Discovering the Characteristics of Community Structures and Functional Properties of Epiphytic Bacteria on Spartina alterniflora in the Coastal Salt Marsh Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Physicochemical Properties of Sampling Water
2.3. DNA Extraction and Sequencing
2.4. Sequence Processing and Analysis
2.5. Statistical Analyses
3. Results
3.1. Physicochemical Properties of Sampling Water in Different Seasons
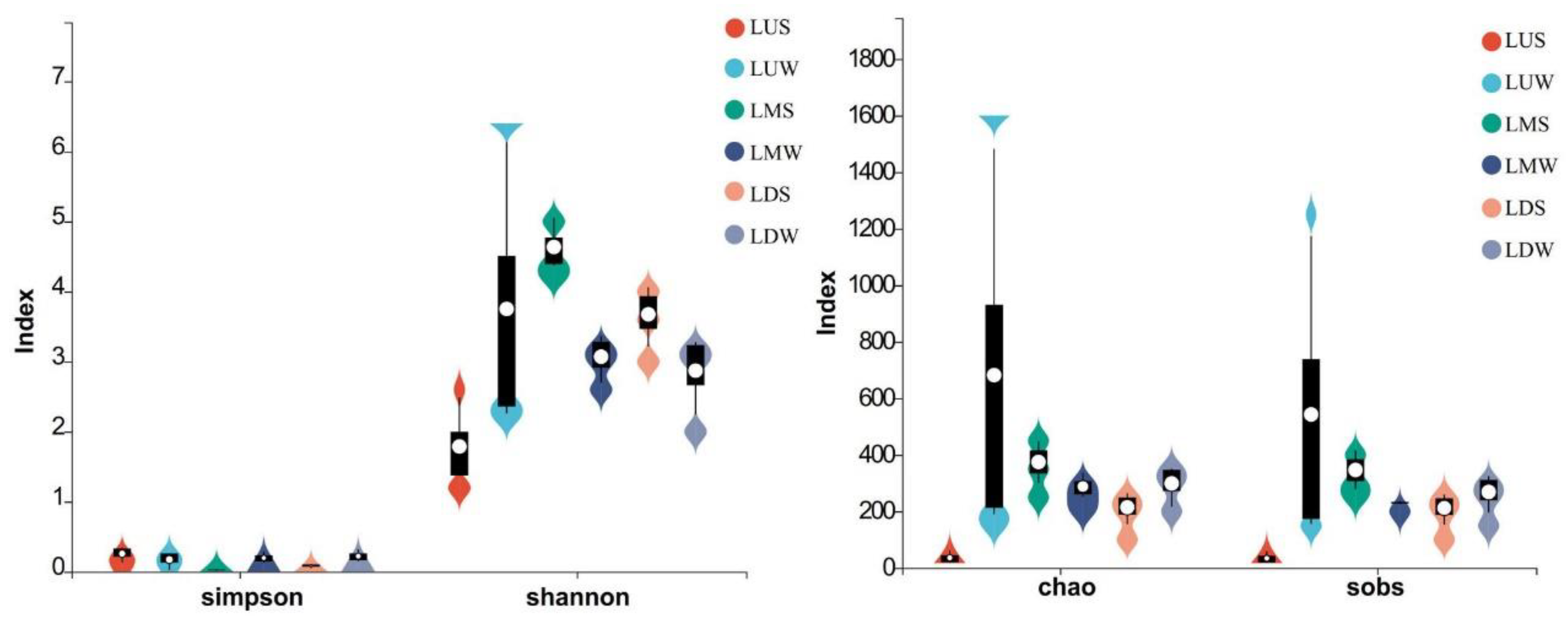
3.2. Diversity of the Prokaryotic Community
3.3. Structure and Distribution Patterns of Prokaryotic Communities
3.4. Potential Factors Influencing the Prokaryotic Communities
3.5. Functional Characteristics of the Bacterial Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wails, C.N.; Baker, K.; Blackburn, R.; Del Vallé, A.; Heise, J.; Herakovich, H.; Holthuijzen, W.A.; Nissenbaum, M.P.; Rankin, L.; Savage, K.; et al. Assessing Changes to Ecosystem Structure and Function Following Invasion by Spartina Alterniflora and Phragmites Australis: A Meta-Analysis. Biol. Invasions 2021, 23, 2695–2709. [Google Scholar] [CrossRef]
- Chung, C.H.; Zhuo, R.Z.; Xu, G.W. Creation of Spartina Plantations for Reclaiming Dongtai, China, Tidal Flats and Offshore Sands. Ecol. Eng. 2004, 23, 135–150. [Google Scholar] [CrossRef]
- Liu, M.; Mao, D.; Wang, Z.; Li, L.; Man, W.; Jia, M.; Ren, C.; Zhang, Y. Rapid Invasion of Spartina Alterniflora in the Coastal Zone of Mainland China: New Observations from Landsat OLI Images. Remote Sens. 2018, 10, 1933. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y. Spatial Distribution of an Invasive Plant Spartina Alterniflora and Its Potential as Biofuels in China. Ecol. Eng. 2013, 52, 175–181. [Google Scholar] [CrossRef]
- Zuo, P.; Zhao, S.; Liu, C.; Wang, C.; Liang, Y. Distribution of Spartina Spp. along China’s Coast. Ecol. Eng. 2012, 40, 160–166. [Google Scholar] [CrossRef]
- Deng, Z.; Deng, Z.; An, S.; Wang, Z.; Liu, Y.; Ouyang, Y.; Zhou, C.; Zhi, Y.; Li, H. Habitat Choice and Seed–Seedling Conflict of Spartina Alterniflora on the Coast of China. Hydrobiologia 2009, 630, 287–297. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.; Wang, W.; Chen, L.; Lin, G. Interactions between Mangroves and Exotic Spartina in an Anthropogenically Disturbed Estuary in Southern China. Ecology 2012, 93, 588–597. [Google Scholar] [CrossRef]
- Li, S.-H.; Ge, Z.-M.; Tan, L.-S.; Zhou, K.; Hu, Z.-J. Coupling Scirpus Recruitment with Spartina Control Guarantees Recolonization of Native Sedges in Coastal Wetlands. Ecol. Eng. 2021, 166, 106246. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Knief, C.; Frances, L.; Vorholt, J.A. Competitiveness of Diverse Methylobacterium Strains in the Phyllosphere of Arabidopsis Thaliana and Identification of Representative Models, Including M. Extorquens PA1. Microb. Ecol. 2010, 60, 440–452. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial Life in the Phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, E.J.N.; Vogel, C.M.; Ueoka, R.; Schäfer, M.; Ryffel, F.; Müller, D.B.; Probst, S.; Kreuzer, M.; Piel, J.; Vorholt, J.A. Bipartite Interactions, Antibiotic Production and Biosynthetic Potential of the Arabidopsis Leaf Microbiome. Nat. Microbiol. 2018, 3, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Gu, L.; Sun, B.; Cai, W.; Zhang, S.; Zhuang, G.; Bai, Z.; Zhuang, X. Seasonal Variation of Epiphytic Bacteria in the Phyllosphere of Gingko Biloba, Pinus Bungeana and Sabina Chinensis. FEMS Microbiol. Ecol. 2020, 96, fiaa017. [Google Scholar] [CrossRef]
- Qian, X.; Li, S.; Wu, B.; Wang, Y.; Ji, N.; Yao, H.; Cai, H.; Shi, M.; Zhang, D. Mainland and Island Populations of Mussaenda Kwangtungensis Differ in Their Phyllosphere Fungal Community Composition and Network Structure. Sci. Rep. 2020, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Cordier, T.; Robin, C.; Capdevielle, X.; Desprez-Loustau, M.-L.; Vacher, C. Spatial Variability of Phyllosphere Fungal Assemblages: Genetic Distance Predominates over Geographic Distance in a European Beech Stand (Fagus Sylvatica). Fungal Ecol. 2012, 5, 509–520. [Google Scholar] [CrossRef]
- Peñuelas, J.; Rico, L.; Ogaya, R.; Jump, A.S.; Terradas, J. Summer Season and Long-Term Drought Increase the Richness of Bacteria and Fungi in the Foliar Phyllosphere of Quercus Ilex in a Mixed Mediterranean Forest. Plant Biol. 2012, 14, 565–575. [Google Scholar] [CrossRef]
- Rastogi, G.; Sbodio, A.; Tech, J.J.; Suslow, T.V.; Coaker, G.L.; Leveau, J.H.J. Leaf Microbiota in an Agroecosystem: Spatiotemporal Variation in Bacterial Community Composition on Field-Grown Lettuce. ISME J. 2012, 6, 1812–1822. [Google Scholar] [CrossRef]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Rei, F.; do Rosário Félix, M. Spatial and Temporal Variation of Fungal Endophytic Richness and Diversity Associated to the Phyllosphere of Olive Cultivars. Fungal Biol. 2019, 123, 66–76. [Google Scholar] [CrossRef]
- Beattie, G.A. Water Relations in the Interaction of Foliar Bacterial Pathogens with Plants. Annu. Rev. Phytopathol. 2011, 49, 533–555. [Google Scholar] [CrossRef]
- Joung, Y.S.; Ge, Z.; Buie, C.R. Bioaerosol Generation by Raindrops on Soil. Nat. Commun. 2017, 8, 14668. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Ors, S.; Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Influence of Seasonal Changes and Salinity on Spinach Phyllosphere Bacterial Functional Assemblage. PLoS ONE 2021, 16, e0252242. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Sun, Y.; Liu, P.; Wang, Y.; Huang, Y.; Gao, Y.; Hu, X. Invasion of Spartina Alterniflora on Zostera Japonica Enhances the Abundances of Bacteria by Absolute Quantification Sequencing Analysis. Ecol. Evol. 2022, 12(5), e8939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, Y.; Liu, M.; Chang, Y.; Yan, X.; Bu, R.; Zhao, D.; Li, Z. Introduction and Spread of an Exotic Plant, Spartina Alterniflora, Along Coastal Marshes of China. Wetlands 2017, 37, 1181–1193. [Google Scholar] [CrossRef]
- Li, H.; Mao, D.; Wang, Z.; Huang, X.; Li, L.; Jia, M. Invasion of Spartina Alterniflora in the Coastal Zone of Mainland China: Control Achievements from 2015 to 2020 towards the Sustainable Development Goals. J. Environ. Manag. 2022, 323, 116242. [Google Scholar] [CrossRef]
- Gnanamanickam, S.S.; Immanuel, J.E. Epiphytic Bacteria, Their Ecology and Functions. Plant-Assoc. Bact. 2007, 131–153. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Jia, J.; Wang, W.; Wang, X.; Zhao, Q.; Lu, Q. Shifts of Soil Microbial Community Composition along a Short-Term Invasion Chronosequence of Spartina Alterniflora in a Chinese Estuary. Sci. Total Environ. 2019, 657, 222–233. [Google Scholar] [CrossRef]
- Zheng, J.; Li, J.; Lan, Y.; Liu, S.; Zhou, L.; Luo, Y.; Liu, J.; Wu, Z. Effects of Spartina Alterniflora Invasion on Kandelia Candel Rhizospheric Bacterial Community as Determined by High-Throughput Sequencing Analysis. J. Soils Sediments 2019, 19, 332–344. [Google Scholar] [CrossRef]
- Bringel, F.; Couée, I. Pivotal Roles of Phyllosphere Microorganisms at the Interface between Plant Functioning and Atmospheric Trace Gas Dynamics. Front. Microbiol. 2015, 6, 486. [Google Scholar] [CrossRef]
- He, Q.; Cui, B.; Cai, Y.; Deng, J.; Sun, T.; Yang, Z. What Confines an Annual Plant to Two Separate Zones along Coastal Topographic Gradients? Hydrobiologia 2009, 630, 327–340. [Google Scholar] [CrossRef]
- He, Q.; Cui, B.; An, Y. The Importance of Facilitation in the Zonation of Shrubs along a Coastal Salinity Gradient: Zonation and Facilitation along Salinity Gradients. J. Veg. Sci. 2011, 22, 828–836. [Google Scholar] [CrossRef]
- Nasanit, R.; Krataithong, K.; Tantirungkij, M.; Limtong, S. Assessment of Epiphytic Yeast Diversity in Rice (Oryza Sativa) Phyllosphere in Thailand by a Culture-Independent Approach. Antonie Van Leeuwenhoek 2015, 107, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S rRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Yang, L.; Huang, P.; Li, W.; Wang, S.; Zhao, G.; Zhang, M.; Pang, X.; Yan, Z.; et al. Structural Modulation of Gut Microbiota in Life-Long Calorie-Restricted Mice. Nat. Commun. 2013, 4, 2163. [Google Scholar] [CrossRef]
- Liang, S.; Deng, J.; Jiang, Y.; Wu, S.; Zhou, Y.; Zhu, W. Functional Distribution of Bacterial Community under Different Land Use Patterns Based on FaProTax Function Prediction. Pol. J. Environ. Stud. 2020, 29, 1245–1261. [Google Scholar] [CrossRef]
- Shenoy, D.M.; Suresh, I.; Uskaikar, H.; Kurian, S.; Vidya, P.J.; Shirodkar, G.; Gauns, M.U.; Naqvi, S.W.A. Variability of Dissolved Oxygen in the Arabian Sea Oxygen Minimum Zone and Its Driving Mechanisms. J. Mar. Syst. 2020, 204, 103310. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant Beneficial Endophytic Bacteria: Mechanisms, Diversity, Host Range and Genetic Determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, P.; Sáenz de Miera, L.E.; Ansola, G. Influence of Environmental Variables on the Structure and Composition of Soil Bacterial Communities in Natural and Constructed Wetlands. Sci. Total Environ. 2015, 506–507, 380–390. [Google Scholar] [CrossRef]
- Yang, W.; Jeelani, N.; Xia, L.; Zhu, Z.; Luo, Y.; Cheng, X.; An, S. Soil Fungal Communities Vary with Invasion by the Exotic Spartina Alternifolia Loisel. in Coastal Salt Marshes of Eastern China. Plant Soil 2019, 442, 215–232. [Google Scholar] [CrossRef]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community Proteogenomics Reveals Insights into the Physiology of Phyllosphere Bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [PubMed]
- Knief, C.; Delmotte, N.; Chaffron, S.; Stark, M.; Innerebner, G.; Wassmann, R.; von Mering, C.; Vorholt, J.A. Metaproteogenomic Analysis of Microbial Communities in the Phyllosphere and Rhizosphere of Rice. ISME J. 2012, 6, 1378–1390. [Google Scholar] [CrossRef]
- Pan, Y.; Kang, P.; Hu, J.; Song, N. Bacterial Community Demonstrates Stronger Network Connectivity than Fungal Community in Desert-Grassland Salt Marsh. Sci. Total Environ. 2021, 798, 149118. [Google Scholar] [CrossRef]
- Kim, M.; Singh, D.; Lai-Hoe, A.; Go, R.; Abdul Rahim, R.; Ainuddin, A.; Chun, J.; Adams, J.M. Distinctive Phyllosphere Bacterial Communities in Tropical Trees. Microb. Ecol. 2012, 63, 674–681. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial Communities Associated with the Leaves and the Roots of Arabidopsis Thaliana. PLoS ONE 2013, 8, e56329. [Google Scholar] [CrossRef]
- Berg, G.; Krechel, A.; Ditz, M.; Sikora, R.A.; Ulrich, A.; Hallmann, J. Endophytic and Ectophytic Potato-Associated Bacterial Communities Differ in Structure and Antagonistic Function against Plant Pathogenic Fungi. FEMS Microbiol. Ecol. 2005, 51, 215–229. [Google Scholar] [CrossRef]
- Dong, C.-J.; Wang, L.-L.; Li, Q.; Shang, Q.-M. Bacterial Communities in the Rhizosphere, Phyllosphere and Endosphere of Tomato Plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef]
- Lindow, S.E.; Leveau, J.H.J. Phyllosphere Microbiology. Curr. Opin. Biotechnol. 2002, 13, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Kasirajan, L.; Adams, Z.; Couto-Rodriguez, R.L.; Gal, D.; Jia, H.; Mondragon, P.; Wassel, P.C.; Yu, D.; Uthandi, S.; Maupin-Furlow, J.A. Chapter Twelve-High-Level Synthesis and Secretion of Laccase, a Metalloenzyme Biocatalyst, by the Halophilic Archaeon Haloferax Volcanii. In Methods in Enzymology; Kelman, Z., O’Dell, W.B., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 659, pp. 297–313. [Google Scholar]
- He, D.; Ren, L.; Wu, Q. Epiphytic Bacterial Communities on Two Common Submerged Macrophytes in Taihu Lake: Diversity and Host-Specificity. Chin. J. Oceanol. Limnol. 2012, 30, 237–247. [Google Scholar] [CrossRef]
- Murphy, C.L.; Biggerstaff, J.; Eichhorn, A.; Ewing, E.; Shahan, R.; Soriano, D.; Stewart, S.; VanMol, K.; Walker, R.; Walters, P.; et al. Genomic Characterization of Three Novel Desulfobacterota Classes Expand the Metabolic and Phylogenetic Diversity of the Phylum. Environ. Microbiol. 2021, 23, 4326–4343. [Google Scholar] [CrossRef]
- Qin, Y.; Hou, J.; Deng, M.; Liu, Q.; Wu, C.; Ji, Y.; He, X. Bacterial Abundance and Diversity in Pond Water Supplied with Different Feeds. Sci. Rep. 2016, 6, 35232. [Google Scholar] [CrossRef]
- Schlechter, R.O.; Miebach, M.; Remus-Emsermann, M.N.P. Driving Factors of Epiphytic Bacterial Communities: A Review. J. Adv. Res. 2019, 19, 57–65. [Google Scholar] [CrossRef]
- Innerebner, G.; Knief, C.; Vorholt, J.A. Protection of Arabidopsis Thaliana against Leaf-Pathogenic Pseudomonas Syringae by Sphingomonas Strains in a Controlled Model System. Appl. Environ. Microbiol. 2011, 77, 3202–3210. [Google Scholar] [CrossRef]
- Zhang, H.; Yoshizawa, S.; Sun, Y.; Huang, Y.; Chu, X.; González, J.M.; Pinhassi, J.; Luo, H. Repeated Evolutionary Transitions of Flavobacteria from Marine to Non-Marine Habitats. Environ. Microbiol. 2019, 21, 648–666. [Google Scholar] [CrossRef]
- Feng, X.; Chu, X.; Qian, Y.; Henson, M.W.; Lanclos, V.C.; Qin, F.; Barnes, S.; Zhao, Y.; Thrash, J.C.; Luo, H. Mechanisms Driving Genome Reduction of a Novel Roseobacter Lineage. ISME J. 2021, 15, 3576–3586. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Wu, Y.; Gao, T.; Liu, C. Community Structure and Function of Epiphytic Bacteria Associated With Myriophyllum Spicatum in Baiyangdian Lake, China. Front. Microbiol. 2021, 12, 705509. [Google Scholar] [CrossRef]
- Puyol, D.; Barry, E.M.; Hülsen, T.; Batstone, D.J. A Mechanistic Model for Anaerobic Phototrophs in Domestic Wastewater Applications: Photo-Anaerobic Model (PAnM). Water Res. 2017, 116, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, G.; Rajendran, L.; Sendhilvel, V.; Prabakar, K.; Raguchander, T. 22-Diversity and Functions of Secondary Metabolites Secreted by Epi-Endophytic Microbes and Their Interaction with Phytopathogens. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 495–517. ISBN 978-0-12-822919-4. [Google Scholar]
- Martínez-Espinosa, C.; Sauvage, S.; Al Bitar, A.; Green, P.A.; Vörösmarty, C.J.; Sánchez-Pérez, J.M. Denitrification in Wetlands: A Review towards a Quantification at Global Scale. Sci. Total Environ. 2021, 754, 142398. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Xia, P.; Song, X.; Lin, T.; Cao, H. Community Structure and Functional Diversity of Epiphytic Bacteria and Planktonic Bacteria on Submerged Macrophytes in Caohai Lake, Southwest of China. Ann. Microbiol. 2019, 69, 933–944. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Huang, Y.; Liu, Q.; Hu, X. Discovering the Characteristics of Community Structures and Functional Properties of Epiphytic Bacteria on Spartina alterniflora in the Coastal Salt Marsh Area. J. Mar. Sci. Eng. 2022, 10, 1981. https://doi.org/10.3390/jmse10121981
Song Z, Huang Y, Liu Q, Hu X. Discovering the Characteristics of Community Structures and Functional Properties of Epiphytic Bacteria on Spartina alterniflora in the Coastal Salt Marsh Area. Journal of Marine Science and Engineering. 2022; 10(12):1981. https://doi.org/10.3390/jmse10121981
Chicago/Turabian StyleSong, Zenglei, Yanyan Huang, Qing Liu, and Xiaoke Hu. 2022. "Discovering the Characteristics of Community Structures and Functional Properties of Epiphytic Bacteria on Spartina alterniflora in the Coastal Salt Marsh Area" Journal of Marine Science and Engineering 10, no. 12: 1981. https://doi.org/10.3390/jmse10121981
APA StyleSong, Z., Huang, Y., Liu, Q., & Hu, X. (2022). Discovering the Characteristics of Community Structures and Functional Properties of Epiphytic Bacteria on Spartina alterniflora in the Coastal Salt Marsh Area. Journal of Marine Science and Engineering, 10(12), 1981. https://doi.org/10.3390/jmse10121981




