenness. Sociometry 1977, 40, 35–41. [Google Scholar] [CrossRef]
- Girvan, M.; Newman, M.E.J. Community structure in social and biological networks. Proc. Natl. Acad. Sci. USA 2002, 99, 7821–7826. [Google Scholar] [CrossRef] [PubMed]
- Lusseau, D.; Newman, M.E.J. Identifying the role that animals play in their social networks. Proc. R. Soc. B Biol. Sci. 2004, 271, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.E.J.; Girvan, M. Finding and evaluating community structure in networks. Phys. Rev. E 2004, 69, 026113. [Google Scholar] [CrossRef] [PubMed]
- Efron, B. Computers and the theory of statistics: Thinking the unthinkable. SIAM Rev. 1979, 21, 460–480. [Google Scholar] [CrossRef]
- Chen, J.; Zaїane, O.R.; Goebel, R. Detecting communities in social networks using max–min modularity. In Proceedings of the 2009 SIAM International Conference on Data Mining (SDM), Sparks, NV, USA, 30 April–2 May 2009; pp. 978–989. [Google Scholar] [CrossRef]
- Mohr, C.O. Table of equivalent populations of North American small mammals. Am. Midl. Nat. 1947, 37, 223–249. [Google Scholar] [CrossRef]
- Otis, D.L.; Burnham, K.P.; White, G.C.; Anderson, D.R. Statistical inference from capture data on closed animal populations. Wildl. Monogr. 1978, 62, 3–135. [Google Scholar]
- White, G.C.; Anderson, D.R.; Burnham, K.P.; Otis, D.L. Capture-Recapture and Removal Methods for Sampling Closed Populations; Los Alamos National Lab, LA-8787NERP: Los Alamos, NM, USA, 1982.
- Rexstad, E.; Burnham, K. User’s Guide for Interactive Program Capture; Colorado Cooperative Fish and Wildlife Research Unit, Colorado State University: Fort Collins, CO, USA, 1991. [Google Scholar]
- White, G.C.; Burnham, K.P. Program Mark: Survival estimation from populations of marked animals. Bird Study 1999, 46, 120–138. [Google Scholar] [CrossRef]
- Chilvers, B.L.; Corkeron, P.J. Trawling and bottlenose dolphins’ social structure. Proc. R. Soc. Lond. 2001, 268, 1901–1905. [Google Scholar] [CrossRef]
- Williams, J.A.; Dawson, S.M.; Slooten, E. The abundance and distribution of bottlenose dolphins (Tursiops truncatus) in Doubtful Sound, New Zealand. Can. J. Zool. 1993, 71, 2080–2088. [Google Scholar] [CrossRef]
- Rossi, A.; Scordamaglia, E.; Bellingeri, M.; Gnone, G.; Nuti, S.; Salvioli, F.; Manfredi, P.; Santangelo, G. Demography of the bottlenose dolphin Tursiops truncatus (Mammalia: Delphinidae) in the Eastern Ligurian Sea (NW Mediterranean): Quantification of female reproductive parameters. Eur. Zool. J. 2017, 84, 294–302. [Google Scholar] [CrossRef]
- Hammond, P.S. Estimating the size of naturally marked whale population using capture-recapture techniques. Rep. Int. Whal. Comm. 1986, 8, 253–282. [Google Scholar]
- Chao, A.; Lee, S.M.; Jeng, S.L. Estimating population size for capture–recapture data when capture probabilities vary by time and individual animal. Biometrics 1992, 48, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Batzli, G.O.; Henttonen, H. Home range and social organization of the singing vole (Microtus miurus). J. Mammal. 1993, 74, 868–878. [Google Scholar] [CrossRef]
- Maher, C.R.; Burger, J.R. Intraspecific variation in space use, group size, and mating systems of caviomorph rodents. J. Mammal. 2011, 92, 54–64. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Liu, W.; Wang, G.; Wan, X.; Zhong, W. Home-range sizes of social groups of Mongolian gerbils Meriones unguiculatus. J. Arid Environ. 2011, 75, 132–137. [Google Scholar] [CrossRef]
- Diaz Lopez, B. Bottlenose dolphins and aquaculture: Interaction and site fidelity on the north-eastern coast of Sardinia (Italy). Mar. Biol. 2012, 159, 2161–2172. [Google Scholar] [CrossRef]
- Connor, R.C.; Wells, R.S.; Mann, J.; Read, A.J. The bottlenose dolphin: Social relationships in a fission-fusion society. In Cetacean Societies: Field Studies of Dolphins and Whales; Mann, J., Conner, R.C., Tyack, P.L., Whitehead, H., Eds.; University of Chicago Press: Chicago, IL, USA, 2000; pp. 91–126. [Google Scholar]
- Gygax, L. Evolution of group size in the dolphins and porpoises: Interspecific consistency of intraspecific patterns. Behav. Ecol. 2002, 13, 583–590. [Google Scholar] [CrossRef]
- Bearzi, M. Aspects of the ecology and behaviour of bottlenose dolphins (Tursiops truncatus) in Santa Monica Bay, California. J. Cetacean Res. Manag. 2005, 7, 75–83. [Google Scholar]
- Barker, J.; Berrow, S. Temporal and spatial variation in group size of bottlenose dolphins (Tursiops truncatus) in the Shannon Estuary, Ireland. Biol. Environ. Proc. R. Ir. Acad. 2016, 116, 63–70. [Google Scholar] [CrossRef]
- Bárcena, M.Á.; Flores, J.A.; Sierro, F.J.; Perez-Folgado, M.; Fabresb, J.; Calafat, A.; Canals, M. Planktonic response to main oceanographic changes in the AlboranSea (Western Mediterranean) as documented in sedimenttraps and surface sediments. Mar. Micropaleontol. 2004, 53, 423–445. [Google Scholar] [CrossRef]
- Tintore, J.; La Violette, P.E.; Blade, I.; Cruzado, A. A study of an intense density front in the eastern Alboran Sea: The Almeria–Oran Front. J. Phys. Oceanogr. 1988, 18, 1384–1397. [Google Scholar] [CrossRef]
- Heithaus, M.; Dill, L. Food availability and predation risk influence bottlenose dolphin habitat use. Ecology 2002, 83, 480–491. [Google Scholar] [CrossRef]
- Marshall, A.J.; Leighton, M. How does food availability limit the population density of white-bearded gibbons? In Feeding Ecology in Apes and Other Primates. Ecological, Physical and Behavioral Aspects; Hohmann, G., Robbins, M.M., Boesch, C., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 311–334. [Google Scholar]
- Fertl, D.; Leatherwood, S. Cetacean interactions with trawls: A preliminary review. J. Northwest Atl. Fish. Sci. 1997, 22, 219–248. [Google Scholar] [CrossRef]
- Bearzi, G.; Politi, E.; Notarbartolo di Sciara, G. Diurnal behavior of free-ranging bottlenose dolphins in Kvarneric (northern Adriatic Sea). Mar. Mammal Sci. 1999, 15, 1065–1097. [Google Scholar] [CrossRef]
- Lauriano, G.; Fortuna, C.M.; Moltedo, G.; Notarbartolo di Sciara, G. Interactions between common bottlenose dolphins (Tursiops truncatus) and the artisanal fishery in Asinara Island National Park (Sardinia): Assessment of catch damage and economic loss. J. Cetacean Res. Manag. 2004, 6, 165–173. [Google Scholar]
- Brotons, J.M.; Grau, A.M.; Rendell, L. Estimating the impact of interactions between bottlenose dolphins and artisanal fisheries around the Balearic Islands. Mar. Mammal Sci. 2008, 24, 112–127. [Google Scholar] [CrossRef]
- Buscaino, G.; Buffa, G.; Sara’, G.; Bellante, A.; Tonello, A.; Sliva Hardt, S.A.; Cremer, M.; Bonanno, A.; Cuttitta, A.; Mazzola, S. Pinger affects fish catch efficiency and damage to bottom gill nets related to bottlenose dolphins. Fish. Sci. 2009, 75, 537–544. [Google Scholar] [CrossRef]
- Piroddi, C.; Bearzi, G.; Christensen, V. Marine open cage aquaculture in the eastern Mediterranean Sea: A new trophic resource for bottlenose dolphins. Mar. Ecol. Prog. Ser. 2011, 440, 255–266. [Google Scholar] [CrossRef]
- Blasi, M.; Boitani, L. Modelling fine-scale distribution of the bottlenose dolphin Tursiops truncatus using physiographic features on Filicudi (southern Thyrrenian Sea, Italy). Endag. Species Res. 2012, 17, 269–288. [Google Scholar] [CrossRef]
- Bonizzoni, S.; Furey, N.; Pirotta, E.; Valavanis, V.D.; Würsig, B.; Bearzi, G. Fish farming and its appeal to common bottlenose dolphins: Modelling habitat use in a Mediterranean embayment. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 696–711. [Google Scholar] [CrossRef]
- Genov, T.; Centrih, T.; Kotnjek, P.; Hace, A. Behavioural and temporal partitioning of dolphin social groups in the northern Adriatic Sea. Mar. Biol. 2019, 166, 11. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Vella, A.; Vidoris, P.; Christidis, A.; Kamidis, N.; Lefkaditou, E. Interactions between fisheries and cetaceans in the Thracian Sea (Greece) and management proposals. Fish. Manag. Ecol. 2019, 26, 374–388. [Google Scholar] [CrossRef]
- Papale, E.; Alonge, G.; Grammauta, R.; Ceraulo, M.; Giacoma, C.; Mazzola, S.; Buscaino, G. Year-round acoustic patterns of dolphins and interaction with anthropogenic activities in the Sicily Strait, central Mediterranean Sea. Ocean Coast. Manag. 2020, 197, 105320. [Google Scholar] [CrossRef]
- Andrés, C.; Cardona, L.; Gonzalvo, J. Common bottlenose dolphin (Tursiops truncatus) interaction with fish farms in the Gulf of Ambracia, western Greece. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2229–2240. [Google Scholar] [CrossRef]
- Gonzalvo, J.; Valls, M.; Cardona, L.; Aguilar, A. Factors determining the interactions between bottom trawlers off the Balearic Archipelago (western Mediterranean Sea). J. Exp. Mar. Biol. Ecol. 2008, 367, 47–52. [Google Scholar] [CrossRef]
- Díaz López, B. Interactions between Mediterranean bottlenose dolphins (Tursiops truncatus) and gillnets off Sardinia, Italy. ICES J. Mar. Sci. 2006, 63, 946–951. [Google Scholar] [CrossRef]
- Díaz López, B. Temporal variability in predator presence around a fin fish farm in the Northwestern Mediterranean Sea. Mar. Ecol. 2017, 38, e12378. [Google Scholar] [CrossRef]
- Díaz López, B. “Hot deals at sea”: Responses of a top predator (Bottlenose dolphin, Tursiops truncatus) to human-induced changes in the coastal ecosystem. Behav. Ecol. 2019, 30, 291–300. [Google Scholar] [CrossRef]
- Díaz López, B.; Bunke, M.; Bernal Shirai, J.A. Marine aquaculture off Sardinia Island (Italy): Ecosystem effects evaluated through a trophic mass-balance model. Ecol. Eng. 2008, 212, 292–303. [Google Scholar] [CrossRef]
- Natoli, A.; Genov, T.; Kerem, D.; Gonzalvo, J.; Holcer, D.; Labach, H.; Marsili, L.; Mazzariol, S.; Moura, A.E.; Öztürk, A.A.; et al. Tursiops truncatus (Mediterranean subpopulation). IUCN Red List. Threat. Species 2021, E.T16369383A50285287. Available online: https://www.iucnredlist.org/species/16369383/215248781 (accessed on 10 May 2022).
- Gonzalvo, J.; Notarbartolo di Sciara, G. Tursiops truncatus (Gulf of Ambracia subpopulation). IUCN Red List. Threat. Species 2021, E.T181208820A181210985. Available online: https://www.iucnredlist.org/species/181208820/181210985 (accessed on 10 May 2022).
- Fortuna, C.M.; Cañadas, A.; Holcer, D.; Brecciaroli, B.; Donovan, G.P.; Lazar, B.; Mo, G.; Tunesi, L.; Mackelworth, P.C. The coherence of the European Union marine Natura 2000 network for wide-ranging charismatic species: A Mediterranean case study. Front. Mar. Sci. 2018, 5, 356. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).