Towards Risk Assessments of Microplastics in Bivalve Mollusks Globally
Abstract
:1. Introduction
2. Methods
2.1. Literature Retrieval and Data Collection
2.2. Estimation of Microplastic Pollution Load Index
2.3. Assessment of Chemical Composition Risk
2.4. Approach for the Estimation of Annual Dietary Intake of Microplastics via Shellfish Consumption
2.5. Statistical Analysis
3. Results and Discussion
3.1. An Overview of the Occurrence of Microplastics in Bivalve Mollusks Globally
3.2. Comparison of Pollution Load Index of Microplastics in Bivalve Mollusks between Countries
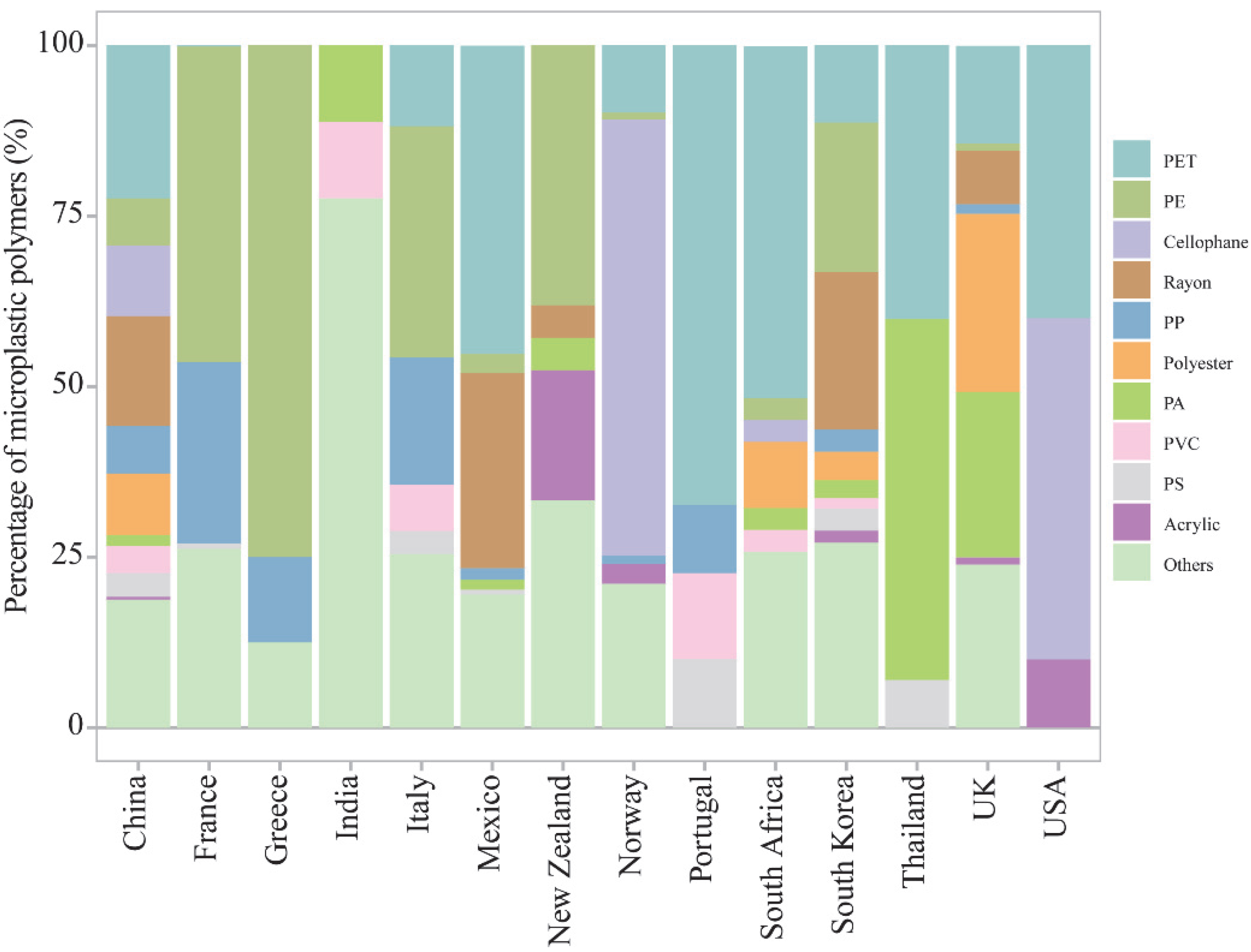
3.3. Comparison of Chemical Composition Risks of Microplastics in Bivalves among Countries
3.4. Human Exposure to Microplastics via Shellfish Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, S.L.; Rowe, D.; Thompson, R.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desforges, J.-P.; Galbraith, M.; Ross, P.S. Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Jiang, F.; Li, J.; Wang, Z.; Sun, C.; Wang, Z.; Fu, L.; Ding, N.X.; He, C. Microplastics in the Coral Reef Systems from Xisha Islands of South China Sea. Environ. Sci. Technol. 2019, 53, 8036–8046. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Marsili, L.; Baini, M.; Giannetti, M.; Coppola, D.; Guerranti, C.; Caliani, I.; Minutoli, R.; Lauriano, G.; Finoia, M.G.; et al. Fin whales and microplastics: The Mediterranean Sea and the Sea of Cortez scenarios. Environ. Pollut. 2016, 209, 68–78. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiol. 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Man, K.Y.; Wong, M.H. Direct and indirect effects of microplastics on bivalves, with a focus on edible species: A mini-review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2109–2143. [Google Scholar] [CrossRef]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; He, C.; Zhang, M.; Ju, P.; Ding, N.X. An examination of the occurrence and potential risks of microplastics across various shellfish. Sci. Total Environ. 2020, 739, 139887. [Google Scholar] [CrossRef]
- Waser, A.M.; Deuzeman, S.; Kangeri, A.K.W.; van Winden, E.; Postma, J.; de Boer, P.; van der Meer, J.; Ens, B.J. Impact on bird fauna of a non-native oyster expanding into blue mussel beds in the Dutch Wadden Sea. Biol. Conserv. 2016, 202, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Z.; Rotchell, J.M.; Shen, X.; Li, Q.; Zhu, J. Where are we? Towards an understanding of the selective accumulation of microplastics in mussels. Environ. Pollut. 2021, 286, 117543. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Adam, V.; Yang, T.; Nowack, B. Toward an ecotoxicological risk assessment of microplastics: Comparison of available hazard and exposure data in freshwaters. Environ. Toxicol. Chem. 2019, 38, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Burns, E.E.; Boxall, A.B. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everaert, G.; Van Cauwenberghe, L.; De Rijcke, M.; Koelmans, A.A.; Mees, J.; Vandegehuchte, M.; Janssen, C.R. Risk assessment of microplastics in the ocean: Modelling approach and first conclusions. Environ. Pollut. 2018, 242, 1930–1938. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Xu, P.; Peng, G.; Su, L.; Gao, Y.; Gao, L.; Li, D. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef]
- Zhang, X.; Leng, Y.; Liu, X.; Huang, K.; Wang, J. Microplastics’ Pollution and Risk Assessment in an Urban River: A Case Study in the Yongjiang River, Nanning City, South China. Expo. Health 2020, 12, 141–151. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Ding, H.; Ding, J.; Jiang, F.; Ding, N.X.; Sun, C. Distribution Characteristics and Influencing Factors of Microplastics in Urban Tap Water and Water Sources in Qingdao, China. Anal. Lett. 2019, 53, 1312–1327. [Google Scholar] [CrossRef]
- Fang, C.; Zheng, R.; Chen, H.; Hong, F.; Lin, L.; Lin, H.; Guo, H.; Bailey, C.; Segner, H.; Mu, J.; et al. Comparison of microplastic contamination in fish and bivalves from two major cities in Fujian province, China and the implications for human health. Aquac. 2019, 512, 734322. [Google Scholar] [CrossRef]
- Angulo, E. The Tomlinson Pollution Load Index applied to heavy metal, ‘Mussel-Watch’ data: A useful index to assess coastal pollution. Sci. Total Environ. 1996, 187, 19–56. [Google Scholar] [CrossRef]
- Sparks, C.; Awe, A.; Maneveld, J. Abundance and characteristics of microplastics in retail mussels from Cape Town, South Africa. Mar. Pollut. Bull. 2021, 166, 112186. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Bour, A.; Avio, C.G.; Gorbi, S.; Regoli, F.; Hylland, K. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ. Pollut. 2018, 243, 1217–1225. [Google Scholar] [CrossRef]
- Klasios, N.; De Frond, H.; Miller, E.; Sedlak, M.; Rochman, C.M. Microplastics and other anthropogenic particles are prevalent in mussels from San Francisco Bay, and show no correlation with PAHs. Environ. Pollut. 2021, 271, 116260. [Google Scholar] [CrossRef]
- Leslie, H.; Brandsma, S.; Van Velzen, M.; Vethaak, A. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Teichert, S.; Löder, M.G.J.; Pyko, I.; Mordek, M.; Schulbert, C.; Wisshak, M.; Laforsch, C. Microplastic contamination of the drilling bivalve Hiatella arctica in Arctic rhodolith beds. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Anselmi, S.; Menconi, V.; Bertoli, M.; Dondo, A.; Pizzul, E.; Renzi, M. Use of the Zebra Mussel Dreissena polymorpha (Mollusca, Bivalvia) as a Bioindicator of Microplastics Pollution in Freshwater Ecosystems: A Case Study from Lake Iseo (North Italy). Water 2021, 13, 434. [Google Scholar] [CrossRef]
- Murphy, C.L. A comparison of microplastics in farmed and wild shellfish. Master’s Thesis, Royal Roads University, Victoria, Canada, 2018. [Google Scholar]
- Kuczenski, B.; Geyer, R. Material flow analysis of polyethylene terephthalate in the US, 1996–2007. Resour. Conserv. Recycl. 2010, 54, 1161–1169. [Google Scholar] [CrossRef]
- Suaria, G.; Perold, V.; Lee, J.; Lebouard, F.; Aliani, S.; Ryan, P.G. Floating macro- and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020, 136, 105494. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastic-the Facts 2020: An Analysis of European Plastics Production, Demand and Waste Data (Brussels, Belgium). Available online: https://plasticseurope.org/wp-content/uploads/2021/09/Plastics_the_facts-WEB-2020_versionJun21_final.pdf (accessed on 28 November 2021).
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Nationwide monitoring of microplastics in bivalves from the coastal environment of Korea. Environ. Pollut. 2021, 270, 116175. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, C.; Dong, L.; Liu, L.; Li, H.; Wu, J.; Ye, C. Microplastic pollution in the Yangtze River Basin: Heterogeneity of abundances and characteristics in different environments. Environ. Pollut. 2021, 287, 117580. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, L.; Xue, B.; Wang, Y. Abundance and characteristics of microplastics in the mangrove sediment of the semi-enclosed Maowei Sea of the south China sea: New implications for location, rhizosphere, and sediment compositions. Environ. Pollut. 2019, 244, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhang, L.; Li, R.; Wang, Y.; Guo, J.; Yu, K.; Wang, S. Underestimated Microplastic Pollution Derived from Fishery Activities and “Hidden” in Deep Sediment. Environ. Sci. Technol. 2020, 54, 2210–2217. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, D.; Yang, X.; Teng, J.; Liu, Y.; Zhang, C.; Zhao, J.; Yin, X.; You, L.; Liu, Y.; et al. Microplastic pollution in the surface sediments collected from Sishili Bay, North Yellow Sea, China. Mar. Pollut. Bull. 2019, 141, 9–15. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.M.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, Isolating and Identifying Microplastics Ingested by Fish and Invertebrates. Anal. Methods-U.K. 2017, 9, 1346–1360. [Google Scholar] [CrossRef] [Green Version]
- Birnstiel, S.; Soares-Gomes, A.; da Gama, B.A. Depuration reduces microplastic content in wild and farmed mussels. Mar. Pollut. Bull. 2019, 140, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Boots, B.; Sigwart, J.; Jiang, S.; Rocha, C. Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ. Pollut. 2016, 208, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 11 June 2021).
- Danopoulos, E.; Jenner, L.C.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Seafood Intended for Human Consumption: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2020, 128, 126002. [Google Scholar] [CrossRef]
- Leslie, H.A.; Depledge, M.H. Where is the evidence that human exposure to microplastics is safe? Environ. Int. 2020, 142, 105807. [Google Scholar] [CrossRef]



| Country | Mollusk Supply (g/Capita/Year) | Yearly Uptake (Microplastics/Capita/Year) |
|---|---|---|
| Argentina | 236 | 59 |
| Belgium | 1039 | 291 |
| Chile | 222 | 71 |
| China | 3269 | 8369 |
| France | 1814 | 1070 |
| Germany | 158 | 57 |
| Greece | 351 | 1370 |
| India | 25 | 49 |
| Iran | 0 | 0 |
| Italy | 2077 | 561 |
| Mexico | 296 | 18 |
| Netherlands | 653 | 85 |
| New Zealand | 328 | 13 |
| Norway | 287 | 279 |
| Portugal | 1462 | 819 |
| South Africa | 36 | 1 |
| South Korea | 3475 1 | 1703 |
| Thailand | 575 | 233 |
| Tunisia | 52 | 64 |
| UK | 305 | 591 |
| USA | 994 | 199 |
| Vietnam | 844 | 245 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Sun, Y.; He, C.; Li, J.; Li, F. Towards Risk Assessments of Microplastics in Bivalve Mollusks Globally. J. Mar. Sci. Eng. 2022, 10, 288. https://doi.org/10.3390/jmse10020288
Ding J, Sun Y, He C, Li J, Li F. Towards Risk Assessments of Microplastics in Bivalve Mollusks Globally. Journal of Marine Science and Engineering. 2022; 10(2):288. https://doi.org/10.3390/jmse10020288
Chicago/Turabian StyleDing, Jinfeng, Yunmei Sun, Changfei He, Jingxi Li, and Fengmin Li. 2022. "Towards Risk Assessments of Microplastics in Bivalve Mollusks Globally" Journal of Marine Science and Engineering 10, no. 2: 288. https://doi.org/10.3390/jmse10020288
APA StyleDing, J., Sun, Y., He, C., Li, J., & Li, F. (2022). Towards Risk Assessments of Microplastics in Bivalve Mollusks Globally. Journal of Marine Science and Engineering, 10(2), 288. https://doi.org/10.3390/jmse10020288






