Mapping of Greek Marine Finfish Farms and Their Potential Impact on the Marine Environment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steiner-Asiedu, M.; Julshamn, K. Lie, Øyvind Effect of local processing methods (cooking, frying and smoking) on three fish species from Ghana: Part I. Proximate composition, fatty acids, minerals, trace elements and vitamins. Food Chem. 1991, 40, 309–321. [Google Scholar] [CrossRef]
- Ågren, J.; Hänninen, O. Effects of cooking on the fatty acids of three freshwater fish species. Food Chem. 1993, 46, 377–382. [Google Scholar] [CrossRef]
- Hanson, G.D.; Rauniyar, G.P.; Herrmann, R.O. Using consumer profiles to increase the U.S. market for seafood: Implications for aquaculture. Aquaculture 1994, 127, 303–316. [Google Scholar] [CrossRef]
- Lall, S.P. Macro and trace elements in fish and shellfish. In Fish and Fishery Products: Composition, Nutritive Properties and Stability; Ruiter, A., Ed.; CAB International: Wallingford, CN, USA, 1995; pp. 187–214. [Google Scholar]
- Kapetsky, J.M.; Aguilar-Manjarrez, J.; Jenness, J. A Global Assessment of Offshore Mariculture Potential from a Spatial Perspective; FAO: Rome, Italy, 2013. [Google Scholar]
- Borja, Á.; Rodriguez, J.; Black, K.; Bodoy, A.; Emblow, C.; Fernandes, T.; Forte, J.; Karakassis, I.; Muxika, I.; Nickell, T.D.; et al. Assessing the suitability of a range of benthic indices in the evaluation of environmental impact of fin and shellfish aquaculture located in sites across Europe. Aquaculture 2009, 293, 231–240. [Google Scholar] [CrossRef]
- Karakassis, I.; Papageorgiou, N.; Kalantzi, I.; Sevastou, K.; Koutsikopoulos, C. Adaptation of fish farming production to the environmental characteristics of the receiving marine ecosystems: A proxy to carrying capacity. Aquaculture 2013, 408–409, 184–190. [Google Scholar] [CrossRef]
- Tomassetti, P.; Gennaro, P.; Lattanzi, L.; Mercatali, I.; Persia, E.; Vani, D.; Porrello, S. Benthic community response to sediment organic enrichment by Mediterranean fish farms: Case studies. Aquaculure 2016, 450, 262–272. [Google Scholar] [CrossRef]
- Karakassis, I.; Tsapakis, M.; Hatziyanni, E.; Papadopoulou, K.-N.; Plaiti, W. Impact of cage farming of fish on the seabed in three Mediterranean coastal areas. ICES J. Mar. Sci. 2000, 57, 1462–1471. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.N.; Yeats, P.A.; Milligan, T.G. Sediment geochronologies for fish farm contaminants in Lime Kiln Bay, Bay of Fundy. In The Handbook of Environmental Chemistry; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2005; pp. 221–238. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Kalantzi, I.; Karakassis, I. Effects of fish farming on the biological and geochemical properties of muddy and sandy sediments in the Mediterranean Sea. Mar. Environ. Res. 2010, 69, 326–336. [Google Scholar] [CrossRef]
- Kalantzi, I.; Shimmield, T.M.; Pergantis, S.A.; Papageorgiou, N.; Black, K.D.; Karakassis, I. Heavy metals, trace elements and sediment geochemistry at four Mediterranean fish farms. Sci. Total Environ. 2013, 444, 128–137. [Google Scholar] [CrossRef]
- Giannoulaki, M.; Machias, A.; Somarakis, S.; Karakassis, I. Wild fish spatial structure in response to presence of fish farms. J. Mar. Biol. Assoc. U. K. 2005, 85, 1271–1277. [Google Scholar] [CrossRef]
- Dempster, T.; Uglem, I.; Sanchez-Jerez, P.; Fernandez-Jover, D.; Bayle-Sempere, J.T.; Nilsen, R.; Bjørn, P. Coastal salmon farms attract large and persistent aggregations of wild fish: An ecosystem effect. Mar. Ecol. Prog. Ser. 2009, 385, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Uglem, I.; Dempster, T.; Bjørn, P.A.; Sanchez-Jerez, P.; Økland, F. High connectivity of salmon farms revealed by aggregation, residence and repeated movements of wild fish among farms. Mar. Ecol. Prog. Ser. 2009, 384, 251–260. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Sanchez-Jerez, P.; Bayle-Sempere, J.; Fernandez-Jover, D.; Martinez-Rubio, L.; Lopez-Jimenez, J.A.; Martinez-Lopez, F.J. Direct interaction between wild fish aggregations at fish farms and fisheries activity at fishing grounds: A case study with Boops boops. Aquac. Res. 2011, 42, 996–1010. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Uglem, I.; Sanchez-Jerez, P.; Fernandez-Jover, D.; Bayle-Sempere, J.; Nilsen, R. Movements of grey mullet Liza aurata and Chelon labrosus associated with coastal fish farms in the western Mediterranean Sea. Aquac. Environ. Interact. 2012, 1, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Arechavala-Lopez, P.; Izquierdo-Gomez, D.; Uglem, I.; Sanchez-Jerez, P. Aggregations of bluefish Pomatomus saltatrix (L.) at Mediterranean coastal fish farms: Seasonal presence, daily patterns and influence of farming activity. J. Appl. Phycol. 2015, 98, 499–510. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Boudouresque, C.-F.; Pasqualini, V.; Pergent, G. Impact of fish farming facilities on Posidonia oceanica meadows: A review. Mar. Ecol. 2006, 27, 310–319. [Google Scholar] [CrossRef]
- Yokoyama, H.; Abo, K.; Ishihi, Y. Quantifying aquaculture-derived organic matter in the sediment in and around a coastal fish farm using stable carbon and nitrogen isotope ratios. Aquaculture 2006, 254, 411–425. [Google Scholar] [CrossRef]
- Akyol, O.; Özgül, A.; Düzbastılar, F.O.; Şen, H.; de Urbina, J.M.O.; Ceyhan, T. Seasonal variations in wild fish aggregation near sea-cage fish farms in the Turkish Aegean Sea. Aquac. Rep. 2020, 18, 100478. [Google Scholar] [CrossRef]
- Dempster, T.; Sanchez-Jerez, P.; Uglem, I.; Bjørn, P.-A. Species-specific patterns of aggregation of wild fish around fish farms. Estuar. Coast. Shelf Sci. 2010, 86, 271–275. [Google Scholar] [CrossRef]
- Holmer, M.; Argyrou, M.; Dalsgaard, T.; Danovaro, R.; Diaz-Almela, E.; Duarte, C.M.; Frederiksen, M.; Grau, A.; Karakassis, I.; Marbà, N.; et al. Effects of fish farm waste on Posidonia oceanica meadows: Synthesis and provision of monitoring and management tools. Mar. Pollut. Bull. 2008, 56, 1618–1629. [Google Scholar] [CrossRef] [Green Version]
- Mangion, M.; Borg, J.A.; Sanchez-Jerez, P. Differences in magnitude and spatial extent of impact of tuna farming on benthic macroinvertebrate assemblages. Reg. Stud. Mar. Sci. 2018, 18, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Dempster, T.; Sanchez-Jerez, P.; Bayle-Sempere, J.T.; Casalduero, F.G.; Valle, C. Attraction of wild fish to sea-cage fish farms in the south-western Mediterranean Sea: Spatial and short-term temporal variability. Mar. Ecol. Prog. Ser. 2002, 242, 237–252. [Google Scholar] [CrossRef]
- Sara’, G.; Scilipoti, D.; Milazzo, M.; Modica, A. Use of stable isotopes to investigate dispersal of waste from fish farms as a function of hydrodynamics. Mar. Ecol. Prog. Ser. 2006, 313, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Machias, A.; Karakassis, I.; Labropoulou, M.; Somarakis, S.; Papadopoulou, K.; Papaconstantinou, C. Changes in wild fish assemblages after the establishment of a fish farming zone in an oligotrophic marine ecosystem. Estuar. Coast. Shelf Sci. 2004, 60, 771–779. [Google Scholar] [CrossRef]
- Machias, A.; Giannoulaki, M.; Somarakis, S.; Maravelias, C.; Neofitou, C.; Koutsoubas, D.; Papadopoulou, K.; Karakassis, I. Fish farming effects on local fisheries landings in oligotrophic seas. Aquaculture 2006, 261, 809–816. [Google Scholar] [CrossRef]
- Zaucha, J.; Gee, K. Maritime Spatial Planning: Past, Present, Future; Palgrave Macmillan: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, J.V. Application of geographical information systems. J. Int. Inf. Manag. 1998, 7, 9. [Google Scholar]
- Trujillo, P.; Piroddi, C.; Jacquet, J. Fish farms at sea: The ground truth from Google Earth. PLoS ONE 2012, 7, e30546. [Google Scholar] [CrossRef]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [Green Version]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Opportunities and challenges for the estimation of aquaculture production based on earth observation data. Remote Sens. 2018, 10, 1076. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.A.; Ottinger, M.; Wei, C.; Leinenkugel, P. Assessment of coastal aquaculture for India from Sentinel-1 SAR time series. Remote Sens. 2019, 11, 357. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Guo, X.; Chen, R. Automatic extraction of aquaculture ponds based on Google Earth engine. Ocean Coast. Manag. 2020, 198, 105348. [Google Scholar] [CrossRef]
- Fu, Y.; Deng, J.; Wang, H.; Comber, A.; Yang, W.; Wu, W.; You, S.; Lin, Y.; Wang, K. A new satellite-derived dataset for marine aquaculture areas in China’s coastal region. Earth Syst. Sci. Data 2021, 13, 1829–1842. [Google Scholar] [CrossRef]
- Sarà, G.; Gouhier, T.C.; Brigolin, D.; Porporato, E.M.D.; Mangano, M.C.; Mirto, S.; Mazzola, A.; Pastres, R. Predicting shifting sustainability trade-offs in marine finfish aquaculture under climate change. Glob. Chang. Biol. 2018, 24, 3654–3665. [Google Scholar] [CrossRef]
- Gernez, P.; Palmer, S.C.J.; Thomas, Y.; Forster, R. Editorial: Remote sensing for aquaculture. Front. Mar. Sci. 2021, 7, 1258. [Google Scholar] [CrossRef]
- Meaden, G.J.; Aguilar-Manjarrez, J. Advances in geographic information systems and remote sensing for fisheries and aquaculture. FAO Fish. Aquac. Tech. Pap. 2013, 552, 1. [Google Scholar]
- Theodorou, J.A.; Perdikaris, C.; Filippopoulos, N.G. Evolution through innovation in aquaculture: A critical review of the Greek mariculture industry. J. Appl. Aquac. 2015, 27, 160–181. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/fishery/statistics/global-production/query/en (accessed on 12 November 2021).
- FGM. FGM Annual Report 2020. Available online: https://www.fgm.com.gr/english/article.php?id=77 (accessed on 12 November 2021).
- ASC. ASC–Programme Improvements. Available online: https://www.asc-aqua.org/programme-improvements/ (accessed on 12 November 2021).
- Petrou, C. Study of Spatial Distribution and Technical Characteristics of Mediterranean Marine Aquaculture Farms via Free Distributed Satellite Images from GoogleTM Earth. Master’s thesis, University of Thessaly, School of Health Sciences, Faculty of Veterinary Medicine, Karditsa, Greece, 2013. [Google Scholar] [CrossRef]
- Karampoula, H.; Tsolakos, K.; Katselis, G. Mapping of potential impacts of marine fish farms in Central Ionian Sea (W. GREECE). In Proceedings of the Hydromedit 2021, Mytilini, Greece, 4–6 November 2021. [Google Scholar]
- Zar, J. Biostatistical Analysis, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 1999. [Google Scholar]
- QGIS.org. QGIS Geographic Information System. QGIS Association. 2021. Available online: http://www.qgis.org (accessed on 9 February 2022).
- EEA (European Environment Agency). Late Lessons from Early Warnings: Science, Precaution, Innovation. EEA Report No 1/2013. EEA. Available online: http://www.eea.europa.eu/publications/late-lessons-2 (accessed on 12 November 2021).
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Factor Analysis. Multivariate Data Analysis; Prentice-Hall: Hoboken, NJ, USA, 1998; Volume 3, pp. 98–99. [Google Scholar]
- Macías-Rivero, J.C.; Avila Zaragozá, P.; Karakassis, I.; Sanchez-Jerez, P.; Massa, F.; Fezzardi, D.; Gier, G.Y.; Franičevič, V.; Borg, J.A.; Chapela Pérez, R.M. Allocated Zones for Aquaculture: A Guide for the Establishment of Coastal Zones Dedicated to Aquaculture in the Mediterranean and the Black Sea; FAO: Rome, Italy, 2019; Volume 97. [Google Scholar]
- Theodorou, J.A. Current and future technological trends of European seabass-seabream culture. Rev. Fish. Sci. 2002, 10, 529–543. [Google Scholar] [CrossRef]
- Lopez, P.A.; Uglem, I.; Fernandez-Jover, D.; Bayle-Sempere, J.T.; Sanchez-Jerez, P. Post-escape dispersion of farmed seabream (Sparus aurata L.) and recaptures by local fisheries in the Western Mediterranean Sea. Fish. Res. 2012, 121–122, 126–135. [Google Scholar] [CrossRef]
- Lopez, P.A.; Sanchez-Jerez, P.; Bayle-Sempere, J.T.; Uglem, I.; Mladineo, I. Reared fish, farmed escapees and wild fish stocks—A triangle of pathogen transmission of concern to Mediterranean aquaculture management. Aquac. Environ. Interact. 2013, 3, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Jover, D.; Faliex, E.; Sanchez-Jerez, P.; Sasal, P.; Bayle-Sempere, J.T. Coastal fish farming does not affect the total parasite communities of wild fish in SW Mediterranean. Aquaculture 2010, 300, 10–16. [Google Scholar] [CrossRef]
- Scottish Executive. Final Report of the Joint Government/Industry Working Group on ISA. Available online: https://www.webarchive.org.uk/wayback/archive/20180514134843mp_/http://www.gov.scot/Uploads/Documents/JGIWGReport.pdf (accessed on 25 December 2021).
- Henderson, A.R.; Davies, I.M. Review of aquaculture, its regulation and monitoring in Scotland. J. Appl. Ichthyol. 2000, 16, 200–208. [Google Scholar] [CrossRef]
- Tzanatos, E.; Dimitriou, E.; Katselis, G.; Georgiadis, M.; Koutsikopoulos, C. Composition, temporal dynamics and regional characteristics of small-scale fisheries in Greece. Fish. Res. 2005, 73, 147–158. [Google Scholar] [CrossRef]
- Dimitriou, E.; Katselis, G.; Moutopoulos, D.K.; Akovitiotis, C.; Koutsikopoulos, C. Possible influence of reared gilthead sea bream (Sparus aurata, L.) on wild stocks in the area of the Messolonghi lagoon (Ionian Sea, Greece). Aquac. Res. 2007, 38, 398–408. [Google Scholar] [CrossRef]
- Katselis, G.; Koukou, K.; Moutopoulos, D. Yield per recruit and spawning stock biomass models for the management of four Mugilidae species in Mesolonghi–Aitoliko lagoon (W. Greece). Int. Aquat. Res. 2010, 2, 155–162. Available online: http://submission.intelaquares.com/article_677699_8077c753f638ca9b5745ba401c56a6c4.pdf (accessed on 12 November 2021).
- Katselis, G.N.; Moutopoulos, D.K.; Dimitriou, E.N.; Koutsikopoulos, C. Long-term changes of fisheries landings in enclosed gulf lagoons (Amvrakikos gulf, W Greece): Influences of fishing and other human impacts. Estuar. Coast. Shelf Sci. 2013, 131, 31–40. [Google Scholar] [CrossRef]
- Modica, A.; Scilipoti, D.; La Torre, R.; Manganaro, A.; Sarà, G. The effect of mariculture facilities on biochemical features of suspended organic matter (Southern Tyrrhenian, Mediterranean). Estuar. Coast. Shelf Sci. 2006, 66, 177–184. [Google Scholar] [CrossRef]
- Sara’, G. Hydrodynamic effects on the origin and quality of organic matter for bivalves: An integrated isotopic, biochemical and transplant study. Mar. Ecol. Prog. Ser. 2006, 328, 65–73. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, C.; Moltschaniwskyj, N.; Crawford, C.; Forbes, S. Biological recovery from organic enrichment: Some systems cope better than others. Mar. Ecol. Prog. Ser. 2007, 342, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Jusup, M.; Geček, S.; Legović, T. Impact of aquacultures on the marine ecosystem: Modelling benthic carbon loading over variable depth. Ecol. Model. 2007, 200, 459–466. [Google Scholar] [CrossRef]
- Lampadariou, N.; Akoumianaki, I.; Karakassis, I. Use of the size fractionation of the macrobenthic biomass for the rapid assessment of benthic organic enrichment. Ecol. Indic. 2008, 8, 729–742. [Google Scholar] [CrossRef]
- Kletou, D.; Kleitou, P.; Savva, I.; Attrill, M.J.; Antoniou, C.; Hall-Spencer, J.M. Seagrass recovery after fish farm relocation in the eastern Mediterranean. Mar. Environ. Res. 2018, 140, 221–233. [Google Scholar] [CrossRef] [PubMed]
- ESA. Sentinel Online. Available online: https://sentinels.copernicus.eu/web/sentinel/technical-guides/sentinel-2-msi/performance (accessed on 12 November 2021).

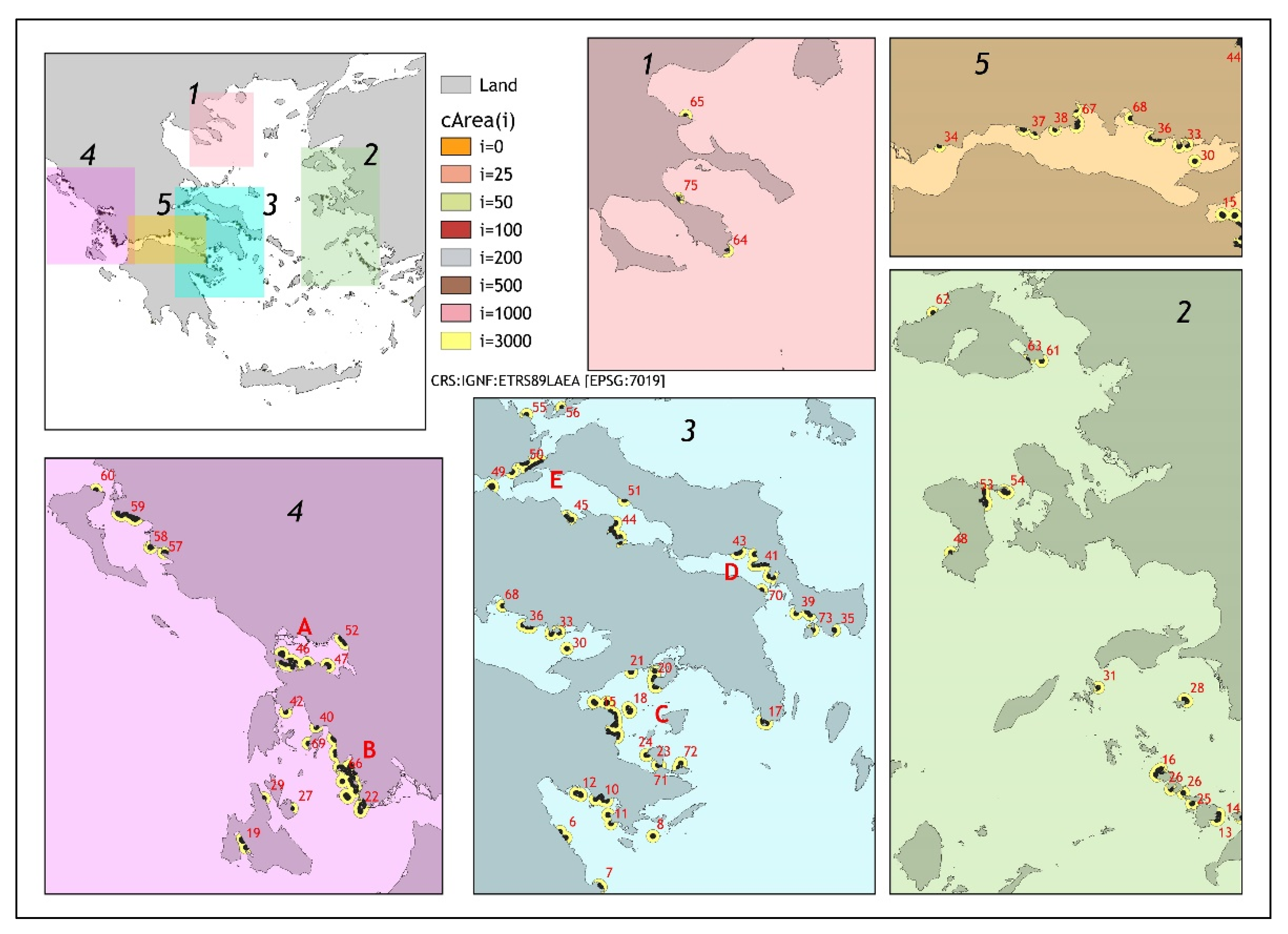

| Distance from the Cages (m) | Sediment | Water Column | Biodiversity | Wild Stocks |
|---|---|---|---|---|
| 0 (under the cage) | Anoxic sediment beneath the cages (methane CH4 and hydrogen sulphide H2S) [9]. Enrichment in organic matter (8%) at the level of the deepest layer of sediment (10–15 cm) [19]. | High values of the organic matter ratio (>50%) and organic carbon and nitrogen contents (>31 and >4.7 mg/g, respectively) were found within 24 m of the cages [20]. | Unacceptable status of all ecological indicators (BQI-species; BQI-family; H’; BENTIX; M-AMBI; BOPA; BENTIXfamily) [7]. | Sparids, Carangids, and Mugilids show the greatest fish diversity beneath sea cages [21]. Boops boops and Pollachius virens are the most abundant fish species beneath sea cages [21,22]. |
| 25 | TOC and TON values, oxygen consumption, and PO4 release are higher than the control station [11]. The concentrations of most elements (organic matter; Cu, Zn, P, U and coarse sediment; Pd, Pb, Sr, Mg, Ca, Na) directly beneath and close to the fish cages were significantly higher [12]. | BENTIX and M-AMBI indicators (28% and 44%, respectively) vs. 83% control station [7]. Significant effect on macrofauna [9]. | Greatest concentrations of wild fish occurred immediately beneath farms, with a steep decline in the abundance of fish just 10–100s of meters away [22]. | |
| 50 | Organic matter ratio and organic carbon and nitrogen contents are reduced to ca. 16%, 4.0 mg/g and 0.6 mg/g at a site 200 m from the fish cage while high acid volatile sulfide values indicated enrichment effects [20]. | BENTIX and M-AMBI indicators (33%, 56%) vs. 83% at the control station [7]. Increased macrofaunal abundance [6]. | ||
| 100 | Sedimentation rates 5–10% compared with the rates just beneath the cages [23]. | Increase in the meiofaunal abundance [23]. Significant variation in the abundance of polychaetes and amphipod families decreased significantly in number and diversity [24]. | ||
| 200 | Contaminant levels for Zn and Cu approaching background levels at distances greater than 200 m from the original cage locations [10]. Spatial extent of waste dispersal [20]. | Abundance (52 to 2837×), biomass (2.8 to 1126×), and number of species (1.6 to 14×) were greater in fish farms than control sites 200 m from farms [25]. | ||
| 500 | Sedimentation rate 40% higher than the control station [23]. | Dispersal of waste. Particulate organic matter (POM), mean isotopic values of carbon (δ13C), and nitrogen (δ15N); significant differences between distance categories (0; 500; 1000) [26]. | Fish farms are connected through attraction to wild fish populations (transmission of diseases and parasites, change in genetic cohesion (hybridization) and reduce the survival capacity of wild stocks [13,15,16,17,18,27,28]. | |
| 1000 | ||||
| 3000 |
| Mean | SD | Sum | ||
|---|---|---|---|---|
| NCl | 16.44 | 12.69 | 7122 | 9477 |
| NSl | 5.43 | 10.22 | 2355 | |
| ANCSl | 1.18 | 0.54 | - | - |
| Vl (× 103 m3) | 38.62 | 26.88 | 16,726.6 | - |
| Dst (m) | 154.55 | 177.23 | - | - |
| l | 433 | - | - | - |
| Impacted Zones i (m from Array’s Edge) | N | cAreai,j (km2) | l | Vsl (m3/m2) | Sum of cAreai,j (km2) |
|---|---|---|---|---|---|
| 0 | 433 | 0.015 (0.013) | 1.00 (0.00) | 3.80 (2.49) | 6.51 |
| 25 | 363 | 0.04 (0.04) | 1.19 (0.62) | 1.08 (0.51) | 16.36 |
| 50 | 308 | 0.10 (0.13) | 1.40 (0.96) | 0.56 (0.32) | 33.04 |
| 100 | 275 | 0.13 (0.06) | 1.57 (1.14) | 0.27 (0.17) | 37.26 |
| 200 | 230 | 0.31 (0.18) | 1.88 (1.47) | 0.11 (0.08) | 73.37 |
| 500 | 140 | 1.42 (1.17) | 3.09 (3.29) | 0.02 (0.02) | 199.87 |
| 1000 | 102 | 4.77 (3.91) | 4.24 (5.09) | 0.007 (0.008) | 487.44 |
| 3000 | 75 | 32.78 (29.52) | 5.77 (8.65) | 0.0009 (0.0010) | 2459.15 |
| total | 1926 | - | - | - | - |
| Factors | |||||
|---|---|---|---|---|---|
| Log−MV | F1 | F2 | F3 | F4 | F5 |
| cArea0 | 0.601 | 0.037 | 0.183 | 0.705 | 0.183 |
| cArea25 | 0.378 | 0.088 | 0.291 | 0.353 | 0.712 |
| cArea50 | 0.086 | 0.022 | 0.737 | 0.073 | 0.483 |
| cArea100 | −0.043 | 0.065 | 0.908 | 0.080 | 0.171 |
| cArea200 | −0.116 | 0.303 | 0.785 | 0.229 | −0.209 |
| cArea500 | −0.075 | 0.756 | 0.320 | 0.225 | −0.115 |
| cArea1000 | −0.006 | 0.876 | 0.136 | 0.177 | 0.038 |
| cArea3000 | 0.081 | 0.782 | 0.076 | −0.027 | 0.180 |
| Vs0 | −0.094 | −0.018 | −0.052 | −0.902 | −0.168 |
| Vs25 | 0.710 | −0.034 | 0.015 | −0.084 | −0.595 |
| Vs50 | 0.868 | −0.001 | −0.235 | 0.187 | −0.270 |
| Vs100 | 0.913 | −0.033 | −0.277 | 0.167 | −0.004 |
| Vs200 | 0.876 | −0.183 | −0.245 | 0.065 | 0.217 |
| Vs500 | 0.700 | −0.563 | −0.030 | 0.030 | 0.174 |
| Vs1000 | 0.629 | −0.672 | 0.113 | 0.054 | 0.093 |
| Vs3000 | 0.566 | −0.649 | 0.165 | 0.171 | 0.027 |
| Vl | 0.892 | −0.016 | 0.273 | 0.207 | 0.163 |
| ANCS | 0.151 | 0.124 | 0.130 | 0.736 | −0.025 |
| %ExpVar | 30.17 | 18.32 | 14.19 | 12.63 | 8.38 |
| %CExpVar | 30.17 | 48.49 | 62.68 | 75.31 | 83.69 |
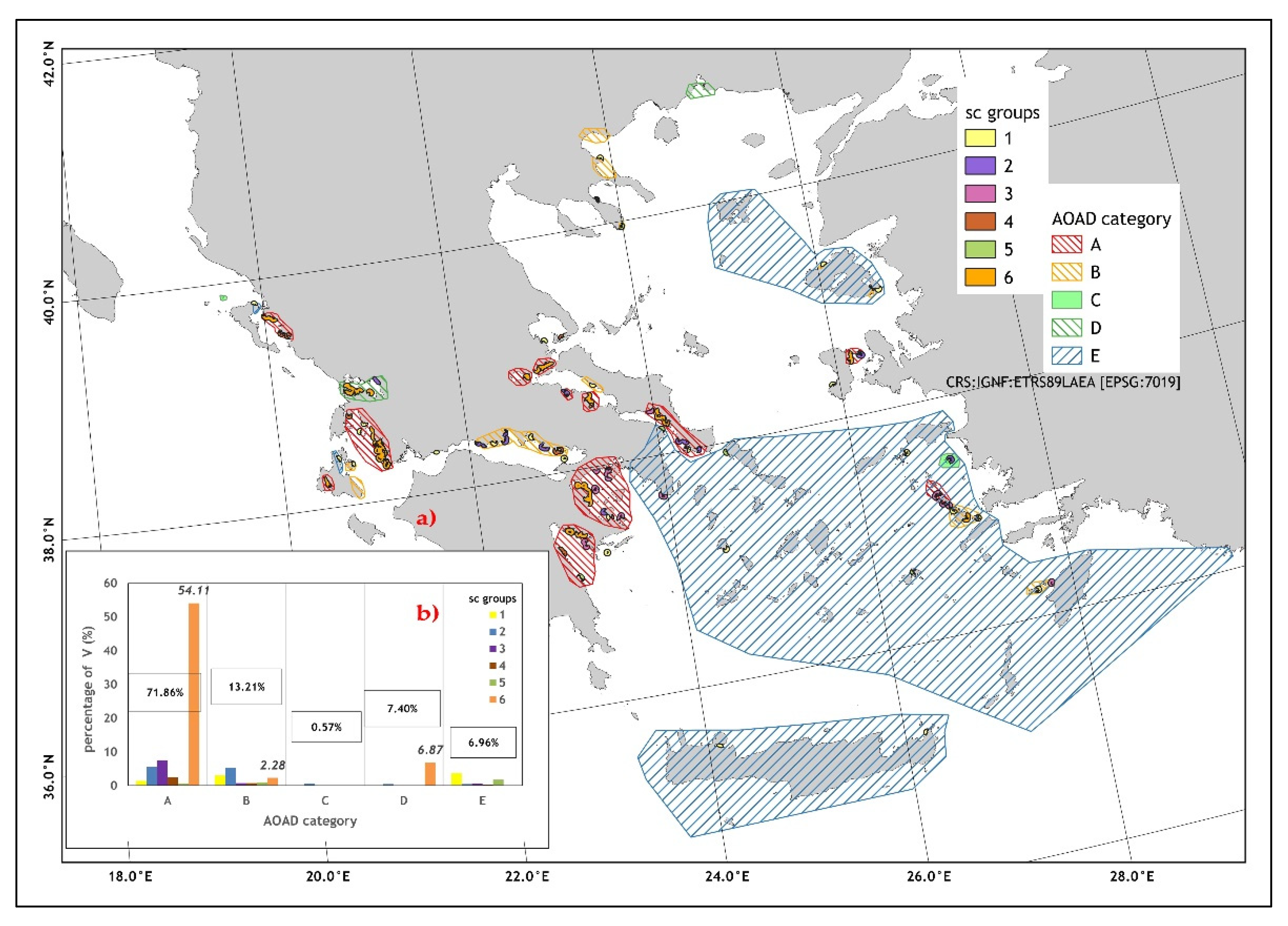
| Sc Groups | Total | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Vl [×103 m3] (%) | 1338.13 (8.00%) | 2083.01 (12.45%) | 1546.3 (9.24%) | 602.31 (3.6%) | 574.25 (3.43%) | 10,582.55 (63.27%) | 16,726.56 |
| mean number of cage arrays (range) | 1 (1–1) 1 | 3.63 (2–7) 2 | 3.9 (2–8) 2 | 5.14 (2–11) 2 | 2.2 (2–3) 2 | 18.6 (5–48) 3 | 5.77 (1–48) |
| l | 27 | 40 | 39 | 36 | 11 | 280 | 433 |
| cArea3000 (SD) [km2] | 17.86 (4.72) a | 31.18 (8.37) b | 36.37 (10.40) b | 22.84 (11.20) a | 17.05 (2.56) a | 68.31 (49.50) c | 32.78 (29.50) |
| Dst [m] | 110.11 (72.71) 1,2 | 140.32 (129.41) 1,2 | 90.59 (42.06) 1 | 96.65 (38.38) 1 | 119.71 (43.02) 1,2 | 178.59 (208.46) 2 | 154.55 (177.23) |
| Number of sc | 27 | 11 | 10 | 7 | 5 | 15 | 75 |
| total cArea3000 [km2] | 482.48 (19.62%) | 343.04 (13.95%) | 363.75 (14.79%) | 159.89 (6.5%) | 85.26 (3.47%) | 1024.73 (41.67%) | 2459.15 |
| Description | Sc Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ANOVA | ||
| F1 mFscores | sc’s intensity of impacts | 3 | 3 | 2 | 1 | 2, 3 | 2 | * |
| F1 SDFscores | Heterogeneity of sc’s intensity of impacts | np | 1 | 1 | 1 | 1 | 1 | ns |
| F2 mFscores | High distance (i > 200 m) impacted areas and their intensity of impacts | 1 | 2, 3 | 3 | 2 | 1 | 4 | * |
| F2 SDFscores | Heterogeneity of high distance (i > 200 m) impacted areas and their intensity of impacts | np | 1 | 1 | 1 | 1 | 1 | ns |
| F3 mFscores | Mid distances (50 < i ≤ 200 m) ipmacted areas | 1 | 1 | 1 | 1 | 2 | 2 | * |
| F3 SDFscores | Heterogeneity of mid distances (50 < i ≤ 200 m) impacted areas | np | 1, 2 | 1, 2 | 1 | 1 | 2 | * |
| F4 mFscores | Local impacted area (under cages: i = 0 m), its intensity of impactand type of cages | 2, 3 | 2 | 3 | 1 | 2, 3 | 2 | * |
| F4 SDFscores | Heterogeneity of local impacted area (under cages: i = 0 m), its intensity of impact and type of cages | np | 2, 3 | 1 | 3 | 1, 2 | 1, 2 | * |
| F5 mFscores | Low distance (i = 25 m) impacted area and its intensity of impact | 1, 2 | 1 | 1, 2 | 1, 2 | 1, 2 | 2 | * |
| F5 SDFscores | Heterogeneity of low distance (i = 25 m) ipmacted area and its intensity of impact | np | 1 | 1 | 1 | 1 | 1 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katselis, G.; Tsolakos, K.; Theodorou, J.A. Mapping of Greek Marine Finfish Farms and Their Potential Impact on the Marine Environment. J. Mar. Sci. Eng. 2022, 10, 286. https://doi.org/10.3390/jmse10020286
Katselis G, Tsolakos K, Theodorou JA. Mapping of Greek Marine Finfish Farms and Their Potential Impact on the Marine Environment. Journal of Marine Science and Engineering. 2022; 10(2):286. https://doi.org/10.3390/jmse10020286
Chicago/Turabian StyleKatselis, George, Konstantinos Tsolakos, and John A. Theodorou. 2022. "Mapping of Greek Marine Finfish Farms and Their Potential Impact on the Marine Environment" Journal of Marine Science and Engineering 10, no. 2: 286. https://doi.org/10.3390/jmse10020286
APA StyleKatselis, G., Tsolakos, K., & Theodorou, J. A. (2022). Mapping of Greek Marine Finfish Farms and Their Potential Impact on the Marine Environment. Journal of Marine Science and Engineering, 10(2), 286. https://doi.org/10.3390/jmse10020286








