Zooplankton Population and Community Structure Changes in Response to a Harmful Algal Bloom Caused by Prorocentrum donghaiense in the East China Sea
Abstract
:1. Introduction
2. Materials and Methods
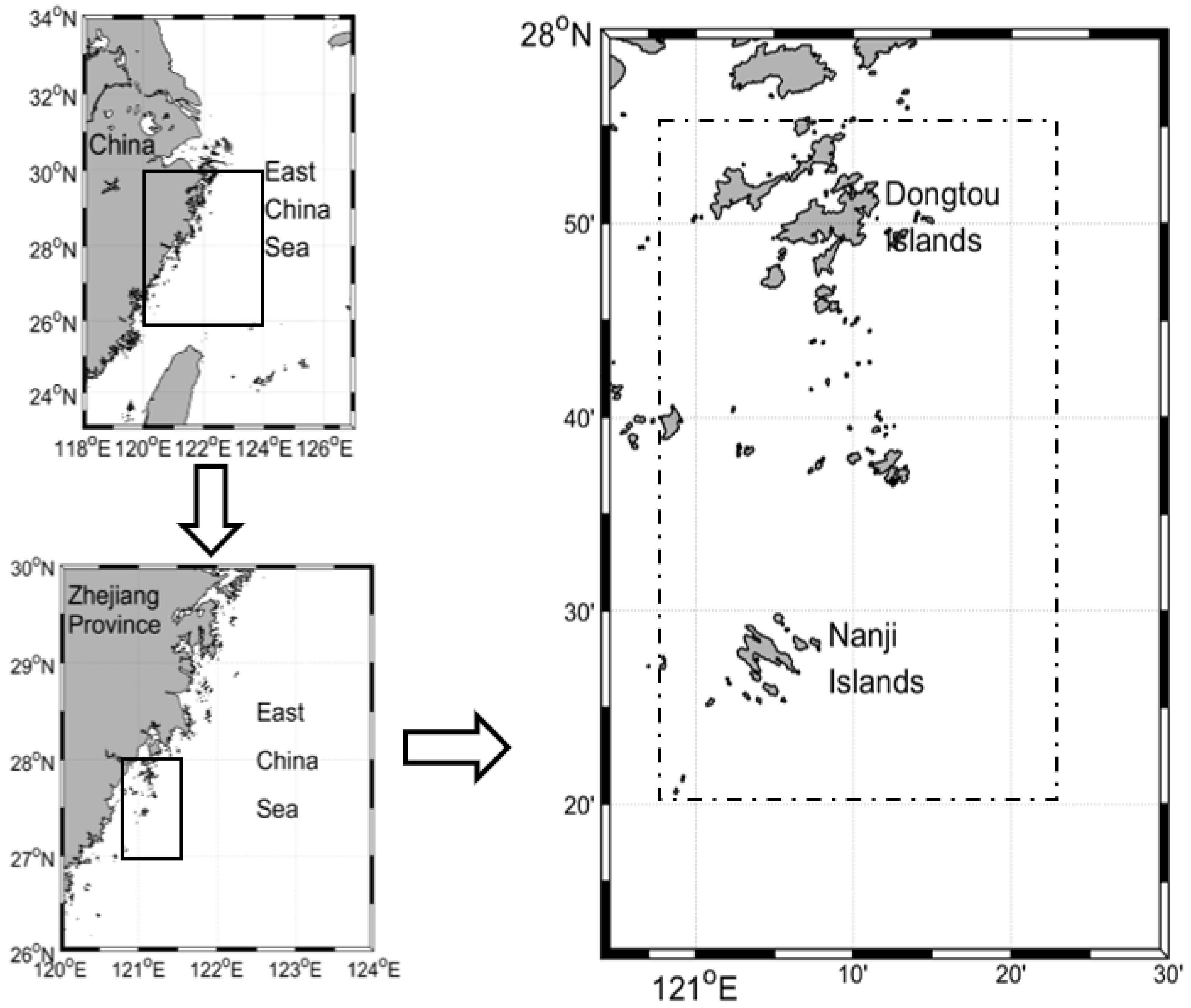
2.1. Study Locations
2.2. Sample Collections and Analysis
2.3. Statistical Analyses
3. Results
3.1. Changes in P. donghaiense Abundance and the Environmental Variables of Seawater during the Algal Bloom Process
3.2. Identification of Dominant Species
3.2.1. Muggiaea atlantica
3.2.2. Sagitta nagae
3.2.3. Calanus sinicus
3.3. Zooplankton Community Structure
3.4. Correlation between Abundance of Zooplankton and Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffy, J.E.; Stachowicz, J.J. Why biodiversity is important to oceanography: Potential roles of genetic, species, and trophic diversity in pelagic ecosystem processes. Mar. Ecol. Prog. Ser. 2006, 311, 179–189. [Google Scholar] [CrossRef] [Green Version]
- de Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahe, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, J.; Katakura, S.; Kasai, H.; Nagai, S. Cryptic zooplankton diversity revealed by a metagenetic approach to monitoring metazoan communities in the coastal waters of the Okhotsk Sea, northeastern hokkaido. Front. Mar. Sci. 2017, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal bloom: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.N.; Song, J.J.; Yan, T.; Zhang, Q.C.; Zhou, M.J. Large-scale dinoflagellate bloom species Prorocentrum donghaiense and Karenia mikimotoi reduce the survival and reproduction of copepod Calanus sinicus. J. Mar. Biol. Assoc. U. K. 2015, 95, 1071–1079. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Buttino, I.; Romano, G.; Poulet, S.A. First evidence of some dinoflagellates reducing male copepod fertilization capacity. Limnol. Oceanogr. 1999, 44, 147–153. [Google Scholar] [CrossRef]
- Jiang, X.D.; Tang, Y.Z.; Lonsdale, D.J.; Gobler, C.J. Deleterious consequences of a red tide dinoflagellate Cochlodinium polykrikoides for the calanoid copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 2009, 390, 105–116. [Google Scholar] [CrossRef]
- Yu, J.; Yang, G.P.; Tian, J.Y. The effects of the harmful alga Heterosigma akashiwo on cultures of Schmackeria inopinus (Copepoda, Calanoida). J. Sea Res. 2010, 64, 287–294. [Google Scholar] [CrossRef]
- Silva, N.J.; Tang, K.W.; Lopes, R.M. Effects of microalgal exudates and intact cells on subtropical marine zooplankton. J. Plankton Res. 2013, 35, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Li, X.D.; Yan, T.; Lin, J.N.; Yu, R.C.; Zhou, M.J. Detrimental impacts of the dinoflagellate Karenia mikimotoi in Fujian coastal waters on typical marine organisms. Harmful Algae 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Xu, J.Y.; Kiørboe, T. Toxic dinoflagellates produce true grazer deterrents. Ecology 2018, 99, 2240–2249. [Google Scholar] [CrossRef] [Green Version]
- Chai, Z.Y.; Wang, H.; Deng, Y.Y.; Hu, Z.X.; Tang, Y.Z. Harmful algal blooms significantly reduce the resource use efficiency in a coastal plankton community. Sci. Total Environ. 2020, 704, 135381. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.N.; Yan, T.; Zhang, Q.C.; Wang, Y.F.; Liu, Q.; Zhou, M.J. In situ detrimental impacts of Prorocentrum donghaiense blooms on zooplankton in the East China Sea. Mar. Poll. Bull. 2014, 88, 302–310. [Google Scholar] [CrossRef]
- Zhou, J.; Richlen, M.L.; Sehein, T.R.; Kulis, D.M.; Anderson, D.M.; Cai, Z. Microbial community structure and associations during a marine dinoflagellate bloom. Front. Microbiol. 2018, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, M.; Grégori, G.J.; Grisoni, J.M.; Pedrotti, M.L.; Mousseau, L.; Artigas, L.F.; Marro, S.; Garcia, N.; Passafiume, O.; Denis, M.J. Onset of the spring bloom in the northwestern Mediterranean Sea: Influence of environmental pulse events on the in situ hourly-scale dynamics of the phytoplankton community structure. Front. Microbiol. 2014, 5, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, A.L.; Ishizaka, J.; Yang, M.M.; Ouyang, L.L.; Yin, Y.E.; Ma, Z.L. Changes in community structure and photosynthetic activities of total phytoplankton species during the growth, maintenance, and dissipation phases of a Prorocentrum donghaiense bloom. Harmful Algae 2019, 82, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.C.; Zhang, Q.C.; Kong, F.Z.; Zhou, Z.X.; Chen, Z.F.; Zhao, Y.; Gen, H.X.; Dai, L.; Yan, T.; Zhou, M.J. Status, impacts and long-term changes of harmful algal blooms in the sea area adjacent to the Changjiang River estuary. Oceanol. Limnol. Sin. 2017, 48, 1178–1186, (In Chinese with English abstract). [Google Scholar]
- Chen, N.S.; Chen, Y. Advances in the study of biodiversity of phytoplankton and red tide species in China (II): The East China Sea. Oceanol. Limnol. Sin. 2021, 52, 363–384, (In Chinese with English abstract). [Google Scholar]
- Zhou, M.J.; Shen, Z.L.; Yu, R.C. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Cont. Shelf Res. 2008, 28, 1483–1489. [Google Scholar] [CrossRef]
- Lu, D.D.; Goebel, J.; Qi, Y.Z.; Zou, J.Z.; Han, X.T.; Gao, Y.H.; Li, Y.G. Morphological and genetic study of Prorocentrum donghaiense Lu from the East China Sea, and comparison with some related Prorocentrum species. Harmful Algae 2005, 4, 493–505. [Google Scholar] [CrossRef]
- Tao, B.Y.; Mao, Z.H.; Lei, H.; Pan, D.L.; Shen, Y.Z.; Bai, Y.; Zhu, Q.K.; Li, Z. A novel method for discriminating Prorocentrum donghaiense from diatom blooms in the East China Sea using MODIS measurements. Remote Sens. Environ. 2015, 158, 267–280. [Google Scholar] [CrossRef]
- Yu, R.C.; Lü, S.H.; Qi, Y.Z.; Zhou, M.J. Progress and perspectives of harmful algal bloom studies in China. Oceanol. Limnol. Sin. 2020, 51, 768–788, (In Chinese with English abstract). [Google Scholar]
- Glibert, P.M.; Burkholder, J.M.; Kana, T.M. Recent insights about relationships between nutrient availability, forms, and stoichiometry, and the distribution, ecophysiology, and food web effects of pelagic and benthic Prorocentrum species. Harmful Algae 2012, 14, 231–259. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Y.; Zhou, M.J. Effects of Prorocentrum donghaiense and Alexandrium catenella on the material transfer in a simulated marine food chain. Acta Ecol. Sin. 2007, 27, 3964–3972, (In Chinese with English abstract). [Google Scholar]
- Tester, P.A.; Steidinger, K.A. Gymnodinium breve red tide blooms: Initiation, transport, and consequences of surface circulation. Limnol. Oceanogr. 1997, 42, 1039–1051. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.X.; Chen, J.H.; Huang, K.G. Chinese Marine Planktonic Diatoms; Shanghai Science Press: Shanghai, China, 1965; pp. 1–230. (In Chinese) [Google Scholar]
- Yamaji, I. Illustrations of the Marine Plankton in Japan; Hoikusha Publishing Co., LTD: Osaka, Japan, 1979; pp. 1–537. (In Japanese) [Google Scholar]
- Yang, S.M.; Dong, S.G. Atlas of Common Planktonic Diatoms in China Seas; China Ocean University Press: Qingdao, China, 2016; pp. 1–264. (In Chinese) [Google Scholar]
- Chen, Q.C.; Zhang, F.Z. Planktonic copepods in the Yellow Sea and East China Sea I: Calanoida. Stud. Mar. Sin. 1965, 7, 20–131. (In Chinese) [Google Scholar]
- Xiao, Y.C. Fauna Sinica, Invertebrates, Vol. 38, Chaetognatha, Sagittaria; China Science Press: Beijing, China, 2004; pp. 1–201. (In Chinese) [Google Scholar]
- Shu, Y.F.; Han, M.S. Atlas of Marine Plankton in China; China Ocean Press: Beijing, China, 1993; pp. 1–236. (In Chinese) [Google Scholar]
- Xu, Z.L.; Chen, Y.Q. Aggregated intensity of dominant species of zooplankton in autumn in the East China Sea and Yellow Sea. Chin. J. Ecol. 1989, 8, 13–15, (In Chinese with English abstract). [Google Scholar]
- Sun, J.; Liu, D.Y. The application of diversity indices in marine phytoplankton studies. Oceanol. Limnol. Sin. 2004, 26, 62–75, (In Chinese with English abstract). [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 1–117. [Google Scholar]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Tseng, L.C.; Kumar, R.; Chen, Q.C.; Hwang, J.S. Summer distribution of Noctiluca scintillans and mesozooplankton in the western and southern East China Sea prior to the Three Gorges Dam operation. Hydrobiologia 2011, 666, 239–256. [Google Scholar] [CrossRef]
- Koch, F.; Burson, A.; Tang, Y.Z.; Collier, J.L.; Fisher, N.S.; Sañudo-Wilhelmy, S.; Gobler, C.J. Alteration of plankton communities and biogeochemical cycles by harmful Cochlodinium polykrikoides (Dinophyceae) blooms. Harmful Algae 2014, 33, 41–54. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, X.X.; Sun, S. The grazing of Aurelia sp.1 on Skeletonema costatum and Prorocentrum donghaiense. Oceanol. Limnol. Sin. 2012, 43, 445–450, (In Chinese with English abstract). [Google Scholar]
- Sun, S.; Li, Y.; Sun, X. Changes in the small-jellyfish community in recent decades in Jiaozhou Bay, China. Chin. J. Oceanol. Limnol. 2012, 30, 507–518. [Google Scholar] [CrossRef]
- Giesecke, R.; Gonzàlez, H.E. Distribution and feeding of chaetognaths in the epipelagic zone of the Lazarev Sea (Antarctica) during austral summer. Polar Biol. 2012, 35, 689–703. [Google Scholar] [CrossRef]
- Chen, X.C.; Shen, Y.; Sun, Y.; Hao, Y. Effects of environmental factors on chaetognatha and their ecological studies. Oceanol. Limnol. Sin. 2019, 4, 156–160, (In Chinese with English abstract). [Google Scholar]
- Wang, L.L.; Hu, S.M.; Guo, M.L.; Li, T.; Wang, Y.J.; Huang, H.; Liu, S. In situ feeding differences between adults and juveniles of chaetognath (Flaccisagitta enflata) in Sanya Bay. J. Trop. Oceanogr. 2020, 39, 57–65, (In Chinese with English abstract). [Google Scholar]
- Zheng, Y.; Dam, H.G.; Avery, D.E. Differential responses of populations of the copepod Acartia hudsonica to toxic and nutritionally insufficient food algae. Harmful Algae 2011, 10, 723–731. [Google Scholar] [CrossRef]
- Tammilehto, A.; Nielsen, T.G.; Krock, B.; Møller, E.F.; Lundholm, N. Induction of domoic acid production in the toxic diatom Pseudo-nitzschia seriata by calanoid copepods. Aquat. Toxicol. 2015, 159, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dam, H.G.; Colin, S.P. Prorocentrum minimum (clone Exuv) is nutritionally insufficient, but not toxic to the copepod Acartia tonsa. Harmful Algae 2005, 4, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Du, N.S. Crustacea; Science Press: Beijing, China, 1993; Volume I, pp. 1–341. (In Chinese) [Google Scholar]
- McGrady-Steed, J.; Harris, P.M.; Morin, P.J. Biodiversity regulates ecosystem predictability. Nature 1997, 390, 162–164. [Google Scholar] [CrossRef]
- Steiner, C.F.; Long, Z.T.; Krumins, J.A.; Morin, P.J. Temporal stability of aquatic food webs: Partitioning the effects of species diversity, species composition and enrichment. Ecol. Lett. 2005, 8, 819–828. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.L.; Ouyang, L.L.; Yin, Y.E.; Zhou, Q.; Ma, Z.L. Effects of Akashiwo sanguinea-dominated algal blooms on the plankton community as observed from the coastal waters of southern Zhejiang, China. Mar. Environ. Sci. 2018, 37, 625–630, (In Chinese with English abstract). [Google Scholar]
- Nagai, S.; Chen, H.; Kawakami, Y.; Yamamoto, K.; Sildever, S.; Kanno, N.; Oikawa, H.; Yasuike, M.; Nakamura, Y.; Hongo, Y.; et al. Monitoring of the toxic dinoflagellate Alexandrium catenella in Osaka Bay, Japan using a massively parallel sequencing (MPS)-based technique. Harmful Algae 2019, 89, 101660. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Scienctific Classification | Growth Phase | Maintenance Phase | Dissipation Phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | OR | Y | A | OR | Y | A | OR | Y | |
| Muggiaea atlantica | 47.56 | 100.00 | 0.421 | 37.14 | 100.00 | 0.562 | 5.05 | 83.33 | 0.095 |
| Clytia sp. | 6.75 | 82.35 | 0.049 | - | - | - | - | - | - |
| Calanus sinicus | 18.91 | 88.24 | 0.148 | - | - | - | - | - | - |
| Zonosagitta nagae | 8.64 | 76.47 | 0.058 | 19.32 | 100.00 | 0.292 | 6.49 | 91.67 | 0.135 |
| Doliolum denticulatum | 12.13 | 41.18 | 0.044 | - | - | - | - | - | - |
| Zonosagitta larvae | 10.29 | 64.71 | 0.059 | - | - | - | - | - | - |
| Copepoda larvae | - | - | - | - | - | - | 28.73 | 66.67 | 0.433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, A.; Chen, W.; Xu, Y.; Ho, K.-C. Zooplankton Population and Community Structure Changes in Response to a Harmful Algal Bloom Caused by Prorocentrum donghaiense in the East China Sea. J. Mar. Sci. Eng. 2022, 10, 291. https://doi.org/10.3390/jmse10020291
Shen A, Chen W, Xu Y, Ho K-C. Zooplankton Population and Community Structure Changes in Response to a Harmful Algal Bloom Caused by Prorocentrum donghaiense in the East China Sea. Journal of Marine Science and Engineering. 2022; 10(2):291. https://doi.org/10.3390/jmse10020291
Chicago/Turabian StyleShen, Anglu, Wenwen Chen, Yongjiu Xu, and Kin-Chung Ho. 2022. "Zooplankton Population and Community Structure Changes in Response to a Harmful Algal Bloom Caused by Prorocentrum donghaiense in the East China Sea" Journal of Marine Science and Engineering 10, no. 2: 291. https://doi.org/10.3390/jmse10020291
APA StyleShen, A., Chen, W., Xu, Y., & Ho, K.-C. (2022). Zooplankton Population and Community Structure Changes in Response to a Harmful Algal Bloom Caused by Prorocentrum donghaiense in the East China Sea. Journal of Marine Science and Engineering, 10(2), 291. https://doi.org/10.3390/jmse10020291





