Structural and Functional Analyses of Motile Fauna Associated with Cystoseira brachycarpa along a Gradient of Ocean Acidification in a CO2-Vent System off Panarea (Aeolian Islands, Italy)
Abstract
:1. Introduction
2. Materials and Methods
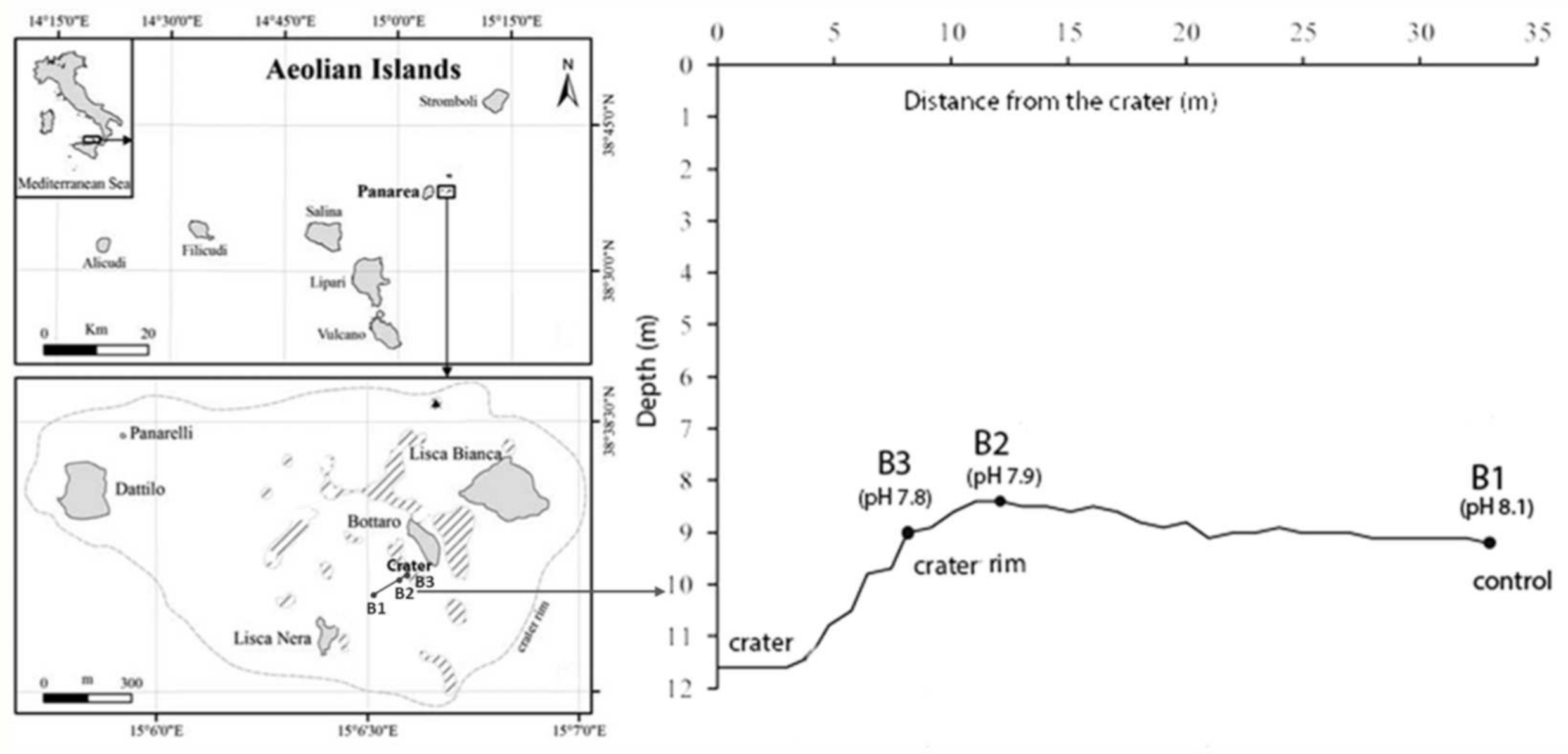
2.1. Study Area and Sampling Activities
2.2. Data Analysis
3. Results
3.1. Physico-Chemical Variables
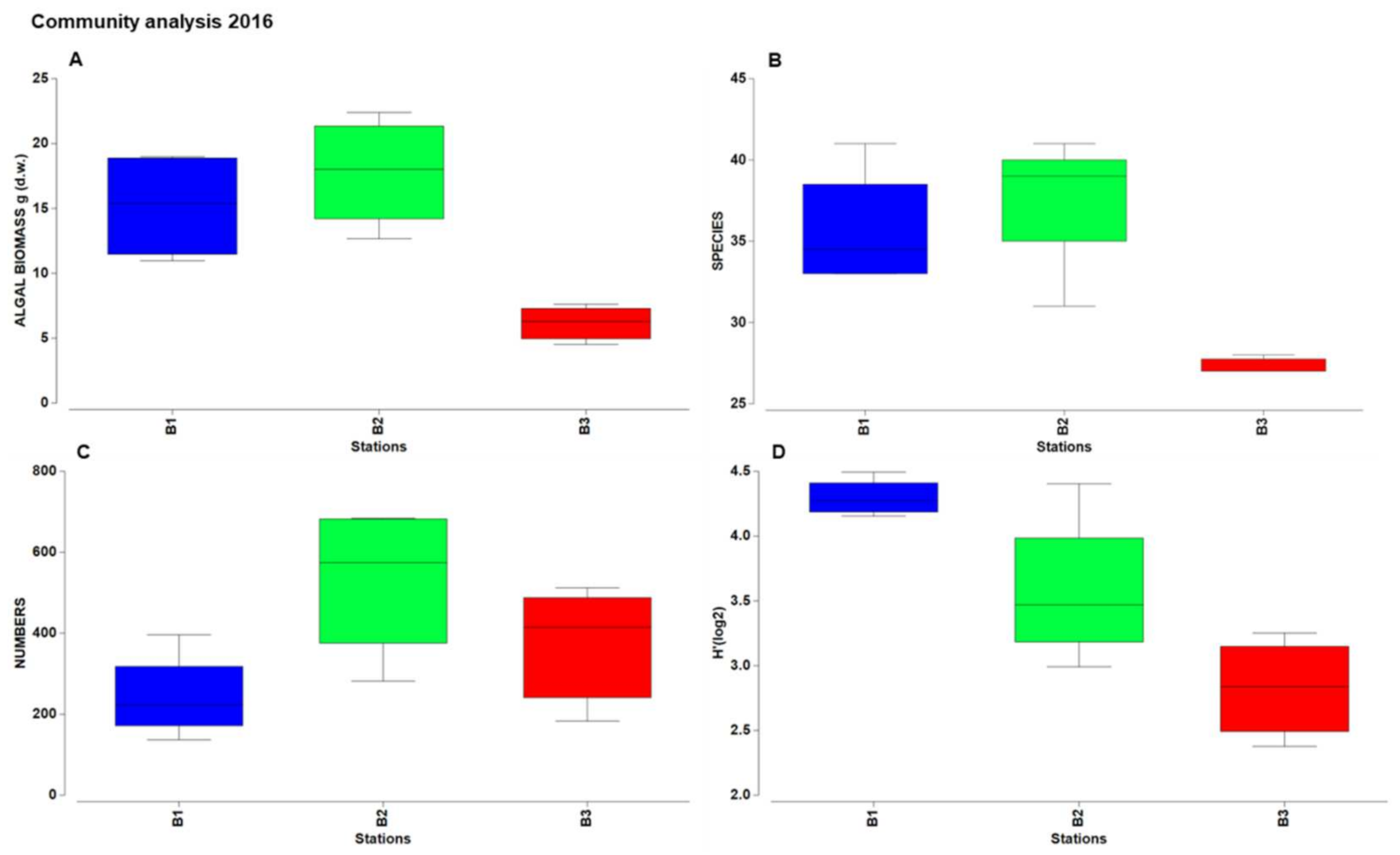
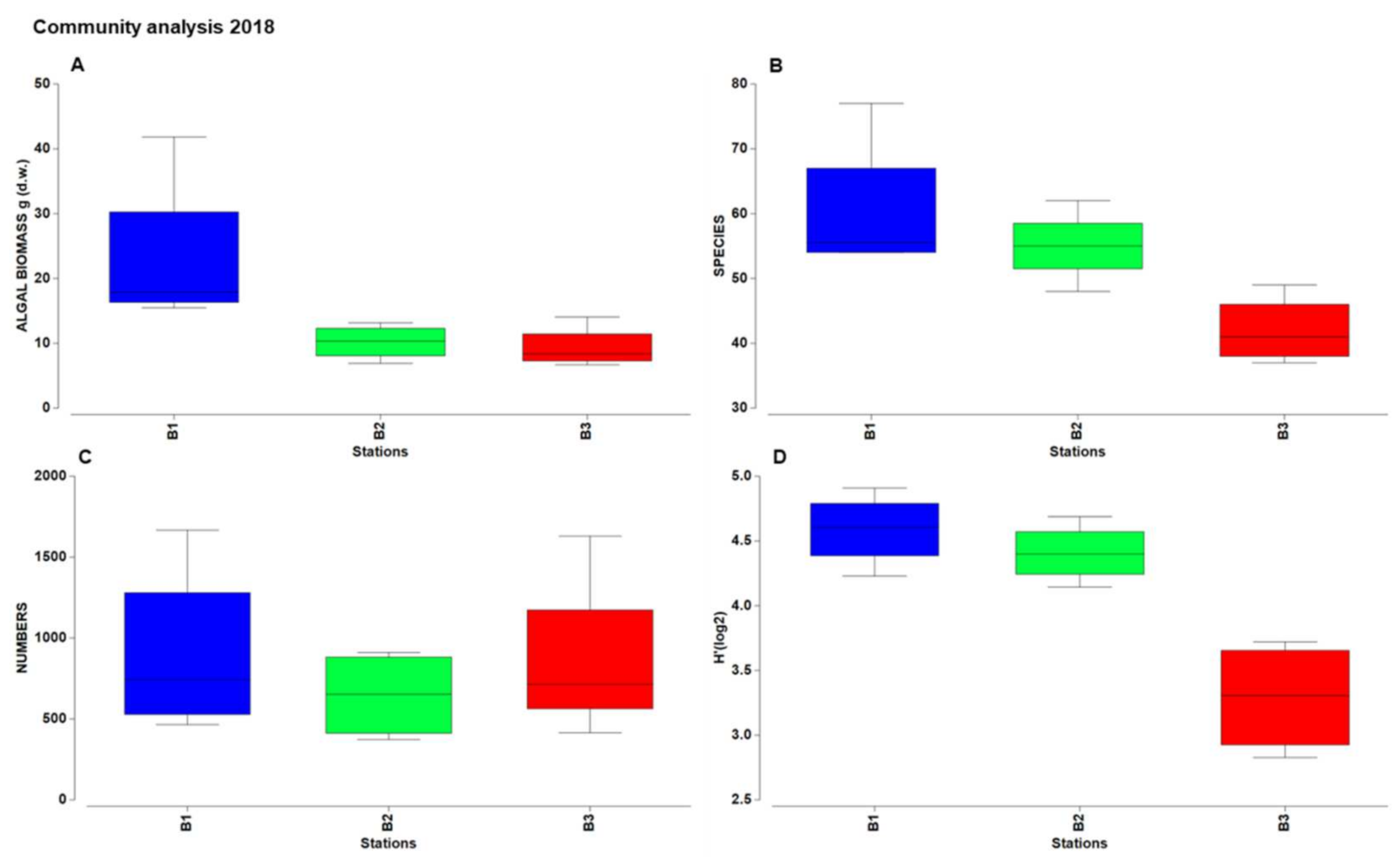
3.2. Taxonomic and Structural Analyses
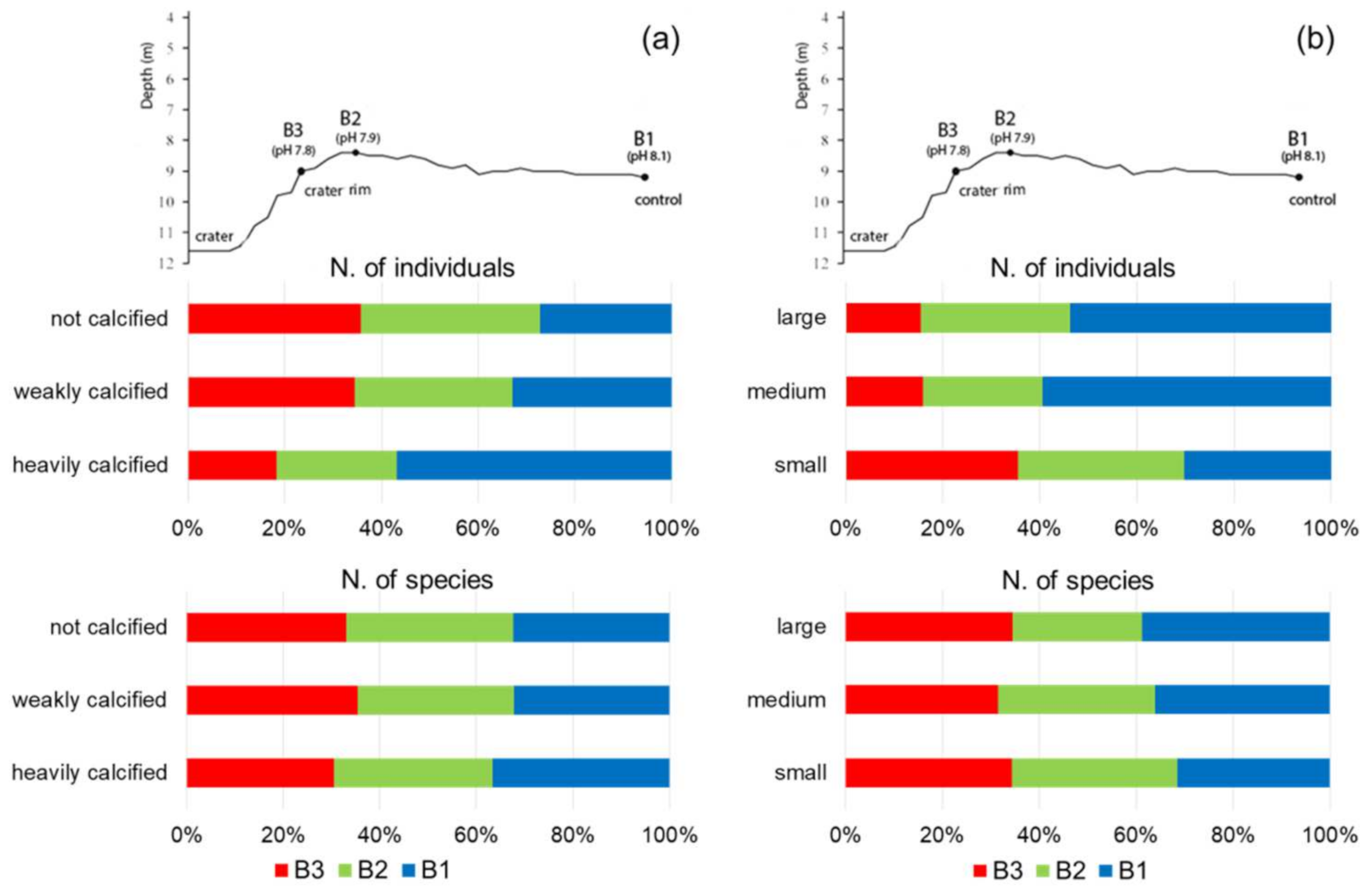
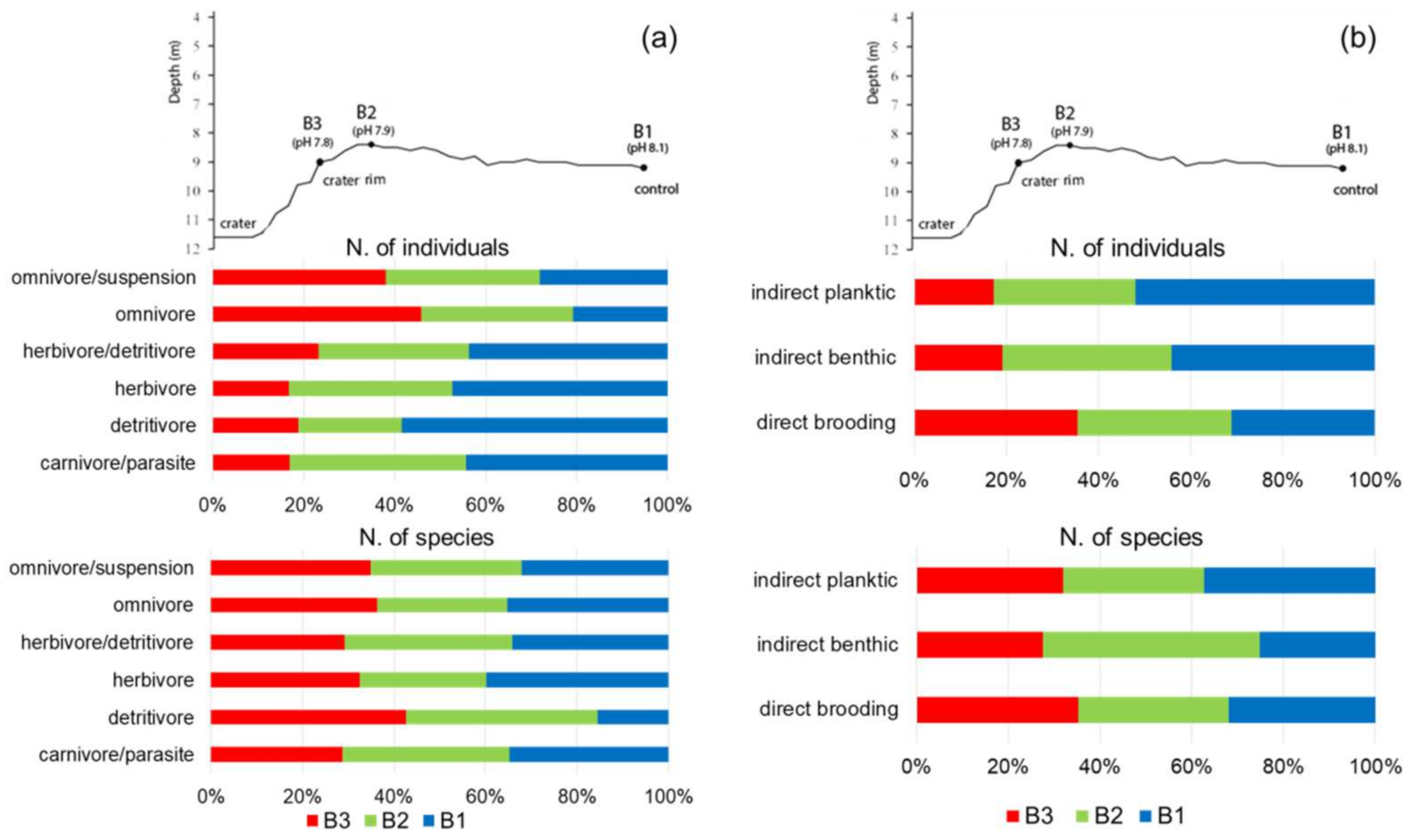
3.3. Analyses of Functional Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, K.; Wickett, M.E. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. Ocean. 2005, 110, C09S04. [Google Scholar] [CrossRef] [Green Version]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 424–432. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Micheli, F.; Gambi, M.C.; Martz, T.R. Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl. Acad. Sci. USA 2011, 108, 14515–14520. [Google Scholar] [CrossRef] [Green Version]
- Kroeker, K.J.; Gambi, M.C.; Micheli, F. Community dynamics and ecosystem simplification in a high-CO2 ocean. Proc. Natl. Acad. Sci. USA 2013, 110, 12721–12726. [Google Scholar] [CrossRef] [Green Version]
- Kroeker, K.J.; Micheli, F.; Gambi, M.C. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat. Clim. Chang. 2013, 3, 256–259. [Google Scholar] [CrossRef]
- Tarasov, V.G.; Gebruk, A.V.; Mironov, A.N.; Moskalev, L.I. Deep-sea and shallow-water hydrothermal vent communities: Two different phenomena? Chem. Geol. 2005, 224, 5–39. [Google Scholar] [CrossRef]
- Hall-Spencer, J.; Rodolfo-Metalpa, R.; Martin, S.; Ransome, S.; Fine, M.; Turner, S.M.; Rowley, S.J.; Tedesco, D.; Buia, M.C. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 2008, 454, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Foo, S.A.; Byrne, M.; Ricevuto, E.; Gambi, M.C. The carbon dioxide vents of Ischia, Italy, a natural laboratory to assess impacts of ocean acidification on marine ecosystems: An overview of research and comparisons with other vent systems. Oceanogr. Mar. Biol. Ann. Rev. 2018, 56, 237–320. [Google Scholar]
- Rastrick, S.S.; Graham, H.; Azetsu-Scott, K.; Calosi, P.; Chierici, M.; Fransson, A.; Hop, H.; Hall-Spencer, J.; Milazzo, M.; Thor, P.; et al. Using natural analogues to investigate the effects of climate change and ocean acidification on Northern ecosystems. ICES J. Mar. Sci. 2018, 75, 2299–2311. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, S.; Hernandez, J.C. The importance of natural acidified systems in the study of Ocean Acidification: What have we learned? Adv. Mar. Biol. 2018, 80, 57–99. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Delgado, S.; Gonzalez Santana, D.; Santana Casiano, M.; Gonzalez Davila, M.; Hernández, C.A.; Sangil, C.; Hernández, J.C. Chemical characterization of Punta de Fuencaliente CO2 seeps system (La Palma Island, NE Atlantic Ocean): A new natural laboratory for ocean acidification studies. Biogeosceinces 2020, 18, 1673–1687. [Google Scholar] [CrossRef]
- Vizzini, S.; Di Leonardo, R.; Costa, V.; Tramati, C.D.; Luzzu, F.; Mazzola, A. Trace element bias in the use of CO2 vents as analogues for low pH environments: Implications for contamination levels in acidified oceans. Estuar. Coast. Shelf Sci. 2013, 134, 19–30. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Harvey, B.P. Ocean acidification impacts on coastal ecosystem services due to habitat degradation. Emerg. Top. Life Sci. 2019, 3, 197–206. [Google Scholar] [PubMed]
- Porzio, L.; Buia, M.C.; Hall-Spencer, J.M. Effects of ocean acidification on macroalgal communities. J. Exp. Mar. Biol. Ecol. 2011, 400, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Fabricius, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, G.; Okazaki, R.; Muehllehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Fabricius, K.E.; De’ath, G.; Noonan, S.; Uthicke, S. Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132479. [Google Scholar] [CrossRef] [Green Version]
- Vizzini, S.; Martínez-Crego, B.; Andolina, C.; Massa-Gallucci, A.; Connell, S.D.; Gambi, M.C. Ocean acidification as a driver of community simplification via the collapse of higher-order and rise of lower-order consumers. Sci. Rep. 2017, 7, 4018. [Google Scholar] [CrossRef] [Green Version]
- Auriemma, R.; De Vittor, C.; Esposito, V.; Gaglioti, M.; Gambi, M.C. Motile Fauna associated to Cystoseira brachycarpa along a gradient of Ocean Acidification at a vent system off Panarea (Aeolian Islands, Italy). Biol. Mar. Mediterr. 2019, 26, 216–219. [Google Scholar]
- Plaisance, L.; Matterson, K.; Fabricius, K.; Drovetski, S.; Meyer, C.; Knowlton, N. Effects of low pH on the coral reef cryptic invertebrate communities near CO2 vents in Papua New Guinea. PLoS ONE 2021, 16, e0258725. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Giuseppe, B.; Morihiko, T.; Porzio, L.; Harvey, B.P.; Agostini, S.; Kon, K. Decreased Diversity and Abundance of Marine Invertebrates at CO2 Seeps in Warm-Temperate Japan. Zool. Sci. 2022, 39, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Gambi, M.C.; Musco, L.; Giangrande, A.; Badalamenti, F.; Micheli, F.; Kroeker, K.J. Distribution and functional traits of polychaetes in a CO2 vent system: Winners and losers among closely related species. Mar. Ecol. Prog. Ser. 2016, 550, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Lucey, N.M.; Lombardi, C.; De Marchi, L.; Schulze, A.; Gambi, M.C.; Calosi, P. To brood or not to brood: Are marine organisms that protect their offspring more resilient to ocean acidification? Sci. Rep. 2015, 5, 12009. [Google Scholar] [CrossRef] [Green Version]
- Garilli, V.; Rodolfo-Metalpa, R.; Scuderi, D.; Brusca, L.; Parrinello, D.; Rastrick, S.P.S.; Foggo, A.; Twitchett, R.J.; Hall-Spencer, J.M.; Milazzo, M. Physiological advantages of dwarfing in surviving extinctions in high-CO2 oceans. Nat. Clim. Chang. 2015, 5, 678–682. [Google Scholar] [CrossRef]
- Teixidó, N.; Gambi, M.C.; Parravacini, V.; Kroeker, K.; Micheli, F.; Villéger, S.; Ballesteros, E. Functional biodiversity loss along natural CO2 gradients. Nat. Commun. 2018, 9, 5149. [Google Scholar] [CrossRef] [PubMed]
- Esposito, V.; Andaloro, F.; Canese, S.; Bortoluzzi, G.; Bo, M.; Di Bella, M.; Italiano, F.; Sabatino, G.; Battaglia, P.; Consoli, P.; et al. Exceptional discovery of a shallow-water hydrothermal site in the SW area of Basiluzzo islet (Aeolian archipelago, South Tyrrhenian Sea): An environment to preserve. PLoS ONE 2018, 13, e0190710. [Google Scholar] [CrossRef] [PubMed]
- Vizzini, S.; Tomasello, A.; Di Maida, G.; Pirrotta, M.; Mazzola, A.; Calvo, S. Effect of explosive shallow hydrothermal vents on δ13C and growth performance in the seagrass Posidonia oceanica. J. Ecol. 2010, 98, 1284–1291. [Google Scholar] [CrossRef]
- Goffredo, S.; Prada, F.; Caroselli, E.; Capaccioni, B.; Zaccanti, F.; Fantazzini, P.; Fermani, S.; Reggi, M.; Levy, O.; Fabricius, K.E.; et al. Biomineralization control related to population density under ocean acidification. Nat. Clim. Chang. 2014, 4, 293–297. [Google Scholar] [CrossRef] [Green Version]
- Rogelja, M.; Cibic, T.; Pennesi, C.; De Vittor, C. Microphytobenthic community composition and primary production at gas and thermal vents in the Aeolian Islands (Tyrrhenian Sea, Italy). Mar. Environ. Res. 2016, 118, 31–44. [Google Scholar] [CrossRef]
- Guilini, K.; Weber, M.; De Beer, D.; Schneider, M.; Molari, M.; Lott, C.; Bodnar, W.; Mascart, T.; De Troch, M.; Vanreusel, A. Response of Posidonia oceanica seagrass and its epibiont communities to ocean acidification. PLoS ONE 2017, 12, e0181531. [Google Scholar] [CrossRef] [Green Version]
- Piazzi, L.; Bonaviri, C.; Castelli, A.; Ceccherelli, G.; Costa, G.; Curini-Galletti, M.; Langeneck, J.; Manconi, R.; Montefalcone, M.; Pipitone, C.; et al. Biodiversity in canopy-forming algae: Structure and spatial variability of the Mediterranean Cystoseira assemblages. Estuar. Coast. Shelf Sci. 2018, 207, 132–141. [Google Scholar] [CrossRef]
- Chiarore, A.; Bertocci, I.; Fioretti, S.; Meccariello, A.; Saccone, G.; Crocetta, F.; Patti, F.P. Syntopic Cystoseira taxa support different molluscan assemblages in the Gulf of Naples (southern Tyrrhenian Sea). Mar. Freshw. Res. 2019, 70, 1561–1575. [Google Scholar] [CrossRef]
- Gianni, F.; Bartonili, F.; Pey, A.; Laurent, M.; Martins, G.M.; Airoldi, L.; Mangialajo, L. Threats to large brown algal forests in temperate seas: The overlooked role of native herbivorous fish. Sci. Rep. 2017, 7, 6012. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo, R.; Rull Lluch, J.; Tomasello, A.; Calvo, S. The community of Cystoseira brachycarpa J. Agardh emend Giaccone (Fucales, Phaeophyceae) in a shallow hydrothermal vent area of the Aeolian Islands. Plant. Biosyst. 2014, 148, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Wahl, M.; Schneider Covach, S.V.; Saderne, V.; Hiebenthal, S.C.; Mueller, J.D.; Pansch, C.; Sawall, Y. Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol. Oceanogr. 2018, 63, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Greiner, C.M.; Klingerb, T.; Ruesink, J.L.; Barbera, J.S.; Horwithd, M. Habitat effects of macrophytes and shell on carbonate chemistry and juvenile clam recruitment, survival, and growth. J. Exp. Mar. Biol. Ecol. 2018, 509, 8–15. [Google Scholar] [CrossRef]
- Poore, A.G.B.; Graba-Landry, A.; Favret, M.; Sheppard Brennard, H.; Byrne, M.; Dworjanym, S.A. Direct and indirect effects of ocean acidification and warming on a marine plant–herbivore interaction. Oecologia 2013, 173, 1113–1124. [Google Scholar] [CrossRef]
- Gambi, M.C.; De Vittor, C.; Bigi, S.; Italiano, F. Third School of Scientific Diving at Panarea (Aeolian Islands, Tyrrhenian Sea, Italy): The first international edition. Notiz. SIBM 2018, 74, 104–110. [Google Scholar]
- Esposito, A.; Giordano, G.; Anzidei, M. The 2002–2003 submarine gas eruption at Panarea volcano (Aeolian Islands, Italy): Volcanology of the seafloor and implications for the hazard scenario. Mar. Geol. 2006, 227, 119–134. [Google Scholar] [CrossRef]
- Hughes, R.N. A Functional Biology of Marine Gastropods; Croom Helm: London, UK; Sydney, NSW, Australia, 1986; p. 245. ISBN 0-7099-3746-6. [Google Scholar]
- Krapp-Schickel, G. Do algal-dwelling amphipods react to the ‘critical zones’ of a coastal slope? J. Nat. Hist. 1993, 27, 883–900. [Google Scholar] [CrossRef]
- Fretter, V.; Graham, A. Their functional anatomy and ecology. In British Prosobranch Molluscs; The Ray Society: London, UK, 1994; ISBN 0-903874-23-7. [Google Scholar]
- Voultsiadou, E.; Pyrounaki, M.M.; Chintiroglou, C. The habitat engineering tunicate Microcosmus sabatieri Roule, 1885 and its associated peracarid epifauna. Estuar. Coast. Shelf Sci. 2007, 74, 197–204. [Google Scholar] [CrossRef]
- Scipione, M.B. Do studies of functional groups give more insight to amphipod biodiversity. Crustaceana 2013, 86, 955–1006. [Google Scholar] [CrossRef]
- Guerra-Garcia, J.M.; Tierno de Figueroa, J.M.; Navarro-Barranco, C.; Ros, M.; Sánchez-Moyano, J.E.; Moreira, J. Dietary analysis of the marine Amphipoda (Crustacea: Peracarida) from the Iberian Peninsula. J. Sea Res. 2014, 85, 508–517. [Google Scholar] [CrossRef]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 2015, 17, 497–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giangrande, A.; Putignano, M.; Licciano, M.; Gambi, M.C. The Pandora’s box: Morphological diversity within the genus Amphiglena Claparède, 1864 (Sabellidae, Annelida) in the Mediterranean Sea with the description of nine new species. Zootaxa 2021, 4949, 201–239. [Google Scholar] [CrossRef]
- Gaglioti, M.; Ricevuto, E.; Gambi, M.C. Pattern and map of biodiversity related to ocean acidification in CO2 vents of Ischia. Biol. Mar. Mediterr. 2018, 25, 235–236. [Google Scholar]
- Ricevuto, E.; Lorenti, M.; Patti, F.P.; Scipione, M.B.; Gambi, M.C. Temporal trends of benthic invertebrate settlement along a gradient of ocean acidification at natural CO2 vents (Tyrrhenian Sea). Biol. Mar. Mediter. 2012, 19, 49–52. [Google Scholar]
- Scipione, M.B.; Kroeker, K.J.; Ricevuto, E.; Gambi, M.C. Amphipod assemblages along shallow water natural pH gradients: Data from artificial substrata (Island of Ischia, Italy). Biodiv. J. 2017, 8, 469–470. [Google Scholar]
- Barruffo, A.; Ciaralli, L.; Ardizzone, G.; Gambi, M.C.; Casoli, E. Ocean Acidification and Molluscs Settlement in Posidonia oceanica Meadows: Does the Seagrass Buffer Low pH Effects at CO2 Vents? Diversity 2021, 13, 311. [Google Scholar] [CrossRef]
- Garrard, S.L.; Gambi, M.C.; Scipione, M.B.; Patti, F.P.; Lorenti, M.; Zupo, V.; Paterson, D.M.; Buia, M.C. Indirect effects may buffer negative responses of seagrass invertebrate communities to ocean acidification. J. Exp. Mar. Biol. Ecol. 2014, 462, 32–38. [Google Scholar] [CrossRef]
- Kapsenberg, L.; Cyronak, T. Ocean acidification refugia in variable environments. Glob. Chang. Biol. 2019, 25, 3201–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstrom, H.; Silva, J.; Martins, C.; Horta, P. Seagrass can mitigate negative ocean acidification effects on calcifying algae. Sci. Rep. 2019, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Ricart, A.M.; Ward, M.; Hill, T.M.; Sanford, E.; Kroeker, K.J.; Takeshita, Y.; Merolla, S.; Shukla, P.; Ninokawa, A.T.; Elsmore, K.; et al. Coast-wide evidence of low pH amelioration by seagrass ecosystems. Glob. Chang. Biol. 2021, 27, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Doo, S.S.; Leplastrier, A.; Graba-Landry, A.; Harianto, J.; Coleman, R.A.; Byrne, M. Amelioration of ocean acidification and warming effects through physiological buffering of a macroalgae. Ecol. Evol. 2020, 10, 8465–8475. [Google Scholar] [CrossRef]
- Molari, M.; Guilini, K.; Lins, L.; Ramette, A.; Vanreusel, A. CO2 leakage can cause loss of benthic links open overlay panel. Mar. Environ. Res. 2019, 144, 213–229. [Google Scholar] [CrossRef]
- Linares, C.; Vidal, M.; Canals, M.; Kersting, D.K.; Amblas, D.; Aspillaga, E.; Cebrian, E.; Delgado-Huertas, A.; Diaz, D.; Garrabou, J.; et al. Persistent natural acidification drives major distribution shifts in marine benthic ecosystems. Proc. R. Soc. B 2015, 282, 20150587. [Google Scholar] [CrossRef]
- Mecca, S.; Casoli, E.; Ardizzone, G.; Gambi, M.C. Effects of ocean acidification on phenology and epiphytes of the seagrass Posidonia oceanica at two CO2 vent systems of Ischia (Italy) Mediterr. Mar. Sci. 2020, 21, 70–83. [Google Scholar] [CrossRef]
- Calosi, P.; Rastrick, S.P.; Lombardi, C.; de Guzman, H.J.; Davidson, L.; Jahnke, M.; Giangrande, A.; Hardege, J.D.; Schulze, A.; Spicer, J.I.; et al. Adaptation and acclimatization to ocean acidification in marine ectotherms: An in situ transplant experiment with polychaetes at a shallow CO2 vent system. Philos Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120444. [Google Scholar] [CrossRef] [Green Version]
- Agostini, S.; Harvey, B.P.; Milazzo, M.; Wada, S.; Kon, K.; Floc’h, N.; Komatsu, K.; Kuroyama, M.; Hall-Spencer, J.M. Simplification, not “tropicalization”, of temperate marine ecosystems under ocean warming and acidification. Glob. Chang. Biol. 2021, 27, 4771–4784. [Google Scholar] [CrossRef]


| Year/Station | 2016 B1 | 2016 B2 | 2016 B3 | 2019 B1 | 2019 B2 | 2019 B3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | |
| T (°C) in situ | 23.1 | 0.4 | 22.9 | - | 23.1 | - | - | - | - | - | - | - |
| Salinity | 39.55 | - | 39.49 | - | 39.53 | - | - | - | - | - | - | - |
| N-NO2 (µmol L−1) | 0.008 | 0.004 | 0.012 | 0.003 | 0.019 | 0.009 | 0.008 | 0.001 | 0.011 | - | <0006 | - |
| N-NO3 (µmol L−1) | 0.048 | 0.010 | 0.035 | 0.003 | 0.128 | 0.148 | 0.040 | 0.005 | 0.035 | 0.006 | 0.060 | 0.029 |
| P-PO4 (µmol L−1) | 0.057 | 0.004 | 0.010 | 0.001 | 0.008 | 0.004 | 0.036 | 0.020 | 0.021 | 0.016 | 0.038 | - |
| N-NH4 (µmol L−1) | 0.070 | 0.009 | 0.163 | 0.017 | 0.127 | 0.026 | 0.068 | 0.006 | 0.066 | 0.039 | 0.111 | 0.050 |
| Si-Si(OH)4 (µmol L−1) | 5.592 | 0.433 | 6.306 | 2.447 | 2.014 | 0.877 | 1.147 | 0.083 | 1.084 | 0.025 | 1.110 | 0.055 |
| pHT 25 °C | 7.990 | - | 7.857 | - | 7.785 | 0.002 | 7.991 | 0.030 | 7.995 | 0.025 | 7.988 | 0.072 |
| AT (µmol L−1) | 2543 | 2.7 | 2538 | 4.4 | 2540 | 3.4 | 2552 | 1.6 | 2555 | 4.4 | 2554 | 1.2 |
| TCO2 (µmol L−1) | 2222 | 2.4 | 2297 | 4.3 | 2338 | 2.4 | - | - | - | - | - | - |
| pCO2 (µatm) | 462 | 6.8 | 661 | 1.6 | 810 | 2.5 | - | - | - | - | - | - |
| Ω Ca | 5.31 | 0.01 | 4.14 | 0.01 | 3.61 | 0.02 | - | - | - | - | - | - |
| Ω Ar | 3.50 | 0.01 | 2.75 | 0.01 | 2.37 | 0.01 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, V.; Auriemma, R.; De Vittor, C.; Relitti, F.; Urbini, L.; Kralj, M.; Gambi, M.C. Structural and Functional Analyses of Motile Fauna Associated with Cystoseira brachycarpa along a Gradient of Ocean Acidification in a CO2-Vent System off Panarea (Aeolian Islands, Italy). J. Mar. Sci. Eng. 2022, 10, 451. https://doi.org/10.3390/jmse10040451
Esposito V, Auriemma R, De Vittor C, Relitti F, Urbini L, Kralj M, Gambi MC. Structural and Functional Analyses of Motile Fauna Associated with Cystoseira brachycarpa along a Gradient of Ocean Acidification in a CO2-Vent System off Panarea (Aeolian Islands, Italy). Journal of Marine Science and Engineering. 2022; 10(4):451. https://doi.org/10.3390/jmse10040451
Chicago/Turabian StyleEsposito, Valentina, Rocco Auriemma, Cinzia De Vittor, Federica Relitti, Lidia Urbini, Martina Kralj, and Maria Cristina Gambi. 2022. "Structural and Functional Analyses of Motile Fauna Associated with Cystoseira brachycarpa along a Gradient of Ocean Acidification in a CO2-Vent System off Panarea (Aeolian Islands, Italy)" Journal of Marine Science and Engineering 10, no. 4: 451. https://doi.org/10.3390/jmse10040451
APA StyleEsposito, V., Auriemma, R., De Vittor, C., Relitti, F., Urbini, L., Kralj, M., & Gambi, M. C. (2022). Structural and Functional Analyses of Motile Fauna Associated with Cystoseira brachycarpa along a Gradient of Ocean Acidification in a CO2-Vent System off Panarea (Aeolian Islands, Italy). Journal of Marine Science and Engineering, 10(4), 451. https://doi.org/10.3390/jmse10040451









