Evaluating the Shark Deterrent Effects of the Novel Exclusion Barrier in Comparison to the Rigorously Tested Sharksafe Barrier Technology
Abstract
:1. Introduction
2. Materials and Methods
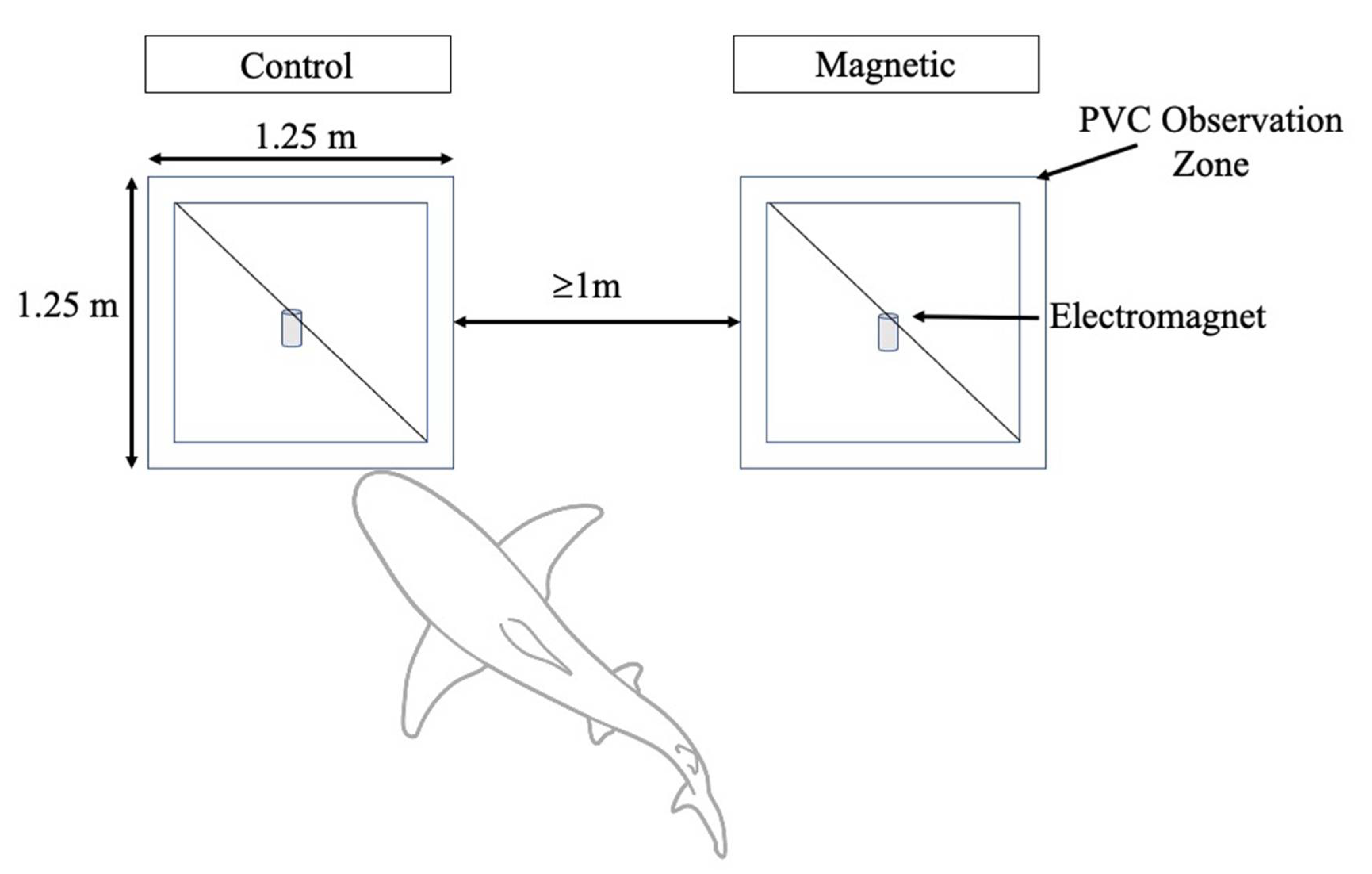
2.1. Bait Experiment
Statistical Analysis
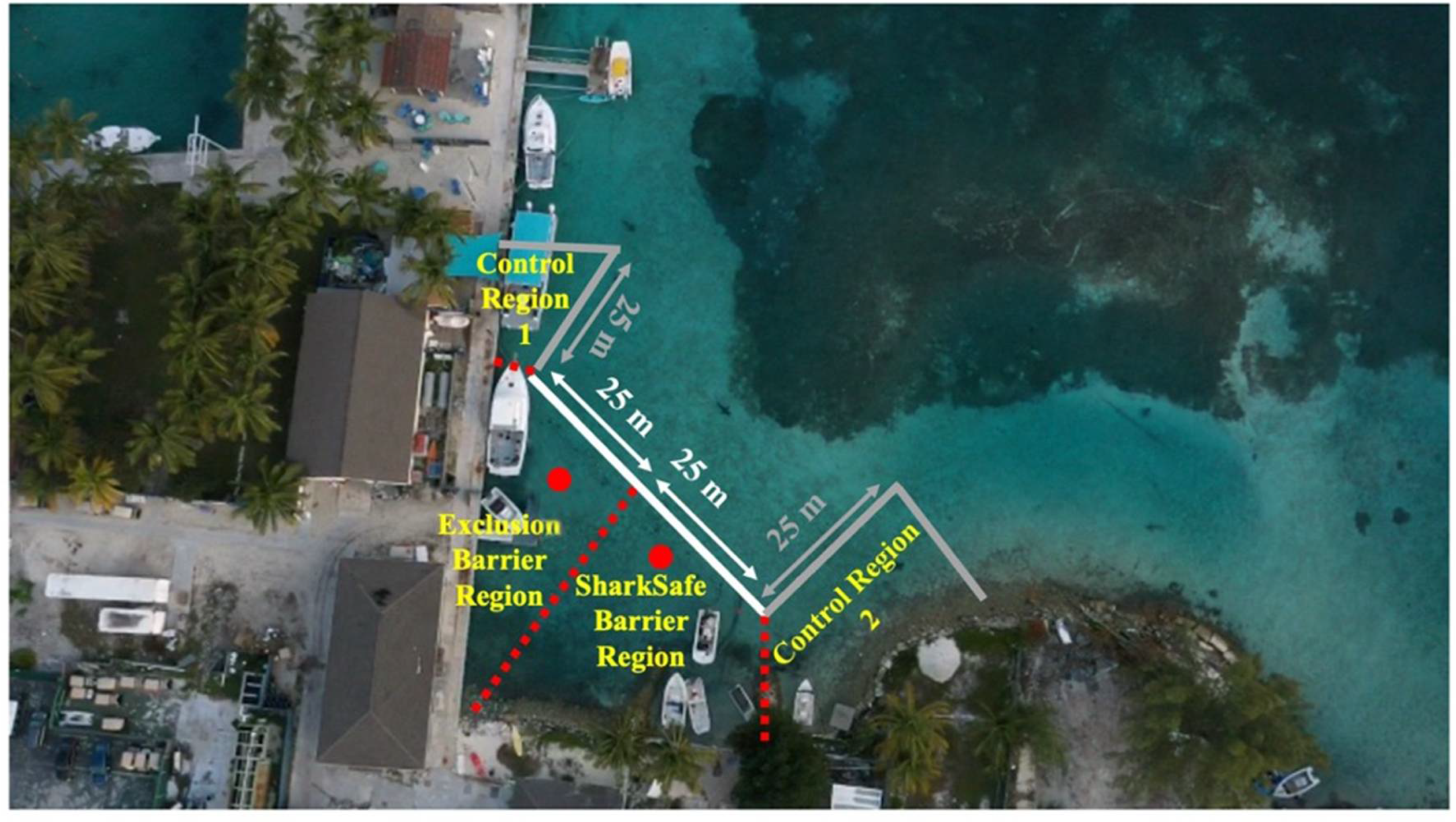
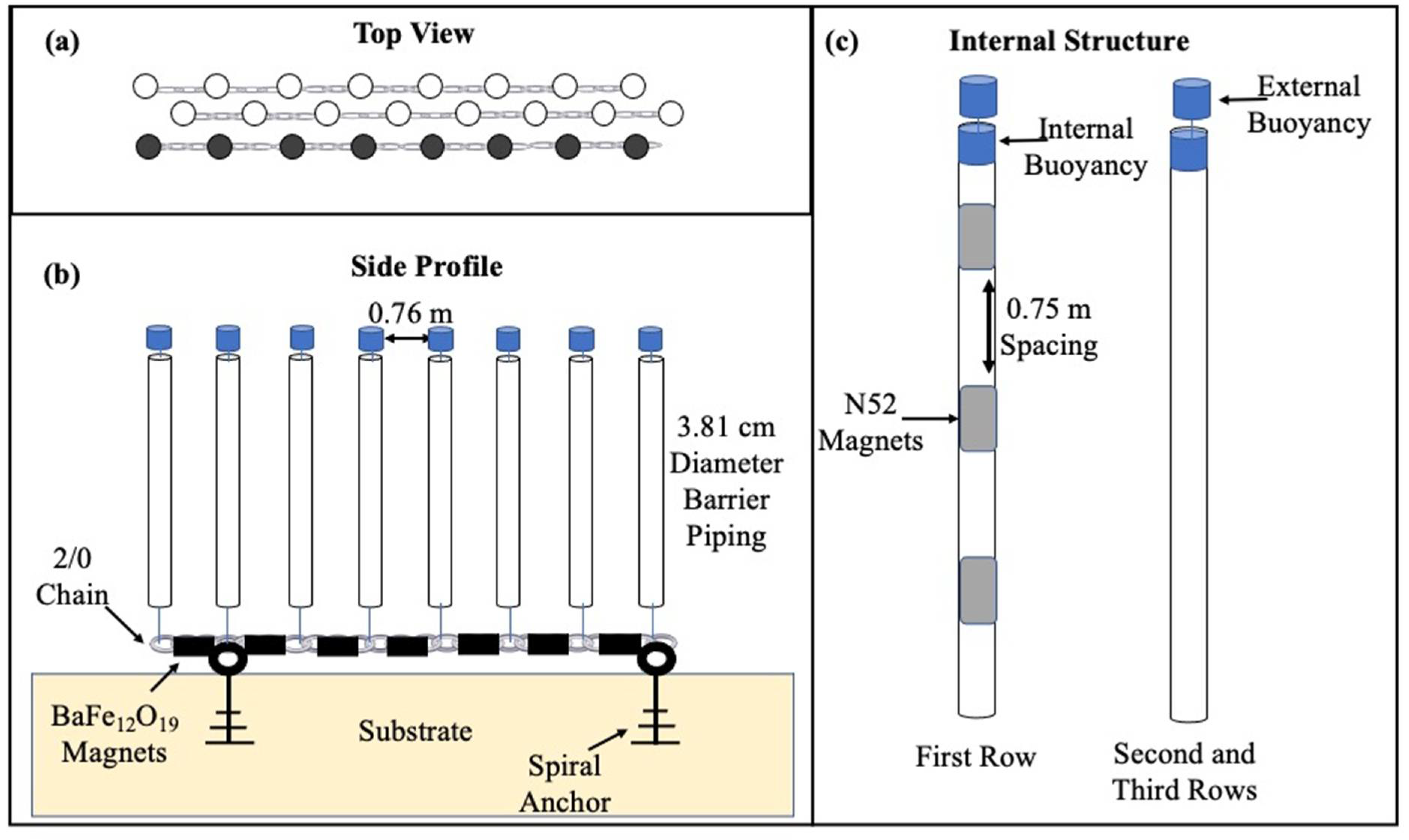
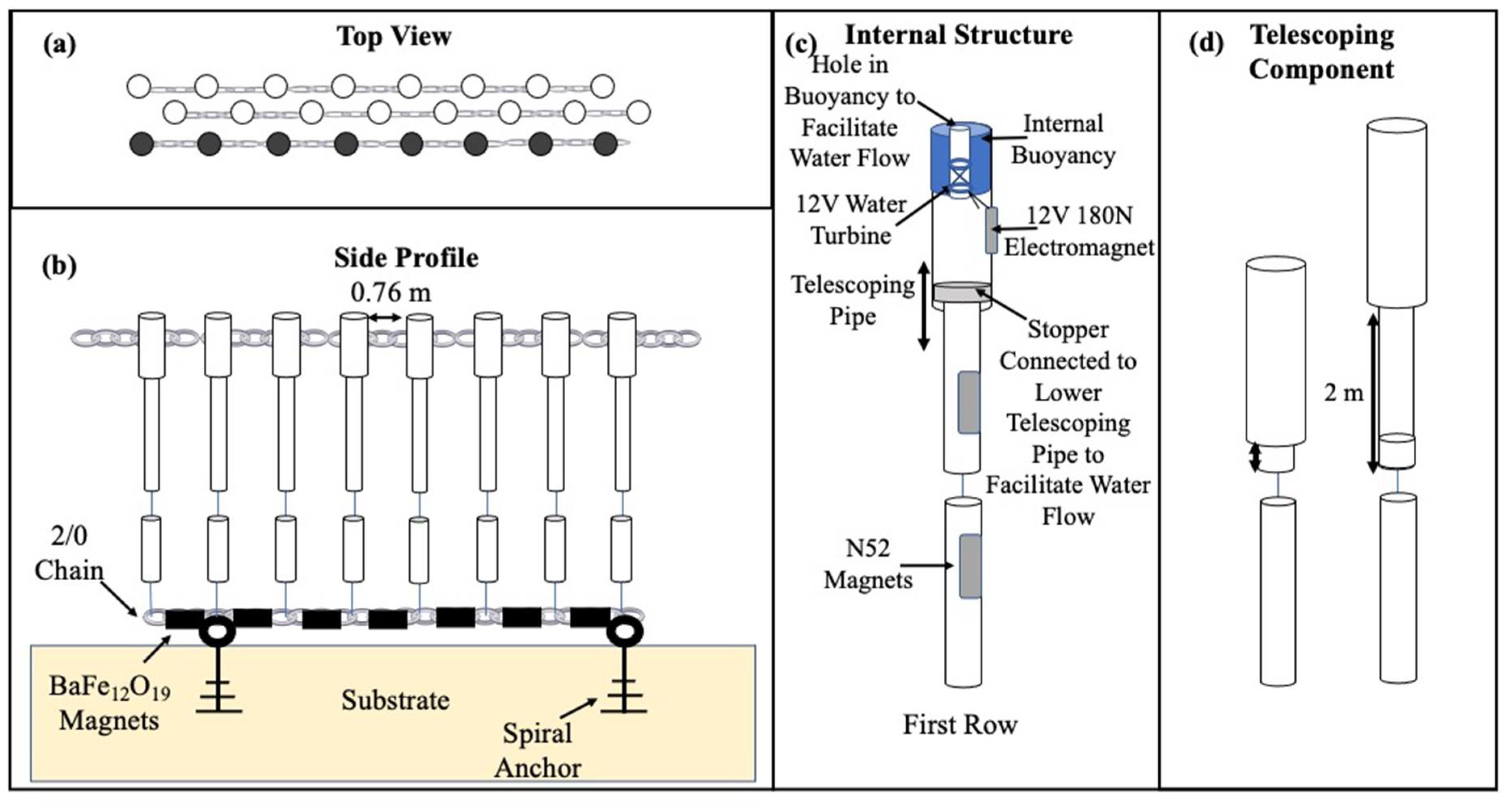
2.2. Barrier Experiment
Statistical Analysis
3. Results
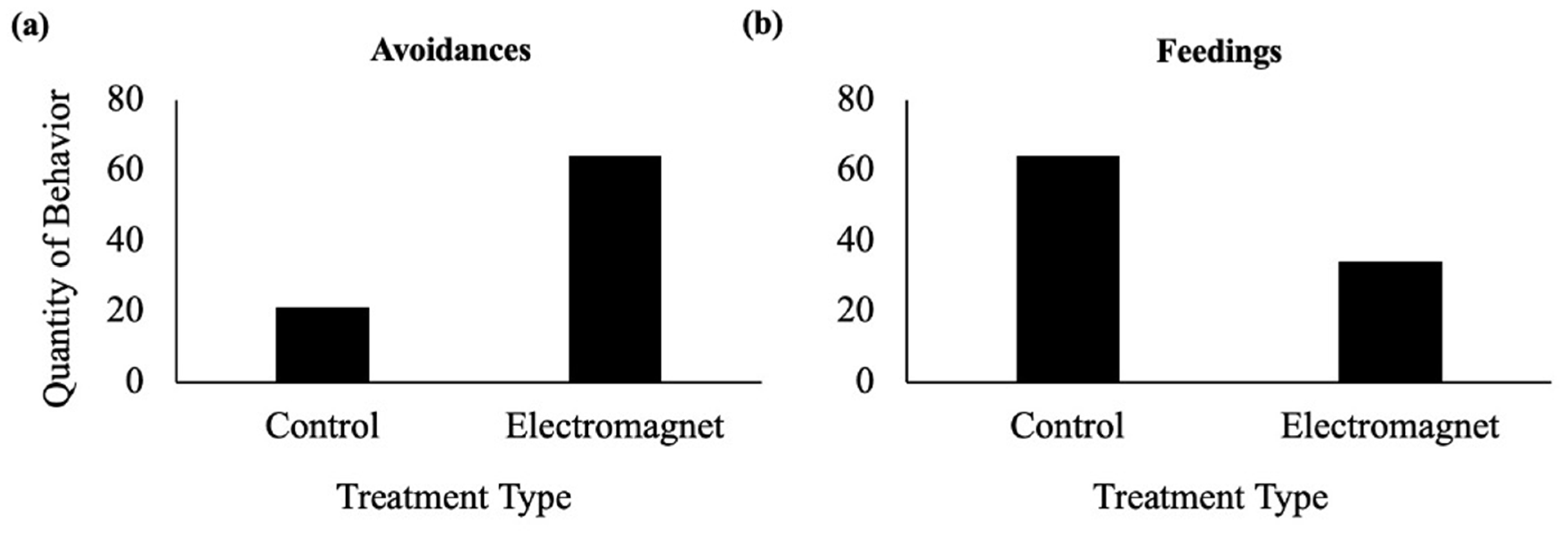
3.1. Bait Experiment
3.1.1. Avoidance Frequency
3.1.2. Feeding Frequency
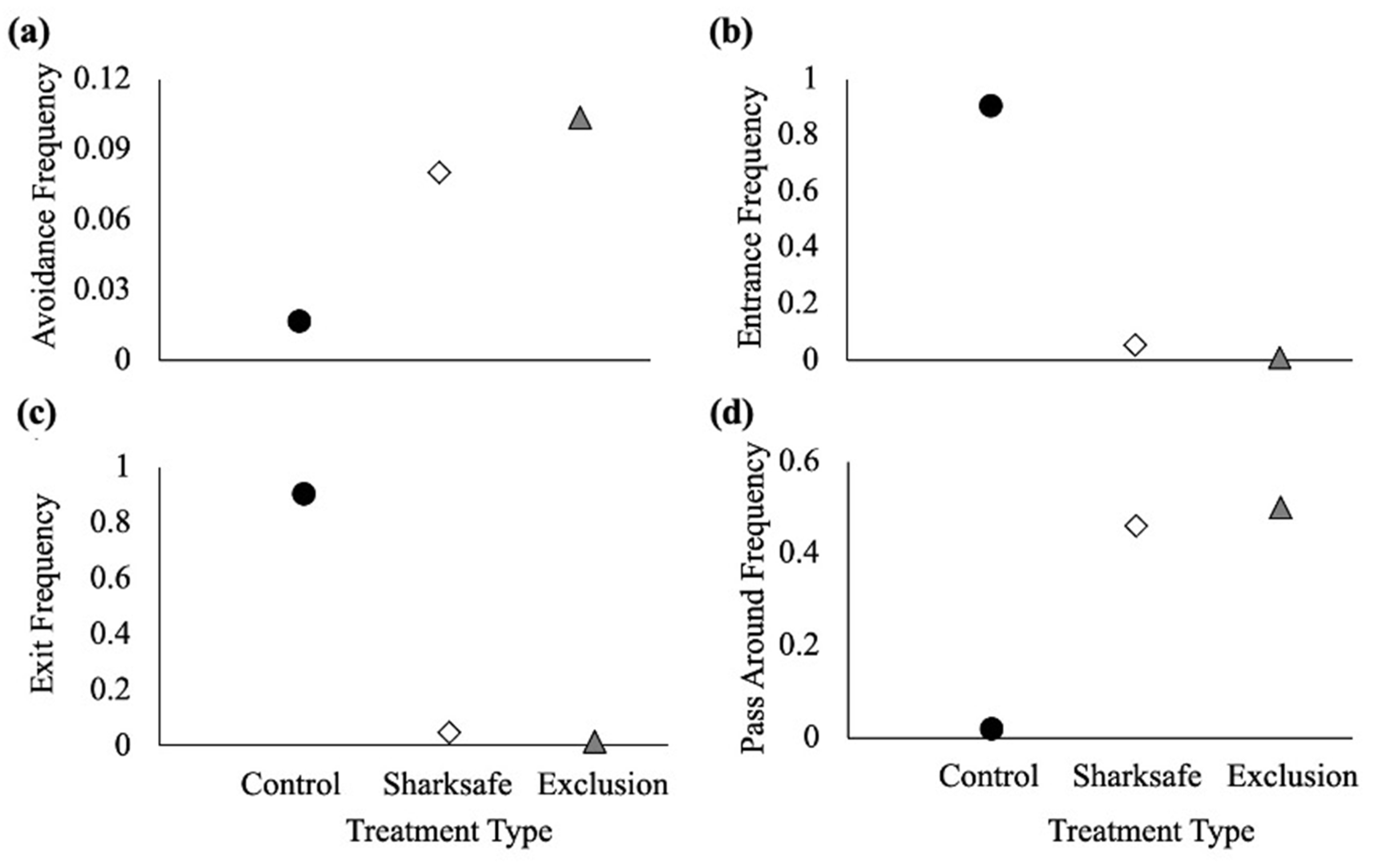
3.2. Barrier Experiment
3.2.1. Avoidance Frequency
3.2.2. Entrance Frequency
3.2.3. Exit Frequency
3.2.4. Pass around Frequency
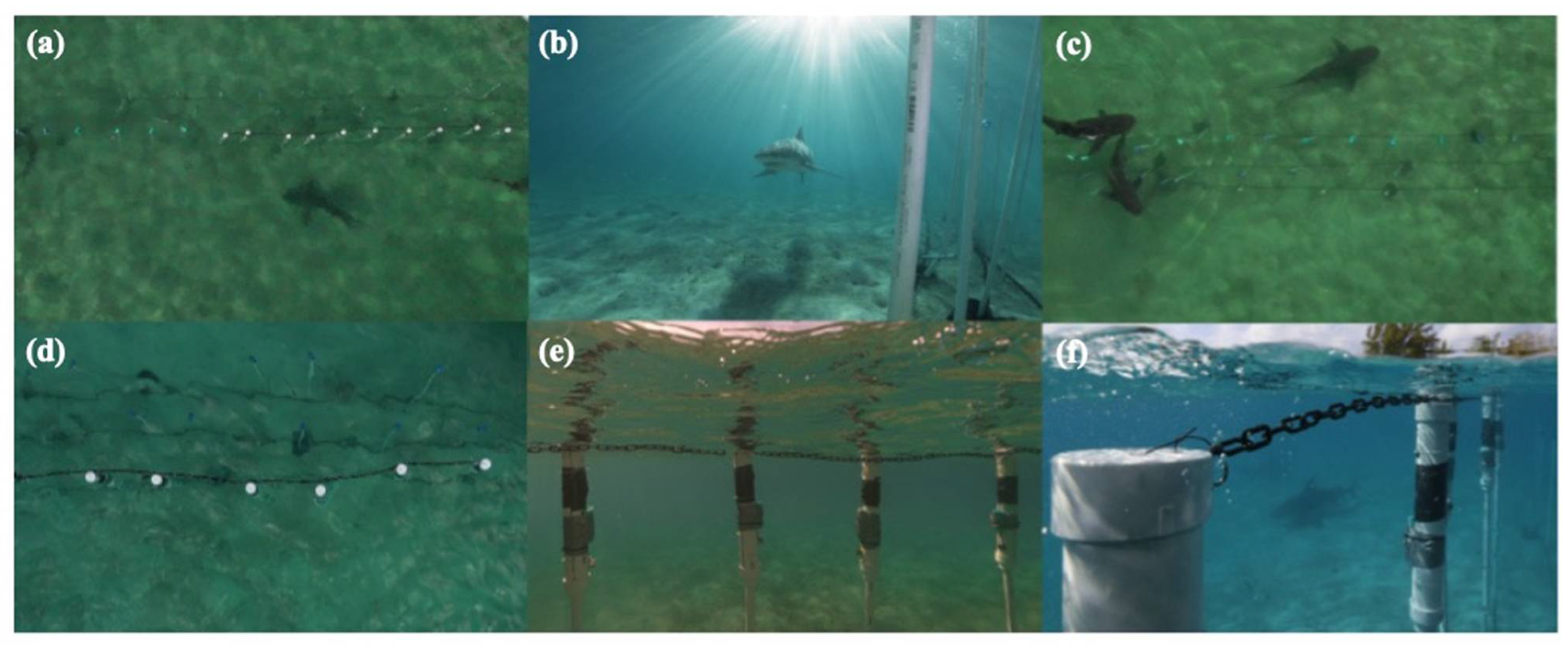
3.2.5. Descriptive Behaviors
4. Discussion
4.1. Basic Behavioural Interactions
4.1.1. Bait Experiment
4.1.2. Barrier Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forstall, R.L. California: Population of Counties by Decennial Census: 1900 to 1990. United States Bureau. 1995. Available online: http://www.census.gov/population/cencounts/ca190090.txt (accessed on 1 March 2021).
- Center for International Earth Science Information Network—CIESIN—Columbia University; United Nations Food and Agriculture Programme—FAO and Centro Internacional de Agricultura Tropical—CIAT; NASA Socioeconomic Data and Applications Center. Coastal Zone Population Method: Percentage of total Population Living in Coastal Areas. 2007. Available online: http://sedac.ciesin.columbia.edu/es/papers/Coastal_Zone_Pop_Method.pdf (accessed on 1 March 2021).
- Midway, S.R.; Wagner, T.; Burgess, G.H. Trends in global shark attacks. PLoS ONE 2019, 14, e0211049. [Google Scholar] [CrossRef] [PubMed]
- Government of Western Australia. New Measures to Combat WA Shark Risks. 2014. Available online: http://www.fish.wa.gov.au/Pages/news_archive/New-measures-to-combat-WA-shark-risks.aspx (accessed on 1 March 2021).
- Neff, C. Australian Beach Safety and the Politics of Shark Attacks. Coast. Manag. 2012, 40, 88–106. [Google Scholar] [CrossRef]
- NSW Department of Primary Industries. SMART Drumlines. 2017. Available online: http://www.dpi.nsw.gov.au/fishing/sharks/shark-management/smart-drumlines (accessed on 1 March 2021).
- Dudley, S.F. A comparison of the shark control programs of New South Wales and Queensland (Australia) and KwaZulu-Natal (South Africa). Ocean Coast. Manag. 1997, 34, 1–27. [Google Scholar] [CrossRef]
- Wetherbee, B.M.; Lowe, C.G.; Crow, G.L. A review of shark control in Hawaii with recommendations for future research. Pac. Sci. 1994, 48, 95–115. [Google Scholar]
- Dudley, S.F.J.; Cliff, G. Some effects of shark nets in the Natal nearshore environment. Environ. Biol. Fish 1993, 36, 243–255. [Google Scholar] [CrossRef]
- Atkins, S.; Cliff, G.; Pillay, N. Humpback dolphin bycatch in the shark nets in KwaZulu-Natal, South Africa. Biol. Conserv. 2013, 159, 442–449. [Google Scholar] [CrossRef]
- Burkholder, D.A.; Heithaus, M.R.; Fourqurean, J.W.; Wirsing, A.; Dill, L.M. Patterns of top-down control in a seagrass ecosystem: Could a roving apex predator induce a behaviour-mediated trophic cascade? J. Anim. Ecol. 2013, 82, 1192–1202. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- Ruppert, J.L.W.; Travers, M.J.; Smith, L.L.; Fortin, M.-J.; Meekan, M. Caught in the middle: Combines impacts of shark removal and coral loss on the fish communities of coral reefs. PLoS ONE 2013, 8, e74648. [Google Scholar] [CrossRef]
- Kock, A.; Titley, S.; Petersen, W.; Sikweyiya, M.; Tsotsobe, S.; Colenbrander, D.; Gold, H.; Oelofse, G. A Pioneering Shark Safety Program in Cape Town, South Africa. In Global Perspectives on the Biology and Life History of the White Shark; CRC Press: Boca Raton, FL, USA, 2012; pp. 447–465. [Google Scholar]
- O’Connell, C.P.; Gressle, J.; Crews, J.; King, A.A.; He, P. Evaluating the effects of a SharkSafe Barrier TM shoreline deployment on bull shark (Carcharhinus leucas) behaviour. Aquat. Conserv. 2022, 32, 55–65. [Google Scholar] [CrossRef]
- Huveneers, C.; Rogers, P.J.; Semmens, J.; Beckmann, C.; Kock, A.A.; Page, B.; Goldsworthy, S.D. Effects of the Shark ShieldTM electric deterrent on the behaviour of white sharks (Carcharodon carcharias). In Final Report to SafeWork South Australia, Version 2; SARDI Publication No. F2012/000123-1. SARDI Research Report Series No. 632; South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2012; p. 66. [Google Scholar]
- Guyomard, D.; Perry, C.; Tournoux, P.U.; Cliff, G.; Peddemors, V.; Jaquemet, S. An innovative fishing gear to enhance the release of non-target species in coastal shark-control programs: The SMART (shark management alert in real-time) drumline. Fish. Res. 2019, 216, 6–17. [Google Scholar] [CrossRef]
- McPhee, D.P. Likely Effectiveness of Netting or Other Capture Programs as a Shark Hazard Mitigation Strategy under Western Australian Conditions; Department of Fisheries: Perth, WA, Australia, 2012.
- O’Connell, C.P.; Andreotti, S.; Rutzen, M.; Matthee, C.A.; Meyer, M.; He, P. Effects of the Sharksafe barrier on white shark (Carcharodon carcharias) behavior and its implications for future conservation technologies. J. Exp. Mar. Biol. Ecol. 2014, 460, 37–46. [Google Scholar] [CrossRef]
- Davison, A.; Kock, A. Fish Hoek exclusion net evaluation report. Shark Spotter Rep. 2014. Available online: https://sharkspotters.org.za/wp-content/uploads/2016/10/FINAL-Exclusion-net-report-24-06-14.pdf (accessed on 1 March 2021).
- Rigg, D.P.; Peverell, S.C.; Hearndon, M.; Seymour, J.E. Do elasmobranch reactions to magnetic fields in water show promise for bycatch mitigation? Mar. Freshw. Res. 2009, 60, 942–948. [Google Scholar] [CrossRef]
- Robbins, W.D.; Peddemors, V.M.; Kennelly, S.J. Assessment of permanent magnets and electropositive metals to reduce the line-based capture of Galapagos sharks, Carcharhinus galapagensis. Fish. Res. 2011, 109, 100–106. [Google Scholar] [CrossRef]
- O’Connell, C.P.; Gruber, S.H.; Abel, D.C.; Stroud, E.M.; Rice, P.H. The responses of juvenile lemon sharks, Negaprion brevirostris, to a magnetic barrier. Ocean Coast. Manag. 2011, 54, 225–230. [Google Scholar] [CrossRef]
- Brill, R.; Bushnell, P.; Smith, L.; Speaks, C.; Sundaram, R.; Stroud, E.; Wang, J. The repulsive and feeding deterrent effects of electropositive metals on juvenile sandbar sharks (Cacharhiuns plumbeus). Fish. Bull. 2009, 107, 298–307. [Google Scholar]
- O’Connell, C.P.; Guttridge, T.L.; Finger, J.S.; Gruber, S.H.; He, P. Behavioral modification of visually deprived lemon sharks (Negaprion brevirostris) toward magnetic fields. J. Exp. Mar. Biol. Ecol. 2013, 453, 131–137. [Google Scholar] [CrossRef]
- O’Connell, C.P.; Hyun, S.-Y.; Rillahan, C.B.; He, P. Bull shark (Carcharhinus leucas) exclusion properties of the Sharksafe barrier and behavioral validation using the ARIS sonar technology. Globl. Ecol. Conserv. 2014, 2, 300–314. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, C.P.; He, P. A large-scale field analysis examining the effect of magnetically treated baits and barriers on teleost and elasmobranch behavior. Ocean Coast. Manag. 2014, 96, 130–137. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B. Lme4: Linear Mixed-Effects Models Using S4 Classes; R Package, 2012. Available online: http://lme4.r-forge.r-project.org (accessed on 1 March 2021).
- Hyun, S.-Y.; Cadrin, S.X.; Roman, S. Fixed and mixed effect models for fishery data on depth distribution of Georges Bank yellowtail flounder. Fish. Res. 2014, 157, 180–186. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006.
- Keller, B.A.; Putman, N.F.; Grubbs, R.D.; Portnoy, D.S.; Murphy, T.P. Map-like use of Earth’s magnetic field in sharks. Curr. Biol. 2021, 31, 2881–2886. [Google Scholar] [CrossRef]
- Meyer, C.G.; Holland, K.N.; Papastamatiou, Y.P. Sharks can detect changes in the geomagnetic field. J. R. Soc. Interface 2004, 2, 129–130. [Google Scholar] [CrossRef] [Green Version]
- Kalmijn, A.J. Biophysics of geomagnetic field detection. IEEE Trans. Magn. 1981, 17, 1113–1124. [Google Scholar] [CrossRef]
- Kalmijn, A.J. Electric and magnetic field detection in elasmobranch fishes. Science 1982, 218, 916–918. [Google Scholar] [CrossRef]
- Kalmijn, A.J. Theory of electromagnetic orientation: A further analysis. In Comparative Physiology of Sensory Systems; Cambridge Univesity Press: Cambridge, UK, 1984; pp. 25–560. [Google Scholar]
- Klimley, A.P. Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar. Biol. 1993, 117, 1–22. [Google Scholar] [CrossRef]
- Tallack, S.M.; Mandelman, J.W. Do rare-earth metals deter spiny dogfish? A feasibility study on the use of electropositive “mischmetal” to reduce the bycatch of Squalus acanthias by hook gear in the Gulf of Maine. ICES J. Mar. Sci. 2011, 66, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Boord, R.L.; Campbell, C.B.G. Structural and Functional Organization of the lateral line system of sharks. Am. Zool. 1977, 17, 442–449. [Google Scholar] [CrossRef]
- Roberts, B.L. Mechanoreceptors and the behavior of elasmobranch fishes with special reference to the acoustic-lateralis system. In Sensory Biology of Sharks, Skates, and Rays; Office of Naval Research: Arlington, VA, USA, 1978; pp. 331–390. [Google Scholar]
- Kasumyan, A.O. The lateral line in fish: Stricture, function and role in behaviour. J. Ichthyol. 2003, 43, S175–S203. [Google Scholar]
- Maruska, K.; Tricas, T. Test of the mechanotactile hypothesis: Neuromast morphology and response dynamics of mechanosensory lateral line primary afferents in the stingray. J. Exp. Biol. 2004, 207, 3463–3476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myrberg, A.A.; Banner, A.; Richard, J.D. Shark attraction using a video-acoustic system. Mar. Biol. 1969, 2, 264–276. [Google Scholar] [CrossRef]
- Myrberg, A.A.; Gordan, C.R.; Klimley, A.P. Rapid withdrawal from a sound source by open-ocean sharks. J. Acoust. Soc. 1978, 64, 1289–1297. [Google Scholar] [CrossRef]
- Maruska, K.P. Morphology of the Mechansensory Lateral Line System in Elasmobranch Fishes: Ecological and Behavioral Considerations. Environ. Biol. Fishes 2001, 60, 47–75. [Google Scholar] [CrossRef]
- Gardiner, J.M.; Atema, J. Flow Sensing in Sharks: Lateral Line Contributions to Navigation and Prey Capture. In Flow Sensing in Air and Water: Behavioral, Neural, and Engineering Princples of Operation; Springer: New York, NY, USA, 2014; pp. 127–146. [Google Scholar]
- Brazier, W.; Nel, R.; Cliff, G.; Dudley, S. Impact of protective shark nets on sea turtles in KwaZulu-Natal, South Africa, 1981–2008. Afri. J. Mar. Sci. 2012, 34, 49–257. [Google Scholar] [CrossRef]
- Reid, D.D.; Robbins, W.D.; Peddemors, V.M. Decadal trends in shark catches and effort from the New South Wales, Australia, Shark Meshing Program 1950–2010. Mar. Freshw. Res. 2011, 62, 676–693. [Google Scholar] [CrossRef]
- Bornatowski, H.; Navia, A.F.; Braga, R.R.; Abilhoa, V.; Corrêa, M.F.M. Ecological importance of sharks and rays in a structural foodweb analysis in southern Brazil. ICES J. Mar. Sci. 2014, 71, 1586–1592. [Google Scholar] [CrossRef] [Green Version]
- Roff, G.; Doropoulos, C.; Rogers, A.; Bozec, Y.-M.; Krueck, N.C.; Aurellado, E.; Priest, M.; Birrell, C.; Mumby, P.J. The ecological role of sharks on coral reefs. Trends Ecol. Evol. 2016, 31, 395–407. [Google Scholar] [CrossRef] [PubMed]
| Behavior | Definition of Behavior |
|---|---|
| Bait Experiment Behaviors | |
| Visit | Shark swam within one body length of an observation zone |
| Avoidances | Shark abruptly changed direction, such as a sudden turn and/or acceleration away, after visiting the observation zone |
| Feeding | Shark was observed to bite or remove bait from apparatus |
| Barrier Experiment Behaviors | |
| Visit | Shark swam within one body length of an observation zone |
| Avoidances | Shark abruptly changed direction, such as a 45°, 90°, or 180° turn and/or acceleration away, after visiting an observation zone |
| Entrances | Shark visited an observation zone and swam through the PVC pipes |
| Exits | Shark entered an observation zone and swam back out through the PVC pipes |
| Pass Arounds | A visit followed by a shark swimming adjacent to an entire experimental region, but not avoiding or entering through the treatment region |
| Model | Log Likelihood | −2(Δl) Deviance | ΔPAR | p-Value | AIC | |
|---|---|---|---|---|---|---|
| Avoidance Frequency | ||||||
| A1 | Null | −198.91 | - | - | - | 401.82 |
| A2 | 1+Treat | −155.84 | 553.66 | 2 | <0.001 | 319.68 |
| A3 | 1+Con | −197.28 | 595.10 | 2 | 0.19 | 402.56 |
| A4 | 1+Vis | −198.11 | 595.93 | 1 | 0.21 | 402.22 |
| A5 | 1+Treat+Con | −154.44 | 466.12 | 2 | 0.25 | 320.88 |
| A6 | 1+Treat+Vis | −155.07 | 466.75 | 1 | 0.22 | 320.15 |
| A7 | 1+Treat+Con+Treat x Con | −150.41 | 459.29 | 4 | 0.10 | 320.83 |
| A8 | 1+Treat+Vis+Treat x Vis | −154.62 | 464.76 | 2 | 0.63 | 323.23 |
| Entrance Frequency | ||||||
| B1 | Null | −1086.78 | 2177.57 | |||
| B2 | 1+Treat | −136.82 | 2310.38 | 2 | <0.001 | 281.63 |
| B3 | 1+Con | −1086.00 | 3259.61 | 2 | 0.48 | 2180.10 |
| B4 | 1+Vis | −1086.60 | 3260.17 | 1 | 0.55 | 2179.20 |
| B5 | 1+Treat+Con | −136.40 | 410.04 | 2 | 0.66 | 284.81 |
| B6 | 1+Treat+Vis | −136.75 | 410.38 | 1 | 0.72 | 283.50 |
| B7 | 1+Treat+Con+Treat x Con | −132.77 | 405.58 | 4 | 0.12 | 285.54 |
| B8 | 1+Treat+Vis+Treat x Vis | −136.31 | 409.81 | 2 | 0.64 | 286.61 |
| Exit Frequency | ||||||
| C1 | Null | −1081.69 | 2167.40 | |||
| C2 | 1+Treat | −143.45 | 2306.83 | 2 | <0.001 | 294.90 |
| C3 | 1+Con | −1081.00 | 3244.33 | 2 | 0.48 | 2169.90 |
| C4 | 1+Vis | −1081.50 | 3244.89 | 1 | 0.55 | 2169.00 |
| C5 | 1+Treat+Con | −143.03 | 429.93 | 2 | 0.66 | 298.06 |
| C6 | 1+Treat+Vis | −143.38 | 430.28 | 1 | 0.71 | 296.76 |
| C7 | 1+Treat+Con+Treat x Con | −139.09 | 425.14 | 4 | 0.10 | 298.18 |
| C8 | 1+Treat+Vis+Treat x Vis | −142.42 | 429.19 | 2 | 0.38 | 298.84 |
| Pass Around Frequency | ||||||
| D1 | Null | −567.04 | 1138.08 | |||
| D2 | 1+Treat | −194.38 | 1328.46 | 2 | <0.001 | 396.76 |
| D3 | 1+Con | −567.04 | 1701.12 | 2 | 0.994 | 1142.10 |
| D4 | 1+Vis | −565.88 | 1699.96 | 1 | 0.13 | 1137.80 |
| D5 | 1+Treat+Con | −194.31 | 583.06 | 2 | 0.93 | 400.61 |
| D6 | 1+Treat+Vis | −193.46 | 582.21 | 1 | 0.17 | 396.91 |
| D7 | 1+Treat+Con+Treat x Con | −193.30 | 581.91 | 4 | 0.73 | 406.60 |
| D8 | 1+Treat+Vis+Treat x Vis | −193.05 | 579.97 | 2 | 0.67 | 400.11 |
| Explanatory Variable | Coefficient | Standard Error | t | p-Value |
|---|---|---|---|---|
| Avoidance Frequency | ||||
| Intercept | −4.18 | 0.25 | −16.99 | <0.001 |
| Exclusion Barrier | 1.85 | 0.24 | 7.53 | <0.001 |
| SharkSafe Barrier | 1.59 | 0.25 | 6.40 | <0.001 |
| Entrance Frequency | ||||
| Intercept | −0.10 | 0.03 | −3.28 | 0.001 |
| Exclusion Barrier | −4.29 | 0.27 | −15.95 | <0.001 |
| Sharksafe Barrier | −2.98 | 0.14 | −21.57 | <0.001 |
| Exit Frequency | ||||
| Intercept | −0.10 | 0.03 | −3.28 | 0.001 |
| Exclusion Barrier | −4.98 | 0.38 | −13.14 | <0.001 |
| Sharksafe Barrier | −2.86 | 0.13 | −21.91 | <0.001 |
| Pass Around Frequency | ||||
| Intercept | −4.03 | 0.22 | −18.24 | <0.001 |
| Exclusion Barrier | 3.33 | 0.22 | 14.99 | <0.001 |
| Sharksafe Barrier | 3.24 | 0.22 | 14.60 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connell, C.P.; Crews, J.; King, A.; Gressle, J. Evaluating the Shark Deterrent Effects of the Novel Exclusion Barrier in Comparison to the Rigorously Tested Sharksafe Barrier Technology. J. Mar. Sci. Eng. 2022, 10, 634. https://doi.org/10.3390/jmse10050634
O’Connell CP, Crews J, King A, Gressle J. Evaluating the Shark Deterrent Effects of the Novel Exclusion Barrier in Comparison to the Rigorously Tested Sharksafe Barrier Technology. Journal of Marine Science and Engineering. 2022; 10(5):634. https://doi.org/10.3390/jmse10050634
Chicago/Turabian StyleO’Connell, Craig P, Julia Crews, Andre King, and Juliet Gressle. 2022. "Evaluating the Shark Deterrent Effects of the Novel Exclusion Barrier in Comparison to the Rigorously Tested Sharksafe Barrier Technology" Journal of Marine Science and Engineering 10, no. 5: 634. https://doi.org/10.3390/jmse10050634
APA StyleO’Connell, C. P., Crews, J., King, A., & Gressle, J. (2022). Evaluating the Shark Deterrent Effects of the Novel Exclusion Barrier in Comparison to the Rigorously Tested Sharksafe Barrier Technology. Journal of Marine Science and Engineering, 10(5), 634. https://doi.org/10.3390/jmse10050634






