The Landscape of Genome-Wide and Gender-Specific Microsatellites in Indo-Pacific Humpback Dolphin and Potential Applications in Cetacean Resource Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microsatellites Detection and Primer Designing
2.2. Microsatellites Statistics
2.3. Microsatellites Distribution and GO (Gene Ontology) Function Enrichment
2.4. Microsatellite Markers Identification
2.5. Male S. chinensis Specific Markers Development
3. Results and Discussion
3.1. Isolation and Characterization of Genomic Microsatellites in S. chinensis
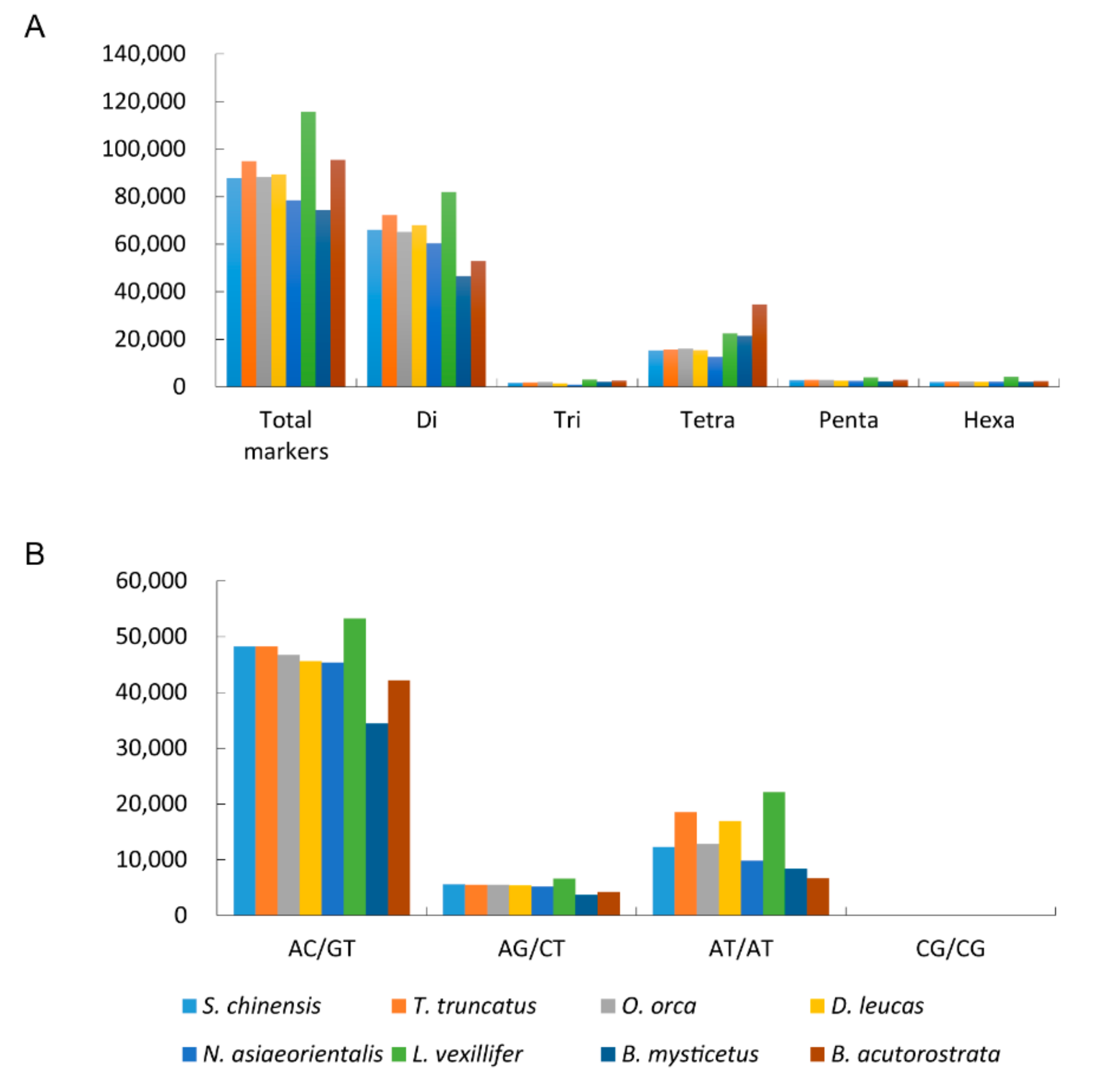
3.2. The Microsatellites Characteristics Comparison among Cetacean Genomes
3.3. Microsatellites Distribution in the S. chinensis Genome
3.4. Validation of Microsatellites in S. chinensis
3.5. Polymorphic Microsatellites Detection Based on Male and Female Markers in S. chinensis
3.6. Specific Markers of Male S. chinensis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, W.; Machray, G.C.; Provan, J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996, 1, 215–222. [Google Scholar] [CrossRef]
- Li, Y.C.; Korol, A.B.; Fahima, T.; Beiles, A.; Nevo, E. Microsatellites: Genomic distribution, putative functions and mutational mechanisms: A review. Mol. Ecol. 2002, 11, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muneer, P.M. Application of microsatellite markers in conservation genetics and fisheries management: Recent advances in population structure analysis and conservation strategies. Genet. Res. Int. 2014, 2014, 691759. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; Munhoz, C.d.F. Microsatellite markers: What they mean and why they are so useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-F.; Wang, B.-X.; Guo, Y.-J.; Deng, C.-L.; Gao, W.-J. Genome-wide characterization of microsatellites and genetic diversity assessment of spinach in the Chinese germplasm collection. Breed. Sci. 2018, 68, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Misra, G.; Kumari, K.; Gupta, S.; Parida, S.K.; Chattopadhyay, D.; Prasad, M. Genome-wide development and use of microsatellite markers for large-scale genotyping applications in foxtail millet [Setaria italica (L.)]. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2013, 20, 197–207. [Google Scholar] [CrossRef]
- Khajuria, Y.P.; Saxena, M.S.; Gaur, R.; Chattopadhyay, D.; Jain, M.; Parida, S.K.; Bhatia, S. Development and Integration of Genome-Wide Polymorphic Microsatellite Markers onto a Reference Linkage Map for Constructing a High-Density Genetic Map of Chickpea. PLoS ONE 2015, 10, e0125583. [Google Scholar] [CrossRef]
- Abdelkrim, J.; Robertson, B.C.; Stanton, J.-A.L.; Gemmell, N.J. Fast, cost-effective development of species-specific microsatellite markers by genomic sequencing. BioTechniques 2009, 46, 185–192. [Google Scholar] [CrossRef]
- Sharma, P.C.; Grover, A.; Kahl, G. Mining microsatellites in eukaryotic genomes. Trends Biotechnol. 2007, 25, 490–498. [Google Scholar] [CrossRef]
- Taheri, S.; Lee Abdullah, T.; Yusop, M.R.; Hanafi, M.M.; Sahebi, M.; Azizi, P.; Shamshiri, R.R. Mining and Development of Novel SSR Markers Using Next Generation Sequencing (NGS) Data in Plants. Molecules 2018, 23, 399. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, L.; Chen, J.; Hu, Y.; Si, Z.; Wang, S.; Chang, L.; Guo, W.; Zhang, T. Genome-Wide Mining, Characterization and Development of Microsatellite Markers in Gossypium Species. Sci. Rep. 2015, 5, 10638. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Kuntal, H.; Shanker, A.; Sharma, S.N. Mining and analysis of simple sequence repeats in the chloroplast genomes of genus Vigna. Biotechnol. Res. Innov. 2018, 2, 9–18. [Google Scholar] [CrossRef]
- Dettori, M.T.; Micali, S.; Giovinazzi, J.; Scalabrin, S.; Verde, I.; Cipriani, G. Mining microsatellites in the peach genome: Development of new long-core SSR markers for genetic analyses in five Prunus species. SpringerPlus 2015, 4, 337. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liao, X.; Cheng, L.; Yu, X.; Tong, J. Development of novel EST-SSR markers in common carp by data mining from public EST sequences. Aquaculture 2007, 271, 558–574. [Google Scholar] [CrossRef]
- Srivastava, S.; Avvaru, A.K.; Sowpati, D.T.; Mishra, R.K. Patterns of microsatellite distribution across eukaryotic genomes. BMC Genom. 2019, 20, 153. [Google Scholar] [CrossRef]
- Valsecchi, E.; Amos, W. Microsatellite markers for the study of cetacean populations. Mol. Ecol. 1996, 5, 151–156. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Domingoroura, X.; Takenaka, O. Microsatellites in the bottlenose dolphin Tursiops truncatus. Mol. Ecol. 1997, 6, 695–696. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. A set of polymorphic dinucleotide and tetranucleotide microsatellite markers for the Indo-Pacific humpback dolphin (Sousa chinensis) and cross-amplification in other cetacean species. Conserv. Genet. 2008, 10, 697–700. [Google Scholar] [CrossRef]
- Nater, A.; Kopps, A.M.; Krutzen, M. New polymorphic tetranucleotide microsatellites improve scoring accuracy in the bottlenose dolphin Tursiops aduncus. Mol. Ecol. Resour. 2009, 9, 531–534. [Google Scholar] [CrossRef]
- Chen, I.; Nishida, S.; Yang, W.C.; Isobe, T.; Tajima, Y.; Hoelzel, A.R. Genetic diversity of bottlenose dolphin (Tursiops sp.) populations in the western North Pacific and the conservation implications. Mar. Biol. 2017, 164, 202. [Google Scholar] [CrossRef]
- Perez-Alvarez, M.J.; Olavarria, C.; Moraga, R.; Baker, C.S.; Hamner, R.M.; Poulin, E. Microsatellite markers reveal strong genetic structure in the endemic Chilean dolphin. PLoS ONE 2015, 10, e0123956. [Google Scholar] [CrossRef]
- Sumiyama, D.; Kitamura, S.; Terasawa, F.; Hori, Y.; Murata, K.; Kulski, J.K.; Inoko, H. Paternity determination of captive bottlenose dolphins (Tursiops truncatus) using microsatellite DNA analysis. J. Vet. Med. Sci. 2008, 70, 711–713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gui, D.; Jia, K.; Xia, J.; Yang, L.; Chen, J.; Wu, Y.; Yi, M. De novo assembly of the Indo-Pacific humpback dolphin leucocyte transcriptome to identify putative genes involved in the aquatic adaptation and immune response. PLoS ONE 2013, 8, e72417. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chantra, R.; Kittiwattanawong, K.; Zhao, L.; Sakornwimon, W.; Aierken, R.; Wu, F.; Wang, X. Genetic structure of the endangered Irrawaddy dolphin (Orcaella brevirostris) in the Gulf of Thailand. Genet. Mol. Biol. 2021, 44, e20200365. [Google Scholar] [CrossRef]
- Chen, B.Y.; Zheng, D.M.; Wang, L.; Xu, X.R.; Yang, G. The northernmost distribution of Indo-Pacific humpback dolphin (Sousa chinensis) in the world: Evidence from preliminary survey in Ningde, China. Pak. J. Zool. 2012, 44, 1209–1214. [Google Scholar]
- Hung, S.K.; Würsig, B.; Wang, J.; Sims, M.P.; Piwetz, M.S. Monitoring of Marine Mammals in Hong Kong Waters (2011–12); Agriculture, Fisheries and Conservation Department of Hong Kong SAR Government: Hong Kong, China, 2012.
- Xu, X.; Song, J.; Zhang, Z.; Li, P.; Yang, G.; Zhou, K. The world’s second largest population of humpback dolphins in the waters of Zhanjiang deserves the highest conservation priority. Sci. Rep. 2015, 5, 8147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Yang, F.; Li, Y.; Li, L.; Lin, D.; He, T.; Liang, B.; Zhang, T.; Lin, Y. A framework for the assessment of the spatial and temporal patterns of threatened coastal delphinids. Sci. Rep. 2016, 6, 19883. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Jian, J.; Yu, X.; Wang, J.; Liu, W. The genome resources for conservation of Indo-Pacific humpback dolphin, Sousa chinensis. Sci. Data 2019, 6, 68. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Li, C.; Lin, M.; Dong, L.; Zhang, R.; Liu, M.; Li, K.; Zhang, H.; Liu, X.; et al. An Indo-Pacific Humpback Dolphin Genome Reveals Insights into Chromosome Evolution and the Demography of a Vulnerable Species. iScience 2020, 23, 101640. [Google Scholar] [CrossRef]
- Thiel, T.; Michalek, W.; Varshney, R.K.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J. BLAT—The BLAST-like alignment tool. Genome Res. 2002, 12, 656–664. [Google Scholar] [PubMed]
- Bourret, V.; Macé, M.; Bonhomme, M.; Crouau-Roy, B. Microsatellites in cetaceans: An overview. Open Mar. Biol. J. 2008, 2, 38–42. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Weber, J.L. Survey of human and rat microsatellites. Genomics 1992, 12, 627–631. [Google Scholar] [CrossRef]
- Jurka, J.; Pethiyagoda, C. Simple repetitive DNA sequences from primates: Compilation and analysis. J. Mol. Evol. 1995, 40, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Moran, C. Microsatellite repeats in pig (Sus domestica) and chicken (Gallus domesticus) genomes. J. Hered. 1993, 84, 274–280. [Google Scholar] [CrossRef]
- Nadir, E.; Margalit, H.; Gallily, T.; Ben-Sasson, S.A. Microsatellite spreading in the human genome: Evolutionary mechanisms and structural implications. Proc. Natl. Acad. Sci. USA 1996, 93, 6470–6475. [Google Scholar] [CrossRef]
- Manee, M.M.; Algarni, A.T.; Alharbi, S.N.; Al-Shomrani, B.M.; Ibrahim, M.A.; Binghadir, S.A.; Al-Fageeh, M.B. Genome-wide characterization and analysis of microsatellite sequences in camelid species. Mammal Res. 2020, 65, 359–373. [Google Scholar] [CrossRef]
- Song, X.; Yang, T.; Zhang, X.; Yuan, Y.; Yan, X.; Wei, Y.; Zhang, J.; Zhou, C. Comparison of the Microsatellite Distribution Patterns in the Genomes of Euarchontoglires at the Taxonomic Level. Front. Genet. 2021, 12, 227. [Google Scholar] [CrossRef]
- Getino-Mamet, L.N.; Valdivia-Carrillo, T.; Gomez Daglio, L.; Garcia-De Leon, F.J. Isolation and characterization of 14 tetranucleotide microsatellite loci for the cannonball jellyfish (Stomolophus sp.) by next generation sequencing. Mol. Biol. Rep. 2017, 44, 257–260. [Google Scholar] [CrossRef]
- Landinez-Garcia, R.M.; Marquez, E.J. Development and characterization of 24 polymorphic microsatellite loci for the freshwater fish Ichthyoelephas longirostris (Characiformes: Prochilodontidae). PeerJ 2016, 4, e2419. [Google Scholar] [CrossRef]
- Guo, W.; Guo, C.; Wang, Y.; Hu, W.; Mei, J. Population structure and genetic diversity in yellow catfish (Pelteobagrus fulvidraco) assessed with microsatellites. J. Genet. 2019, 98, 1–4. [Google Scholar] [CrossRef]
- Li, Z.; Chen, F.; Huang, C.; Zheng, W.; Yu, C.; Cheng, H.; Zhou, R. Genome-wide mapping and characterization of microsatellites in the swamp eel genome. Sci. Rep. 2017, 7, 3157. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhou, Y.; Price, M.; Song, Z. Genome-wide characterization of microsatellite DNA in fishes: Survey and analysis of their abundance and frequency in genome-specific regions. BMC Genom. 2021, 22, 421. [Google Scholar] [CrossRef]
- Toth, G.; Gaspari, Z.; Jurka, J. Microsatellites in different eukaryotic genomes: Survey and analysis. Genome Res. 2000, 10, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Ellegren, H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435–445. [Google Scholar] [CrossRef]
- Rajendrakumar, P.; Biswal, A.K.; Balachandran, S.M.; Srinivasarao, K.; Sundaram, R.M. Simple sequence repeats in organellar genomes of rice: Frequency and distribution in genic and intergenic regions. Bioinformatics 2007, 23, 1–4. [Google Scholar] [CrossRef]
- Li, Y.-C.; Korol, A.B.; Fahima, T.; Nevo, E. Microsatellites within genes: Structure, function, and evolution. Mol. Biol. Evol. 2004, 21, 991–1007. [Google Scholar] [CrossRef]
- Metzgar, D.; Bytof, J.; Wills, C. Selection against frameshift mutations limits microsatellite expansion in coding DNA. Genome Res. 2000, 10, 72–80. [Google Scholar]
- Wang, Z.; Weber, J.L.; Zhong, G.; Tanksley, S. Survey of plant short tandem DNA repeats. Theor. Appl. Genet. 1994, 88, 1–6. [Google Scholar] [CrossRef]
- Van Lith, H.; Van Zutphen, L. Characterization of rabbit DNA micros extracted from the EMBL nucleotide sequence database. Anim. Genet. 1996, 27, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Pardue, M.L.; Gall, J.G. Chromosomal localization of mouse satellite DNA. Science 1970, 168, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. Chromosome organization and genic expression. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 1951; pp. 13–47. [Google Scholar]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Teixeira, F.K.; Colot, V. Repeat elements and the Arabidopsis DNA methylation landscape. Heredity 2010, 105, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Joyce, B.T.; Liu, L.; Zhang, Z.; Kibbe, W.A.; Zhang, W.; Hou, L. Prediction of genome-wide DNA methylation in repetitive elements. Nucleic Acids Res. 2017, 45, 8697–8711. [Google Scholar] [CrossRef]
- Bollati, V.; Galimberti, D.; Pergoli, L.; Dalla Valle, E.; Barretta, F.; Cortini, F.; Scarpini, E.; Bertazzi, P.A.; Baccarelli, A. DNA methylation in repetitive elements and Alzheimer disease. Brain Behav. Immun. 2011, 25, 1078–1083. [Google Scholar] [CrossRef]
- Kriangwanich, W.; Buddhachat, K.; Poommouang, A.; Chomdej, S.; Thitaram, C.; Kaewmong, P.; Kittiwattanawong, K.; Nganvongpanit, K. Feasibility of melting fingerprint obtained from ISSR-HRM curves for marine mammal species identification. PeerJ 2021, 9, e11689. [Google Scholar] [CrossRef]
- Silva, V.S.; Skueresky, N.; Lopes, F.; Koch, T.K.; Ott, P.H.; Siciliano, S.; Barreto, A.S.; Secchi, E.R.; de Meirelles, A.C.O.; Carvalho, V.L. Integrating morphology and DNA barcoding to assess cetacean diversity in Brazil. Mammal Res. 2021, 66, 349–369. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, X.; Zhang, Q.; Li, Y.; Tan, S.; Li, D.; Yang, Z.; Wang, J. Cetaceans and microplastics: First report of microplastic ingestion by a coastal delphinid, Sousa chinensis. Sci. Total Environ. 2019, 659, 649–654. [Google Scholar] [CrossRef]
- Ziemniczak, K.; Traldi, J.B.; Nogaroto, V.; De Almeida, M.C.; Artoni, R.F.; Moreirafilho, O.; Vicari, M.R. In situ Localization of (GATA)n and (TTAGGG)n Repeated DNAs and W Sex Chromosome Differentiation in Parodontidae (Actinopterygii: Characiformes). Cytogenet. Genome Res. 2015, 144, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Jian, J.; Yu, F.; Yu, X.; Wang, J.; Liu, W. Molecular footprints of inshore aquatic adaptation in Indo-Pacific humpback dolphin (Sousa chinensis). Genomics 2018, 111, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
| GO ID | GO Term | GO Class | Corrected p-Value |
|---|---|---|---|
| GO:0010468 | regulation of gene expression | BP | 1.76 × 10−19 |
| GO:0051252 | regulation of RNA metabolic process | BP | 1.73 × 10−17 |
| GO:2000112 | regulation of cellular macromolecule biosynthetic process | BP | 2.08 × 10−17 |
| GO:0006355 | regulation of transcription, DNA-templated | BP | 2.41 × 10−17 |
| GO:0006351 | transcription, DNA-templated | BP | 2.41 × 10−17 |
| GO:0051171 | regulation of nitrogen compound metabolic process | BP | 1.77 × 10−16 |
| GO:0080090 | regulation of primary metabolic process | BP | 2.23 × 10−16 |
| GO:0031323 | regulation of cellular metabolic process | BP | 4.73 × 10−16 |
| GO:0016070 | RNA metabolic process | BP | 5.11 × 10−14 |
| GO:0090304 | nucleic acid metabolic process | BP | 2.73 × 10−11 |
| GO:0005634 | nucleus | CC | 8.34 × 10−9 |
| GO:0006725 | cellular aromatic compound metabolic process | BP | 2.00 × 10−8 |
| GO:1901360 | organic cyclic compound metabolic process | BP | 3.74 × 10−8 |
| GO:0003676 | nucleic acid binding | MF | 9.51 × 10−8 |
| GO:0010467 | gene expression | BP | 1.57 × 10−7 |
| GO:0005488 | binding | MF | 5.25 × 10−7 |
| GO:0003677 | DNA binding | MF | 8.27 × 10−7 |
| GO:0044451 | nucleoplasm part | CC | 8.87 × 10−6 |
| GO:0031981 | nuclear lumen | CC | 1.07 × 10−5 |
| GO:0034645 | cellular macromolecule biosynthetic process | BP | 1.11 × 10−5 |
| GO:0043227 | membrane-bounded organelle | CC | 1.93 × 10−5 |
| GO:0043565 | sequence-specific DNA binding | MF | 2.44 × 10−5 |
| GO:0044271 | cellular nitrogen compound biosynthetic process | BP | 2.59 × 10−5 |
| GO:0050789 | regulation of biological process | BP | 3.32 × 10−5 |
| GO:0004402 | histone acetyltransferase activity | MF | 4.15 × 10−5 |
| GO:0043231 | intracellular membrane-bounded organelle | CC | 6.23 × 10−5 |
| GO:0050794 | regulation of cellular process | BP | 6.46 × 10−5 |
| GO:0140110 | transcription regulator activity | MF | 1.21 × 10−4 |
| GO:0044428 | nuclear part | CC | 1.74 × 10−4 |
| GO:0065007 | biological regulation | BP | 2.80 × 10−4 |
| GO:0016573 | histone acetylation | BP | 2.80 × 10−4 |
| GO:0006325 | chromatin organization | BP | 3.29 × 10−4 |
| GO:0016570 | histone modification | BP | 3.43 × 10−4 |
| GO:0035097 | histone methyltransferase complex | CC | 5.69 × 10−4 |
| GO:0009058 | biosynthetic process | BP | 6.57 × 10−4 |
| GO:0018205 | peptidyl-lysine modification | BP | 7.91 × 10−4 |
| GO:0034641 | cellular nitrogen compound metabolic process | BP | 8.80 × 10−4 |
| GO:1901363 | heterocyclic compound binding | MF | 1.27 × 10−3 |
| GO:0097159 | organic cyclic compound binding | MF | 1.30 × 10−3 |
| GO:0003712 | transcription coregulator activity | MF | 1.43 × 10−3 |
| GO:0044260 | cellular macromolecule metabolic process | BP | 1.49 × 10−3 |
| GO:0042800 | histone methyltransferase activity (H3-K4 specific) | MF | 4.83 × 10−3 |
| GO:0016286 | small conductance calcium-activated potassium channel activity | MF | 4.83 × 10−3 |
| GO:0043170 | macromolecule metabolic process | BP | 7.22 × 10−3 |
| GO:0005515 | protein binding | MF | 9.68 × 10−3 |
| GO:0016592 | mediator complex | CC | 0.015 |
| GO:1990234 | transferase complex | CC | 0.019 |
| GO:0003700 | DNA-binding transcription factor activity | MF | 0.023 |
| GO:0018024 | histone-lysine N-methyltransferase activity | MF | 0.023 |
| GO:0003713 | transcription coactivator activity | MF | 0.024 |
| GO:0005516 | calmodulin binding | MF | 0.029 |
| GO:0071339 | MLL1 complex | CC | 0.029 |
| Published Marker | Repeat Type | SSR ID in S. chinensis | Repeat Type | Note |
|---|---|---|---|---|
| SGATA42 | (GATA)11 | scaffold428.1_57256 | (GATA)11 | + |
| SCA6 | (CA)17 | scaffold113.1_88883 | (CA)19 | + |
| SGATA47 | (TATC)5 | / | (TAGA)5 | ※ |
| SGATA45 | (AGAT)8 | scaffold2.1_32807 | (ATCT)9 | − |
| SGATA35 | (TCTA)4 | / | (GATA)4 | ※ |
| SGATA33 | (ATAG)7 | scaffold470.1_58268 | (TCTA)8 | − |
| SGATA30 | (AGAT)7 | scaffold162.1_47075 | (TATC)7 | − |
| SGATA28 | (AT)4 | / | (AT)4 | ※ |
| SGATA25 | (GATA)5 | / | (AGAT)5 | ※ |
| SGATA21 | (TAGA)10 | scaffold35.1_72245 | (ATAG)10 | + |
| SGATA18 | (GATA)3GAT(GATA)3AATA(GATA)2 | / | / | # |
| SGATA13 | (TCTA)4 | / | (TCTA)4 | ※ |
| SCA66 | (CA)11 | scaffold85.1_89945 | (GT)11 | − |
| SCA64 | (CA)23 | scaffold77.1_66768 | (CA)22 | + |
| SCA58 | (AC)12 | scaffold18.1_1347 | (GT)12 | − |
| SCA56 | (CA)15 | scaffold284.1_42084 | (CA)13 | + |
| SCA55 | (AC)14 | scaffold249.1_12650 | (AC)14 | + |
| SCA54 | (CA)20 | scaffold199.1_4149 | (AC)18 | + |
| SCA48 | (CA)16 | scaffold319.1_1085 | (TG)16 | − |
| SCA39 | (AC)20 | scaffold196.1_39358 | (AC)24 | + |
| SCA37 | (AC)23 | scaffold57.1_92416 | (GT)23 | − |
| SCA33 | (AC)22 | scaffold34.1_17501 | (AC)17 | + |
| SCA30 | (CA)9 | / | (TG)9 | ※ |
| SCA27 | (AC)21 | scaffold70.1_71178 | (TG)22 | − |
| SCA22 | (CT)7TTCT(CA)36 | scaffold54.1_109484 | (GT)28GAGAAA(GA)7 | − |
| SCA17 | (AC)16 | scaffold66.1_63598 | (AC)19 | + |
| SCA12 | (AC)13 | scaffold20.1_86731 | (AC)13 | + |
| SCA9 | (CA)23 | scaffold329.1_58395 | (AC)37 | − |
| Sch5878 | (CAAC)12 | scaffold108.1_36155 | (TTGG)11 | − |
| Sch6660 | (AAGG)13 | scaffold10.1_97587 | (CCTT)13 | − |
| Sch443 | (CCAT)12 | scaffold10.1_97555 | (GGAT)12 | − |
| Sch10207 | (CATC)12 | scaffold240.1_14114 | (GATG)12 | − |
| Sch843 | (AAAT)11 | scaffold25.1_94818 | (TTTA)11 | − |
| Sch7424 | (ATGG)13 | scaffold89.1_41509 | (CCAT)13 | − |
| Sch7357 | (ATGG)11 | scaffold20.1_86885 | (ATGG)11 | + |
| Sch193 | (AGAGA)12 | scaffold26.1_104752 | (TCTCT)11 | − |
| Sch8186 | (CCAT)11 | scaffold15.1_6749 | (CCAT)11 | + |
| Sch123 | (CCTAAC)7 | scaffold66.1_64097 | (CCTAAC)7 | + |
| Sch4657 | (TTCC)11 | scaffold304.1_101153 | (AGGA)11 | − |
| Sch5373 | (GATG)11 | scaffold91.1_67861 | (TCCA)11 | − |
| Sch9144 | (ATCT)14 | scaffold11.1_40258 | (TAGA)15 | − |
| Sch2513 | (CATC)13 | scaffold165.1_13454 | (CATC)13 | + |
| Sch5685 | (AGGA)12 | scaffold9.1_107855 | (AGGA)13 | + |
| Sch974 | (GTTTT)13 | scaffold32.1_92270 | (AAAAC)10 | − |
| Sch5094 | (TCTA)11 | scaffold28.1_42911 | (GATA)11 | − |
| Sch8947 | (CTAT)12 | scaffold11.1_40359 | (CTAT)12 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, Y.; Yu, X.; Liu, W.; Wang, J.; Liu, W. The Landscape of Genome-Wide and Gender-Specific Microsatellites in Indo-Pacific Humpback Dolphin and Potential Applications in Cetacean Resource Investigation. J. Mar. Sci. Eng. 2022, 10, 834. https://doi.org/10.3390/jmse10060834
Ming Y, Yu X, Liu W, Wang J, Liu W. The Landscape of Genome-Wide and Gender-Specific Microsatellites in Indo-Pacific Humpback Dolphin and Potential Applications in Cetacean Resource Investigation. Journal of Marine Science and Engineering. 2022; 10(6):834. https://doi.org/10.3390/jmse10060834
Chicago/Turabian StyleMing, Yao, Xueying Yu, Wei Liu, Jingzhen Wang, and Wenhua Liu. 2022. "The Landscape of Genome-Wide and Gender-Specific Microsatellites in Indo-Pacific Humpback Dolphin and Potential Applications in Cetacean Resource Investigation" Journal of Marine Science and Engineering 10, no. 6: 834. https://doi.org/10.3390/jmse10060834
APA StyleMing, Y., Yu, X., Liu, W., Wang, J., & Liu, W. (2022). The Landscape of Genome-Wide and Gender-Specific Microsatellites in Indo-Pacific Humpback Dolphin and Potential Applications in Cetacean Resource Investigation. Journal of Marine Science and Engineering, 10(6), 834. https://doi.org/10.3390/jmse10060834





