Strengthening Angel Shark Conservation in the Northeastern Mediterranean Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Sources
2.3. Species Distribution Modeling
Spatial Prioritization Modeling
3. Results
3.1. Data Collection
3.2. Species Distribution Modeling
3.3.Spatial Prioritization Modeling
4. Discussion
Species Distributions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Title: Survey on the Presence of Angel Sharks in Greece |
|---|
|
References
- Ebert, D.A.; Fowler, S.L.; Compagno, L.J. Sharks of the World: A Fully Illustrated Guide; Wild Nature Press: Plymouth, UK, 2013; pp. 1–528. [Google Scholar]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef] [PubMed]
- Long, D.J.; Ebert, D.A.; Tavera, J.; Acero, P.A.; Robertson, D.R. Squatina mapama n. sp., a new cryptic species of angel shark (Elasmobranchii: Squatinidae) from the southwestern Caribbean Sea. J. Ocean Sci. Found. 2021, 38, 113–130. [Google Scholar]
- Compagno, L.J.V. Sharks of the world: An annotated and illustrated catalogue of shark species known to date. Part 1—Hexanchiformes to Lamniformes. In FAO Species Catalogue; FAO: Rome, Italy, 1984; Volume 4. [Google Scholar]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Ragonese, S.; Vitale, S.; Dimech, M.; Mazzola, S. Abundances of demersal sharks and chimaera from 1994–2009 scientific surveys in the central Mediterranean Sea. PLoS ONE 2013, 8, e74865. [Google Scholar] [CrossRef] [PubMed]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787.e8. [Google Scholar] [CrossRef] [PubMed]
- Morey, G.; Barker, J.; Hood, A.; Gordon, C.; Bartolí, A.; Meyers, E.K.M.; Ellis, J.; Sharp, R.; Jimenez-Alvarado, D.; Pollom, R. Squatina squatina. The IUCN Red List of Threatened Species 2019; e.T39332A117498371; International Union for Conservation of Nature: Fontainebleau, France, 2019. [Google Scholar]
- Morey, G.; Barker, J.; Bartolí, A.; Gordon, C.; Hood, A.; Meyers, E.K.M.; Pollom, R. Squatina oculata. The IUCN Red List of Threatened Species; T61418A116782036; International Union for Conservation of Nature: Fontainebleau, France, 2019. [Google Scholar] [CrossRef]
- Morey, G.; Barker, J.; Bartolí, A.; Gordon, C.; Hood, A.; Jimenez-Alvarado, D.; Meyers, E.K.M. Squatina aculeata. The IUCN Red List of Threatened Species 2019; e.T61417A116768915; International Union for Conservation of Nature: Fontainebleau, France, 2019. [Google Scholar] [CrossRef]
- Ferretti, F.; Morey, G.; Serena, F.; Mancusi, C.; Fowler, S.L.; Dipper, F.; Ellis, J.R. Squatina squatina The IUCN Red List of Threatened Species 2016; e.T39332A101695971; (Errata Version Published in 2016); International Union for Conservation of Nature: Fontainebleau, France, 2019. [Google Scholar]
- Ferretti, F.; Morey, G.; Serena, F.; Mancusi, C.; Coelho, R.P.; Seisay, M.; Litvinov, F.; Buscher, E. Squatina oculata. The IUCN Red List of Threatened Species 2016; e.T61418A16570000; International Union for Conservation of Nature: Fontainebleau, France, 2016. [Google Scholar]
- Soldo, A.; Bariche, M. Squatina aculeata. The IUCN Red List of Threatened Species 2016; e.T61417A16569265; International Union for Conservation of Nature: Fontainebleau, France, 2016. [Google Scholar]
- Gordon, C.A.; Hood, A.R.; Al Mabruk, S.A.A.; Barker, J.; Bartolí, A.; Ben Abdelhamid, S.; Bradai, M.N.; Dulvy, N.K.; Fortibuoni, T.; Giovos, I.; et al. Mediterranean Angel Sharks: Regional Action Plan; The Shark Trust: Plymouth, UK, 2019; p. 36. [Google Scholar]
- Papaconstantinou, C. Fauna Graeciae: An Updated Checklist of the Fishes in the Hellenic Seas; Hellenic Centre for Marine Research (HCMR): Athens, Greece, 2014; pp. 1–340. [Google Scholar]
- Giovos, I.; Stoilas, V.O.; Al-Mabruk, S.A.; Doumpas, N.; Marakis, P.; Maximiadi, M.; Moutopoulos, D.; Kleitou, P.; Keramidas, I.; Tiralongo, F.; et al. Integrating local ecological knowledge, citizen science and long-term historical data for endangered species conservation: Additional records of angel sharks (Chondrichthyes: Squatinidae) in the Mediterranean Sea. Aquat. Conserv. 2019, 29, 881–890. [Google Scholar] [CrossRef]
- Lawson, J.M.; Pollom, R.A.; Gordon, C.A.; Barker, J.; Meyers, E.K.; Zidowitz, H.; Ellis, J.R.; Bartolí, Á.; Morey, G.; Fowler, S.L.; et al. Extinction risk and conservation of critically endangered angel sharks in the Eastern Atlantic and Mediterranean Sea. ICES J. Mar. Sci. 2020, 77, 12–29. [Google Scholar] [CrossRef]
- Moutopoulos, D.K.; Koutsikopoulos, C. Fishing strange data in national fisheries statistics of Greece. Mar. Policy 2014, 48, 114–122. [Google Scholar] [CrossRef]
- Cashion, M.S.; Bailly, N.; Pauly, D. Official catch data underrepresent shark and ray taxa caught in Mediterranean and Black Sea fisheries. Mar. Policy 2019, 105, 1–9. [Google Scholar] [CrossRef]
- Giovos, I.; Arculeo, M.; Doumpas, N.; Katsada, D.; Maximiadi, M.; Mitsou, Ε.; Paravas, V.; Aga-Spyridopoulou, R.N.; Stoilas, V.O.; Tiralongo, F.; et al. Assessing multiple sources of data to detect illegal fishing, trade and mislabelling of elasmobranchs in Greek markets. Mar. Policy 2020, 112, 103730. [Google Scholar] [CrossRef]
- Giovos, I.; Spyridopoulou, R.A.; Doumpas, N.; Glaus, K.; Kleitou, P.; Kazlari, Z.; Katsada, D.; Loukovitis, D.; Mantzouni, I.; Papapetrou, M.; et al. Approaching the “real” state of elasmobranch fisheries and trade: A case study from the Mediterranean. Ocean Coast. Manag. 2021, 211, 105743. [Google Scholar] [CrossRef]
- Pazartzi, T.; Siaperopoulou, S.; Gubili, C.; Maradidou, S.; Loukovitis, D.; Chatzispyrou, A.; Griffiths, M.; Minos, G.; Imsiridou, A. High levels of mislabeling in shark meat–Investigating patterns of species utilization with DNA barcoding in Greek retailers. Food Control 2019, 98, 179–186. [Google Scholar] [CrossRef]
- Yığın, C.Ç.; Işmen, A.; Daban, B.; Cabbar, K.; Önal, U. Recent findings of rare sharks, Squatina oculata Bonaparte, 1840 and Squatina squatina (Linnaeus, 1758) from Gökçeada Island, Northern Aegean Sea, Turkey. J. Black Sea/Mediterr. Environ. 2019, 25, 305–314. [Google Scholar]
- Bengil, E.G.T.; Başusta, N. Chondrichthyan species as by-catch: A review on species inhabiting Turkish waters. J. Black Sea/Mediterr. Environ. 2018, 24, 288–305. [Google Scholar]
- Kabasakal, H. Finally, under protection! Status of the angel shark, Squatina squatina (Linnaeus, 1758) in Turkish Seas, with notes on a recent sighting and incidental captures. Ann. Ser. Hist. Nat. 2019, 29, 17–24. [Google Scholar]
- Meyers, E.K.; Tuya, F.; Barker, J.; Jiménez Alvarado, D.; Castro-Hernández, J.J.; Haroun, R.; Rödder, D. Population structure, distribution and habitat use of the Critically Endangered Angelshark, Squatina squatina, in the Canary Islands. Aquat. Conserv. 2017, 27, 1133–1144. [Google Scholar] [CrossRef]
- Gordon, C.A.; Hood, A.R.; Giovos, I.; Naasan Aga—Spyridopoulou, R.; Ozturk, A.A.; Yigin, C.C.; Fakioğlu, E.; Ibrahim, D.; Oruc, A.; Niedermüller, S. Mediterranean Angel Sharks: SubRegional Action Plan (SubRAP) GSAs 22/23 (Aegean Sea and Crete); The Shark Trust: Plymouth, UK, 2020; p. 12. [Google Scholar]
- Sakellariou, D.; Lykousis, V.; Karageorgis, A.; Anagnostou, C. Geomorpholgy and tectonic structure. In State of the Hellenic Marine Environment; Papathanassiou, E., Zenetos, A., Eds.; Hellenic Centre for Marine Research Publications: Athens, Greece, 2005; pp. 16–20. [Google Scholar]
- Theocharis, A.; Balopoulos, E.; Kioroglou, S.; Kontoyiannis, H.; Iona, A. A synthesis of the circulation and hydrography of the South Aegean Sea and the Straits of the Cretan Arc (March 1994–January 1995). Prog. Oceanogr. 1999, 44, 469–509. [Google Scholar] [CrossRef]
- Sini, M.; Katsanevakis, S.; Koukourouvli, N.; Gerovasileiou, V.; Dailianis, T.; Buhl-Mortensen, L.; Damalas, D.; Dendrinos, P.; Dimas, X.; Frantzis, A.; et al. Assembling ecological pieces to reconstruct the conservation puzzle of the Aegean Sea. Front. Mar. Sci. 2017, 4, 347. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Tsiros, V.Z.; Stergiou, K.I. Assessing the state of Greek marine fisheries resources. Fish. Manag. Ecol. 2013, 20, 34–41. [Google Scholar] [CrossRef]
- Sini, M.; Vatikiotis, K.; Thanopoulou, Z.; Katsoupis, C.; Maina, I.; Kavadas, S.; Karachle, P.K.; Katsanevakis, S. Small-scale coastal fishing shapes the structure of shallow rocky reef fish in the Aegean Sea. Front. Mar. Sci. 2019, 6, 599. [Google Scholar] [CrossRef]
- DCF (2021) Annual Reports 2004–2019. Available online: https://datacollection.jrc.ec.europa.eu/ars (accessed on 2 November 2021).
- Guisan, A.; Thuiller, W.; Zimmermann, N. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017; pp. 1–478. [Google Scholar]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Assis, J.; Tyberghein, L.; Bosch, S.; Verbruggen, H.; Serrão, E.A.; De Clerck, O. Bio-ORACLE v2. 0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 2018, 27, 277–284. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Modell. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiello-Lammens, M.E.; Muscarella, R.; Merow, C.; Anderson, R.P. Wallace: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods Ecol. Evol. 2018, 9, 1151–1156. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecol. Modell. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, M.; Moilanen, A. Design of reserve networks and the persistence of biodiversity. Trends Ecol. Evol. 2001, 16, 242–248. [Google Scholar] [CrossRef]
- Lehtomäki, J.; Moilanen, A. Methods and workflow for spatial conservation prioritization using zonation. Environ. Model. Softw. 2013, 47, 128–137. [Google Scholar] [CrossRef]
- Moilanen, A.; Leathwick, J.R.; Quinn, J.M. Spatial prioritization of conservation management. Conserv. Lett. 2011, 4, 383–393. [Google Scholar] [CrossRef]
- Moilanen, A.; Wilson, K.A.; Possingham, H.P. Spatial Conservation Prioritization: Quantitative Methods and Computational Tools; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Moilanen, A.; Pouzols, F.; Meller, L.; Veach, V.; Arponen, A.; Leppänen, J.; Kujala, H. Zonation—Spatial Conservation Planning Methods and Software; Version 4, User Manual; University of Helsinski: Helsinski, Finland, 2014. [Google Scholar]
- Petza, D.; Maina, I.; Koukourouvli, N.; Dimarchopoulou, D.; Akrivos, D.; Kavadas, S.; Tsikliras, A.; Karachle, P.; Katsanevakis, S. Where not to fish—reviewing and mapping fisheries restricted areas in the Greek Aegean Sea. Med. Mar. Sci. 2017, 18, 310–323. [Google Scholar] [CrossRef]
- Corsini, M.; Zava, B. Recent capture of Squatina oculata and Squatina aculeata from Dodecanese Islands (SE Aegean Sea, Eastern Mediterranean). Biol. Mar. Mediterr. 2007, 14, 352–353. [Google Scholar]
- Filiz, H.; Irmak, E.; Mater, S. Occurrence of Squatina aculeata Cuvier, 1829 (Elasmobranchii, Squatinidae) from the Aegean Sea, Turkey. J. Fish. Aquat. Sci. 2005, 22, 451–452. [Google Scholar]
- Kabasakal, H.; Kabasakal, E. Sharks captured by commercial fishing vessels off the coast of Turkey in the northern Aegean Sea. Ann. Ser. Hist. Nat. 2004, 14, 171–180. [Google Scholar]
- Karakulak, F.S.; Erk, H.; Bilgin, B. Length–weight relationships for 47 coastal fish species from the northern Aegean Sea, Turkey. J. Appl. Ichthyol. 2006, 22, 274–278. [Google Scholar] [CrossRef]
- Ismen, A.; Cigdem Yigin, C.; Altinagac, U.; Ayaz, A.D.N.A.N. Length–weight relationships for ten shark species from Saros Bay (North Aegean Sea). J. Appl. Ichthyol. 2009, 25, 109–112. [Google Scholar] [CrossRef]
- Kara, A.; Saglam, C.; Acarli, D.; Cengiz, Ö. Length-weight relationships for 48 fish species of the Gediz estuary, in Izmir Bay (Central Aegean Sea, Turkey). J. Mar. Biol. Assoc. UK 2017, 98, 879. [Google Scholar] [CrossRef]
- Öğretmen, F.; Yılmaz, F.; Torcu Koç, H. An Investigation on Fishes of Gökova Bay (Southern Aegean Sea). Balıkesir Üniv. Fen Bilim. Enst. Dergisi 2005, 7, 19–36. [Google Scholar]
- Akyol, O.; Ünal, V.; Capapé, C. Occurrence and biological observations on angel shark Squatina squatina (Chondrichthyes: Squatinidae) from the Turkish waters (Eastern Mediterranean). Turkish J. Fish. Aquat. Sci. 2015, 15, 931–935. [Google Scholar]
- Jones, M.C.; Dye, S.R.; Fernandes, J.A.; Frölicher, T.L.; Pinnegar, J.K.; Warren, R.; Cheung, W.W.L. Predicting the impact of climate change on threatened species in UK waters. PLoS ONE 2013, 8, e54216. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, M.; Giovos, I. New records of the critically endangered Squatina squatina (Linnaeus, 1758) from Corsica, France. Acta Adriat. 2019, 60, 205–209. [Google Scholar] [CrossRef]
- Fortibuoni, T.; Borme, D.; Franceschini, G.; Giovanardi, O.; Raicevich, S. Common, rare or extirpated? Shifting baselines for common angelshark, Squatina squatina (Elasmobranchii: Squatinidae), in the Northern Adriatic Sea (Mediterranean Sea). Hydrobiologia 2016, 772, 247–259. [Google Scholar] [CrossRef]
- Holcer, D.; Lazar, B. New data on the occurrence of the critically endangered common angelshark, Squatina squatina, in the Croatian Adriatic Sea. Nat. Croat. 2017, 26, 313–320. [Google Scholar] [CrossRef]
- Al-Mabruk, S.A.A.; Rizgalla, J.; Giovos, I. Preliminary evidences of illegal elasmobranch fishing in Libya. In Proceedings of the 23rd Annual Conference, European Elasmobranch Association, Rende, Italy, 16–18 October 2019. [Google Scholar]
- Zava, B.; Insacco, G.; Corsini-Foka, M.; Serena, F. Updating records of Squatina aculeata (Elasmobranchii: Squatiniformes: Squatinidae) in the Mediterranean Sea. Acta Ichthyol. Piscat. 2020, 50, 401–411. [Google Scholar] [CrossRef]
- SPA/RAC–UN Environment/MAP. Action Plan for the Conservation of Cartilaginous Fishes (Chondrichtyans) in the Mediterranean Sea; Bradai, M.N., Ed.; SPA/RAC: Tunis, Tunisia, 2020; p. 18. [Google Scholar]
- Milazzo, M.; Cattano, C.; Al Mabruk, S.A.; Giovos, I. Mediterranean sharks and rays need action. Science 2021, 371, 355–356. [Google Scholar] [CrossRef]
- WWF. Mediterranean Marine Initiative, “Sharks and Rays: A Deadly Harvest”. 2020. Available online: https://wwfeu.awsassets.panda.org/downloads/wwf_iuu_sharks_and_rays_briefing_2020_v6__1_.pdf (accessed on 1 September 2021).
- Noviello, N.; McGonigle, C.; Jacoby, D.M.; Meyers, E.K.; Jiménez-Alvarado, D.; Barker, J. Modelling Critically Endangered marine species: Bias-corrected citizen science data inform habitat suitability for the angelshark (Squatina squatina). Aquat. Conserv. 2021, 31, 3451–3465. [Google Scholar] [CrossRef]
- Agardy, M.T. Advances in marine conservation: The role of marine protected areas. Trends Ecol. Evol. 1994, 9, 267–270. [Google Scholar] [CrossRef]
- Evans, P.G. Marine Protected Areas and marine spatial planning for the benefit of marine mammals. J. Mar. Biol. Assoc. UK 2018, 98, 973–976. [Google Scholar] [CrossRef]
- MacKeracher, T.; Diedrich, A.; Simpfendorfer, C.A. Sharks, rays and marine protected areas: A critical evaluation of current perspectives. Fish Fish. 2019, 20, 255–267. [Google Scholar] [CrossRef]
- MPAtlas. Marine Protected Atlas. 2016. Available online: www.mpatlas.org (accessed on 1 September 2021).
- Katsanevakis, S.; Coll, M.; Fraschetti, S.; Giakoumi, S.; Goldsborough, D.; Mačić, V.; Mackelworth, P.; Rilov, G.; Stelzenmüller, V.; Albano, P.G.; et al. Twelve recommendations for advancing marine conservation in European and contiguous seas. Front. Mar. Sci. 2020, 7, 879. [Google Scholar] [CrossRef]
- Pressey, R.L.; Bottrill, M.C. Approaches to landscape-and seascape-scale conservation planning: Convergence, contrasts and challenges. Oryx 2009, 43, 464–475. [Google Scholar] [CrossRef]
- Mazor, T.; Possingham, H.P.; Edelist, D.; Brokovich, E.; Kark, S. The crowded sea: Incorporating multiple marine activities in conservation plans can significantly alter spatial priorities. PLoS ONE 2014, 9, e104489. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pilgrim, J.D.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar]
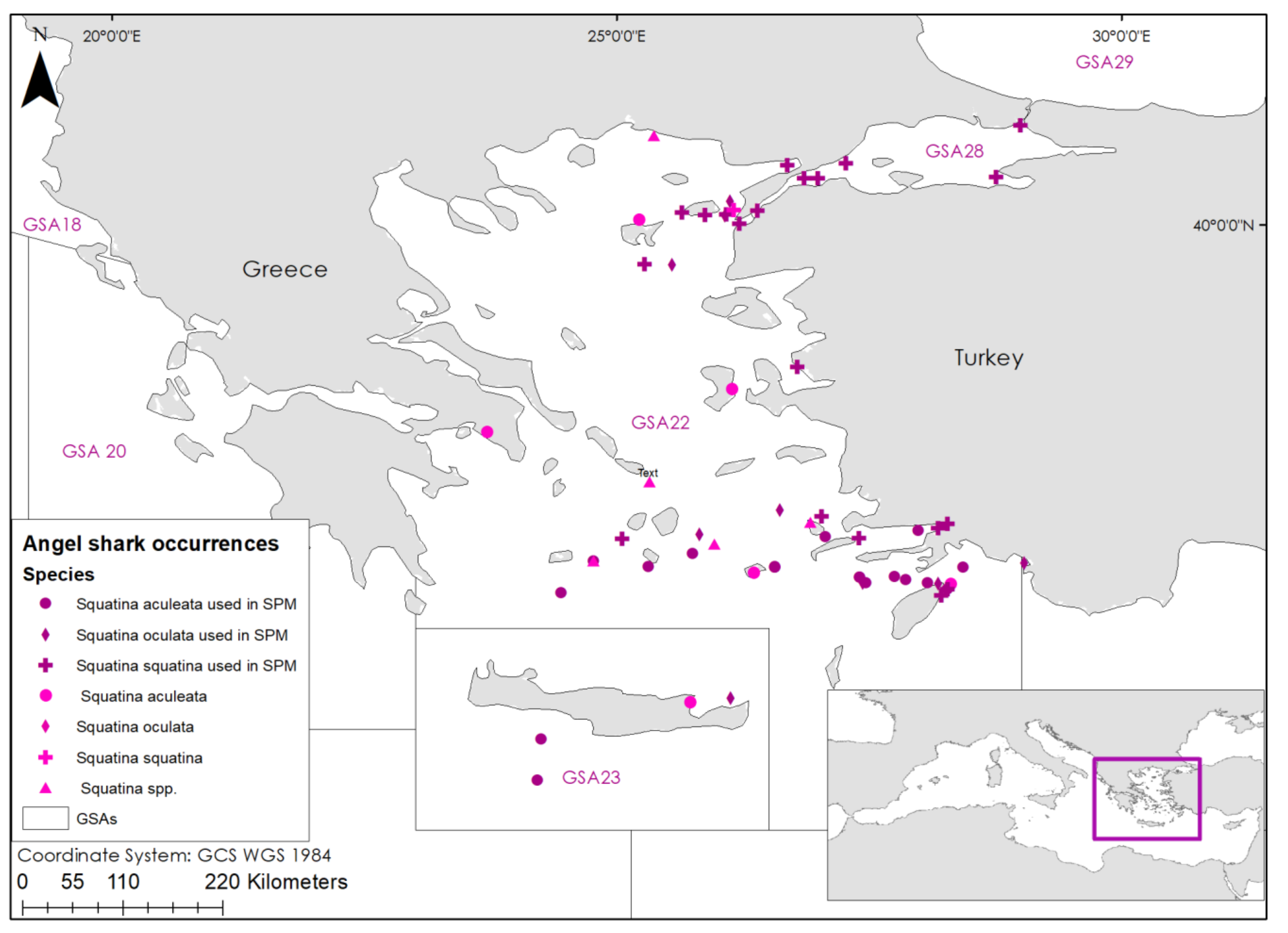

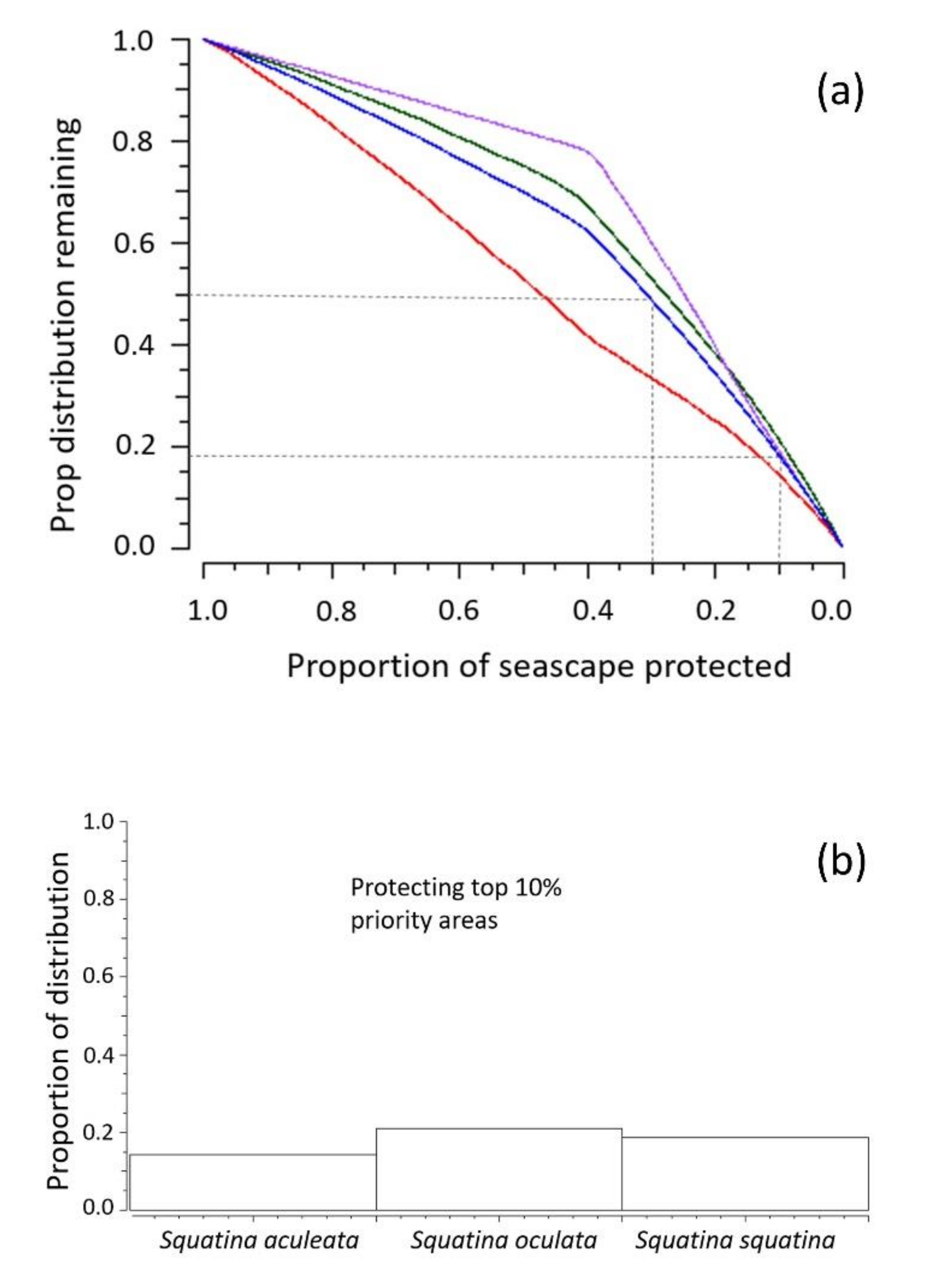
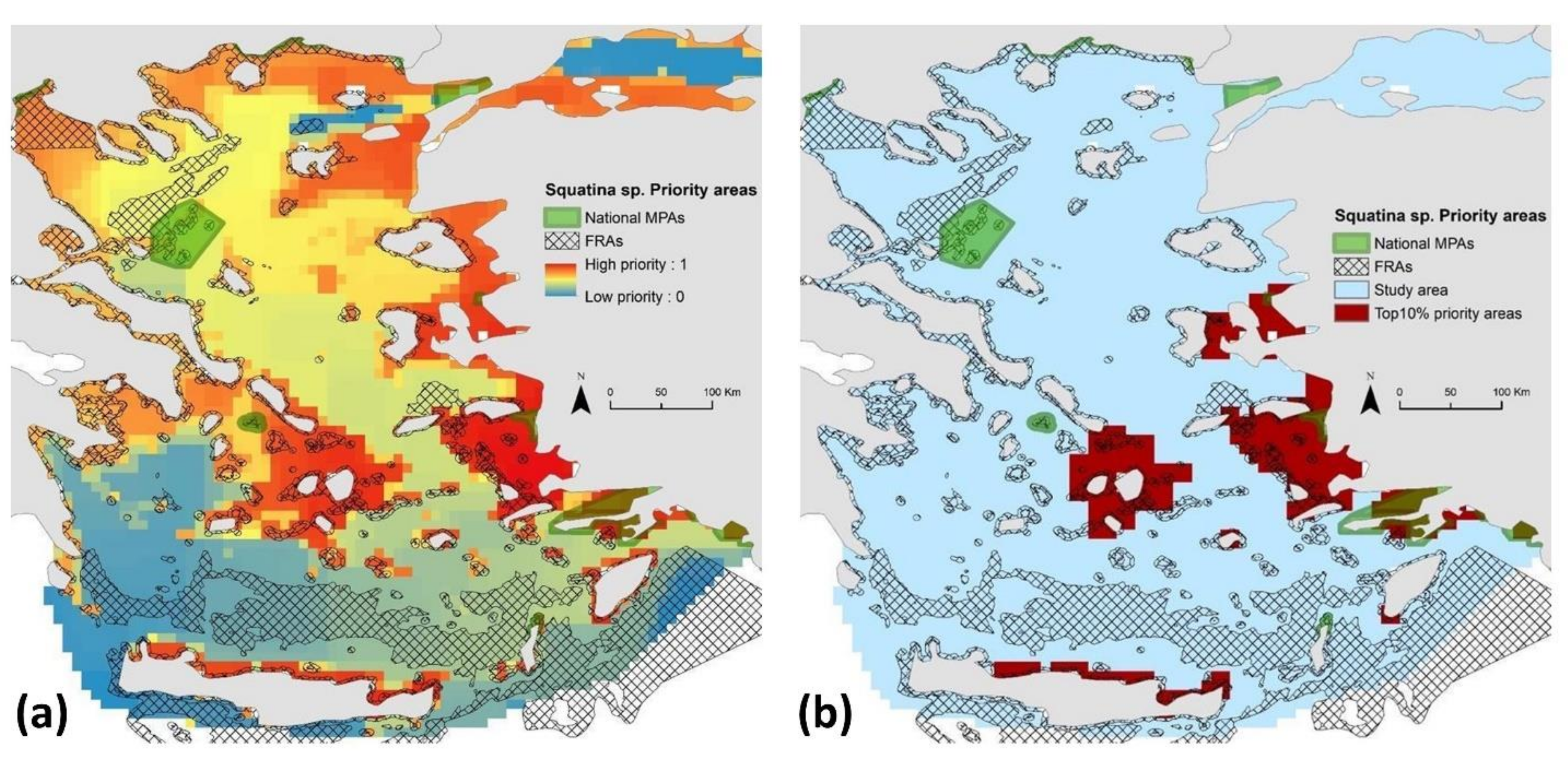
| N | Species | Year of Sighting | Type of Report |
|---|---|---|---|
| 1 | Squatina aculeata | 2020 | The M.E.C.O. Project |
| 2 * | Squatina aculeata | 2018 | [16] |
| 3 * | Squatina aculeata | 2018 | [16] |
| 4 | Squatina aculeata | 2020 | The M.E.C.O. Project |
| 5 * | Squatina aculeata | 2013 | The M.E.C.O. Project |
| 6 * | Squatina aculeata | 2004 | [53] |
| 7 * | Squatina aculeata | 2006 | [53] |
| 8 * | Squatina aculeata | 2018 | [16] |
| 9 * | Squatina aculeata | 2015–2016 | [30] |
| 10 * | Squatina aculeata | 2015–2016 | [30] |
| 11 * | Squatina aculeata | 2015–2016 | [30] |
| 12 * | Squatina aculeata | 2015–2016 | [30] |
| 13 * | Squatina aculeata | 2015–2016 | [30] |
| 14 * | Squatina aculeata | 2015–2016 | [30] |
| 15 * | Squatina aculeata | 2015–2016 | [30] |
| 16 * | Squatina aculeata | 2015–2016 | [30] |
| 17 * | Squatina aculeata | 2015–2016 | [30] |
| 18 * | Squatina aculeata | 2005 | [54] |
| 19 * | Squatina aculeata | 2015 | [32] |
| 20 * | Squatina aculeata | 2015 | [32] |
| 21 * | Squatina aculeata | 2016 | [32] |
| 22 * | Squatina aculeata | 2020 | [32] |
| 23 | Squatina aculeata | 1979 | The M.E.C.O. Project |
| 24 | Squatina aculeata | 2020 | Current project |
| 25 | Squatina aculeata | 2020 | Current project |
| 26 | Squatina aculeata | 2019 | The M.E.C.O. Project |
| 27 * | Squatina oculata | 1999 | [55] |
| 28 * | Squatina oculata | 2015 | The M.E.C.O. Project |
| 29 * | Squatina oculata | 2004 | [53] |
| 30 * | Squatina oculata | 2020 | ASSM |
| 31 * | Squatina oculata | 2015–2016 | [30] |
| 32 * | Squatina oculata | 2015–2016 | [30] |
| 33 * | Squatina oculata | 2015–2016 | [30] |
| 34 * | Squatina oculata | 2015–2016 | [30] |
| 35 * | Squatina oculata | 1997 | [55] |
| 36 | Squatina oculata | 2018 | [23] |
| 37 | Squatina spp. | 2018 | [16] |
| 38 | Squatina spp. | 2018 | [16] |
| 39 | Squatina spp. | 1983 | The M.E.C.O. Project |
| 40 | Squatina spp. | 2020 | Current project |
| 41 | Squatina spp. | 2019 | Current project |
| 42 * | Squatina squatina | 2018 | The M.E.C.O. Project |
| 43 * | Squatina squatina | 2020 | Angel Shark Conservation Network |
| 44 * | Squatina squatina | 2017 | SharkPulse |
| 45 * | Squatina squatina | 2015–2016 | [30] |
| 46 * | Squatina squatina | 1997 | [55] |
| 47 * | Squatina squatina | 2004–2005 | [56] |
| 48 * | Squatina squatina | 1996 | [55] |
| 49 * | Squatina squatina | 2020 | ASSM |
| 50 * | Squatina squatina | 2013 | [25] |
| 51 * | Squatina squatina | 2005–2008 | [57] |
| 52 * | Squatina squatina | 2010–2014 | [58] |
| 53 * | Squatina squatina | 2015 | [25] |
| 54 * | Squatina squatina | 2018 | [25] |
| 55 * | Squatina squatina | 2015 | [25] |
| 56 * | Squatina squatina | 2018 | [25] |
| 57 * | Squatina squatina | 2015 | [25] |
| 58 * | Squatina squatina | 2000–2001 | [59] |
| 59 * | Squatina squatina | 2015 | [60] |
| 60 * | Squatina squatina | 2014 | [25] |
| 61 * | Squatina squatina | 2012 | [25] |
| 62 | Squatina squatina | 2014 | SharkPulse |
| 63 | Squatina squatina | 2018 | [23] |
| 64 | Squatina squatina | 2020 | The M.E.C.O Project |
| Species | Settings | Features | rm | Train.AUC | Avg.Test.AUC | Occurrence Points Used in the Model |
|---|---|---|---|---|---|---|
| Squatina aculeata | H_3.5 | H | 3.5 | 0.72 | 0.71 | 14 |
| Squatina squatina | H_3.5 | H | 3.5 | 0.84 | 0.83 | 19 |
| Squatina oculata | H_3.5 | H | 3.5 | 0.90 | 0.83 | 9 |
| Actions to Secure the Conservation of Angel Sharks in the Aegean Sea | |
|---|---|
| Greece |
|
| |
| |
| |
| |
| |
| |
| Turkey |
|
| |
| |
| |
| |
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovos, I.; Katsada, D.; Spyridopoulou, R.N.A.; Poursanidis, D.; Doxa, A.; Katsanevakis, S.; Kleitou, P.; Oikonomou, V.; Minasidis, V.; Ozturk, A.A.; et al. Strengthening Angel Shark Conservation in the Northeastern Mediterranean Sea. J. Mar. Sci. Eng. 2022, 10, 269. https://doi.org/10.3390/jmse10020269
Giovos I, Katsada D, Spyridopoulou RNA, Poursanidis D, Doxa A, Katsanevakis S, Kleitou P, Oikonomou V, Minasidis V, Ozturk AA, et al. Strengthening Angel Shark Conservation in the Northeastern Mediterranean Sea. Journal of Marine Science and Engineering. 2022; 10(2):269. https://doi.org/10.3390/jmse10020269
Chicago/Turabian StyleGiovos, Ioannis, Dimitra Katsada, Roxani Naasan Aga Spyridopoulou, Dimitrios Poursanidis, Aggeliki Doxa, Stelios Katsanevakis, Periklis Kleitou, Vasiliki Oikonomou, Vasileios Minasidis, Ayaka A. Ozturk, and et al. 2022. "Strengthening Angel Shark Conservation in the Northeastern Mediterranean Sea" Journal of Marine Science and Engineering 10, no. 2: 269. https://doi.org/10.3390/jmse10020269
APA StyleGiovos, I., Katsada, D., Spyridopoulou, R. N. A., Poursanidis, D., Doxa, A., Katsanevakis, S., Kleitou, P., Oikonomou, V., Minasidis, V., Ozturk, A. A., Petza, D., Sini, M., Yigin, C. C., Meyers, E. K. M., Barker, J., Jiménez-Alvarado, D., & Hood, A. R. (2022). Strengthening Angel Shark Conservation in the Northeastern Mediterranean Sea. Journal of Marine Science and Engineering, 10(2), 269. https://doi.org/10.3390/jmse10020269












