Abstract
Oil spills in open waters pose a significant threat to marine life. The application of dispersant as an oil-spill response is a promising approach to minimize the environmental burden caused by these accidental events. Dispersants have been accepted and applied by many countries around the world as a countermeasure in responding to oil spills due to their great success and advancements in recent years. This review covers different approaches for design and development of chemical formulas of oil dispersants with the aim to improve dispersing efficiencies, followed by formulating non-chemical dispersants, which are more environmentally friendly approaches. The encouraging properties motivate scientific communities to research and develop these non-chemical-based dispersants. In general, this review intends to offer a multi-perspective overall picture of progress made in recent years to develop and apply different dispersants suitable for combating oil spills.
1. Background
Petroleum oil has historically been the largest major energy source for global energy consumption. Oil reserves are distributed unevenly, with the greatest share in the Middle East, followed by Africa and Latin America. The imbalanced distribution of oil resources accelerated the rapid growth of oil trade in early 19th century, which in turn boosted the unprecedented development of marine oil transportation. Crude oil tankers had been an essential way to transport crude oils from production sites to refiners worldwide. With the rising maritime transportation volume of oil, oil leaks occurred from time to time despite the best efforts of the oil and shipping industries [1].
Decades ago, tanker accidents contributed the increase of oil released to the sea. Depending on the tonnage of tankers, such spills could release huge amounts of oil within a few days. Figure 1 shows the average number of global oil spills from tankers from 1970 to 2016 [2]. Tanker accidents dominated marine oil spills in 1970s, when the averaged total oil spills were in excess of 70 per year, about 35% of which were large oil spills, leaking over 700 metric tons of oil to the sea in a single spill. Thanks to the advancement of oil tanker transportation technologies and the increased attentions given by global maritime authorities, the frequency of oil-spill accidents and total oil spillage have decreased significantly despite steady growth in the crude trade.
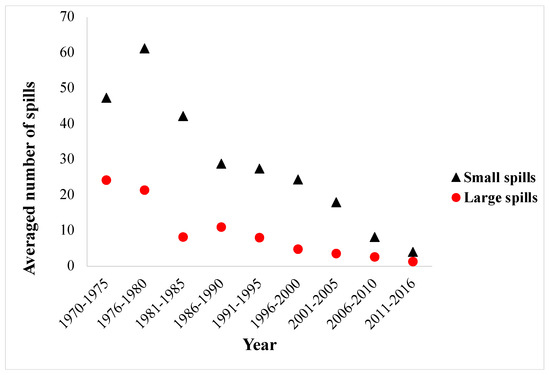
Figure 1.
Averaged number of global oil spills from tankers from 1970–2016 [2].
However, the number of pipeline ruptures and leakages show the opposite trend. Substantial increase in the number and total length of oil pipelines and the ageing of pipelines and pumping stations are responsible for the increased leakage. The Gulf War oil spill in January 1991 is the largest global oil spill to ever take place since commercial drilling took off. An estimated 380 to 520 million gallons of oil were intentionally released into the ocean. The Deepwater Horizon well blowouts in 2010 is the second largest oil spill. Over 200 million gallons of oil were released into the Gulf of Mexico, while 11 people were killed in the accident [3]. Marine blowouts can cause large losses, especially in deep waters. The safety of oil transportation has become increasingly important.
Oil spills have been and will continue to be a pressing issue and source of pollution because the petroleum products are moved around the world, and exploration of oil from oceanic resources is steadily on the rise. Once oil is leaked to open waters, it is of paramount importance to employ various cleanup methods to minimize the risk that it could pose to the marine ecosystem. In 1967, the sinking of the Torrey Canyon on the Isles of Scilly of Cornwall was the first reportedly largest accident, releasing 119,000 tons of crude oil. At the time, there were not either appropriate experience or techniques to treat large-scale oil spills. It resulted in serious and huge natural and economic losses for both Britain and France. During the handling of the spill, the so-called first-generation dispersants, containing toxic alkylphenol surfactants, were applied in a large scale [4]. Unfortunately, these chemicals proved be of limited effectiveness in dispersing oil; even worse, they caused considerably more ecological damage—particularly in the intertidal regions—than the spilled oil itself. This awkward history of chemical dispersants made their roles controversial [5].
Since the Torrey Canyon spill, both oil-spill treatment protocols and clean-up technologies have undergone significant development. Typically, the countermeasure techniques are categorized as mechanical/physical, chemical, and biological methods. The mechanical/physical approach includes oil booms/barriers, skimmers, adsorbents, and oil recovery vessels. They are commonly used to control oil spills and to recover oil from water surface without changing oil properties so that oil can be reused. This response method is usually constrained by equipment availability, severely limited by weather and seas. It may not be appropriate to deal with large-scale oil spills on open waters. Chemical response technologies may involve the application of chemicals and can change the physical and chemical properties of oil, including in situ burning and use of chemical agents, as such herders, dispersants, solidifiers, oil sinking agents, etc. Chemical approaches are commonly used in combination with physical methods [6]. Each chemical agent has its own window of opportunity and specific spill conditions in which it can be used. A chemical agent itself may also have adverse impact to the environment. Since the aim of oil-spill response is to minimize environmental damage, it is important to first assess the environmental risk of an oil spill in order to select the most suitable response option.
Biological approach is a process where microorganisms are used to degrade the spilled oil and restore environment quality. Biodegradation of oil compounds is an important natural attenuation process.
Thus, bioremediation is a favorable approach in the treatment of marine oil spills and has many advantages because of its environmentally friendly and economic properties. The lengthy treatment process and strong dependency on environmental factors are the primary constraints. There are a few excellent reviews that commented the advantages and disadvantages of the oil-spill response techniques along with assessment of their performance and costs [6,7,8]. An overview of the development of dispersant formulation is presented in this review.
2. Brief History of Oil Dispersants
Petroleum crude oils except for extra-heavy oils, such as bitumen, have a density lower than water, and therefore, spilled oils frequently float on water surface. Floating oil could pose health risks for seabirds and air-breathing marine species, such as sea turtles and marine mammals. Additionally, wind and tidal currents may drive floating oil towards the shoreline and its highly inhabited sensitive habitats. For instance, once contaminated, the spilled oil in salt marshes cannot be cleaned up without causing additional damage. At this situation, it may be advantageous to disperse unrecoverable oils.
Spilled oil can be dispersed by natural processes, while application of chemical dispersants and mechanical forces can accelerate the dispersion process. Chemical dispersants are composed of amphiphilic compounds with the purpose of lowering the interfacial tension between water and crude oil. The oil slicks are then quickly broken into small oil droplets that are easily entrained and diluted into the local water column. This action enhances the water accommodated fraction (WAF) of oil so that the risks of contaminating shorelines and damaging marine fowls are minimized. The dispersed oil droplets increase the biodegradation rate of the oil and thus reduce the environmental and economic impact of spilled oil [9]. However, the chemical dispersants available on the market are chemically stable, and thus, they remain longer in the marine environment. The use of dispersants in oil-spill response involves trade-offs between the direct exposal of coastal life to oil and the effects of dispersants and dispersed oil to deep-sea environments. Being one of several options available to responders during oil spill occurrences, chemical dispersants are more frequently used on large, offshore spills when environmental conditions impede either mechanical recovery or other chemical responses, such as in situ burning, or allowing natural processes to control the fate and effects of the oil [10].
However, the impacts of dispersants to environment have been a concern since the Torrey Canyon oil spill, which also landmarked the initiation of the development of modern dispersants [11]. The research to formulate a less-toxic dispersant was then promoted. Many advances were made in the development of modern dispersants, in particular in lowering the toxicity. Steen and Findley (2008) reported that dispersants were applied to 213 incidents at the sea surface between 1968 and 2007, many of which were on a relatively limited spatial scale, with small amounts of dispersant being used [12]. Due to the small scales, the environmental impacts of dispersants were not hugely investigated. In the 2010, at the Deepwater Horizon oil spill, 4.1 million liters of the dispersants (Corexit dispersants 9500 and 9527, developed by Exxon) were applied at the sea surface. In addition, 2.9 million liters were injected, for the first time, at the wellhead about 1500 m below water surface to reduce the vertical oil transport and emergence of oil at the surface [10]. The unparalleled, large-scale application of dispersants received much attention from the public and the research community. More than 100 peer-reviewed articles have addressed dispersant-relevant issues, in particular its impact on a wide range of habitats, organisms, and ecological functions [13]. Compared to the first generation of dispersant, the Corexit dispersants were very effective and less toxic. However, no consensus was reached on the role of dispersants in oil-spill response. Intense disagreements were expressed regarding the environmental costs due to the use of dispersants. There are huge knowledge gaps on the fate of dispersants and their long-term impact.
3. Regulations on Application of Oil Dispersants
Decisions on dispersant use will be site-/situation-specific. Selection of oil-spill dispersant depends on many factors: the availability of material, cost, material safety, and surface tension and interfacial tension properties. Timing is a key factor to the successful application of dispersants. Thus, pre-established and well-understood regulations concerning dispersant approval and application are critical to support the timely decision-making process. Each country has its own policy on uses of dispersants. Canada does not have a written guideline on dispersant application, and decisions are made on a case-by-case basis. If dispersants are determined to be beneficial to mitigate the impact of the spill on the environment and marine life, dispersants would be approved for use that are legally approved for use in Canada by Environment and Climate Change Canada. The USA allows great flexibility for responders. Many States in the USA have pre-approved the use of dispersants outside three nautical miles from shore and/or in depths greater than 10 m. The National Product Schedule acts as a preapproval mechanism, allowing the Federal On-Scene Coordinator, working with state and local governments, to respond quickly to a spill situation using the best available technology [14]. Currently, 15 dispersants are listed on the National Product Schedule (the list can be accessed via referred link) [14]. Assessment of the risks and benefits of using dispersant in waters less than three nautical miles offshore is currently in progress. Similar to the USA, Australian Maritime Safety Authority recognizes and allows to use dispersants in responding marine spills. The means for recognition and acceptance of a dispersant is through the Register. To date, four dispersant products, including Ardrox, Corexist, Finasol, and Slickgone, are listed. A decision-making tool is in place to ensure the Register-listed products are considered for operational deployment.
In Europe, policies for the use of dispersants vary greatly. Except for the UK, which has listed the use of preferred dispersants, most European countries have regulated the use of dispersant to be secondary to mechanical recovery countermeasures with certain restrictions on spraying based on the distance from shore and/or the water depth. Dispersants are generally not used by Baltic states, which have coastlines bordering the Baltic Sea, due to the sensitive ecological conditions and low water exchange. Norway has made a notable change in policy from mechanical recovery only to a permission of use of dispersants when the application of the dispersant presents the best environmental results as judged by Net Environmental Benefit Analysis (NEBA).
In Asia, dispersants have been frequently used lately. Most countries have recognized and accepted dispersants as an option in responding to marine oil spills. An approved list of dispersants may be available for each Asian country. Unfortunately, the authorities have failed to promote their uses at the planning level. There have been occasions when dispersant has been used without due consideration of oil type, weathering effects, or water exchange [15]. It is of prime importance to have a well-defined regulation by the policy maker and clear guidelines/procedures regarding their use.
4. Chemical Dispersants and Its Application
4.1. Mechanism of Oil Dispersion by Dispersant
Typically, oil dispersants are formulated to be a mixture of surfactant(s) dissolved in one or more solvents. Containing chemical functional groups affinity for both oil (lipophilic) and water (hydrophilic), surfactants are the active ingredients that lower the interfacial tension of the oil slick and promote and stabilize oil-in-water dispersions. Canevari proposed how a dispersant disperses spilled oil, which is illustrated in Figure 2 [16]. After oil dispersant is applied to oil slicks, the oil–water interface is diffused due to the amphipathic nature of the surfactant component. The lipophilic ends of the surfactant molecules are attached to the oil phase, while the hydrophilic ends are soluble in the water phase, which reduces the interfacial surface tension between water and oil. With agitation provided by waves, oil slicks break up into discrete, micro-sized droplets ranging from 1 to 70 microns, which float in the top 5–10 m of the water column [17]. The formed oil droplets are stabilized by the adsorbed surfactant molecules and then diluted in the water column, where the oil in the droplets is subsequently degraded by various micro-organisms present in the sea.
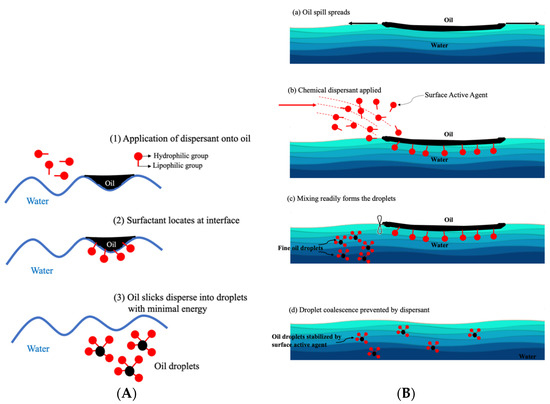
Figure 2.
(A) How dispersants work: (1) oil and water do not mix. (2) Dispersants are applied to the surface of the oil film, and surfactant diffuses the oil/water interface where they align themselves. (3) The interfacial tension is reduced, and oil is dispersed as tiny droplets. (B) Mechanism of chemical dispersion [16].
4.2. Solvents
The solvent in oil dispersant has a huge impact on the effectiveness of oil dispersants. More than serving as a carrier system to solubilize the surfactants and to deliver a liquid solution in the dispersant response, the solvent penetrates the oil slick and enhances the diffusion of surfactants to the oil/water interface. The solvents’ ability to promote oil dispersion may be attributed to its affinity with petroleum oil, which encourages solvents to stay within the oil slick rather than leaching off into the water column. The affinity to oil further assists the surfactants to remain within oil slicks [18]. It is critical that solvents can remain in the oil film and resist extraction by seawater long enough to enable the surfactants to be effective [17].
Oil dispersants have developed from toxic products, such as the aromatic solvent-based detergent products used in the Torrey Canyon spill half a century ago to the modern dispersant with low toxicity. To lower the toxicity of dispersant, early generation of dispersant products used either water-based surfactant systems or nonaromatic hydrocarbon as solvent. However, high application rates, such as 1:3 dispersant-to-oil ratio (DOR), plus additional mechanical agitation were generally required [11]. To lower the DOR and to improve the application effectiveness, oxygenated compounds with low toxicity, such as propylene/isopropylene glycols and glycol ethers, were introduced to the solvent carrier system, blending with petroleum-derived nonaromatic hydrocarbons. Fernandes et al. (2019) studied a series of oxygenated chemicals and suggested that the chemicals with appropriate hydrophilicity and hydrophobicity have potentials to be ideal solvents [18]. The short-chain alcohols, such as methanol and ethanol, are poor solvents due to their strong affinity to water. In contrast, long-chained alcohol, such as 1-octanol, showed excellence performance. Specific examples of solvents for dispersants include ethylene glycol monobutyl ether, dipropylene glycol monomethyl ether, de-aromatized kerosene, and isoparaffinic solvents [11]. The use of oxygenated compounds in solvent allows to solubilize much more surfactant contents. Modern dispersants can have up to about 65 wt % surfactant. The concentrate dispersants, therefore, are effective at lower DOR rates, typically 1:20 DOR.
Noteworthy is the flash point in selection of solvents for dispersant [18]. Low flash point implies high vapor pressure at room temperature. Solvent vapor could pose a risk of igniting during storage and application. Thus, solvents with a flash point lower than 60 °C are inappropriate to be used for dispersants. Density and viscosity of solvent are other factors to consider. The solvents expected to be light enough to float at the water–oil interface instead of sinking into the water column. Solvent viscosity should be appropriate to ensure the final dispersant products can be delivered as a spray.
4.3. Surfactants
Oil dispersants usually consist of several nonionic and anionic surfactants, mixed between lipophilicity and hydrophilicity and dissolved in one or more organic solvents. Nonionic surfactants frequently used in oil dispersants include sorbitan esters, polyalkoxylated fatty alcohols, ethoxylated sorbitan of oleic or lauric acids, and polyethylene glycol fatty alcohol ethers. Typical anionic type surfactants mostly applied in current dispersant formulations are sulfosuccinate esters and oxyalkylated C12–C15 alcohols. Solvents in currently marketed dispersants include ethylene glycol, glycol ethers, and nonaromatic hydrocarbons [19]. For instance, the widely used COREXIT dispersants were formulated with one anionic surfactant, bis-(2-ethylhexyl) sulfosuccinate (DOSS), and three nonionic surfactants, including sorbitan monooleate (Span 80), sorbitan monooleate polyethoxylate (Tween 80), and sorbitan trioleate polyethoxylate (Tween 85), which were reported by the United State Environmental Protection Agency (U.S. EPA) [20]. The anionic and nonionic surfactants present in Corexit interact synergistically to enhance its dispersion effectiveness. Although claimed as low to no toxicity, the Corexit dispersants received criticism shortly after the Deep Horizon oil spill. A major loss to the marine biota was observed and was from the combined effects of Corexit application, crude oil toxicity, and oxygen depletion [21]. White et al. tracked the fate of the dispersant products from environments known to contain oil persisting from the DWH oil spill. DOSS, one key ingredient of Corexit, was found to persist in deep sea coral communities about four years after the spill, indicating that the applied dispersant underwent degradation much slower than expectation [22]. In addition, Asadov et al. reported the synthesis of “Dodecyl Isopropylolamine and Derived Surfactants”. In their study, dodecylisopropylolammoniumacetate and dodecylisopropylolammonium propionate in undiluted forms (both ionic) manifested dispersing capability in sea water [23]. The application of ionic liquid (IL) surfactants to disperse crude oil have also gained considerable attention in the past years as discussed in a book chapter by Shah et al. [24].
4.4. Factors to Affect Dispersion Effectiveness
Crude oils can be very different in terms of physical properties and chemical compositions, which determine the behavior of spilled oil. Some crudes are lighter and more readily dispersed than others. Oils with high viscosities and pour points tend to be much less dispersible. For instance, the crudes with high contents of wax and/or asphaltene are more difficult to disperse than light crude oils. Asphaltenes are of an important component of crude oils. It has a complex molecular structure that represents the heaviest and most polar components in crude oils. As the content of asphaltene increases, the crudes become more viscous. Besides, asphaltene can facilitate the formation of stable oil–water emulsion, representing a great challenge to oil-spill cleanup.
Modern dispersants can be effective on a wide range of oil types given the right conditions and proper application ratios. In general, one part dispersant will disperse about 20–30 parts oil. If the oil is light, and the sea has high energy, over 100 parts of oil per part of dispersant may be dispersed [17]. Heavy crudes with API gravity of less than 22 and weathered oils are more resistant to dispersion and may require a higher ratio of dispersant to oil.
Sea state and weather conditions also play an important role in achieving a successful dispersion response. On a very windy day, accurate application of dispersant on the oil slick becomes difficult. Even landed on the oil, dispersant may also be washed off by rough waves or blown off by strong wind into the sea before it has a chance to penetrate the oil–water interface. On the contrary, in a very calm condition, little wave energy may result in ineffective oil dispersion. It was reported that 5 m/s was the minimal wind speed needed to generate sufficient wave energy for effective dispersion of oil [25].
Salinity is another important factor. Most commercially available dispersants are formulated for use in normal marine salinities of about 3% or higher [9]. Their effectiveness generally decreases when salinities are lowered. This is because surfactant becomes more soluble when salinity is reduced, and thus, less is available to interact with oil. For example, Corexit 9500, designed for use in marine environments, is often ineffective in fresh water. Dispersants optimized for use in fresh water are less sensitive to salinity [26]. However, there is not a fixed relationship between dispersion effectiveness and salinity, which is highly associated with the different dispersant–oil combinations. In generally, dispersant is not suggested for use in waters of low salinity. Dispersant is restricted in the Baltic Sea due to its low salinity in addition to many other factors, such as the abovementioned factors of low mixing energy, limited potential for dilution, etc.
4.5. Application of Dispersant
Once spilled on open waters, light components of the spilled oils evaporate naturally (which is called weathering), and the evaporation rates are highly associated with the properties of the spilled oils and weather conditions, such as wind, temperature, etc. The weathering process increases the viscosity of the oil. Thus, the longer the oil is left on water, the less likely it is to disperse effectively. There exists an appropriate “window of opportunity” for optimizing a dispersant application strategy for crude oils. It varies with each spill depending upon oil type, degree of mixing energy, degree of oil weathering, and strength of the dispersant used. The first few hours after a typical spill are critical to a successful dispersant operation.
To avoid delaying oil-spill response or losing the window of opportunity, resources and logistics are essential to ensure a successful dispersant response. Hard lessons learnt from the Deepwater Horizon oil spill in the Gulf of Mexico led to an in-depth look into readiness for worst-case scenarios in the future. Dispersant supply became an apparent constraint during the catastrophic oil spill. This gives rise to the establishment of the Global Dispersant Stockpile (GDS) as part of a post-Macondo Joint Industry Project through Oil Spill Response Limited (OSRL) [27]. Five stockpile locations were selected to reflect major global areas of exploration and production, which are the Gulf of Mexico, Malaysia, South and West Africa, and the eastern coast of Southern America. It aims for an easily accessible and deployed dispersants for industry use. The dispersants chosen for the stockpile cover the widest global approvals included Finasol® OSR 52 (Total), Corexit® EC9500A (Nalco), and Slickgone® NS (Dasic) [15].
5. Factors to Determine the Effectiveness of Oil Dispersants
The role of dispersants is to enhance the formation of a fine emulsion of crude oil in seawater by lowering the interfacial tension. This emulsion should also be rendered sufficiently stable to avoid coalescence of the droplets before dilution by sea currents. The concepts, emulsion, hydrophilic–lipophilic balance (HLB), hydrophilic–lipophilic deviation (HLD), and equivalent alkane carbon number (EACN) are frequently used in determining effectiveness of oil dispersants and in the design of oil dispersant formulations.
5.1. Emulsion
An emulsion, defined by IUPAC, is a dispersion of droplets of one liquid in another liquid with which it is incompletely miscible. Emulsions of fine droplets of oil in water are indicated by the symbol O/W and emulsions of water droplets dispersing in oil as W/O. This heterogeneous system is usually formed by a mechanical agitation process. However, emulsion is thermodynamically unstable since the dispersed and continuous phases can revert back as separate phases, oil and water, by fusion or coalescence of droplets. In general, a microemulsion is considered as thermodynamically stable. Surfactants are commonly used to enhance the formation of fine emulsion droplets and to stabilize them.
Winsor [28] proposed four general types of emulsions based on phase equilibria. Figure 3 is a schematic of four types of emulsion: type I, the surfactant is preferentially soluble in water and coexists with oil phase to form oil-in-water (O/W) emulsions; type II, the surfactant mainly stays in the oil phase and coexists with the water phase to develop water-in-oil (w/o) emulsions; type III, a three-phase system where a surfactant-rich middle-phase coexists with both water and oil phases; and type IV, a single (isotropic) micellar phase. Inter-conversion among the four types of phases can be achieved by adjusting proportions of the constituents.
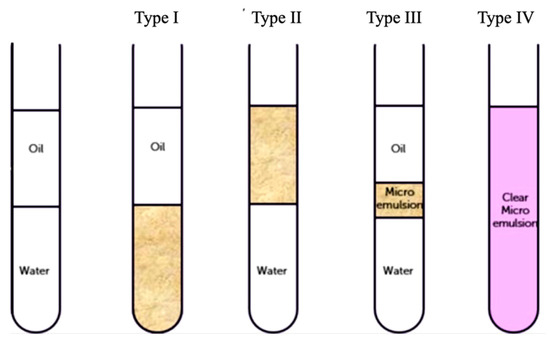
Figure 3.
Different types of emulsions proposed by Winsor.
In an event of oil spill in open waters, wind or waves, on one hand, work as mechanical forces to naturally disperse spilled oil into finely divided droplets to form type I or type III emulsions, whose dispersion rates depend mainly on sea state. On the other hand, the wave action can also accelerate the formation of a water-in-oil emulsion, known as “mousse”, containing 20–80% water, which is very unfavorable, as it represents an expansion in volume of spilled oil up to 3–5 times the original volume and an increase in viscosity from a few Pa.s to thousands Pa.s [29]. The viscous “mousse” can be very stable and render a cleanup process difficult. It also inhibits biodegradation because the water trapped in the oil keeps out essential nutrients and oxygen. Early treatment of an oil spill with dispersants can prevent the formation of mousse and small oil droplets to permit natural weather to continue.
5.2. Hydrophilic–Lipophilic Balance
Hydrophilic–lipophilic balance (HLB) was initially proposed as a tool to classify surfactants. It is calculated from the weight percentage of the hydrophilic groups to the hydrophobic groups in a surfactant molecule, with values ranging from 1–20. A widely used empirical equation (Equation (1)) for determining HLB was proposed by Davies [30].
where H and L are constants assigned to hydrophilic and hydrophobic groups, respectively, and nH and nL are the number of these groups per surfactant molecule. The HLB of a surfactant mixture is determined by the mass-weighted average of the HLB of the individual surfactants. A surfactant with an HLB of 10 suggests that the forces of the molecule is equally balanced between hydrophilic and lipophilic tendencies. Lower HLB values are an indication of high oil affinity. A high HLB value, on the other hand, indicates high water solubility. Surfactants favor forming w/o emulsions with HLB between 3 and 6 and developing O/W emulsions when the HLB value is between 8 and 16 [31]. Traditionally, dispersants for oil spills designed for use in seawater have an overall HLB in the range of 9–11 to promote the formation of O/W emulsions. This is often achieved by combining several surfactants of higher and lower HLB [4].
HLB = (nH × H − nL × L) + 7
HLB is frequently used to describe the characteristics and effectiveness of dispersants as well as guiding surfactant selection for emulsion and microemulsion systems. Wrenn et al. in 2009 applied the HLB concept to optimize the mixture composition from commercially available surfactants to achieve an effective freshwater dispersant. They found that the sorbitan ester surfactants formulated with HLB of between 8 and 10 exhibited the best performance in dispersing a weathered Mars crude oil in the synthetic lake water [26]. A large fraction of floating crude oil was observed to be dispersed into the water column as small droplets in fresh water when the hydrophilic–lipophilic balance of the dispersant was optimized. Some research groups tend to modify the structure of surfactants or terminal functional groups to adjust the HLB to reach appropriate value for dispersing oils. For example, incorporation of hydroxyl groups to surfactant molecules can increase the hydrophilicity of the surfactant so that the HLB value is increased to a desirable value [32].
However, the HLB scale alone is not a sufficient formulation tool since it fails to take into account the strong molecular interactions in a surfactant/oil/water system and the system conditions, such as temperature and salinity. In the late 1990s, combination of chemometric tools and experimental design with the HLB value were applied to optimize surfactant blends for various crude oils and different weathering degrees [33]. Statistical methodologies were proven to be a powerful tool to efficiently formulate a desirable dispersant for dispersing crude oils. To date, a variety of statistical design methods have been reported in the literature to optimize oil-spill dispersants, including multivariate approach, uniform design, and Monte Carlo method [34,35].
5.3. Hydrophilic–Lipophilic Difference (HLD)
Based on the HLB concept, Salager and co-workers [36] proposed the hydrophilic-lipophilic deviation (HLD) from optimum formulation concept, and it measures the deviation of a system from the optimum formulation. The HLD overcomes the weakness of HLB by using the information extracted from the entire surfactant oil−water system to predict the emulsion/microemulsion behavior of mixed surfactants.
The semi-empirical equations for determining the HLD for non-ionic and ionic surfactants are shown in Equations (2) and (3), respectively [37].
where S is the salinity of the aqueous phase based on g of electrolyte/100 mL, Cc the characteristic curvature of the surfactant, and T the actual temperature of the system (°C). k is an empirical constant depending on the type of surfactant head group, with k ≅ 0.6 for most surfactants. CT and αT are characteristic parameters of surfactants, with CT ≅ 0.06 °C−1 for most alkyl ethoxylates and αT ≅ 0.01 for most ionic surfactants. Other variables, b is the sensitivity of the formulation balance to the presence of salt, with b ≅ 0.13 (100 mL/g NaCl). EACN is the equivalent alkane carbon number, which was introduced by Wade et al. (1977) [38]. It is a dimensionless number that reflects the “hydrophobicity” of oil. The EACN of an oil is determined experimentally by comparing its phase behavior with that of a well-defined linear hydrocarbon in the same surfactant/oil/water system. For the normal alkane case, EACN number is the same as the carbon number. For example, hexane has an EACN of 6.
HLD = Cc − (k)(EACN) + bS + CT (T − 25 °C)
HLD = Cc − (k)(EACN) + ln(S) − αT (T − 25 °C)
The characteristic curvature of a surfactant was proposed by Acosta et al. [39]. It reflects the tendency of a surfactant to form normal micelles, reverse micelles, or intermediate aggregates. The value of the characteristic curvature ranges from negative to positive. A negative Cc suggests that the surfactant is hydrophilic and likely to form O/W microemulsions (normal micelles). On the other hand, a positive value of Cc refers to a hydrophobic surfactant, forming W/O microemulsions (reverse micelles). For a mixture of surfactants, the Cc of the mixture can be calculated using the following equation:
where Xi and Cci represent the mole fraction and characteristic curvature of surfactant i in the mixture.
Cc mix =Σ(XiCci)
When the HLD reaches zero, the surfactant is equally soluble in oil and water, and middle phase bicontinuous microemulsions (Winsor type III or type IV) are formed. A negative value of HLD indicates a hydrophilic surfactant system, and O/W microemulsions (Winsor type I) are formed, while a positive value of HLD corresponds to a hydrophobic surfactant system, and W/O microemulsions (Winsor type II) are formed [40].
The hydrophilic-ipophilic deviation (HLD) concept has been successfully applied in formulating an optimal dispersant for oil-spill cleanup since it provides a rapid yet reliable way to formulate an effective dispersant for dispersing oil slicks. In combination of the EACN of hydrocarbons and HLD value, Rongsayamanont et al. successfully designed and optimized a dispersant composed of a bacterially derived lipopeptide biosurfactant and sodium dihexyl sulfocuccinate (SDHS) [41]. Based on the EACN of interested oils and seawater salinity, Equation (3) was used to calculate the fractions of lipopeptides and SDHS that are desirable to allow the HLD of the designed surfactant blend to be zero. The three-phase behavior of Winsor type III microemulsions and the lowest interfacial tension (IFT) were obtained, and thus, an optimal dispersant for a specified oil was then achieved. Application of HLD minimized the experimental tests required in the optimization of the dispersant formulation. Later, the same research group [42] extended their formulation of the same surfactants (lipopeptide and SDHS), targeting to treat heavy oil, such as bunker C fuels. The proportion of each surfactant was calculated based on the HLD concept and verified using the bulk C and brine aqueous solution with different salinities. The formulated bio-based dispersants were further optimized using response surface methodology with Box–Behnken design.
The HLD calculations alone are a good tool for pre-screening dispersant formulations but fail to predict emulsion stability at high water-dilution levels. The Acosta group observed that dispersants with nearly identical HLD produce variable effectiveness [40]. To improve the prediction, the research group integrated the net-average-curvature (NAC) with the HLD calculations to predict thermodynamic properties that play a role in emulsion stability (e.g., interfacial rigidity, microemulsion density and viscosity, and IFT) [43]. Corcoran et al. suggested to consider the three-phase region in the oil–water dimension due to the nature of the dispersant application [37]. They developed a testing protocol that allows the phase behavior to be observed on short time scales (ca. hours) and provides a set of guidelines to interpret the results. The complementary use of HLD calculations and the testing protocol as a predictive model helped the authors to efficiently optimize novel dispersant blends.
6. Alternative Dispersants
Elevated environmental concerns from the public along with strict regulatory rules have forced researchers to use environmentally friendly, natural products to replace chemical surfactants to formulate alternative dispersants that are expected to possess less toxicity and high biodegradability.
6.1. Particle Stabilizers
Ramsden and Pickering first found that fine, solid particles can serve as an alternative option to prevent oil droplets from coalescing, thus stabilizing emulsion droplets [44,45]. Thanks to Pickering’s original work, considerable progress has been made in the area of Pickering emulsions. This has also opened a door to develop environmentally benign dispersants. Many types of solid particles have been studied as emulsion stabilizers, including carbon, silica, various oxides, and organic materials.
Saha et al. [46] demonstrated that carbon black (CB) particles can serve as an alternative to replace surfactant to stabilize crude oil-in-seawater emulsions. They mixed 0.015 wt % CB with 10 vol % BP-MC 252 crude oil in seawater obtained from the Narragansett Bay, and single emulsion droplets were formed as shown in the images (Figure 4), in which the emulsion was approved to be stable for several months. Under the same condition, the oil-droplet emulsion formed by Corexit 9500A destabilized in about an hour. The large specific surface area of CB particles was believed to lead to the superior performance in stabilizing oil droplets. In addition, carbon black particles are an excellent adsorbent for absorbing polycyclic aromatic hydrocarbons.
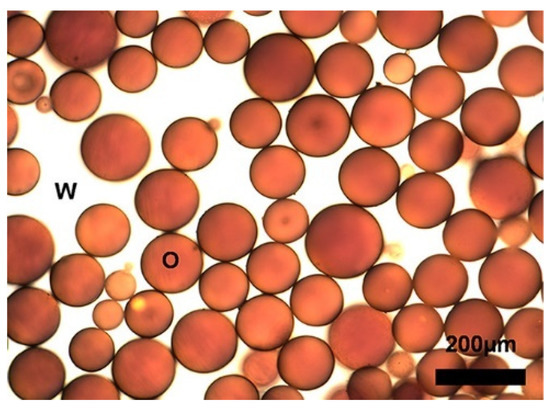
Figure 4.
Optical micrographs show oil-in-water emulsions formed with 10 vol% BP-MC 252 crude oil, 0.015 wt % CB (W: water and O: Oil) [46]. (Copyright 2019 American Chemical Society).
Natural clays, such as montmorillonite (MMT) and kaolinite, offer several distinctive advantages, such as being inexpensive and environmentally benign, and were studied to disperse oil by forming oil mineral aggregates [47]. The hydrophilicity of natural clays make them unsuitable to be a stabilizer of oil emulsion. However, Dong et al. found that, in combination with as low as 0.001% w/v surfactant, clay particles can generate remarkable synergistic effect in stabilizing oil droplets [48]. Introduction of natural clays to the responses of oil spill can significantly reduce the quantity of surfactant required in the oil-spill cleanup.
Silica particles are another popular particle that has been extensively studied as a replacement for chemical surfactants. To reduce the toxicity, silica particles were also paired with biodegradable biosurfactants to produce stable O/W emulsions for oil-spill remediation [49]. Other particles, including alumina, barium sulfate, and calcium carbonate, have also been researched and proven to be effective in formation of Pickering emulsions. Common features of these fine powders are their large specific surface area and surface properties, which make them excellent adsorbent materials. Mixed with a surfactant, these particles adsorb surfactant molecules on particle surfaces so that the hydrophilic surface of these particles turns relatively hydrophobic, obtaining the function to stable o/w or w/o emulsions. Worthen et al. investigated the system of hydrophilic fumed silica particles mixing with a nonionic surfactant (C12E7) [50]. It was evidenced that the adsorption of surfactant was initiated due to hydrogen bonding between silanol (SiOH) groups on silica particles and ethyleneoxy headgroups of the surfactant. Continuous adsorption of surfactant was eventually rendered as the balance was reached as shown in Figure 5. The silica particles with adsorbed surfactant were found to lower the oil-water interfacial tension through measurement of the contact angles at oil-water-solid interfaces. They proposed that there existed a competition between the influence of surfactant and the attachment energy of a particle to the interface. An optimal synergistic stabilization was achieved at an intermediate concentration of surfactant. To date, mineral-based nanoparticles have not commonly been used in the field of oil-spill response in marine environment. The impact of nano-sized minerals on animals are being assessed [51].
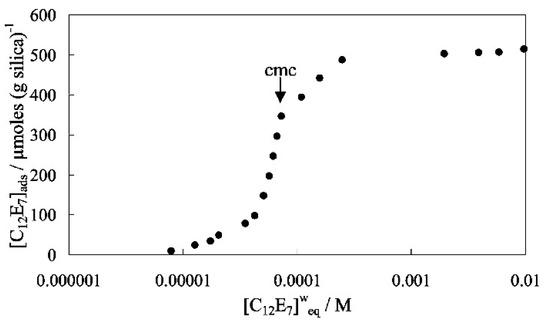
Figure 5.
Adsorption isotherm of C12E7 surfactant onto N20 silica particles (2 wt %) in water.
Biomass-Derived Particles
Biomass-derived particles provide an interesting source of biodegradable alternative, working as green dispersants in the form of nanoparticles. Cellulose nanocrystals and lignin, derived from lignocellulosic materials, have been given special attention recently and have been studied as an alternative to surfactant/emulsion agents, with wide application in wastewater treatment, pharmaceutical industry, and the food sector [52,53]. Ossi et al. studied commercial cellulose nanocrystals and their ability to serve as a marine oil-dispersing agent [54]. They stated that the stability of emulsion was highly relevant to droplet sizes of emulsion. Large droplets indicate low stability and are easy to coalesce. Cellulose nanocrystals stabilized emulsions had mean droplet size less than 10 microns and can remain stable for more than six weeks. At the oil/water interface, cellulose nanocrystals, different from surfactants, interact with oil only via their surfaces but did not penetrate. This was observed using small-angle neutron scattering (SANS) [55]. Different from cellulose, lignin has complex molecular structure containing both hydrophobic and hydrophilic moieties that result in natural amphiphilicity. Lignin has a powerful dispersive capacity and has been demonstrated as a Pickering emulsifier to stabilize O/W emulsions [56]. Lignin stabilizes emulsions by adsorption at the oil/water interface, which prevents droplet coalescence by electrostatic and steric repulsion.
6.2. Oil–Mineral Aggregates
Observation of natural oil-spill cleaning suggested that the spilled oil, with presence of appropriate conditions, may interact with fine particles of minerals to form oil-coated agglomerates, which then sink in the water column. The Braer oil spillage near Shetland, Scotland, in 1993 was a good example of natural OMA processing. A total of 30,000 tons of oil was spilled, about 30% of which were found to form oil–mineral aggregates, which were deposited in subtidal sediments. Oil–mineral interactions are believed to be instrumental in the natural recovery process. The Lee’s group [57] defined it as oil–mineral aggregates (OMA), which has been widely accepted in the literature. An oil-spill countermeasure strategy was proposed that, instead of using chemical dispersant, OMA was applied in response operations for cleaning up residual oils at both open waters and shorelines [58,59]. Fine mineral particles are proposed to be purposely sprayed onto the surface of oil slicks to facilitate OMA formation, which enhances the natural dispersion of oil spilled in the environment and reduces its environmental persistence.
Since the first experiments that reported the interaction between oil droplets and minerals [58,60,61], OMA has been extensively studied through both experimental and computational methodologies. The widely reported finding is that, in the absence of chemical dispersants, OMA can be formed with fine mineral particles, and the formation of OMA is associated with oil properties, particle properties, oil–particle ratio, and environmental conditions. OMA formation increased with oil viscosity and oil polar component content. Lee et al. [62] indicated that oils with a dynamic viscosity greater than 500 cP (high viscosity) tend to bind to sediments rapidly. The droplet size depends on the turbulent kinetic energy available.
Mineral particles play a key in the formation of OMA. Stoffyn-Egli and Lee (2002) observed that quartz and kaolinite give mostly droplet OMA, while montimorillite only forms flake aggregates. They concluded that the type of minerals determined the OMA type formed [63]. Other observations suggested that smaller grain sizes and higher concentration of mineral particles favor OMA formation by coating the oil droplets [64]. Wang et al. [65] studied the evolution of uprising oil droplets and the interaction between oil and kaolin particles of different levels hydrophobicity using particle image velocimetry (PIV). Figure 6 shows that the rising oil droplet was surrounded by a large amount of the modified kaolin particles with higher hydrophobicity compared to the original kaolin particles, which had a hydrophilic surface. This indicated that modified kaolin particles had stronger interaction with the rising oil droplets.
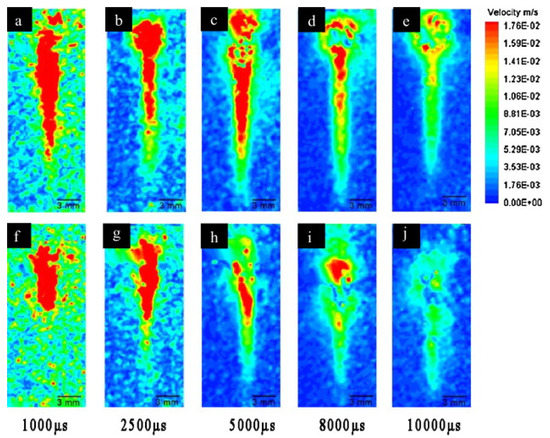
Figure 6.
The interaction between an oil droplet and mineral particles as a function of time. (a–e) Modified kaolin (relative hydrophobic) and Alaska North Slope oil. (f–j) Original kaolin (hydrophilic) and Alaska North Slope [65] (Copyright 2011 Elsevier Ltd.).
Successful formation of OMA is also greatly influenced by the sea state, salinity, and temperatures. Mixing energy is a key in the formation of OMA. Lower than the threshold mixing energy, no OMA can be formed [66]. Temperature also has an impact on the formation of OMA.
Lab studies on the influence of oil type and temperature on the characteristics of OMA concluded that non-spherical (relatively “elongated”) and small OMAs were formed more at higher temperatures. As the asphaltenes-resins content (ARC) of crude oils increases and temperature decreases, the formation of OMA becomes more difficult (Figure 7) [47,67].
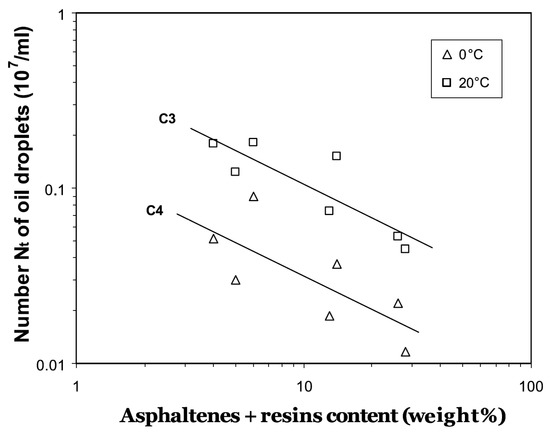
Figure 7.
Variations of number concentration of OMA with asphaltenes + resins content (ARC) (extracted from Khelifa 2002) [67]. (Copyright 2002 Elsevier Ltd.).
6.3. Plant-Based Surfactants
Investigation has been undertaken to apply plant-based surfactants to replace the synthetic surfactants in oil-spill dispersant. Soybean lecithin is one of the most studied plant-derived surfactants. Nyankson et al. reported that the fractionated phospholipid components in soybean lecithin are effective in dispersing spilled crude oil [68]. Blending soybean lecithin (60 wt%) with 40 wt% food-grade surfactant, Tween 80, further advanced the dispersion effectiveness of crude oil due to synergy effects [69]. These biodegradable and low-toxicity biosurfactants are proven efficient in dispersing oil slicks although the costs need to be significantly reduced before they can be applied to oil spills [70].
6.4. Biosurfactants
Biosurfactants are amphiphilic molecules sourced from microorganisms [71]. Biosurfactant-based dispersants (BBDs) offer low toxicity and short environmental half-lives, salinity, and pH. These promising properties combined with their enhanced hydrocarbon solubility place them as good candidates for application as a dispersant for slicks of crude oil [71]. However, the high cost associated with their developments limits their applications [71]. Only a handful of them are developed and known, including those produced using Rhodococcus sp. strains [72,73,74], Bacillus sp. strains [75,76,77], a Candida sp. strain [70], and a Gordonia sp. strain [78]. Therefore, they are often utilized in combination with other solvents or dispersants. As shown in Figure 8, they are still not as active as Corexit9500A, a commercial oil dispersant, when applied in different rations for dispersion of different crude oils.
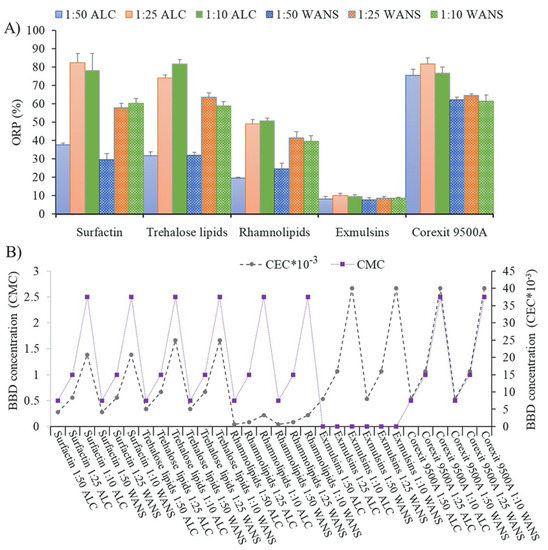
Figure 8.
Oil dispersion effectiveness of BBDs. (A) ORP (oil-removal performance) (%) of various BBDs used at three DORs (dispersant-to-oil ratios) against ALC (Arabian light crude oil) and WANS (weathered Alaska North Slope crude oil) in BFT (Baffled Flask Test); (B) concentrations of BBDs in terms of unit CMC (critical micelle concentration) or unit CEC (critical emulsion concentration) in each BFT [74]. (Copyright 2016 Elsevier Ltd.).
7. Conclusions
The discussed mechanism of action for chemical-based dispersants explains why a well-developed dispersant is composed of different chemicals, such as mixtures of different solvents and surfactants, because every component plays a role in dispersing oil. Therefore, there are significant demands for applying environmentally friendly solvents and strong surfactants with short half-lives. Combining different surfactants to obtain synergized dispersing efficiency appears successful and with tremendous potential. Furthermore, the chemical-based dispersant can also be combined with non-chemical dispersant in a suitable solvent mixture to formulate hybrid dispersant with an extraordinary performance that is inherited from the critical properties of both parent dispersants. The reviewed science in this account aims to provide essential points of view and necessary tools to fulfill the abovementioned strategies to develop highly effective, ecofriendly dispersants with low cost.
Funding
Ecosystems and Oceans Science, Fisheries & Oceans Canada; Canada Research Chair program, CRC-2020-00361.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.; Zhang, W.; Wan, Z.; Li, S.; Huang, T.; Fei, Y. Oil Spills from Global Tankers: Status Review and Future Governance. J. Clean. Prod. 2019, 227, 20–32. [Google Scholar] [CrossRef]
- ITOPF. Oil Tanker Spill Statistics 2020; ITOPF: London, UK, 2021. [Google Scholar]
- Lubchenco, J.; McNutt, M.K.; Dreyfus, G.; Murawski, S.A.; Kennedy, D.M.; Anastas, P.T.; Chu, S.; Hunter, T. Science in support of the Deepwater Horizon response. Proc. Natl. Acad. Sci. USA 2012, 109, 20212. [Google Scholar] [CrossRef] [Green Version]
- Clayton, J.R.; Payne, J.R.; Farlow, J.S.; Sarwar, C. Oil Spill Dispersants: Mechanisms of Action and Laboratory Tests; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Wells, P.G. The Iconic Torrey Canyon Oil Spill of 1967—Marking Its Legacy. Mar. Pollut. Bull. 2017, 115, 1–2. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Remediation Technologies for Marine Oil Spills: A Critical Review and Comparative Analysis. Am. J. Environ. Sci. 2011, 7, 423. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, D.P.; Gschwend, P.M. Assessing the Performance and Cost of Oil Spill Remediation Technologies. J. Clean. Prod. 2014, 78, 233–242. [Google Scholar] [CrossRef]
- Wilkinson, J.; Beegle-Krause, C.J.; Evers, K.-U.; Hughes, N.; Lewis, A.; Reed, M.; Wadhams, P. Oil Spill Response Capabilities and Technologies for Ice-Covered Arctic Marine Waters: A Review of Recent Developments and Established Practices. Ambio 2017, 46, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Chapman, H.; Purnell, K.; Law, R.J.; Kirby, M.F. The Use of Chemical Dispersants to Combat Oil Spills at Sea: A Review of Practice and Research Needs in Europe. Mar. Pollut. Bull. 2007, 54, 827–838. [Google Scholar] [CrossRef]
- Quigg, A.; Farrington, J.W.; Gilbert, S.; Murawski, S.A.; John, V.T. A Decade of Gomri Dispersant Science. Oceanography 2021, 34, 98–111. [Google Scholar] [CrossRef]
- Fiocco, R.J.; Lewis, A. Oil Spill Dispersants. Pure Appl. Chem. 1999, 71, 27–42. [Google Scholar] [CrossRef]
- Steen, A.; Findlay, A. Frequency of Dispersant Use Worldwide. Int. Oil Spill Conf. Proc. 2008, 2008, 645–649. [Google Scholar] [CrossRef]
- Beyer, J.; Trannum, H.C.; Bakke, T.; Hodson, P.V.; Collier, T.K. Environmental Effects of the Deepwater Horizon Oil Spill: A Review. Mar. Pollut. Bull. 2016, 110, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 15 Dispersants Are Listed on the National Product Schedule. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P100W60V.txt?ZyActionD=ZyDocument&Client=EPA&Index=2016%20Thru%202020&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5CZYFILES%5CINDEX%20DATA%5C16THRU20%5CTXT%5C00000011%5CP100W60V.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=2 (accessed on 30 January 2022).
- Coolbaugh, T.; Varghese, G.; Li, L.S. Global Dispersant Approvals in Asia Pacific—Current Status and on Going Challenges. Int. Oil Spill Conf. Proc. 2017, 2017, 657–677. [Google Scholar] [CrossRef]
- Canevari, G.P. Oil Spill Dispersants—Current Status and Future Outlook. Int. Oil Spill Conf. Proc. 1971, 1971, 263–270. [Google Scholar] [CrossRef]
- Lessard, R.R.; DeMarco, G. The Significance of Oil Spill Dispersants. Spill Sci. Technol. Bull. 2000, 6, 59–68. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Agrawal, N.R.; Aljirafi, F.O.; Bothun, G.D.; McCormick, A.V.; John, V.T.; Raghavan, S.R. Does the Solvent in a Dispersant Impact the Efficiency of Crude-Oil Dispersion? Langmuir 2019, 35, 16630–16639. [Google Scholar] [CrossRef]
- Board, M.; Council, N.R. Using Oil Spill Dispersants on the Sea; National Academies Press: Washington, WA, USA, 1989. [Google Scholar]
- Place, B.J.; Perkins, M.J.; Sinclair, E.; Barsamian, A.L.; Blakemore, P.R.; Field, J.A. Trace Analysis of Surfactants in Corexit Oil Dispersant Formulations and Seawater. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 129, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Goodbody-Gringley, G.; Wetzel, D.L.; Gillon, D.; Pulster, E.; Miller, A.; Ritchie, K.B. Toxicity of Deepwater Horizon Source Oil and the Chemical Dispersant, Corexit® 9500, to Coral Larvae. PLoS ONE 2013, 8, e45574. [Google Scholar] [CrossRef]
- White, H.K.; Lyons, S.L.; Harrison, S.J.; Findley, D.M.; Liu, Y.; Kujawinski, E.B. Long-Term Persistence of Dispersants Following the Deepwater Horizon Oil Spill. Environ. Sci. Technol. 2014, 1, 295–299. [Google Scholar] [CrossRef]
- Asadov, Z.H.; Rahimov, R.A.; Mammadova, K.A.; Gurbanov, A.V.; Ahmadova, G.A. Synthesis and Characteristics of Dodecyl Isopropylolamine and Derived Surfactants. J. Surfactants Deterg. 2016, 19, 145–153. [Google Scholar] [CrossRef]
- Shah, M.U.H.; Reddy, A.V.B.; Moniruzzaman, M. Chapter 16—Ionic Liquid–Based Surfactants for Oil Spill Remediation. In Ionic Liquid-Based Technologies for Environmental Sustainability; Jawaid, M., Ahmad, A., Reddy, A.V.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 257–268. [Google Scholar]
- National Reserach Counsil; Ocean Studies Board; Committee On Understanding Oil Spill Dispersants Efficacy And Effects. Oil Spill Dispersants: Efficacy and Effects; National Academies Press: Washington, WA, USA, 2005. [Google Scholar]
- Wrenn, B.A.; Virkus, A.; Mukherjee, B.; Venosa, A.D. Dispersibility of Crude Oil in Fresh Water. Environ. Pollut. 2009, 157, 1807–1814. [Google Scholar] [CrossRef]
- Carter-Groves, M. Global Dispersant Stockpile: Part of the Industry Solution to Worst Case Scenario Readiness. Int. Oil Spill Conf. Proc. 2014, 2014, 504–515. [Google Scholar] [CrossRef]
- Winsor, P.A. Hydrotropy, Solubilisation and Related Emulsification Processes. Trans. Faraday Soc. 1948, 44, 376–398. [Google Scholar] [CrossRef]
- Fingas, M. Water-in-Oil Emulsion Formation: A Review of Physics and Mathematical Modelling. Spill Sci. Technol. Bull. 1995, 2, 55–59. [Google Scholar] [CrossRef]
- Davies, J.T. A Quantitative Kinetic Theory of Emulsion Type, I. Physical Chemistry of the Emulsifying Agent. In Gas/Liquid and Liquid/Liquid Interface, Proceedings of the International Congress of Surface Activity, London, UK, 8–13 January 1957; Citeseer: Princeton, NJ, USA, 1957; pp. 6–438. [Google Scholar]
- Kralova, I.; Sjöblom, J.; Øye, G.; Simon, S.; Grimes, B.A.; Paso, K. Heavy Crude Oils/Particle Stabilized Emulsions. Adv. Colloid Interface Sci. 2011, 169, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Nyankson, E.; Demir, M.; Gonen, M.; Gupta, R.B. Interfacially Active Hydroxylated Soybean Lecithin Dispersant for Crude Oil Spill Remediation. ACS Sustain. Chem. Eng. 2016, 4, 2056–2067. [Google Scholar] [CrossRef]
- Molinier, V.; le Goué, E.; Rondón-Gonzáles, M.; Passade-Boupat, N.; Bourrel, M. Optimization of Chemical Dispersants Effectiveness in Case of Subsurface Oil Spill. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 43–51. [Google Scholar] [CrossRef]
- Brandvik, P.J.; Daling, P. Optimisation of Oil Spill Dispersant Composition by Mixture Design and Response Surface Methods. Chemom. Intell. Lab. Syst. 1998, 42, 63–72. [Google Scholar] [CrossRef]
- Song, D.; Liang, S.; Zhang, Q.; Wang, J.; Yan, L. Development of High Efficient and Low Toxic Oil Spill Dispersants Based on Sorbitol Derivants Nonionic Surfactants and Glycolipid Biosurfactants. J. Environ. Prot. 2013, 4, 16–22. [Google Scholar] [CrossRef]
- Salager, J.-L.; Marquez, N.; Graciaa, A.; Lachaise, J. Partitioning of Ethoxylated Octylphenol Surfactants in Microemulsion−Oil−Water Systems: Influence of Temperature and Relation between Partitioning Coefficient and Physicochemical Formulation. Langmuir 2000, 16, 5534–5539. [Google Scholar] [CrossRef]
- Corcoran, L.G.; Almaraz, B.A.S.; Amen, K.Y.; Bothun, G.D.; Raghavan, S.R.; John, V.T.; McCormick, A.V.; Penn, R.L. Using Microemulsion Phase Behavior as a Predictive Model for Lecithin–Tween 80 Marine Oil Dispersant Effectiveness. Langmuir 2021, 37, 8115–8128. [Google Scholar] [CrossRef]
- Wade, W.H.; Morgan, J.C.; Jacobson, J.K.; Schechter, R.S. Low Interfacial Tensions Involving Mixtures of Surfactants. Soc. Pet. Eng. J. 1977, 17, 122–128. [Google Scholar] [CrossRef]
- Acosta, E.J.; Yuan, J.S.; Bhakta, A.S. The Characteristic Curvature of Ionic Surfactants. J. Surfactants Deterg. 2008, 11, 145–158. [Google Scholar] [CrossRef]
- Sundar, S.; Nouraei, M.; Latta, T.; Acosta, E. Hydrophilic-Lipophilic-Difference (Hld) Guided Formulation of Oil Spill Dispersants with Biobased Surfactants. Tenside Surfactants Deterg. 2019, 56, 417–428. [Google Scholar] [CrossRef]
- Rongsayamanont, W.; Soonglerdsongpha, S.; Khondee, N.; Pinyakong, O.; Tongcumpou, C.; Sabatini, D.A.; Luepromchai, E. Formulation of Crude Oil Spill Dispersants Based on the Hld Concept and Using a Lipopeptide Biosurfactant. J. Hazard. Mater. 2017, 334, 168–177. [Google Scholar] [CrossRef]
- Nawavimarn, P.; Rongsayamanont, W.; Subsanguan, T.; Luepromchai, E. Bio-Based Dispersants for Fuel Oil Spill Remediation Based on the Hydrophilic-Lipophilic Deviation (Hld) Concept and Box-Behnken Design. Environ. Pollut. 2021, 285, 117378. [Google Scholar] [CrossRef]
- Kiran, S.K.; Acosta, E.J. Hld–Nac and the Formation and Stability of Emulsions near the Phase Inversion Point. Ind. Eng. Chem. Res. 2015, 54, 6467–6479. [Google Scholar] [CrossRef]
- Ramsden, W.; Gotch, F. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation).—Preliminary Account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar]
- Pickering, S.U. Cxcvi.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Saha, A.; Nikova, A.; Venkataraman, P.; John, V.T.; Bose, A. Oil Emulsification Using Surface-Tunable Carbon Black Particles. ACS Appl. Mater. Interfaces 2013, 5, 3094–3100. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Lee, K. Chemical Dispersion of Oil with Mineral Fines in a Low Temperature Environment. Mar. Pollut. Bull. 2013, 72, 205–212. [Google Scholar] [CrossRef]
- Dong, J.; Worthen, A.J.; Foster, L.M.; Chen, Y.; Cornell, K.A.; Bryant, S.L.; Truskett, T.M.; Bielawski, C.W.; Johnston, K.P. Modified Montmorillonite Clay Microparticles for Stable Oil-in-Seawater Emulsions. ACS Appl. Mater. Interfaces 2014, 6, 11502–11513. [Google Scholar] [CrossRef] [PubMed]
- Pi, G.; Mao, L.; Bao, M.; Li, Y.; Gong, H.; Zhang, J. Preparation of Oil-in-Seawater Emulsions Based on Environmentally Benign Nanoparticles and Biosurfactant for Oil Spill Remediation. ACS Sustain. Chem. Eng. 2015, 3, 2686–2693. [Google Scholar] [CrossRef]
- Worthen, A.J.; Foster, L.M.; Dong, J.; Bollinger, J.A.; Peterman, A.H.; Pastora, L.E.; Bryant, S.L.; Truskett, T.M.; Bielawski, C.W.; Johnston, K.P. Synergistic Formation and Stabilization of Oil-in-Water Emulsions by a Weakly Interacting Mixture of Zwitterionic Surfactant and Silica Nanoparticles. Langmuir 2014, 30, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of Silica Nanoparticles Depends on Size, Dose, and Cell Type. Nanomedicine 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent Advances on Cellulose Nanocrystals for Pickering Emulsions: Development and Challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Österberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical Lignin Particles: A Review on Their Sustainability and Applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef] [Green Version]
- Laitinen, O.; Ojala, J.; Sirviö, J.A.; Liimatainen, H. Sustainable Stabilization of Oil in Water Emulsions by Cellulose Nanocrystals Synthesized from Deep Eutectic Solvents. Cellulose 2017, 24, 1679–1689. [Google Scholar] [CrossRef] [Green Version]
- Cherhal, F.; Cousin, F.; Capron, I. Structural Description of the Interface of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef]
- Bai, L.; Greca, L.G.; Xiang, W.; Lehtonen, J.; Huan, S.; Nugroho, R.W.N.; Tardy, B.L.; Rojas, O.J. Adsorption and Assembly of Cellulosic and Lignin Colloids at Oil/Water Interfaces. Langmuir 2019, 35, 571–588. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Stoffyn-Egli, P.; Wood, P.A.; Lunel, T. Formation and Structure of Oil-Mineral Fines Aggregates in Coastal Environments. In Proceedings of the twenty-first Arctic and marine oilspill program (AMOP) technical seminar, Edmonton, AB, Canada, 10–12 June 1998; p. 962. [Google Scholar]
- Owens, E.H.; Lee, K. Interaction of Oil and Mineral Fines on Shorelines: Review and Assessment. Mar. Pollut. Bull. 2003, 47, 397–405. [Google Scholar] [CrossRef]
- Niu, H.; Li, Z.; Lee, K.; Kepkay, P.; Mullin, J.V. Modelling the Transport of Oil–Mineral-Aggregates (Omas) in the Marine Environment and Assessment of Their Potential Risks. Environ. Model. Assess. 2011, 16, 61–75. [Google Scholar] [CrossRef]
- Delvigne, G.A.L.; van der Stel, J.A.; Sweeney, C.E.; Laboratorium, W.; Hydraulics, I.E.; Service, U.S.M.M. Measurement of Vertical Turbulent Dispersion and Diffusion of Oil Droplets and Oiled Particles: Final Report; Engineering Hydraulics: Redmond, WA, USA, 1987. [Google Scholar]
- Delvigne, G.A.L.; Van der Stel, J.A.; Sweeney, C.E. Final Report: Ocs Study Mms 87-111; US Department of the Interior, Minerals Management Service: Anchorage, AK, USA, 1987; 501p. [Google Scholar]
- Lee, K.; Boufadel, M.; Chen, B.; Foght, J.; Hodson, P.; Swanson, S.; Venosa, A. Expert Panel Report on the Behaviour and Environmental Impacts of Crude Oil Released into Aqueous Environments; Royal Society of Canada: Ottawa, ON, Canada, 2015. [Google Scholar]
- Stoffyn-Egli, P.; Lee, K. Formation and Characterization of Oil–Mineral Aggregates. Spill Sci. Technol. Bull. 2002, 8, 31–44. [Google Scholar] [CrossRef]
- Ajijolaiya, L.O.; Hill, P.S.; Khelifa, A.; Islam, R.M.; Lee, K. Laboratory Investigation of the Effects of Mineral Size and Concentration on the Formation of Oil–Mineral Aggregates. Mar. Pollut. Bull. 2006, 52, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, Y.; Li, Z.; Lee, K. Piv Investigation of Oil–Mineral Interaction for an Oil Spill Application. Chem. Eng. J. 2011, 170, 241–249. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, X. A Review of Oil-Suspended Particulate Matter Aggregation—a Natural Process of Cleansing Spilled Oil in the Aquatic Environment. J. Environ. Monit. 2009, 11, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Khelifa, A.; Stoffyn-Egli, P.; Hill, P.S.; Lee, K. Characteristics of Oil Droplets Stabilized by Mineral Particles: Effects of Oil Type and Temperature. Spill Sci. Technol. Bull. 2002, 8, 19–30. [Google Scholar] [CrossRef]
- Nyankson, E.; DeCuir, M.J.; Gupta, R.B. Soybean Lecithin as a Dispersant for Crude Oil Spills. ACS Sustain. Chem. Eng. 2015, 3, 920–931. [Google Scholar] [CrossRef]
- Athas, J.C.; Jun, K.; McCafferty, C.; Owoseni, O.; John, V.T.; Raghavan, S.R. An Effective Dispersant for Oil Spills Based on Food-Grade Amphiphiles. Langmuir 2014, 30, 9285–9294. [Google Scholar] [CrossRef]
- Freitas, B.; Brito, J.; Brasileiro, P.; Rufino, R.; Luna, J.; Santos, V.; Sarubbo, L. Formulation of a Commercial Biosurfactant for Application as a Dispersant of Petroleum and by-Products Spilled in Oceans. Front. Microbiol. 2016, 7, 1646. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.U.H.; Moniruzzaman, M.; Sivapragasam, M.; Talukder, M.M.R.; Yusup, S.B.; Goto, M. A Binary Mixture of a Biosurfactant and an Ionic Liquid Surfactant as a Green Dispersant for Oil Spill Remediation. J. Mol. Liq. 2019, 280, 111–119. [Google Scholar] [CrossRef]
- Lv, Z.; Cai, Q.; Zhang, B.; Chen, B. Env-624: A New High-Yielding Bio-Dispersant Producer Mutated from Rhodococcus Erythropolis Strain P6–4p. 2016. Available online: http://www.csce2016.ca/ (accessed on 30 January 2022).
- Cao, T. Generation of Biodispersants for Offshore Oil Spill Response. Master’s Thesis, Memorial University of Newfoundland, Newfoundland and Labrador, NL, Canada, 2015. [Google Scholar]
- Cai, Q.; Zhu, Z.; Chen, B.; Lee, K.; Nedwed, T.J.; Greer, C.; Zhang, B. A Cross-Comparison of Biosurfactants as Marine Oil Spill Dispersants: Governing Factors, Synergetic Effects and Fates. J. Hazard. Mater. 2021, 416, 126122. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, B.; Cai, Q.; Ling, J.; Lee, K.; Chen, B. Fish Waste Based Lipopeptide Production and the Potential Application as a Bio-Dispersant for Oil Spill Control. Front. Bioeng. Biotechnol. 2020, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.E.; Colonna, W.J.; Patra, P.; Zhang, H.; Green, C.; Reznik, G.; Pynn, M.; Jarrell, K.; Nyman, J.A.; Somasundaran, P.; et al. Production and Characterization of Microbial Biosurfactants for Potential Use in Oil-Spill Remediation. Enzyme Microb. Technol. 2014, 55, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Moshtagh, B.; Hawboldt, K.; Zhang, B. Kinetic Modeling of Biosurfactant Production by Bacillus Subtilis N3–1p Using Brewery Waste. Chem. Prod. Process. Model. 2021. [Google Scholar] [CrossRef]
- Saeki, H.; Sasaki, M.; Komatsu, K.; Miura, A.; Matsuda, H. Oil Spill Remediation by Using the Remediation Agent Je1058bs That Contains a Biosurfactant Produced by Gordonia Sp. Strain Je-1058. Bioresour. Technol. 2009, 100, 572–577. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).