Effect of Ultrasound Irradiation on the Properties and Sulfur Contents of Blended Very Low-Sulfur Fuel Oil (VLSFO)
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Analysis Methods
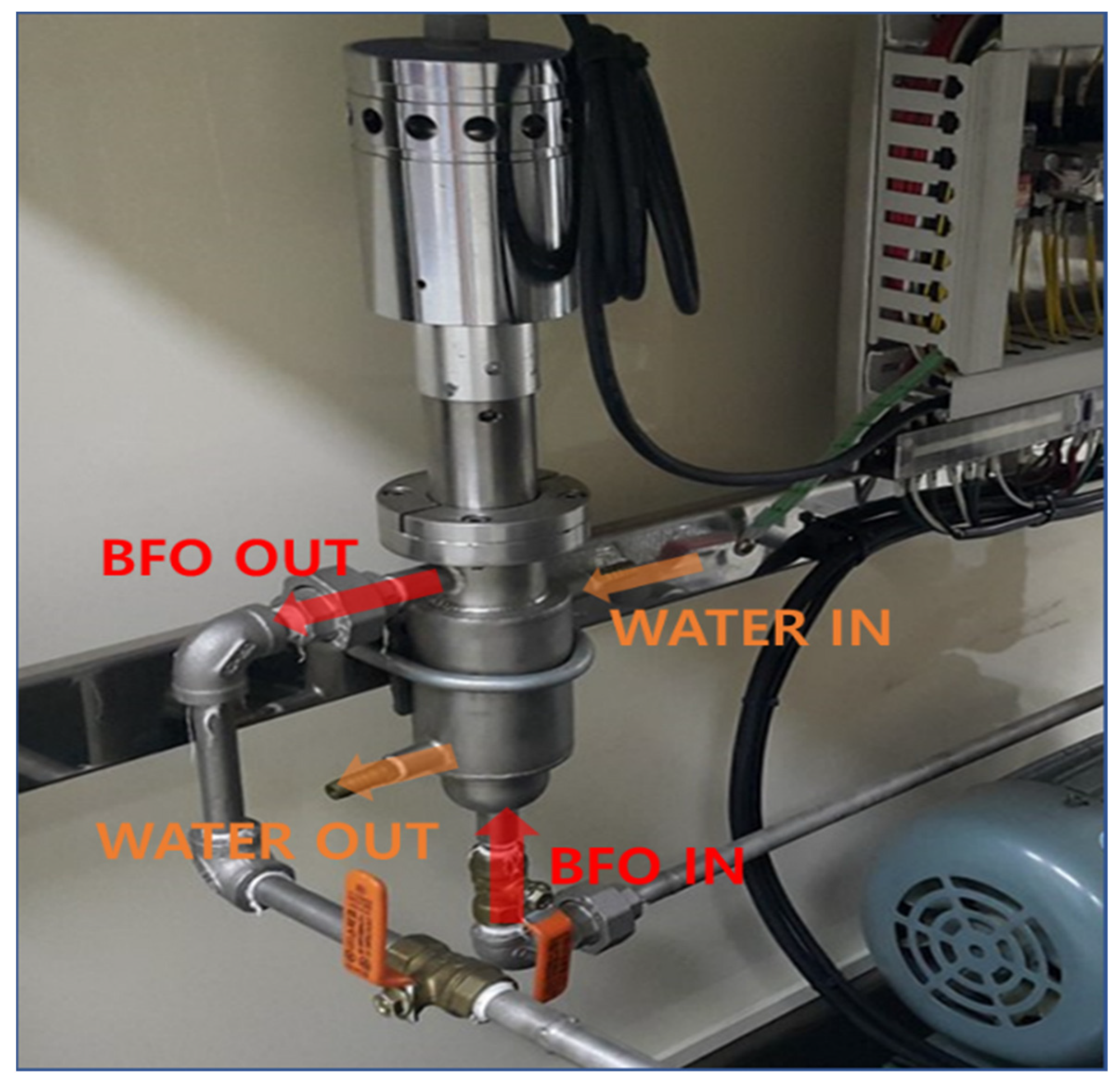
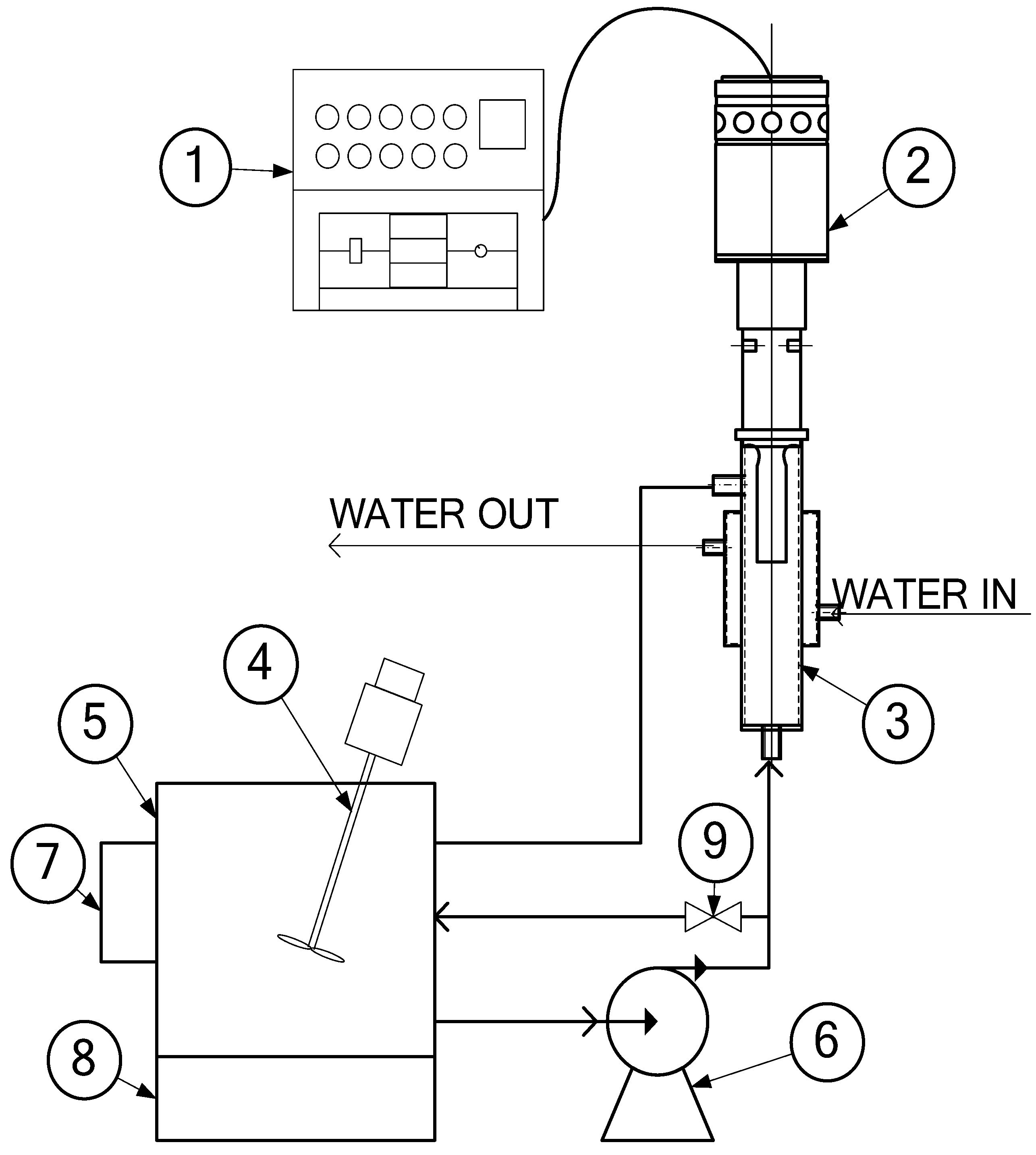
2.2. Equipment
2.3. Ultrasonic Irradiation Experiment
3. Results and Discussion
3.1. Blending
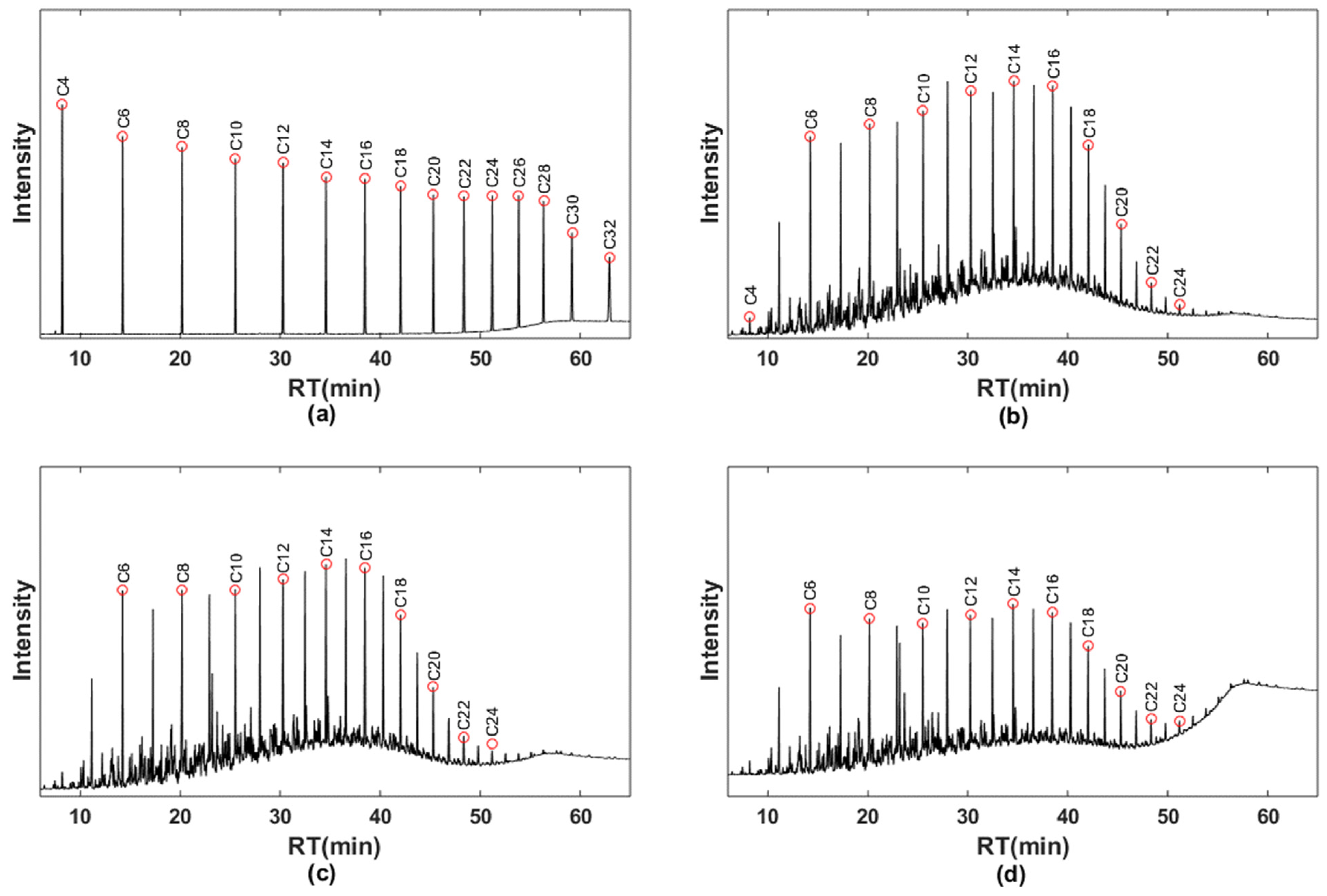
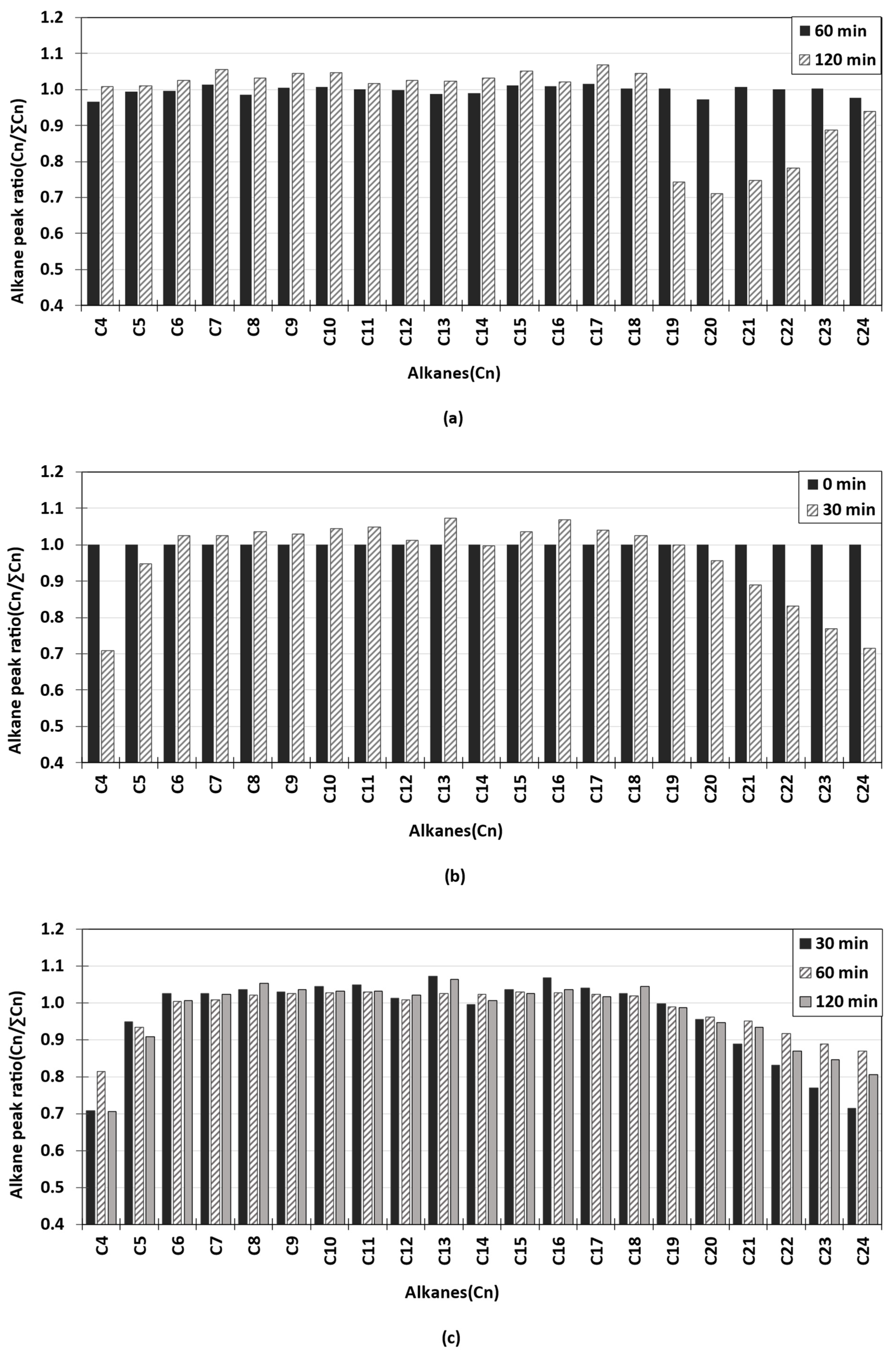
3.2. GC/FID Analysis
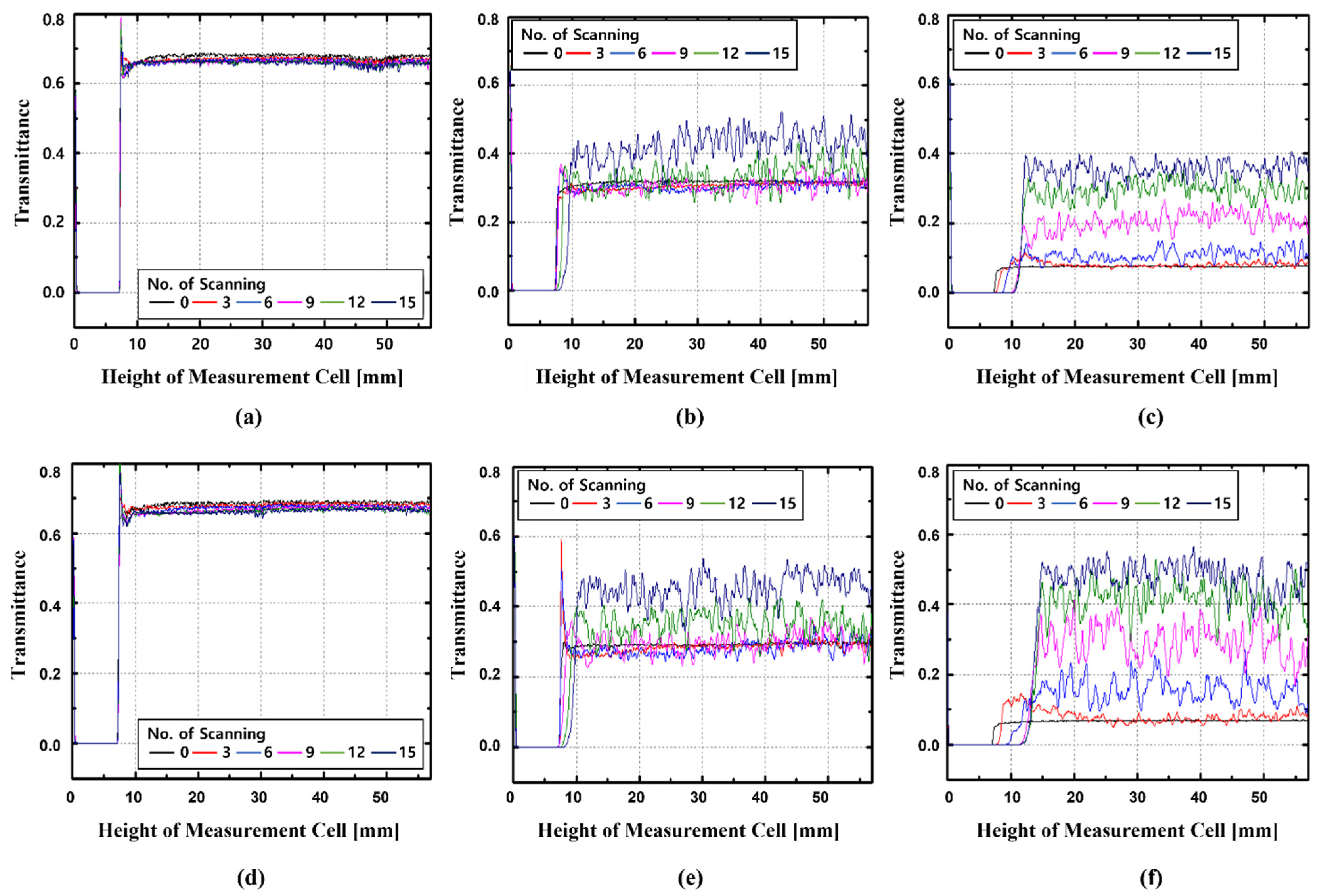
3.3. Dispersion Stability
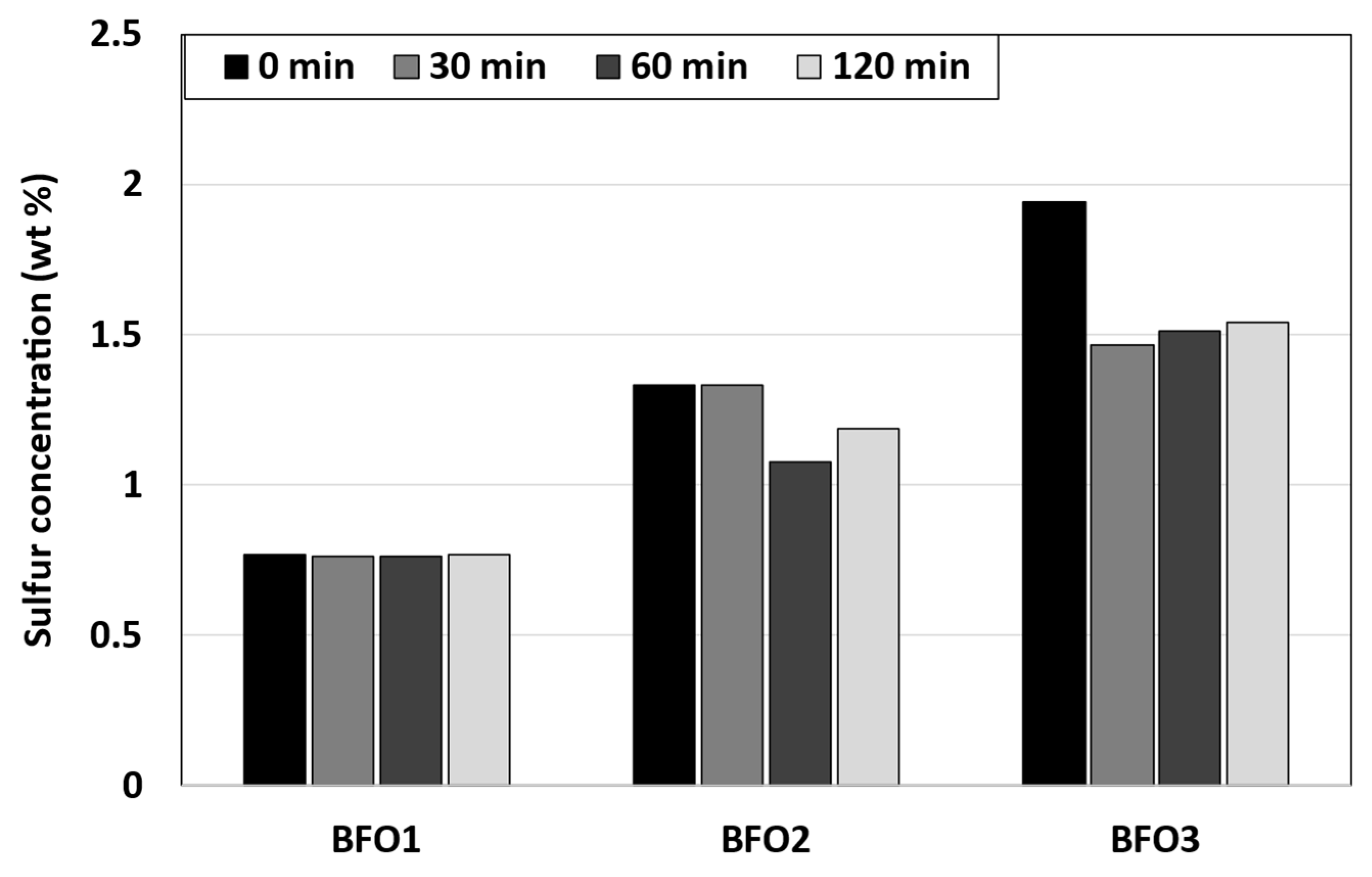
3.4. Sulfur Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BFO | Blended fuel oil |
| BFO1 | Blended fuel oil 1 (mixing ratio 25:75% for ISO-F-DMA and ISO-F-RMG) |
| BFO2 | Blended fuel oil 2 (mixing ratio 50:50% for ISO-F-DMA and ISO-F-RMG) |
| BFO3 | Blended fuel oil 3 (mixing ratio 75:25% for ISO-F-DMA and ISO-F-RMG) |
| DMA | ISO-F-DMA |
| GC/FID | Gas chromatography/Flame ionization detection |
| HDS | Hydrodesulfurization |
| HSFO | High sulfur fuel oil |
| IMO | International maritime organization |
| LNG | Liquefied natural gas |
| ODS | Oxidative desulfurization |
| RMG | ISO-F-RMG |
| RT | Run time for GC/FID |
| SN | Stability number |
| TPH | Total petroleum hydrocarbons |
| UAOD | Ultrasound-assisted oxidation desulfurization |
| UCM | Unresolved complex mixture |
| VLSFO | Very-low-sulfur fuel oil |
References
- Walker, T.R. Green Marine: An environmental program to establish sustainability in marine transportation. Mar. Pollut. Bull. 2016, 105, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lindstad, H.E.; Eskeland, G.S. Environmental regulations in shipping: Policies leaning towards globalization of scrubbers deserve scrutiny. Transp. Res. 2016, 47, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Vedachalam, S.; Baquerizo, N.; Dalai, A.K. Review on impacts of low sulfur regulations on marine fuels and compliance options. Fuel 2022, 310, 122243. [Google Scholar] [CrossRef]
- Corbett, J.J.; Winebrake, J.J.; Carr, E.W.; Jalkanen, J.P.; Johansson, L.; Prank, M.; Sofiev, M. Health Impacts Associated with Delay of MARPOL Global Sulphur Standard, IMO. MEPC 70/INF 34. 2016. Available online: https://wwwcdn.imo.org/localresources/en/MediaCentre/HotTopics/Documents/Finland%20study%20on%20health%20benefits.pdf (accessed on 4 April 2022).
- Park, S.; Kim, T. Implication of IMO Emission Regulation on Korean Shipping Companies; Korea Maritime Institute: Busan, Korea, 2019; Available online: https://www.kmi.re.kr/web/board/view.do?rbsIdx=304&idx=21 (accessed on 4 April 2022).
- Zhang, G.; Yan, H.; Li, T.; Zhu, Y.; Zhou, S.; Feng, Y.; Zhou, W. Relation analysis on emission control and economic cost of SCR system for marine diesels. Sci. Total Environ. 2021, 788, 147856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y.; Kang, M. Recent advances in heighten sulfur resistance of SCR catalysts: A review. Environ. Eng. Res. 2022, 27, 200642. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Xia, C.; Hou, Q. Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission. Atmosphere 2022, 13, 731. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L.; Ampah, J.D. Evaluation of biodiesel on speciated PM2.5, organic compound, ultrafine particle and gaseous emissions from a low-speed EPA Tier II marine diesel engine coupled with DPF, DEP and SCR filter at various loads. Energy 2022, 239, 121837. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Yusuf, D.A.; Jie, Z.; Bello, T.Y.; Tambaya, M.; Abdullahi, B.; Muhammed-Dabo, I.; Yahuza, I.; Dandakouta, H. Influence of waste oil-biodiesel on toxic pollutants from marine engine coupled with emission reduction measures at various loads. Atmos. Pollut. Res. 2022, 13, 101258. [Google Scholar] [CrossRef]
- Gerlitz, L.; Mildenstrey, E.; Prause, G. Ammonia as clean shipping fuel for the Baltic Sea region. Transp. Telecommun. J. 2022, 23, 102–112. [Google Scholar] [CrossRef]
- Smyshlyaeva, K.I.; Rudko, V.A.; Povarov, V.G.; Shaidulina, A.A.; Efimov, I.; Gabdulkhakov, R.R.; Pyagay, I.N.; Speight, J.G. Influence of Asphaltenes on the Low-Sulphur Residual Marine Fuels’ Stability. J. Mar. Sci. Eng. 2021, 9, 1235. [Google Scholar] [CrossRef]
- Lee, B.; Kim, M. Identify the Quality Characteristic of Low Sulfur Fuel Oil to Implement IMO Regulation on Sox. Korea Ind. Chem. News 2019, 22, 41–55. Available online: https://www.cheric.org/research/tech/periodicals/view.php?seq=1754997 (accessed on 4 April 2022).
- Javadli, R.; de Klerk, A. Desulfurization of heavy oil. Appl. Petrochem. Res. 2012, 1, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Mello, P.D.A.; Duarte, F.A.; Nunes, M.A.; Alencar, M.S.; Moreira, E.M.; Korn, M.; Dressler, V.L.; Flores, É.M. Ultrasound-assisted oxidative process for sulfur removal from petroleum product feedstock. Ultrason. Sonochem. 2009, 16, 732–736. [Google Scholar] [CrossRef]
- De Filippis, P.; Scarsella, M. Oxidative Desulfurization: Oxidation Reactivity of Sulfur Compounds in Different Organic Matrixes. Energy Fuels 2003, 17, 1452–1455. [Google Scholar] [CrossRef]
- BIMCO; ICS; INTERCARGO; INTERTANKO. 2020 Fuel Oil Quality and Safety Survey; BIMCO: Copenhagen, Denmark, 2020; Available online: https://www.bimco.org/-/media/bimco/news-and-trends/news/priority-news/2020/2020-fuel-oil-quality-and-safety-survey---report.ashx (accessed on 4 April 2022).
- Sultanbekov, R.; Islamov, S.; Mardashov, D.; Beloglazov, I.; Hemmingsen, T. Research of the Influence of Marine Residual Fuel Composition on Sedimentation Due to Incompatibility. J. Mar. Sci. Eng. 2021, 9, 1067. [Google Scholar] [CrossRef]
- Vráblík, A.; Schlehöfer, D.; Jaklová, K.D.; Herrador, J.M.H.; Cerny, R. Comparative Study of Light Cycle Oil and Naphthalene as an Adequate Additive to Improve the Stability of Marine Fuels. ACS Omega 2022, 7, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- CIMAC. WG7 Fuels Members, Cold Flow Properties of Marine Fuel Oils, International Council on Combustion Engines; CIMAC: Frankfurt, Germany, 2015; Available online: https://www.cimac.com/cms/upload/workinggroups/WG7/CIMAC_WG7_2015_01_Guideline_Cold__Flow_Properties_Marine_Fuel_Oils_final.pdf (accessed on 4 April 2022).
- Luo, X.; Gong, H.; He, Z.; Zhang, P.; He, L. Recent advances in applications of power ultrasound for petroleum industry. Ultrason. Sonochem. 2021, 70, 105337. [Google Scholar] [CrossRef]
- Lin, Y.; Feng, L.; Li, X.; Chen, Y.; Yin, G.; Zhou, W. Study on ultrasound-assisted oxidative desulfurization for crude oil. Ultrason. Sonochem. 2020, 63, 104946. [Google Scholar] [CrossRef]
- Bolla, M.K.; Choudhury, H.A.; Moholkar, V.S. Mechanistic features of ultrasound-assisted oxidative desulfurization of liquid fuels. Ind. Eng. Chem. Res. 2021, 51, 9705–9712. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, D.; Qi, Y. Sono-desulfurization oxidation reactivities of FCC diesel fuel in metal ion/H2O2 systems. Ultrason. Sonochem. 2011, 18, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Ryu, J. A study on relationship between fuel characteristics and combustion characteristics of reformed diesel fuels by ultrasonic energy irradiation (II)- Relationship between chemical structure and cetane number. Trans. KSAE 2003, 11, 64–71. Available online: https://scienceon.kisti.re.kr/commons/util/originalView.do?cn=JAKO200311921499915&oCn=JAKO200311921499915&dbt=JAKO&journal=NJOU00291229 (accessed on 27 June 2022).
- Hosseini, H.; Hamidi, A. Sulfur Removal of Crude Oil by Ultrasound-Assisted Oxidative Method. In Proceedings of the International Conference on Biological, Civil and Environmental Engineering, Dubai, United Arab Emirates, 17–18 March 2014. [Google Scholar] [CrossRef] [Green Version]
- Khodaei, B.; Sobati, M.A.; Shahhosseini, S. Rapid oxidation of dibenzothiophene in model fuel under ultrasound irradiation. Mon. Chem. 2017, 148, 387–396. [Google Scholar] [CrossRef]
- Margeta, D.; Sertić-Bionda, K.; Foglar, L. Ultrasound assisted oxidative desulfurization of model diesel fuel. Appl. Acoust. 2016, 103, 202–206. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Huang, X.; Wu, Y.; Chen, J. Optimization of ultrasonic-assisted oxidative desulfurization of gasoline and crude oil. Chem. Eng. Process.-Process Intensif. 2020, 147, 107789. [Google Scholar] [CrossRef]
- Ja’fari, M.; Ebrahimi, S.L.; Khosravi-Nikou, M.R. Ultrasound-assisted oxidative desulfurization and denitrogenation of liquid hydrocarbon fuels: A critical review. Ultrason. Sonochem. 2018, 40, 955–968. [Google Scholar] [CrossRef]
- ISO8217; Petroleum Products-Fuels (Class-F)-Specification of Marine Fuels. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/64247.html (accessed on 27 June 2022).
- Cho, K. Properties of fuel oil. In Marine Fuel Oil Theory and Practice–I Fuel Oil; Dasom Publish: Busan, Korea, 2012. [Google Scholar]
- Frysinger, G.S.; Gaines, R.B.; Xu, L.; Reddy, C.M. Resolving the unresolved complex mixture in petroleum-contaminated sediments. Environ. Sci. Technol. 2003, 37, 1653–1662. [Google Scholar] [CrossRef]
- AFCONA Technical Team. AFCONA Additives-Dispersing Technology; AFCONA Chemicals: Shah Alam, Malaysia, 2005; Available online: https://www.afcona.com.my/pdf/Dispersing-Technology.pdf (accessed on 27 June 2022).
- Gregory, J. Flocculation Fundamentals. In Encyclopedia of Colloid and Interface Science; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Najafi, I.; Amani, M. Asphaltene flocculation inhibition with ultrasonic wave radiation: A detailed experimental study of the governing mechanisms. Adv. Pet. Explor. Dev. 2011, 2, 32–36. [Google Scholar] [CrossRef]
- Gollapudi, U.K.; Bang, S.S.; Islam, M.R. Ultrasonic Treatment for Removal of Asphaltene Deposits During Petroleum Production. In Proceedings of the SPE Formation Damage Control Symposium, Lafayette, LA, USA, 7–10 February 1994; p. SPE-27377-MS. [Google Scholar] [CrossRef]
- Diallo, M.S.; Cagin, T.; Faulon, J.L.; Goddard, W.A. Thermodynamic properties of asphaltenes: A predictive approach based on computer assisted structure elucidation and atomistic simulations. In Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 2000; Volume 40. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M.R. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment–issues and recommendations. Nanotoxicology 2011, 5, 711–729. [Google Scholar] [CrossRef]
- Chung, S.J.; Leonard, J.P.; Nettleship, I.; Lee, J.K.; Soong, Y.; Martello, D.V.; Chyu, M.K. Characterization of ZnO nanoparticle suspension in water: Effectiveness of ultrasonic dispersion. Powder Technol. 2009, 194, 75–80. [Google Scholar] [CrossRef]
- Ihara, S. Feasibility of hydrogen production by direct water splitting at high temperature. Int. J. Hydrogen Energy 1978, 3, 287–296. [Google Scholar] [CrossRef]
| Case | ISO-F-DMA, vol.% | ISO-F-RMG, vol.% | Total Volume, L |
|---|---|---|---|
| DMA | 100 | 0 | 10 |
| RMG | 0 | 100 | 10 |
| BFO1 | 75 | 25 | 10 |
| BFO2 | 50 | 50 | 10 |
| BFO3 | 25 | 75 | 10 |
| Property | DMA | RMG | BFO 1 | BFO 2 | BFO 3 |
|---|---|---|---|---|---|
| Specific gravity, 15/4 °C | 0.85 | 0.98 | 0.88 | 0.90 | 0.93 |
| Kinematic viscosity, cSt at 50 °C | 3.4 | 169.2 | 6.0 | 24.6 | 35.8 |
| Sulfur content, wt.% | 0.05 | 2.67 | 0.76 | 1.33 | 1.94 |
| Stability number | - | - | 0.6 | 3.6 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, H.-j.; Jeon, S.-k. Effect of Ultrasound Irradiation on the Properties and Sulfur Contents of Blended Very Low-Sulfur Fuel Oil (VLSFO). J. Mar. Sci. Eng. 2022, 10, 980. https://doi.org/10.3390/jmse10070980
Ju H-j, Jeon S-k. Effect of Ultrasound Irradiation on the Properties and Sulfur Contents of Blended Very Low-Sulfur Fuel Oil (VLSFO). Journal of Marine Science and Engineering. 2022; 10(7):980. https://doi.org/10.3390/jmse10070980
Chicago/Turabian StyleJu, Hae-ji, and Soo-kyung Jeon. 2022. "Effect of Ultrasound Irradiation on the Properties and Sulfur Contents of Blended Very Low-Sulfur Fuel Oil (VLSFO)" Journal of Marine Science and Engineering 10, no. 7: 980. https://doi.org/10.3390/jmse10070980
APA StyleJu, H.-j., & Jeon, S.-k. (2022). Effect of Ultrasound Irradiation on the Properties and Sulfur Contents of Blended Very Low-Sulfur Fuel Oil (VLSFO). Journal of Marine Science and Engineering, 10(7), 980. https://doi.org/10.3390/jmse10070980






