Abstract
Food availability is thought to exert a bottom-up control on the population dynamics of small pelagic fish; therefore, studies on trophic ecology are essential to improve their management. Sardina pilchardus is one of the most important commercial species in the Adriatic Sea, yet there is little information on its diet in this area. Adult sardines were caught in the Gulf of Trieste (northern Adriatic) from spring 2006 to winter 2007. Experimental catches conducted over 24-h cycles in May, June and July showed that the sardines foraged mainly in the late afternoon. A total of 96 adult sardines were analysed: the number of prey varied from a minimum of 305 to a maximum of 3318 prey/stomach, with an overall mean of 1259 ± 884 prey/stomach. Prey items were identified to the lowest possible taxonomical level, counted and measured at the stereo-microscope. Overall, sardines fed on a wide range of planktonic organisms (87 prey items from 17 μm to 18.4 mm were identified), with copepods being the most abundant prey (56%) and phytoplankton never exceeding 10% of the prey. Copepods of the Clauso-Paracalanidae group and of the genus Oncaea were by far the most important prey. The carbon content of prey items was indirectly estimated from prey dry mass or body volume. Almost all carbon uptake relied on a few groups of zooplankton. Ivlev’s selectivity index showed that sardines positively selected small preys (small copepods < 1 mm size), but also larger preys (such as teleost eggs, decapod larvae and chaetognaths), confirming their adaptive feeding capacity.
1. Introduction
Small pelagic fish play a crucial role in marine food webs, as key species in energy transfer from plankton to larger predators, marine mammals and seabirds [1,2]. Short lifespans, high fecundity rates and planktivorous feeding behaviour make small pelagic fishes sensitive to changing oceanographic conditions [3]. However, the ability of anchovies and sardines to adapt to different trophic conditions and the plasticity of their feeding behaviour have often been linked to their “ecological success” [4,5,6].
Fish exploitation and environmental changes may exert an important combined effect on small pelagic fish stocks. In the Mediterranean Sea, several authors have supported the hypothesis that food availability and/or trophic competition may exert a bottom-up control on the growth, size and body condition of small pelagic fish [7,8]. Therefore, studies on the trophic ecology of small pelagic fishes represent fundamental knowledge to improve the assessment and management of these species.
Previous studies based on numerical dietary indices described sardines as exclusively or almost exclusively phytophagous fishes. This was especially true for sardines living in upwelling areas, where it is commonly assumed that high phytoplankton production supports short and efficient food chains [9,10,11]. However, this assumption has been challenged following the assessment of carbon uptake by different food items [4,12]. It has been shown that the diets of both sardines and anchovies rely significantly more heavily on zooplankton than on the more numerous cells of phytoplankton for carbon content [12].
There is great intraspecific variability in the morphology of feeding structures between sardine populations from different regions [13]. Sardines live in both highly productive upwelling systems [4,11] and more oligotrophic waters [14,15,16,17], probably thanks to their ability to switch to the most appropriate feeding mode to optimise energy intake from the trophic environment. In particular, sardines from the Mediterranean have fewer gill rakers and greater spacing than those from the Atlantic [15]. This difference has been explained as an adaptation to the higher availability of phytoplankton in the Atlantic, where sardines have an advantage due to their filtration behaviour. In the Mediterranean Sea, the diet of sardines was mainly investigated in the Gulf of Lion [5,15,18,19,20] and in the Aegean Sea [16,21,22,23], but little information is available for the Adriatic Sea, where only a few studies have been conducted on the diet of adult sardines in Croatian waters (central–eastern Adriatic) [24,25,26,27].
The European sardine Sardina pilchardus (Walbaum, 1792) is one of the most important commercially exploited species in the Mediterranean Sea, where it is caught by purse seine fishing with artificial lighting and pelagic trawling. The northern Adriatic is one of the most productive areas of the Mediterranean [28,29], and in the entire basin the average sardine landing from 1975 to 2013 was 45,000 t/year [30], which, together with anchovy, represents over 97% of the small pelagic catches. Landings of small pelagic species in the Adriatic Sea were estimated to be around 74 million (2013), representing almost one fifth of the total fish production in this Sea. Nevertheless, landings of small pelagic fish in the Mediterranean Sea have been characterised by a general downward trend in recent years [31].
The aim of this study is to improve the knowledge of the feeding habits of adult S. pilchardus in the northernmost part of the Adriatic Sea (Gulf of Trieste), and in particular: (i) to describe the diel feeding cycle, (ii) to analyse the diet composition across different seasons, (iii) to assess carbon uptake and (iv) to estimate feeding selectivity. The results of this study will provide important information for modelling studies and contribute to a better understanding of the functioning of the pelagic food web in the northern Adriatic.
2. Materials and Methods
2.1. Study Area
The Gulf of Trieste (Figure 1) is the northernmost gulf of the Adriatic Sea, with an average depth of 20 m (maximum depth: 25 m), a surface area of 600 km2 and a volume of 9.5 km3. This semi-enclosed continental shelf area exhibits large oscillations of temperature (4–29.2 °C) and salinity (10–38.5) [32]. Termohaline stratification occurs during spring and lasts to autumn, while, during winter, the water column is generally homogenized by wind mixing [33]. The principal freshwater input comes from the Isonzo River discharges (mean flow of 82 m3 s −1), which contribute to about 90% of the freshwater inputs [34].
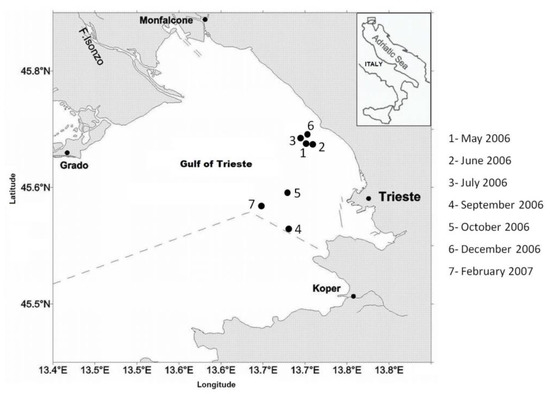
Figure 1.
Map of the study area in the Gulf of Trieste (northeastern Adriatic Sea), with the ending positions of the fishing tows (●) (black dots) used to describe both sardine diet and mesozooplankton in the field.
2.2. Field Sampling
Sardines were collected from May 2006 to February 2007 in the Gulf of Trieste (northeastern Adriatic Sea) (Figure 1). Sampling took place monthly and/or bimonthly and during each sampling day 6 to 8 consecutive tows, at least 2 h from each other, were performed to describe the feeding diel cycle. No sardines were found in samplings carried out in December and February (Table 1). In May, June and July 2006, fish were sampled with 1 or 2 monofilament gillnets, each 4 m high and 400 m long, with a knot-to-knot mesh size of 15.5 mm. In September, October, December 2006 and February 2007, fish were sampled with a semipelagic trawl net equipped with a 12 mm (knot-to-knot) mesh cod-end, towed at an approximate speed of 3.0 knots. Both fishing gears worked in the same water layer, being deployed from the bottom to a height of 4 m above the bottom. Setting and towing times were adapted to seasonal and environmental conditions to maximize the fishing success. Fish from each tow were sorted, immediately frozen on board at −20 °C to stop digestive processes and preserved at the same temperature until laboratory analysis.

Table 1.
Sampling information: date; sunrise and sunset time at each sampling day; fishing gear used; number of performed tows during the 24 h cycle of sampling and in brackets the number of tows with at least 20 sardines. Time is expressed as (GMT + 1).
Zooplankton samples were collected during fishing operations, generally after the retrieval of the fishing net. Vertical plankton tows from about 3 m from the bottom up to the surface were performed with a standard WP2 net (mesh size 200 μm; mouth opening diameter 58 cm). Immediately on retrieval of the net, plankton samples were fixed and preserved in a seawater-buffered formaldehyde solution (4% final concentration).
Temperature profiles were measured using a PNF-300 Profiling Natural Fluorometer probe in May, June and July 2006 and a portable thermometer YSI85 probe in September, October, December 2006 and February 2007.
2.3. Diel Feeding Cycle
At laboratory fish were defrosted, measured to the nearest 1 mm of total length (TL) and weighed on an analytical balance to the nearest 0.0001 g of total wet body mass. A total of 343 sardines (Table 2) were dissected and their stomachs were removed and preserved individually in a buffered 4% formaldehyde–seawater solution. Afterwards the stomachs were dissected and their contents were washed with distilled water on a Petri dish under a stereo-microscope. Subsequently, the stomach contents were filtered through pre-dried and pre-weighed glass microfiber filters (Whatman® grade GF/F, 25 mm Ø) and dried at 60 °C until constant mass. To determine the diel feeding periodicity of sardine, the Fullness index (F) was calculated as
where DM is the dry mass of the stomach content and SWM is the somatic wet mass of fish. Feeding periodicity was described by plotting mean Fullness index (F) calculated in fish from the same tow against the time of day.
F = (DM/SWM) × 1000 g SWM

Table 2.
Information about fish considered for diet composition analysis and for feeding cycle: number (n), total length ± st. dev. (TL), total wet mass ± st. dev. (TWM).
2.4. Diet Composition and Dietary Carbon
Only sardine specimens caught during the period of maximum feeding activity were analysed to describe the diet composition since prey were less digested and easier to identify. Digestive tracts’ dissection took place under a stereo-microscope and the stomach content of each fish was washed out onto a Petri dish. All the material contained both in the cardiac stomach and in the fundulus of the stomach was considered as “stomach content”. Regurgitation during sampling was not observed since no food was found in any oesophagus.
For each sampling period (Table 2), sardines were randomly divided in 3 groups of 8 individuals. The stomach contents of the 8 sardines were pooled and diluted in a known volume of 0.22 μm filtered seawater. After homogenization, subsamples representing one fish (1/8 of the original pool), were analysed under the stereo-microscope (Leica M205-C, up to 160× magnification). This procedure was repeated for 3 pools of 8 sardines each, in order to produce replicates. Prey items were identified to the lowest possible taxonomical level and counted. When specific characters were missing or damaged, copepod specimens of the genera Paracalanus, Ctenocalanus, Clausocalanus and Pseudocalanus were classified as the “Clauso-Paracalanidae” group. The prosoma length of all copepods and the maximum dimension of each other prey were measured using an ocular micrometer (accuracy of 6 μm). The original size of incomplete prey was reconstructed by means of morphometric relationship, obtained by the measurements of whole individuals captured in zooplankton samples. Prey size distribution was represented by grouping sizes in classes of 50 μm.
The carbon content of prey items was indirectly estimated applying relationship between dry mass or body volume and carbon content (Table A1).
2.5. Feeding Selectivity
Feeding selectivity was estimated by Ivlev’s electivity index E [34], calculated as follows
E = (ri − ai)/(ri + ai)
Mesozooplankton samples collected in concomitance with fishing operations, were considered to define the food availability at sea. Taxonomic composition was analysed in subsamples sufficient to count and identify at least 1000 specimens. Mesozooplankton abundance was expressed as number of individuals per cubic meter of seawater. The volume of filtered water for each sample was estimated by multiplying the net-mouth area by the sampling depth.
3. Results
3.1. Temperature and Mesozooplankton in the Field
Sea surface temperature ranged from 10.1 °C to 26.1 °C, recorded in February 2007 and July 2006, respectively. From late spring to the beginning of autumn, a thermocline formed (stratified periods), while in October, December and February the seawater temperature was homogenous from the surface to the bottom (mixing periods) (Figure 2a). Salinity ranged from 36.9 to 38.2, presenting higher values in February. In May and June, the values were constant along the water column. In July, September and October, an alocline formed at a depth of 13–14 m. In December and February, salinity again showed constant values along the water column from the bottom to a depth of 2–3 m (Figure 2b).
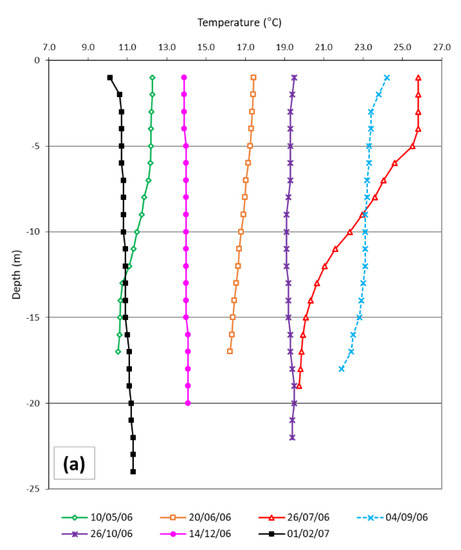
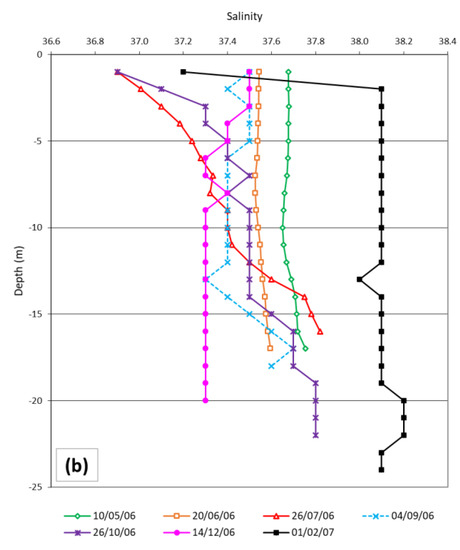
Figure 2.
Temperature (a) and salinity (b) profiles measured close to positions where sardine diet and mesozooplankton were described.
Mesozooplankton composition and abundance were analysed in samples collected in correspondence to the fishing hauls dedicated to fish diet analysis. Mesozooplankton abundance ranged from 2044 to 13,212 ind. m−3, measured in December and July, respectively (Figure 3). Copepods were generally the most abundant group with the exception of July and September when Cladocerans numerically dominated (6961 and 6352 ind. m−3, respectively). Copepods were mainly represented by the order Calanoida from February to September (72–91% of Copepods) and by the Cyclopoida of the suborder Ergasilida in October and December (54% and 42%, respectively). Cnidarians were particularly abundant in June (2404 ind. m−3), mainly with Muggiaea spp. and other Siphonophorans. Meroplankton, composed essentially of Echinoderms and Molluscs, showed its maximum (up to 2525 ind. m−3) in June and July (Figure 3). Chaetognaths’ abundance ranged from the minimum of 4 to the maximum of 78 individuals m−3 in May and December, respectively. Detailed information is available in the Table A2.
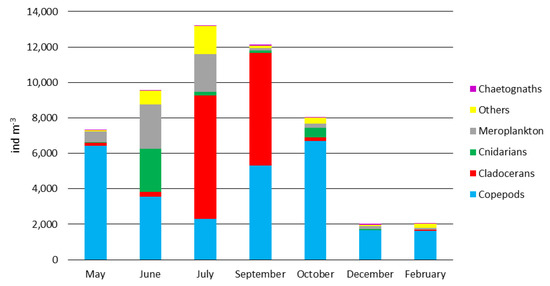
Figure 3.
Composition and abundance (ind. m−3) of mesozooplanktonic taxa in the field during the sampling periods.
3.2. Diel Feeding Cycle
The diel pattern of stomach fullness confirmed the diurnal feeding behaviour of S. pilchardus. The fullness index (F) was generally low in the morning, gradually increased at noon and peaked at dusk. Overnight, feeding activity had nearly ceased by sunrise. In all months studied, the highest values of stomach fullness were observed between 18:00 and 21:00 and the lowest values around 4:00. The mean Fullness index varied from a minimum of 0.04 in June and July to a maximum of 1.9 in May (Figure 4). In May, the maximum fullness value was twice as high as the maximum values observed in June and July. In October, it was not possible to describe feeding periodicity because catches did not yield sufficient numbers throughout the day. No sardines were caught in December and February.
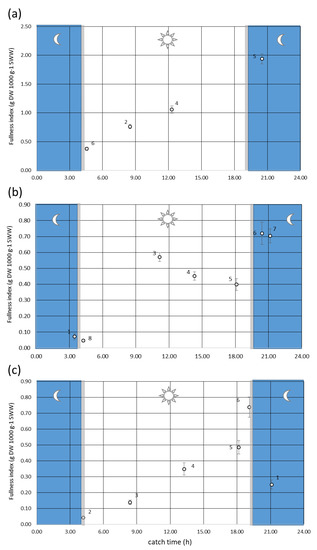
Figure 4.
Sampling time vs. Fullness index, where numbers refer to the tow number, bars to the standard error and grey shaded line to the sunrise and sunset: (a) May; (b) June; (c) July 2006.
3.3. Composition of the Diet
A total of 96 sardines were analysed: 24 specimens collected in the same tow in May, June, July and October (Table 2). The total length (TL) of adult S. pilchardus ranged from a minimum of 138 mm to a maximum of 200 mm, with a mean of 169.5 ± 12.4 mm (Table 2).
A total of 15,109 prey items belonging to 73 taxa were identified (Table 3). The number of prey varied from a minimum of 305 to a maximum of 3318 prey/stomach, with an overall mean of 1259 ± 884 prey/stomach. Large differences were observed between months (Table 4). The numerically most abundant prey categories (N%) were copepods (85.0%) and Dinophyceae (9.5%) in May; tintinnids (56.4%), eggs and larvae of teleosts (12.2%), Dinophyceae (11.3%) and copepods (10.8%) in June; copepods (48.4%) and Crustacea larvae (21.1%) in July; copepods (50.4%) and chaetognaths (44.3%) in October.

Table 3.
Taxonomic composition of identifiable stomach contents expressed as mean ± st. dev. of prey number/stomach.

Table 4.
Composition of Sardina pilchardus diet expressed as prey percentage on numerical (N%) and carbon basis, in May, June, July and October 2006.
Copepods were the most abundant food category in the stomachs of captured sardines in all months, with the sole exception of June when tintinnids predominated numerically. Among copepods, the Clauso-Paracalanidae group and the genus Oncaea were by far the most important, alternately dominating. Copepod nauplii were always found, with relatively small amounts, from 0.38 to 7.35% of copepods. A variable amount of invertebrate eggs with diameters ranging from 33 to 88 µm, probably belonging to copepods, was observed in the gut contents. Nevertheless, we did not consider these eggs as “prey” because it was impossible to determine whether they were ingested intentionally or as egg masses carried by the captured copepods. Chaetognaths represented an important food category in October in terms of numbers (44.3%), immediately after copepods.
We found microphytoplankton in all seasons with 26 taxa representing numerically about 10% of the prey in May, June and July and only 1% in October. In contrast, tintinnids were present with four taxa and were especially present in June when they dominated the number of prey (55.13%), probably related to a bloom of Tintinnopsis radix.
In May, we observed a very high number of pollen grains (4616 ± 637.4 cells/stomach), but they were not included in the diet analysis. In spring, pollen may be very abundant in coastal surface waters, but we have not yet found evidence of the ability of sardines to digest it.
3.4. Food Carbon
An estimation of prey carbon content showed that S. pilchardus obtained almost all carbon from metazoans, especially copepods, chaetognaths, crustacean larvae, and the eggs and larvae of teleosts (Table 4). This was true even when considerable amounts of microphytoplankton and microzooplankton were present in the stomach contents. For example, in June, tintinnids were the most abundant prey in the stomach contents (56.4%); however, they accounted for only 0.3% of the total carbon ingested. Zooplanktonic prey of large size and high carbon content were the most important energetic food source, even when only a few specimens were ingested, such as chaetognaths in July (4.9% by number, 47.3% by carbon content), teleost eggs and larvae in June (12.2% by number, 62.9% by carbon content) or Crustacea larvae in June (5.5% by number, 24.0% by carbon content).
Differences among months were even more pronounced when dietary carbon content was considered. In fact, only one prey category accounted for most of the total carbon intake in each month: 97% of chaetognaths in October, 96% of copepods in May, 63% of teleost eggs and larvae in June, and again 47% of chaetognaths in July.
The overall size spectrum of prey ranged from 17 μm for the diatom Thalassiosira spp. to 18,388 μm for chaetognaths (Figure 5), but in all seasons most prey had sizes from 400 to 1000 μm. Isolated peaks occurred only in May in the 50–100 μm size range (mainly corresponding to Dinophyceae) and in June in the 200–250 μm size range (mainly corresponding to Tintinnids) (Figure 5). Overall, most of the prey carbon was in size classes from 600 to 1000 μm, especially in May and June, with an isolated peak in June and July in the 1700–1750 μm size class (corresponding to Crustacea larvae).
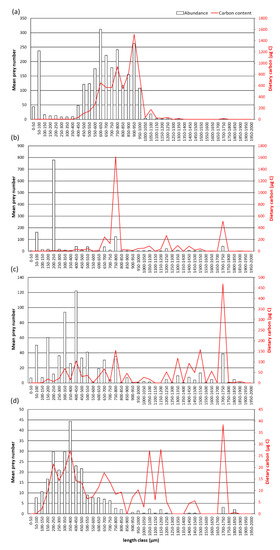
Figure 5.
Distribution of small prey (<2 mm size) in gut contents: mean counted prey/stomach by size classes of 50 µm (histograms) and their relative carbon content (red solid line) in (a) May, (b) June, (c) July and (d) October.
3.5. Selection of the Prey
The Ivlev index was calculated for 16 prey groups, excluding those prey (Bacyllariophyceae, Dinophyceae, Tintinnina, eggs of Invertebrata) that were too small to be effectively retained by the mesh of the WP2 plankton net. All taxa found at abundances <1% in both food and environment (e.g., ctenophores, nemerteans, phoronids, ostracods, stomatopods, amphipods, isopods, mysidaceans, thaliaceans, cephalochordates) were grouped into a category labelled “Others”.
The Ivlev index values (Figure 6) confirmed the preference of adult sardines for large prey such as chaetognaths, decapod larvae, eggs and larvae of teleosts. Nevertheless, some smaller prey such as harpacticoid and cyclopoid (Ergasilida) copepods (with sizes of 150–880 μm and 125–625 μm, respectively) and cirripede larvae (220–660 μm) were also positively selected. Other prey items were selected only occasionally (copepod nauplii, cyclopoid copepods and Cladocera).
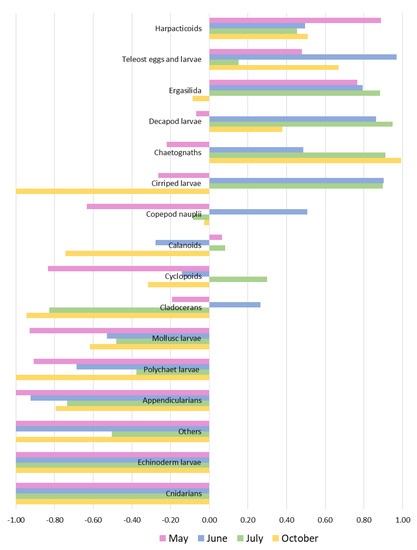
Figure 6.
Graphical representation of Ivlev’s electivity index calculated for prey groups at each sampling period.
Completely avoided potential prey (mesozooplanktonic specimens of taxa found in the sea but never observed in gut contents) that had negative Ivlev index values were cnidarians and echinoderm larvae. Partially avoided prey (mesozooplanktonic specimens of taxa more abundant at sea than in the gut contents, in terms of relative abundance) were: “Others”, Appendicularia, mollusk larvae and polychaetes larvae.
4. Discussion
4.1. Trophic Environment
In the Adriatic Sea, Sardina pilchardus spawns from October to May at water temperatures between 9 and 20 °C, with peaks between 11 °C and 16 °C, and salinity between 35.2 and 38.8 [35]. Gamulin and Hure [36] noted that sardines disappear from fishing grounds from October to March, which corresponds to their spawning season. Tičina et al. [37] suggested that sardines leave the most productive shallow waters of the north in the fall for the deeper waters of the south, where they find the stable and relatively warm waters necessary for spawning. This is probably the reason why we did not catch any fish in winter. Vučetić [23] also reported that she had difficulty obtaining adequate samples of sardines in winter.
According to other authors, sardine migration is favoured by the search for the optimal trophic conditions, with zooplankton concentration being higher in May–June in the shallow coastal areas, especially meroplankton [24,38], while in winter it becomes more available in the upper layers of the offshore areas, especially for large copepods [39,40,41,42]. In any case, migration to open and deeper waters is assumed to be associated with spawning and overwintering [43]. In the study area, copepods were the most abundant mesozooplanktonic organisms, with the only exception being in summer when cladocerans (with the summer swarming species Penilia avirostris) predominated in numbers. Meroplankton, consisting mainly of echinoderms, mollusk and crustacean larvae, were abundant in the spring. This is the typical seasonal outline of the neritic and estuarine plankton community in the northern Adriatic [44].
4.2. Diel Feeding Cycle
In May, June and July, sardines exhibited high stomach fullness during the dusk and early night hours. Evidence of daytime feeding by S. pilchardus has been previously observed in the Adriatic Sea, both in adults [23] and late larvae [45], as well as in the northern Aegean Sea [16] and in the Catalan Sea [19]. The late afternoon feeding peak coincides with the migration of zooplankton to the surface. Zooplankton species are nearly transparent, so only some specific pigmented body parts can be seen by a visual predator [46]. Such prey are difficult to detect in light with a natural angular distribution (low image contrast). Nevertheless, planktivorous fish may increase the contrast of their prey by searching for it at an angle greater than 48.6° to the vertical, as this makes the prey appear bright against a dark background [47]. This finding could explain the feeding peak that occurs when the sun is generally low on the horizon and the angle to the vertical is greater.
When analysing the stomach contents of fish caught by commercial purse seines operated at night under artificial light, a fundamental problem arises because artificial lights attract different species of mesozooplankton in different ways, creating unnatural conditions. This leads to a bias as the natural diet of small pelagic fishes is described under artificial conditions: both qualitative and quantitative aspects are distorted.
4.3. Diet Composition and Seasonality
The decision to analyse only samples caught in the late afternoon, during intense feeding activity, was justified by the possibility to detect prey not yet digested and to describe the composition of the bulk of the diet. We identified an average number of 466 to 2446 prey items per stomach. This abundance is comparable to the results obtained by Nikolioudakis et al. [17] in the northern Aegean (from 83 to 3334) and by Costalago et al. [15] in the western Mediterranean (from 1498 in winter to 4843 in summer). In contrast, the results are quite different when compared to those of Costalago et al. [15] in the Iberian Atlantic (from 40,126 in winter to 1,231,010 in summer). However, when phytoplankton is excluded, the range found by Costalago et al. [15] in the Atlantic (1339 prey/stomach in winter and 4094 prey/stomach in summer) is more similar to that found in this work in the northern Adriatic (from 460 to 2208 prey/stomach).
The results of the present study show that the diet of adult S. pilchardus in the northern Adriatic consists mainly of zooplankton. The diet was numerically based on copepods in all months, with the only exception being in June when the diet was more differentiated. Among the copepods, the Clauso-Paracalanidae group was the most abundant, followed, in decreasing order, by the genus Centropages, unidentified Calanoida, the families Oncaeidae and Corycaeidae, the species Euterpina acutifrons and the genera Temora and Oithona. These copepod groups have also been reported by other authors as important prey on the Spanish Atlantic coast [48], on the Portuguese coast [49], in the northwestern Mediterranean [6,15,19], in the northern Aegean [17], in the eastern Aegean [21] and in the central Adriatic [23,26].
Other prey categories reached high abundances only in a single month: tintinnids (in June), decapod larvae (in July) and chaetognaths (in October). Tintinnids have been confirmed as food for sardine in the Mediterranean [50] and on the Atlantic coast of Spain [49,51,52]. Crustacean larvae have also been found by other authors [15,17,19,21,23,26,49]. In contrast, chaetognaths have only been observed by Vučetić [23] in the central Adriatic. Teleostea eggs were also found in sardine stomachs in previous studies [49,51,53], but only in small quantities in the Mediterranean [15,26].
Dinoflagellates (Dinophyceae) were consistently present in the diet from May to July, while diatoms (Bacillariophyceae) were found in surprisingly low amounts. We observed a mean of 113.6 phytoplanktonic cells/stomach, a value lower than those found by Costalago et al. [15] in the northwestern Mediterranean (from 364.8 to 1192.8) and especially on the Iberian Atlantic coast (from 38,787.1 to 1,226,915.8).
4.4. Dietary Carbon
Early studies on the diet of Sardinops sagax from the Benguela region, based on abundance data or the volumetric method, indicated that sardines were phytoplanktivorous, non-selective filter feeders [54]. Later studies have shown that zooplankton make up the largest proportion of the diet, although phytoplankton play an important role in certain regions (upwelling systems) or in particular periods of the year [55,56]. The contribution of phytoplankton to the carbon content of the diet of adult sardines varies widely, ranging from 14–19% on Portuguese coasts [49] to <3% in the northern Aegean [17]. In the northern Adriatic, we found that the carbon uptake of sardine completely relies on zooplankton, while the contribution of phytoplankton to the total carbon content of the diet was 0.02%. The main food categories in terms of carbon content were: chaetognaths (49.9%), copepods (30.8%), teleost eggs and larvae (10.3%) and decapod larvae (8.0%). It should be noted that in the present study, the contribution of copepods to the carbon content of the diet was underestimated as their egg masses were not taken into account.
The seasonality of the diet, expressed as carbon content, showed even more marked differences than the number of prey. Copepods, chaetognaths, eggs and larvae of teleosts and decapod larvae accounted for almost all the carbon content of the diet in all seasons considered. Similarly, Costalago and Palomera [19] found that, regardless of their numerical importance, decapod larvae and copepods contributed more than any other prey to seasonal differences in diet.
4.5. Feeding Selectivity
Although filter feeding is considered the main feeding mode in S. pilchardus [48,57], the ability to switch to a particulate feeding mode was described by Garrido et al. [49] for sardines under experimental conditions: filtering was adopted when small particles (≤724 μm) were offered as food, while particulate feeding started when bigger prey (≥780 μm) were proposed. When a wide range of prey sizes was offered, both feeding modes were simultaneously adopted. In the studied area, prey showed a wide range of sizes (from 17 μm to 18,388 μm) without a clear distribution (Figure 6), suggesting that adult sardines in the northern Adriatic may use both filter and particulate feeding regardless of the season.
The Ivlev index pointed out that sardines actively selected teleost eggs and larvae, decapod larvae and chaetognaths, prey that were less abundant in the plankton samples than in stomach contents. Nevertheless, not only large prey were positively selected, but also small copepods such as Oncaea spp. and Euterpina acutifrons (whose maximum size is <750 µm) were preferred. The high selectivity for these small copepods reported in this and other works [17,19] and even for anchovies [58,59], could probably be explained by their tendency to associate with detritus and/or gelatinous zooplankton (e.g., [60,61]), which induce them to aggregate into patches, making them easy prey to be pursued and caught by filtering sardines.
Further evidence of selectivity in food intake is the fact that cladocerans were only marginally present in the stomach contents, even though they were dominant in the summer plankton. This result contrasts with Costalago and Palomera [19], who found that cladocerans are highly selected by sardines in the northwestern Mediterranean during summer. The importance of cladocerans in the diet of small pelagic fishes is not clear: some authors emphasise their importance [18,62,63], but others found that high concentrations of cladocerans could even have unfavourable effects on the feeding activity of small planktivores [64].
Some of the most abundant meroplankton (echinoderm larvae) were never found in the stomachs and thus were completely avoided by the sardines. The inconsistency of prey composition in stomach contents in relation to plankton has also been noted by other authors [17,19,23]. In laboratory studies, sardines, when fed with wild mixed prey assemblages, also showed a preference for copepods and decapods over other zooplankton [11]. The constant presence of copepods in gut contents suggests that certain prey characteristics may be more likely to induce predation by fish. In planktivores, prey detection may be strongly influenced by prey movement [65,66], shape and colour of prey body [67], relative orientation between prey and predator [68] and light intensity [69].
The observed low selectivity for calanoid copepods could be due to the ability of these prey to escape sardine predation [17,70]. The swimming behaviour of copepods generally varies between continuous and intermittent locomotion, but can also have even more complex features, as in the genus Clausocalanus, involving a rapid and continuous movement in intertwined small loops [71]. The short pauses of motion may provide copepods with brief moments of invisibility to predators, and the subsequent sinking may increase their perceptual ability [72,73]. In addition, copepod species appear to modify their escape behaviour depending on the strength of the stimulus they encounter [74].
Explaining food selection in small planktivorous fishes is quite difficult as little is known so far about the vertical distribution of planktonic species, their ability to swarm and their escape capacity. This, in turn, complicates considerations about the probability of an encounter between prey and predator. The predator is often able to compensate for the prey’s adaptations, resulting in equal capture success [74]. In any case, for sardines, the factor determining the selection/avoidance of a potential prey, as well as the switch from filter feeding to the particulate feeding mode, is the final energy uptake achieved when the gains from consumption exceed the losses from capture. On the other hand, sardines can influence planktonic community structures and food web functioning thanks to their ability to select for food.
Studying the adaptive capacity of small pelagic fishes is central to better management of fisheries’ resources. Given the importance of mesozooplankton in understanding the ecology of small pelagic fishes, scientific surveys should be carried out with regular environmental monitoring, including plankton sampling.
Author Contributions
Conceptualization, D.B. and V.T.; methodology, D.B., A.d.O. and S.L.; investigation, D.B., S.L. and V.T.; writing—original draft preparation, D.B. and V.T.; writing—review and editing, D.B., A.d.O., S.L. and V.T.; supervision, V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project EcoMAdr (INTERREG IIIA Italy–Slovenia) and the Flagship Project RITMARE—The Italian Research for the Sea—coordinated by the Italian National Research Council and funded by the Italian Ministry of Education, Universities, and Research within the National Research Program 2011–2013. D.B. and S.L. were funded by the Project EcoMAdr (INTERREG IIIA Italy–Slovenia).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during this study are included in this article with the exception of temperature and salinity data which are available on request from the corresponding author.
Acknowledgments
Special thanks to the crew of Cuba and Acquario fishing vessels for their assistance in the field work and to Alenka Goruppi for the revision of the taxonomical list of zooplankton and the help for the editing of figures. We thank the three anonymous reviewers of this manuscript for their useful suggestions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Morphometric relationships used to calculate (a) dry mass (DM) (µg) and (b) carbon content (µg C) of Sardina pilchardus preys. PL: prosome length (µm); L: total length (µm); V: volume. Brackets (a) and (b) indicate which sources the relationships refer to.
Table A1.
Morphometric relationships used to calculate (a) dry mass (DM) (µg) and (b) carbon content (µg C) of Sardina pilchardus preys. PL: prosome length (µm); L: total length (µm); V: volume. Brackets (a) and (b) indicate which sources the relationships refer to.
| Prey item |
|
| References |
|---|---|---|---|
| Cocconeis spp. Coscinodiscus spp. Diploneis spp. Paralia sulcata Pleurosigma spp. Nitzschia spp. Thalassiosira spp. Pennate diatom | volume | C = V × 0.11 | (a) [75,76]; (b) [77] |
| Ceratium candelabrum Ceratium furca Ceratium trichoceros Ceratium tripos Ceratium spp. Diplopsalis spp. Dinophysis caudata Dinophysis fortii Dinophysis sacculus Gonyaulax polygramma Gonyaulax spp. Katodinium spp. Lingulodinium polyedrum Phalacroma spp. Podolampas palmipes Prorocentrum micans Protoperidinium claudicans Protoperidinium conicum Protoperidinium crassipes Protoperidinium depressum Protoperidinium divergens Protoperidinium oblongum Protoperidinium oceanicum Protoperidinium steinii Ornithocercus magnificus Scrippsiella spp. Protoperidinium spp. | volume | C = V × 0.13 | (a) [75,76]; (b) [77] |
| Codonellopsis schabi Eutintinnus fraknoii Stenosemella ventricosa Tintinnopsis radix | volume | C = (444.5 + 0.053 × V) × 10−6 | (b) [78] |
| Gastropoda pediveliger | direct weight | C = (DW × 31.25)/100 | (b) [55] |
| Bivalvia veliger | direct weight | C = (DW × 31.25)/100 | (a) [79]; (b) [55] |
| Polychaeta larvae | direct weight | C = (DW × 40)/100 | (a) [79]; (b) [80] |
| Evadne nordmanni Evadne spinifera Penilia avirostris Pleopis polyphemoides Podon intermedius Podonidae | direct weight | C = (DW × 33.1)/100 | (a) [81]; (b) [82] |
| Acartia clausi Acartia tonsa Acartia spp. | Log C = 3.032 Log PL − 8.556 | (b) [83] | |
| Calanus spp. (ref. Calanus helgolandicus) | Log DW = 2.691 Log PL − 6.883 | C = 0.372 DW − 0.248 | (a) [84]; (b) [85] |
| Centropages ponticus Centropages typicus Centropages spp. | Log DW = 2.451 Log PL − 6.103 | C = (DW × 37.6)/100 | (a) [84]; (b) [82] |
| Clausocalanus sp. Paracalanus parvus Paracalanus spp. Clauso-Paracalanidae Calanoida indet. | Log C = 3.128 Log PL − 8.451 | (b) [86] | |
| Nannocalanus minor (rif. Calanus helgolandicus) | Log DW = 2.691 Log PL − 6.883 | C = 0.372 DW − 0.248 | (a) [84]; (b) [85] |
| Temora longicornis | Log DW = 3.059 Log PL − 7.682 | C = (DW × 46.8)/100 | (a) [84]; (b) [87] |
| Temora stylifera | (Log DW = 2.71 Log L − 3.685)/1000 | C = (DW × 46.8)/100 | (a) [88]; (b) [87] |
| Oithona nana Oithona cf. nana Oithona plumifera Oithona cf. plumifera Oithona setigera Oithona cf. similis Oithona spp. | C = 9.4676 10−7 PL2.16 | (b) [89] | |
| Corycaeus spp. (ref. Cyclopoida) | Ln DW = 1.96 Ln PL − 11.64 | C = (DW × 43.1)/100 | (a) [90]; (b) [87] |
| Oncaea spp. | direct weight | C = (DW × 38.2)/100 | (a) (b) [87] |
| Clytemnestra scutellata | Ln DW = 1.96 Ln PL − 11.64 | C = (DW × 42.4)/100 | (a) [90]; (b) [91] |
| Euterpina acutifrons | DW = 1.389 10−8 L2.857 | C = (DW × 46)/100 | (a) (b) [92] |
| Microsetella rosea Harpacticoida indet. (ref. Microsetella norvegica) | C = 2.65 10−6 L1.95 | (b) [93] | |
| Copepoda eggs | 4/3 π (L/2)3 | C = 140 × 10−9 × V | (b) [94] |
| Copepod nauplii (ref. Acartia nauplii) | Log DW = 2.848 Log L − 7.265 | C = (DW × 42.4)/100 | (a) [95]; (b) [91] |
| Cirripedia nauplii | DW = 80.627 × L4.27 | C = (DW × 39.97)/100 | (a) [55]; (b) [96] |
| Cirripedia cypris | direct weight | C = (DW × 39.97)/100 | (b) [96] |
| Squilla mantis alima (ref. Lucifer reynaudii) | direct weight | C = (DW × 41.1)/100 | (a) (b) [97] |
| Hyperiidae indet. Porcellana zoeae Decapoda zoeae Decapoda mysis Decapoda phyllosoma | direct weight | C = (DW × 43.69)/100 | (a) [79]; (b) [87] |
| Decapoda nauplii (ref. Cirripedia larvae) | direct weight | (a) [98] | |
| Oikopleura spp. | C = 0.04 × L3.29 | (b) [99] | |
| Chaetognatha (ref. Sagitta elegans) | DW = 0.114 × L3.1963 | C = (DW × 35.8)/100 | (a) (b) [100] |
| Engraulis encrasicolus eggs Teleostea spheric eggs (ref. Engraulis mordax) | direct weight | C = 0.457 × DW | (a) [101]; (b) [102] |
| Teleostea larvae (ref. E. encrasicolus larvae) | DW = 6 × 10−15 L4.1229 | C = 0.457 × DW | (a) [58]; (b) [102] |
| Invertebrata spheric eggs (ref. Copepoda eggs) | 4/3 π (L/2)3 | C = 140 × 10−9 × V | (b) [94] |
| Invertebrata elliptical eggs (ref. Copepoda eggs) | 4/3 π (L/2) × (L/4)2 | C = 140 × 10−9 × V | (b) [94] |

Table A2.
Mesozooplankton abundance (ind m−3) and species composition during the studied period: from May 2006 to February 2007. Zooplankton samples were collected at the same time as sardines. Date (day/month/year), time (24 h, GMT + 1).
Table A2.
Mesozooplankton abundance (ind m−3) and species composition during the studied period: from May 2006 to February 2007. Zooplankton samples were collected at the same time as sardines. Date (day/month/year), time (24 h, GMT + 1).
| Date | 10 May 06 | 20 June 06 | 26 July 06 | 04 Sept 06 | 26 Oct 06 | 14 Dec 06 | 01 Feb 07 | |
|---|---|---|---|---|---|---|---|---|
| Time | 22:30 | 21:30 | 18:50 | 16:50 | 17:50 | 15:20 | 18:50 | |
| Group | Sampling depth (m) | 16 | 16 | 16 | 17 | 20 | 12 | 22 |
| Dinophyceae | Noctiluca scintillans | 0 | 20 | 286 | 74 | 0 | 30 | 88 |
| Hydrozoa | Anthomedusae indet. | 27 | 27 | 20 | 85 | 0 | 0 | 0 |
| Obelia spp. | 0 | 12 | 4 | 0 | 6 | 0 | 4 | |
| Leptomedusae indet. | 0 | 4 | 0 | 0 | 0 | 0 | 0 | |
| Siphonophorae | Muggiaea spp. | 0 | 220 | 0 | 0 | 0 | 0 | 0 |
| Siphonophorae indet. | 14 | 2141 | 192 | 41 | 527 | 37 | 16 | |
| Hydrozoa indet. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Scyphozoa | Scyphozoa ephyrae | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Anthozoa | Cerianthus larvae | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ctenophora | Ctenophora larvae | 10 | 31 | 0 | 0 | 0 | 0 | 0 |
| Ctenophora indet. | 0 | 0 | 0 | 0 | 0 | 4 | 1 | |
| Nemertea | Nemertea pilidia | 0 | 20 | 0 | 0 | 0 | 0 | 0 |
| Phoronida | Phoronida actinotrochae | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastropoda | Creseis clava | 0 | 0 | 0 | 0 | 3 | 4 | 0 |
| Gastropoda pediveligers | 239 | 408 | 455 | 0 | 13 | 60 | 17 | |
| Bivalvia | Bivalvia veligers | 6 | 67 | 357 | 37 | 110 | 98 | 34 |
| Polychaeta | Polychaeta larvae | 0 | 12 | 16 | 18 | 56 | 25 | 4 |
| Polychaeta indet. | 24 | 106 | 0 | 0 | 0 | 0 | 0 | |
| Branchiopoda | Pleopis polyphaemoides | 2 | 24 | 35 | 4 | 0 | 0 | 37 |
| Podon intermedius | 2 | 16 | 20 | 0 | 6 | 0 | 10 | |
| Evadne nordmanni | 78 | 8 | 251 | 129 | 0 | 0 | 0 | |
| Evadne spinifera | 73 | 192 | 43 | 122 | 9 | 0 | 0 | |
| Evadne tergestina | 0 | 12 | 78 | 0 | 0 | 0 | 0 | |
| Podonidae indet. | 0 | 0 | 0 | 0 | 3 | 0 | 0 | |
| Penilia avirostris | 0 | 20 | 6533 | 6097 | 198 | 16 | 1 | |
| Ostracoda | Ostracoda indet. | 0 | 8 | 0 | 4 | 0 | 0 | 1 |
| Calanoida | Acartia (Acartiura) clausi | 345 | 298 | 145 | 89 | 41 | 22 | 11 |
| Acartia juv. | 388 | 200 | 184 | 26 | 116 | 93 | 17 | |
| Anomalocera sp. | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Calanus helgolandicus | 200 | 0 | 0 | 0 | 0 | 3 | 10 | |
| Calocalanus spp. | 2 | 0 | 0 | 4 | 0 | 0 | 0 | |
| Candacia juv. | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Centropages ponticus | 39 | 4 | 0 | 7 | 0 | 0 | 0 | |
| Centropages typicus | 86 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Centropages spp. | 839 | 525 | 24 | 81 | 0 | 0 | 0 | |
| Clausocalanus arcuicornis | 6 | 0 | 0 | 7 | 0 | 3 | 30 | |
| Clausocalanus furcatus | 2 | 0 | 0 | 59 | 72 | 0 | 13 | |
| Clausocalanus juv. | 51 | 8 | 8 | 85 | 144 | 3 | 54 | |
| Ctenocalanus vanus | 114 | 0 | 0 | 0 | 0 | 4 | 448 | |
| Diaixis pygmaea | 0 | 0 | 0 | 0 | 0 | 8 | 110 | |
| Euchaeta hebes | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Mecynocera clausi | 0 | 0 | 0 | 4 | 0 | 0 | 0 | |
| Paracalanus denudatus | 649 | 8 | 0 | 55 | 0 | 0 | 0 | |
| Paracalanus parvus s.l. | 2110 | 1173 | 702 | 1358 | 1239 | 150 | 317 | |
| Pseudocalanus elongatus | 120 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Temora longicornis | 171 | 4 | 12 | 0 | 0 | 0 | 3 | |
| Temora stylifera | 2 | 0 | 24 | 1517 | 370 | 41 | 17 | |
| Calanoida copepodites | 739 | 545 | 553 | 698 | 618 | 169 | 147 | |
| Cyclopoida | Oithona nana | 51 | 459 | 75 | 140 | 85 | 34 | 6 |
| Oithona plumifera | 0 | 0 | 4 | 85 | 72 | 33 | 19 | |
| Oithona setigera | 0 | 4 | 4 | 0 | 0 | 0 | 0 | |
| Oithona similis | 153 | 0 | 4 | 15 | 13 | 3 | 3 | |
| Oithona spp. | 200 | 118 | 71 | 114 | 72 | 255 | 33 | |
| Ergasilida | Corycaeidae indet. | 27 | 4 | 35 | 103 | 348 | 170 | 77 |
| Oncaea spp. | 6 | 102 | 267 | 572 | 3250 | 531 | 257 | |
| Sapphirina spp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Harpacticoida | Clytemnestra scutellata | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Euterpina acutifrons | 4 | 4 | 125 | 225 | 191 | 77 | 36 | |
| Harpacticoida indet. | 2 | 35 | 8 | 4 | 0 | 1 | 0 | |
| Copepoda | Copepoda nauplii | 122 | 78 | 59 | 78 | 63 | 60 | 26 |
| Cirripedia | Cirripedia nauplii | 39 | 8 | 75 | 0 | 13 | 1 | 1 |
| Isopoda | Epicaridea indet. | 0 | 0 | 0 | 0 | 3 | 0 | 3 |
| Decapoda | Pisidia larvae | 0 | 12 | 0 | 0 | 0 | 0 | 0 |
| Brachiura zoea | 0 | 0 | 0 | 4 | 0 | 0 | 1 | |
| Decapoda mysis | 2 | 98 | 43 | 44 | 25 | 4 | 1 | |
| Decapoda zoea | 14 | 12 | 4 | 18 | 3 | 0 | 4 | |
| Decapoda nauplii | 0 | 0 | 4 | 0 | 0 | 0 | 0 | |
| Mysida | Mysida indet. | 2 | 0 | 8 | 0 | 3 | 0 | 1 |
| Cumacea | Cumacea indet. | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Chaetognatha | Sagitta spp. | 4 | 39 | 35 | 48 | 13 | 78 | 44 |
| Echinodermata | Asteroidea larvae | 0 | 0 | 27 | 0 | 0 | 0 | 0 |
| Psammechinus larvae | 14 | 4 | 8 | 0 | 0 | 0 | 0 | |
| Echinoidea plutei | 135 | 1733 | 1012 | 4 | 6 | 5 | 41 | |
| Holothuroidea auricularia | 108 | 47 | 16 | 0 | 0 | 0 | 0 | |
| Echinodermata plutei | 39 | 4 | 0 | 0 | 0 | 0 | 0 | |
| Hemichordata | Hemichordata tornariae | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Appendicularia | Oikopleura spp. | 39 | 557 | 1259 | 63 | 314 | 16 | 117 |
| Thaliacea | Doliolum spp. | 0 | 47 | 35 | 0 | 19 | 4 | 4 |
| Cephalochordata | Branchiostoma lanceolatum juv. | 0 | 43 | 0 | 0 | 0 | 0 | 0 |
| Vertebrata | Osteichthyes eggs | 14 | 27 | 35 | 4 | 6 | 0 | 0 |
| Osteichthyes larvae | 4 | 31 | 59 | 4 | 3 | 0 | 1 | |
| TOTAL | 7320 | 9576 | 13,212 | 12,125 | 8035 | 2044 | 2075 |
References
- Bakun, A. Wasp-waist populations and marine ecosystem dynamics: Navigating the ‘predator pit’ topographies. Prog. Oceanogr. 2006, 68, 271–288. [Google Scholar] [CrossRef]
- Coll, M.; Shannon, L.J.; Moloney, C.L.; Palomera, I.; Tudela, S. Comparing trophic flows and fishing impacts of a NW Mediterranean ecosystem with coastal upwelling systems by means of standardized models and indicators. Ecol. Modell. 2006, 198, 53–70. [Google Scholar] [CrossRef]
- Alheit, J.; Roy, C.; Kifani, S. Decadal-scale variability in populations. In Climate Change and Small Pelagic Fish; Checkley, D.M., Jr., Alheit, J., Oozeki, Y., Roy, C., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 64–87. [Google Scholar]
- Van der Lingen, C.D.; Bertrand, A.; Bode, A.; Brodeur, R.; Cubillos, L.A.; Espinoza, P.; Friedland, K.; Garrido, S.; Irigoien, X.; Miller, T.; et al. Trophic dynamics. In Climate Change and Small Pelagic Fish; Checkley, D., Roy, C., Alheit, J., Oozeki, Y., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 112–157. [Google Scholar]
- Costalago, D.; Palomera, I.; Tirelli, V. Seasonal comparison of the diets of juvenile European anchovy Engraulis encrasicolus and sardine Sardina pilchardus in the Gulf of Lions. J. Sea Res. 2014, 89, 64–72. [Google Scholar] [CrossRef]
- Chen, C.T.; Carlotti, F.; Harmelin-Vivien, M.; Guilloux, L.; Bănaru, D. Temporal variation in prey selection by adult European sardine (Sardina pilchardus) in the NW Mediterranean Sea. Progr. Oceanogr. 2021, 196, 102617. [Google Scholar] [CrossRef]
- Brosset, P.; Le Bourg, B.; Costalago, D.; Bănaru, D.; Van Beveren, E.; Bourdeix, J.H.; Fromentin, J.M.; Ménard, F.; Saraux, C. Linking small pelagic dietary shifts with ecosystem changes in the Gulf of Lions. Mar. Ecol. Progr. Ser. 2016, 554, 157–171. [Google Scholar] [CrossRef]
- Saraux, C.; Van Beveren, E.; Brosset, P.; Queiros, Q.; Bourdeix, J.-H.; Dutto, G.; Gasset, E.; Jac, C.; Bonhommeau, S.; Fromentin, J.-M. Small pelagic fish dynamics: A review of mechanisms in the Gulf of Lions. Deep.-Sea Res. Pt. II 2019, 159, 52–61. [Google Scholar] [CrossRef]
- Ryther, J.H. Photosynthesis and fish production in the sea. Science 1969, 166, 72–76. [Google Scholar] [CrossRef]
- Walsh, J.J. A carbon budget for overfishing off Peru. Nature 1981, 290, 300–304. [Google Scholar] [CrossRef]
- Garrido, S.; Marçalo, A.; Zwolinski, J.; van der Lingen, C.D. Laboratory investigations on the effect of prey size and concentration on the feeding behaviour of Sardina pilchardus. Mar. Ecol. Progr. Ser. 2007, 330, 189–199. [Google Scholar] [CrossRef]
- James, A.G. Are clupeoid microphagists herbivorous or omnivorous? A review of the diets of some commercially important clupeids. S. A. J. Mar. Sci. 1988, 7, 161–177. [Google Scholar] [CrossRef]
- Andreu, B. Las branquispinas en la caracterización de las poblaciones de Sardina pilchardus. (Walb.) Inv. Pesq. 1969, 33, 425–607. [Google Scholar]
- Palomera, I.; Olivar, P.; Salat, J.; Sabatés, A.; Coll, M.; García, A.; Morales-Nin, B. Small pelagic fish in the NW Mediterranean Sea: An ecological review. Progr. Oceanogr. 2007, 74, 377–396. [Google Scholar] [CrossRef]
- Costalago, D.; Garrido, S.; Palomera, I. Comparison of the feeding apparatus and diet of European sardines Sardina pilchardus of Atlantic and Mediterranean waters: Ecological implications. J. Fish Biol. 2015, 86, 1348–1362. [Google Scholar] [CrossRef]
- Nikolioudakis, N.; Palomera, I.; Machias, A.; Somarakis, S. Diel feeding intensity and daily ration of the sardine Sardina pilchardus. Mar. Ecol. Progr. Ser. 2011, 437, 215–228. [Google Scholar] [CrossRef]
- Nikolioudakis, N.; Isari, S.; Pitta, P.; Somarakis, S. Diet of sardine Sardina pilchardus: An “end-to-end” field study. Mar. Ecol. Progr. Ser. 2012, 453, 173–188. [Google Scholar] [CrossRef]
- Costalago, D.; Navarro, J.; Álvarez-Calleja, I.; Palomera, I. Ontogenetic and seasonal changes in the feeding habits and trophic levels of two small pelagic fish species. Mar. Ecol. Progr. Ser. 2012, 460, 169–181. [Google Scholar] [CrossRef]
- Costalago, D.; Palomera, I. Feeding of European pilchard (Sardina pilchardus) in the northwestern Mediterranean: From late larvae to adults. Sci. Mar. 2014, 78, 41–54. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Borme, D.; Coll, M.; Tirelli, V.; Palomera, I.; Navarro, J. Trophic ecology of range-expanding round sardinella and resident sympatric species in the NW Mediterranean. Mar. Ecol. Progr. Ser. 2019, 620, 139–154. [Google Scholar] [CrossRef]
- Sever, T.M.; Bayhan, B.; Taskavak, E. A preliminary study on the feeding regime of european pilchard (Sardina pilchardus Walbaum1792) in Izmir Bay, Turkey, Eastern Aegean Sea. NAGA WorldFish Cent. Q. 2005, 28, 41–48. [Google Scholar]
- Nikolioudakis, N.; Isari, S.; Somarakis, S. Trophodynamics of anchovy in a non-upwelling system: Direct comparison with sardine. Mar. Ecol. Progr. Ser. 2014, 500, 215–229. [Google Scholar] [CrossRef]
- Vučetić, T. Ishrana odrasle srdele (Sardina pilchardus Walb.) u srednjem Jadranu. Acta Adriat. 1963, 10, 3–43. [Google Scholar]
- Vučetić, T. O odnosu srdele (Sardina pilchardus Walb.) prema biotskim faktorima sredine–zooplanktonu. Acta Adriat. 1964, 11, 269–276. [Google Scholar]
- Zorica, B.; Keč, V.Č.; Vidjak, O.; Mladineo, I.; Balić, D.E. 2016 Feeding habits and helminth parasites of sardine (S. pilchardus) and anchovy (E. encrasicolus) in the Adriatic Sea. Mediterr. Mar. Sci. 2016, 17, 216–229. [Google Scholar] [CrossRef]
- Zorica, B.; Keč, V.Č.; Vidjak, O.; Kraljević, V.; Brzulja, G. Seasonal pattern of population dynamics, spawning activities, and diet composition of sardine (Sardina pilchardus Walbaum) in the eastern Adriatic Sea. Turk. J. Zool. 2017, 41, 892–900. [Google Scholar] [CrossRef]
- Malačič, V.; Celio, M.; Čermelj, B.; Bussani, A.; Comici, C. Interannual evolution of seasonal thermohaline properties in the Gulf of Trieste (Northern Adriatic) 1991-2003. J. Geophys. Res. 2006, 111, C08009. [Google Scholar] [CrossRef]
- Stambler, N. The Mediterranean Sea—Primary productivity. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 113–121. [Google Scholar]
- Grilli, F.; Accoroni, S.; Acri, F.; Aubry, F.B.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and Interannual Trends of Oceanographic Parameters over 40 Years in the Northern Adriatic Sea in Relation to Nutrient Loadings Using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Santojanni, A.; Leonori, I.; Piccinetti, C.; Fabi, G.; Angelini, S.; Belardinelli, A.; Biagiotti, I.; Canduci, G.; Carpi, P.; Colella, S.; et al. GSA 17—Mare Adriatico settentrionale e centrale. In Annuario Sullo Stato Delle Risorse e Sulle Strutture Produttive dei Mari italiani. Biologia Marina Mediterranea; Mannini, A., Sabatella, R.F., Eds.; Erredi: Genova, Italy, 2015; Volume 22, (Suppl. 1), pp. 116–146. [Google Scholar]
- Vasilakopoulos, P.; Maravelias, C.D.; Tserpes, G. The alarming decline of Mediterranean fish stocks. Curr. Biol. 2014, 24, 1643–1648. [Google Scholar] [CrossRef]
- Mosetti, F. Condizioni idrologiche della costiera triestina. Hydrores 1988, 6, 29–38. [Google Scholar]
- Cozzi, S.; Falconi, C.; Comici, C.; Cermelj, B.; Kovac, N.; Turk, V.; Giani, M. Recent evolution of river discharges in the Gulf of Trieste and their potential response to climate changes and anthropogenic pressure. Est. Coast. Shelf Sci. 2012, 115, 14–24. [Google Scholar] [CrossRef]
- Ivlev, V.S. Experimental Ecology of the Feeding of Fishes; Scott, D., Translator; Yale University Press: New Haven, CT, USA, 1961; p. 302. [Google Scholar]
- Škrivanić, A.; Zavodnik, D. Migrations of the sardine (Sardina pilchardus) in relation to hydrographical conditions of the Adriatic Sea. Neth. J. Sea Res. 1973, 7, 7–18. [Google Scholar] [CrossRef]
- Gamulin, T.; Hure, J. Spawning of the sardine at a definite time of day. Nature 1956, 177, 193–194. [Google Scholar] [CrossRef]
- Tičina, V.; Ivančić, I.; Emrić, V. Relation between the hydrographic properties of the northern Adriatic Sea water and sardine (Sardina pilchardus) population schools. Period. Biolog. 2000, 102, 181–192. [Google Scholar]
- Gamulin, T. La ponte et les aires de ponte de la sardine (Sardina pilchardus Walb.) dans l’Adriatique de 1947 à 1950. In Report of “HVAR” Expedition 4 (4 C); Springer: Berlin/Heidelberg, Germany, 1954; pp. 1–66. [Google Scholar]
- Hure, J. Distribution annuelle vertical du zooplankton sur une station de l’Adriatique méridionale. Acta Adriat. 1955, 7, 1–72. [Google Scholar]
- Hure, J. Dnevna migracija i sezonska vertikalna raspodjela zooplanktona dubljeg mora. Acta Adriat. 1961, 9, 1–59. [Google Scholar]
- Hure, J. Rythme saisonnier de la distribution vertical du zooplancton dams les eaux profondes de l’Adriatique meridionale. Acta Adriat. 1964, 11, 167–172. [Google Scholar]
- Vučetić, T. Vertikalna raspodjela zooplanktona u Velikom jezeru otoka Mljeta. Acta Adriat. 1961, 6, 1–20. [Google Scholar]
- Mužinić, R. Migrations of adult sardines in the central Adriatic. Neth. J. Sea Res. 1973, 7, 19–30. [Google Scholar] [CrossRef]
- Pierson, J.; Camatti, E.; Hood, R.; Kogovšek, T.; Lučić, D.; Tirelli, V.; Malej, A. Mesozooplankton and gelatinous zooplankton in the face of environmental stressors. In Coastal Ecosystems in Transition: A Comparative Analysis of the Northern Adriatic and Chesapeake Bay, 1st ed.; Malone, T., Malej, A., Faganeli, J., Eds.; Geophysical Monograph; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; Volume 256, pp. 105–127. [Google Scholar] [CrossRef]
- Borme, D.; Tirelli, V.; Palomera, I. Feeding habits of European pilchard late larvae in a nursery area in the Adriatic Sea. J. Sea Res. 2013, 78, 8–17. [Google Scholar] [CrossRef]
- Zaret, T.M. Predators, invisible prey, and the nature of polymorphism in the Cladocera (Class Crustacea). Limnol. Oceanogr. 1972, 17, 171–184. [Google Scholar] [CrossRef]
- Janssen, J. Searching for zooplankton just outside Snell’s window. Limnol. Oceanogr. 1981, 26, 1168–1171. [Google Scholar] [CrossRef]
- Varela, M.; Alvarez-Ossorio, M.T.; Valdéz, L. Método para el estudio cuantitativo del contenido estomacal de la sardina. Resultados preliminares. Bol. Instit. Esp. Oceanogr. 1990, 6, 117–126. [Google Scholar]
- Garrido, S.; Ben-Hamadou, R.; Oliveira, P.B.; Cunha, M.E.; Chícharo, M.A.; van der Lingen, C.D. Diet and feeding intensity of sardine Sardina pilchardus: Correlation with satellite derived chlorophyll data. Mar. Ecol. Progr. Ser. 2008, 354, 245–256. [Google Scholar] [CrossRef]
- Massutì, M.; Oliver, M. Estudio de la biometria y biologia de la sardine de Mahón (Baleares), especialmente de su alimentación. Bol. Instit. Esp. Oceanogr. 1948, 3, 1–15. [Google Scholar]
- Varela, M.; Larrañaga, A.; Costas, E.; Rodriguez, B. Contenido estomacal de la sardina (Sardina pilchardus Walbaum) durante la campaña Saracus 871 en la plataformas Cantábrica y de Galicia en febrero de 1971. Bol. Instit. Esp. Oceanogr. 1988, 5, 17–28. [Google Scholar]
- Cunha, M.E.; Garrido, S.; Pissarra, J. The use of stomach fullness and colour indices to assess Sardina pilchardus feeding. J. Mar. Biol. Ass. 2005, 85, 425–431. [Google Scholar] [CrossRef]
- Silva, E. Some notes on the food of the pilchard Sardina pilchardus (Walb.), of the Portuguese coast. Rev. De La Fac. De Cienc. De Lisb. 2 Ser. 1954, 4, 281–294. [Google Scholar]
- Davies, D.H. The South African pilchard (Sardinops ocellata). Preliminary report on feeding off the West Coast, 1953–1956. Inv. Rep. Div. Fish. S. A. 1957, 30, 40. [Google Scholar]
- James, A.G. Feeding ecology, diet and field-based studies on feeding selectivity of the Cape anchovy Engraulis capensis Gilchrist, 1913. S. Afr. J. Mar. Sci. 1987, 5, 673–692. [Google Scholar] [CrossRef]
- Van der Lingen, C.D. Diet of sardine Sardinops sagax in the southern Benguela upwelling ecosystem. S. Afr. J. Mar. Sci. 2002, 24, 301–316. [Google Scholar] [CrossRef]
- Bode, A.; Carrera, P.; Lens, S. The pelagic foodweb in the upwelling ecosystem of Galicia (NW Spain) during spring: Natural abundance of stable carbon and nitrogen isotopes. ICES J. Mar. Sci. 2003, 60, 11–22. [Google Scholar] [CrossRef]
- Borme, D.; Tirelli, V.; Brandt, S.B.; Umani, S.F.; Arneri, E. Diet of Engraulis encrasicolus in the northern Adriatic Sea (Mediterranean): Ontogenetic changes and feeding selectivity. Mar. Ecol. Progr. Ser. 2009, 392, 193–209. [Google Scholar] [CrossRef]
- Tudela, S.; Palomera, I. Trophic ecology of the European anchovy Engraulis encrasicolus in the Catalan Sea (northwest Mediterranean). Mar. Ecol. Progr. Ser. 1997, 160, 121–134. [Google Scholar] [CrossRef][Green Version]
- Green, E.P.; Dagg, M.J. Mesozooplankton associations with medium to large marine snow aggregates in the northern Gulf of Mexico. J. Plankton Res. 1997, 19, 435–447. [Google Scholar] [CrossRef]
- Diaz, E.; Cotano, U.; Villate, F. Reproductive response of Euterpina acutifrons in 2 estuaries of the Basque Country (Bay of Biscay) with contrasting nutritional environment. J. Exp. Mar. Biol. Ecol. 2003, 292, 213–230. [Google Scholar] [CrossRef]
- Štirn, J. The north Adriatic pelagial, its oceanological characteristics, structure and distribution of the biomass during the year 1965. Diss. Acad. Sci. Arts Slov. 1969, 12, 1–92. [Google Scholar]
- Specchi, M.; Orlandi, C.; Radin, B.; Manetti, M.F.; Cassetti, P. Observations on the occurrence of Penilia avirostris Dana and the eggs of Engraulis encrasicolus L. in the Gulf of Trieste (Northern Adriatic Sea). Boll. Soc. Adr. Sci. 1999, 78, 309–316. [Google Scholar]
- Ogawa, Y.; Nakahara, T. Interrelationships between pelagic fishes and plankton in the coastal fishing ground of the southwestern Japan Sea. Mar. Ecol. Progr. Ser. 1979, 1, 115–122. [Google Scholar] [CrossRef]
- Zaret, T.M. The effect of prey motion on planktivore choice. In Evolution and Ecology of Zooplankton Communities; Kerfoot, W.C., Ed.; New England University Press: Hanover, NH, USA, 1980; pp. 599–603. [Google Scholar]
- Wright, D.I.; O’Brien, W.J. Differential location of Chaoborus larvae and Daphnia by fish: The importance of motion and visible size. Am. Mid. Nat. 1982, 108, 68–73. [Google Scholar] [CrossRef]
- Imbrahim, A.A.; Huntingford, F.A. Laboratory and field studies on diet choice of three-spined thicklebacks, Gasterosteus aculeatus L., in relation to profitability and visual features of prey. J. Fish Biol. 1989, 34, 245–258. [Google Scholar] [CrossRef]
- Luecke, C.; O’Brien, W.J. Prey location volume of a planktivorous fish: A new measurement of prey vulnerability. Can. J. Fish. Aquat. Sci. 1981, 38, 1264–1270. [Google Scholar] [CrossRef]
- Vinyard, G.L.; O’Brien, W.J. Effects of light and turbidity on the reactive distance of bluegill (Lepomis macrochirus). J. Fish. Res. Board Can. 1976, 33, 2845–2849. [Google Scholar] [CrossRef]
- Strickler, J.R.; Udvadia, A.J.; Marino, J.; Radabaugh, N.; Ziarek, J.; Nihongi, A. Visibility as a factor in the copepod-planktivorous fish relationship. Sci. Mar. 2005, 69 (Suppl. 1), 111–124. [Google Scholar] [CrossRef]
- Mazzocchi, M.G.; Paffenhöfer, G.A. Swimming and feeding behaviour of the planktonic copepod Clausocalanus furcatus. J. Plankton Res. 1999, 21, 1501–1518. [Google Scholar] [CrossRef]
- Yen, J. Life in transition: Balancing inertial and viscous forces by planktonic copepods. Biol. Bull. 2000, 198, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.L.; McLaughlin, R.L. The behavioral ecology of intermittent locomotion. Am. Zool. 2001, 41, 137–153. [Google Scholar] [CrossRef]
- Waggett, R.J.; Buskey, E.J. Calanoid copepod escape behavior in response to a visual predator. Mar. Biol. 2007, 150, 599–607. [Google Scholar] [CrossRef]
- Hillebrandt, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. HELCOM Balt. Sea Environ. Proc. 2006, 106, 144. [Google Scholar]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Verity, P.G.; Langdon, C. Relationship between lorica volume carbon, nitrogen and ATP content of tintinnids in Narragansett Bay. J. Plankton Res. 1984, 6, 857–869. [Google Scholar] [CrossRef]
- La Mesa, M.; Borme, D.; Tirelli, V.; Di Poi, E.; Legovini, S.; Umani, S.F. Feeding ecology of the transparent goby Aphia minuta (Pisces, Gobiidae) in the northwestern Adriatic Sea. Sci. Mar. 2008, 72, 99–108. [Google Scholar]
- Båmstedt, U. Chemical composition and energy content. In The Biological Chemistry of Marine Copepods; Corner, E.D.S., O’Hara, S.C.M., Eds.; Clarendon Press: Oxford, UK, 1986; pp. 1–58. [Google Scholar]
- Umani, S.F.; Specchi, M.; Buda-Dancevich, M.; Zanolla, F. Lo zooplancton raccolto presso le due bocche principali della Laguna di Grado (Alto Adriatico—Golfo di Trieste). I. Dati quantitativi. Boll. Soc. Adr. Sci. 1979, 63, 83–95. [Google Scholar]
- Gorsky, G.; Dallot, S.; Sardou, J.; Fenaux, R.; Carré, C.; Palazzoli, I. C and N composition of some northwestern Mediterranean zooplankton and micronekton species. J. Exp. Mari. Biol. Ecol. 1988, 124, 133–144. [Google Scholar] [CrossRef]
- Cataletto, B.; Umani, S.F. Seasonal variations in carbon and nitrogen content of Acartia clausi (Copepoda, Calanoida) in the Gulf of Trieste (Northern Adriatic Sea). Hydrobiologia 1994, 292/293, 283–288. [Google Scholar] [CrossRef]
- Hay, S.J.; Kiørboe, T.; Matthews, A. Zooplankton biomass and production in the North Sea during the Autumn Circulation Experiment, October 1987–March 1988. Cont. Shelf Res. 1991, 11, 1453–1476. [Google Scholar] [CrossRef]
- Paffenhöfer, G.-A. Feeding, growth, and food conversion of the marine planktonic copepod Calanus helgolandicus. Limnol. Oceanogr. 1976, 21, 39–50. [Google Scholar] [CrossRef]
- Uye, S.I. Temperature-dependent development and growth of the planktonic copepod Paracalanus sp. in the laboratory. Bull. Plankton Soc. Jpn. 1991, 1, 627–636. [Google Scholar]
- Legovini, S. Ecologia Trofica di Sardina Sardina pilchardus (Walbaum, 1792) e Acciuga Engraulis encrasicolus (Linnaeus, 1758) nel Golfo di Trieste. Ph.D. Thesis, University of Trieste, Trieste, Italy, 2008. [Google Scholar]
- Razouls, C. Estimation de la Production Secondaire (Copepodes Pelagiques) dans une Province Neritique Méditerranéenne (Golfe du Lion). Ph.D. Thesis, University P & M Curie, Paris, France, 1972. Volume VI. [Google Scholar]
- Sabatini, M.; Kiørboe, T. Egg production, growth and development of the cyclopoid copepod Oithona similis. J. Plankton Res. 1994, 16, 1329–1351. [Google Scholar] [CrossRef]
- Chisholm, L.A.; Roff, J.C. Size-weight relationships and biomass of tropical neritic copepods off Kingston, Jamaica. Mar. Biol. 1990, 106, 71–77. [Google Scholar] [CrossRef]
- Van der Lingen, C.D. Gastric evacuation, feeding periodicity and daily ration of sardine Sardinops sagax in the southern Benguela upwelling ecosystem. S. Afr. J. Mar. Sci. 1998, 19, 305–316. [Google Scholar] [CrossRef]
- Ara, K. Temporal variability and production of Euterpina acutifrons (Copepoda: Harpacticoida) in the Cananéia Lagoon estuarine system, Sao Paulo, Brazil. Hydrobiologia 2001, 453/454, 177–187. [Google Scholar] [CrossRef]
- Uye, S.I.; Aoto, I.; Onbe, T. Seasonal population dynamics and production of Microsetella norvegica, a widely distributed but little-studied marine planktonic harpacticoid copepod. J. Plankton Res. 2002, 24, 143–153. [Google Scholar] [CrossRef]
- Kiørboe, T.; Møhlenberg, F.; Riisgård, H.U. In situ feeding rates of planktonic copepods: A comparison of four methods. J. Exp. Mar. Biol. Ecol. 1985, 88, 67–81. [Google Scholar] [CrossRef]
- Durbin, E.G.; Durbin, A.G. Length and weight relationships of Acartia clausi from Narragansett Bay, R.I. Limnol. Oceanogr. 1978, 23, 958–969. [Google Scholar] [CrossRef]
- Lucas, M.I. Studies on Energy Flow in a Barnacle Population. Ph.D. Thesis, University College of North Wales, North Wales, UK, 1979. [Google Scholar]
- Omori, M. Weight and chemical composition of some important oceanic zooplankton in the North Pacific Ocean. Mar. Biol. 1969, 3, 4–10. [Google Scholar] [CrossRef]
- Sautour, B.; Castel, J. Spring zooplankton distribution and production of the copepod Euterpina acutifrons in Marennes-Oléron Bay (France). Hydrobiologia 1995, 310, 163–175. [Google Scholar] [CrossRef]
- Deibel, D. Feeding mechanism and house of the appendicularian Oikopleura vanhoeffeni. Mar. Biol. 1986, 93, 429–436. [Google Scholar] [CrossRef]
- Conway, D.V.P.; Williams, R. Seasonal population structure, vertical distribution and migration of the chaetognath Sagitta elegans in the Celtic Sea. Mar. Biol. 1986, 93, 377–387. [Google Scholar] [CrossRef]
- Hunter, J.R.; Dorr, H. Thresholds for filter feeding in northern anchovy, Engraulis mordax. Cal. Coop. Ocean. Fish. Invest. Rep. 1982, 23, 198–204. [Google Scholar]
- Napier, I.R. The organic carbon content of gravel bed herring spawning grounds and the impact of herring spawn deposition. J. Mar. Biol. Ass. 1993, 73, 863–870. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).