Application and Validation of an Ecological Quality Index, ISEP, in the Yellow Sea
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Field Sampling and Laboratory Analysis
2.3. Index Application and Validation
2.4. Measuring Agreements in SAB Patterns
3. Results
3.1. ISEP Variation with Environmental Factors
3.2. Species-Abundance-Biomass Variation along ISEP Grades
3.3. Matching of SAB between ISEP and the P-R Model
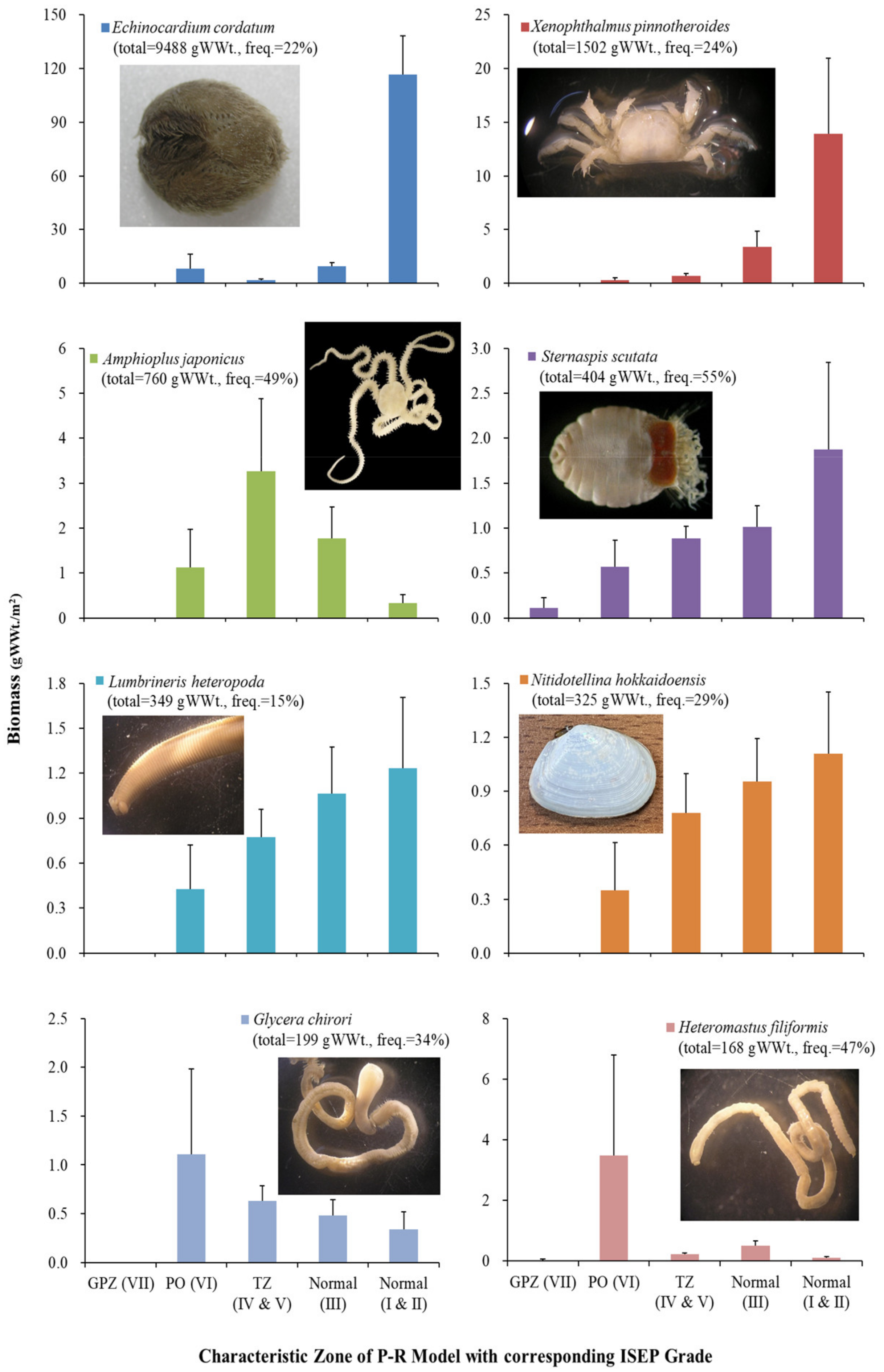
3.4. Patterns of Major Taxa and Species over ISEP Grades
4. Discussion
4.1. Relationship with Natural/Artificial Factors
4.2. Correspondence with the P-R Paradigm
4.3. Compatibility with WFD Habitat Classification
5. Conclusions
- (1)
- We validated the performance of a macrobenthos-based ecological index, the ISEP, in the west coast of Korea. The validation was performed by examination of the linear relationship between the ISEP grades and environmental factors, and the correspondence of species-abundance-biomass (SAB) and taxonomical variation between the ISEP and the Pearson-Rosenberg (P-R) model.
- (2)
- The ISEP responded effectively to bottom-water SS, a key impact source in this area, but was found to be independent of natural habitat condition variability such as salinity and sediment mean grain size. The SAB curves and the variation of major taxa and dominant species along the ISEP grades corresponded well with those of the P-R model.
- (3)
- Conformity to the P-R model confirmed the compatibility of the ISEP index with the European Union’s Water Framework Directive (EU WFD) classification system of ecological status and indicated the potential applicability to other areas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- NOAA. Report on the Delineation of Regional Ecosystems; National Oceanic and Atmospheric Administration Regional Ecosystem Delineation Workgroup: Washington DC, USA, 2004; p. 54.
- Costello, M.J.; Coll, M.; Danovaro, R.; Halpin, P.; Ojaveer, H.; Miloslavich, P. A census of marine biodiversity knowledge, resources, and future challenges. PLoS ONE 2010, 5, e12110. [Google Scholar] [CrossRef] [PubMed]
- MOF. National Investigation of Marine Ecosystem—The Southern Part of the East Sea (Gijang 35.3° N~Yeongdeok 36.5° N) Vol. 1; Ministry of Oceans and Fisheries: Sejong-Si, Republic of Korea, 2013; p. 600. (In Korean)
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 1978, 16, 229–311. [Google Scholar]
- Borja, A.; Franco, J.; Pérez, V. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Dauvin, J.C. Paradox of estuarine quality: Benthic indicators and indices, consensus or debate for the future. Mar. Pollut. Bull. 2007, 55, 271–281. [Google Scholar] [CrossRef]
- Zettler, M.L.; Schiedek, D.; Bobertz, B. Benthic biodiversity indices versus salinity gradient in the southern Baltic Sea. Mar. Pollut. Bull. 2007, 55, 258–270. [Google Scholar] [CrossRef]
- Yoo, J.W.; Lee, Y.W.; Ruesink, J.L.; Lee, C.G.; Kim, C.S.; Park, M.R.; Yoon, K.T.; Hwang, I.S.; Maeng, J.H.; Rosenberg, R.; et al. Environmental quality of Korean coasts as determined by modified Shannon–Wiener evenness proportion. Environ. Monit. Assess. 2010, 170, 141–157. [Google Scholar] [CrossRef]
- Warwick, R.M. A new method for detecting pollution effects on marine macrobenthic communities. Mar. Biol. 1986, 92, 557–562. [Google Scholar] [CrossRef]
- Weisberg, S.B.; Ranasinghe, J.A.; Dauer, D.M.; Schaffner, L.C.; Diaz, R.J.; Frithsen, J.B. An estuarine benthic index of biotic integrity (B-IBI) for Chesapeake Bay. Estuaries 1997, 20, 149–158. [Google Scholar] [CrossRef]
- Teixeira, H.; Weisberg, S.B.; Borja, A.; Ranasinghe, J.A.; Cadien, D.B.; Velarde, R.G.; Lovell, L.L.; Pasko, D.; Phillips, C.A.; Montagne, D.E.; et al. Calibration and validation of the AZTI’s Marine Biotic Index (AMBI) for Southern California marine bays. Ecol. Indic. 2012, 12, 84–95. [Google Scholar] [CrossRef]
- Rodil, I.F.; Lohrer, A.M.; Hewitt, J.E.; Townsend, M.; Thrush, S.F.; Carbines, M. Tracking environmental stress gradients using three biotic integrity indices: Advantages of a locally developed traits-based approach. Ecol. Indic. 2013, 34, 560–570. [Google Scholar] [CrossRef]
- McManus, J.W.; Pauly, D. Measuring ecological stress: Variations on a theme by R.M. Warwick. Mar. Biol. 1990, 106, 305–308. [Google Scholar] [CrossRef]
- Magni, P.; Tagliapietra, D.; Lardicci, C.; Balthis, L.; Castelli, A.; Como, S.; Frangipane, G.; Giordani, G.; Hyland, J.; Maltagliati, F.; et al. Animal-sediment relationships: Evaluating the “Pearson–Rosenberg paradigm” in Mediterranean coastal lagoons. Mar. Pollut. Bull. 2009, 58, 478–486. [Google Scholar] [CrossRef]
- Smith, R.W.; Bergen, M.; Weisberg, S.B.; Cadien, D.; Dalkey, A.; Montagne, D.; Stull, J.K.; Velarde, R.G. Benthic response index for assessing infaunal communities on the Southern California mainland shelf. Ecol. Appl. 2001, 11, 1073–1087. [Google Scholar] [CrossRef]
- Borja, A.; Muxika, I.; Franco, J. The application of a marine biotic index to different impact sources affecting soft-bottom benthic communities along European coasts. Mar. Pollut. Bull. 2003, 46, 835–845. [Google Scholar] [CrossRef]
- Borja, A.; Muxika, I. Do benthic indicator tools respond to all impact sources? The case of AMBI (AZTI marine biotic index). In Proceedings of the International Workshop on the Promotion and Use of Benthic Tools for Assessing the Health of Coastal Marine Ecosystems, IOC Workshop Report 195. Torregrande-Oristano, Italy, 8–9 October 2004; pp. 15–18. [Google Scholar]
- Diaz, R.J.; Solan, M.; Valente, R.M. A review of approaches for classifying benthic habitats and evaluating habitat quality. J. Environ. Manag. 2004, 73, 165–181. [Google Scholar] [CrossRef]
- Hyland, J.; Balthis, L.; Karakassis, I.; Magni, P.; Petrov, A.; Shine, J.; Vestergaard, O.; Warwick, R. Organic carbon content of sediments as an indicator of stress in the marine benthos. Mar. Ecol. Prog. Ser. 2005, 295, 91–103. [Google Scholar] [CrossRef]
- Barry, J.; Rees, H.L. Use of simulated data as a tool for testing the performance of diversity indices in response to an organic enrichment event. ICES J. Mar. Sci. 2008, 65, 1456–1461. [Google Scholar] [CrossRef]
- Heileman, S.; Jiang, Y. X-28 Yellow Sea: LME #48. In The UNEP Large Marine Ecosystems Report: A Perspective on Changing Conditions in LMEs of the World’s Regional Seas UNEP Regional Seas Report and Studies; Sherman, K., Hempel, G., Eds.; United Nations Environmental Programme: Nairobi, Kenya, 2008; Volume 182, pp. 441–451. [Google Scholar]
- Hong, J.S.; Yoo, J.W.; Park, H.S. Niche characterization of the three species of genus Ophiura (Echinodermata, Ophiuroidea) in Korean waters, with special emphasis on the distribution of Ophiura sarsi vadicola Djakonov. J. Korean Soc. Oceanogr. 1995, 30, 442–457, (In Korean with English Abstract). [Google Scholar]
- Hong, J.S.; Yoo, J.W. Distributional patterns of the molluscan assemblages in the Yellow Sea. In Proceedings of the International congress of Ecology, Florence, Italy, 19–25 July 1998; Volume VII, p. 194. [Google Scholar]
- MLTM. National Investigation of Marine Ecosystem—Protocol, 2nd ed.; Ministry of Land, Transport and Maritime Affairs: Sejong-Si, Republic of Korea, 2011; p. 169. (In Korean)
- Yi, S.Y. On the tides, tidal currents and tidal prism at Incheon Harbour. J. Oceanol. Soc. Korea 1972, 7, 86–97, (In Korean with English Abstract). [Google Scholar]
- Seung, Y.H. Water masses and circulations around Korean Peninsula. J. Oceanol. Soc. Korea. 1992, 27, 324–331, (In Korean with English Abstract). [Google Scholar]
- Yoo, J.W.; Hong, J.S.; Lee, J.J. Long-term environmental changes and the interpretations from a marine benthic ecologist’s perspective (I)—Physical environment. Fish. Aquat. Sci. 1999, 2, 199–209. [Google Scholar]
- Hong, J.S.; Seo, I.S.; Yoo, J.W.; Jung, R.H. Soft-bottom benthic communities in North Port, Inchon, Korea. Nat. Conserv. 1994, 88, 34–50, (In Korean with English Abstract). [Google Scholar]
- Hong, J.S.; Jung, R.H.; Seo, I.S.; Yoon, K.T.; Choi, B.M.; Yoo, J.W. How are the spatiotemporal distribution patterns of benthic macrofaunal communities affected by the construction of Shihwa Dike in the west coast of Korea? J. Korean Fish. Soc. 1997, 30, 882–895, (In Korean with English Abstract). [Google Scholar]
- Koh, B.S.; Lee, J.H.; Hong, J.S. Distribution patterns of the benthic macrofaunal community in the coastal area of Inchon, Korea. Sea J. Korean Soc. Oceanogr. 1997, 2, 31–41, (In Korean with English Abstract). [Google Scholar]
- Park, H.S.; Lim, H.S.; Hong, J.S. Spatio- and temporal patterns of benthic environment and macrobenthos community on subtidal soft-bottom in Chonsu Bay, Korea. Korean. J. Fish. Aquat. Sci. 2000, 33, 262–271, (In Korean with English Abstract). [Google Scholar]
- Hyun, S.M.; Kim, E.S.; Paeng, W.H. Heavy metal contamination and spatial differences in redox condition of the artificial Shihwa Lake, Korea. J. Environ. Sci. 2004, 13, 479–488. [Google Scholar]
- Park, S.C.; Lee, H.H.; Han, H.S.; Lee, G.H.; Kim, D.C.; Yoo, D.G. Evolution of late Quaternary mud deposits and recent sediment budget in the southeastern Yellow Sea. Mar. Geol. 2000, 170, 271–288. [Google Scholar] [CrossRef]
- Lee, H.J.; Chu, Y.S. Origin of inner-shelf mud deposit in the southeastern Yellow Sea: Huksan Mud Belt. J. Sediment Res. 2001, 71, 144–154. [Google Scholar] [CrossRef]
- Chough, S.K. Marine Geology of Korean Seas; International Human Resources Development Corporation Publishers: Boston, MA, USA, 1983; p. 157. [Google Scholar]
- Lim, D.I.; Choi, J.Y.; Jung, H.S.; Rho, K.C.; Ahn, K.S. Recent sediment accumulation and origin of shelf mud deposits in the Yellow and East China Seas. Prog. Oceanogr. 2007, 73, 145–159. [Google Scholar] [CrossRef]
- Wells, J.T. Distribution of suspended sediment in the Korea Strait and southeastern Yellow Sea: Onset of winter monsoons. Mar. Geol. 1988, 83, 273–284. [Google Scholar] [CrossRef]
- MLTM. Analytical Standards for Marine Environment; Ministry of Land, Transport and Maritime Affairs: Sejong-Si, Republic of Korea, 2010; p. 495. (In Korean)
- Ingram, R.L. Sieve analysis. In Procedures in Sedimentary Petrology; Carver, R.E., Ed.; Wiley-Inter Science: New York, NY, USA, 1971; pp. 49–67. [Google Scholar]
- Folk, R.L.; Ward, W.C. Brazos River bar [Texas]; A study in the significance of grain size parameters. J. Sediment Res. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Barbieri, M. The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. J. Geol. Geophys. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. A Mathematical Theory of Communication; University of Illinois: Urbana, IL, USA, 1949. [Google Scholar]
- Rosenberg, R.; Blomqvist, M.; Nilsson, H.C.; Cederwall, H.; Dimming, A. Marine quality assessment by use of benthic species-abundance distributions: A proposed new protocol within the European Union water Framework Directive. Mar. Pollut. Bull. 2004, 49, 728–739. [Google Scholar] [CrossRef]
- Clarke, K.R.; Ainsworth, M. A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser. 1993, 92, 205–219. [Google Scholar] [CrossRef]
- Pearson, T.H. The benthic ecology of Loch Linnhe and Loch Eil, a sea-loch system on the west coast of Scotland. IV. Changes in the benthic fauna attributable to organic enrichment. J. Exp. Mar. Biol. Ecol. 1975, 20, 1–41. [Google Scholar] [CrossRef]
- Wilson, J.G.; Jeffrey, D.W. Benthic biological pollution indices in estuaries. In Biomonitoring of Coastal Waters and Estuaries; Kramer, K.J.M., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 311–327. [Google Scholar]
- Bilotta, G.S.; Brazier, R.E. Understanding the influence of suspended solids on water quality and aquatic biota. Water Res. 2008, 42, 2849–2861. [Google Scholar] [CrossRef]
- MLTM. National Investigation of Marine Ecosystem—The Southern Area of the West Coast (35.5° N Younggwang—34.5° N Jindo); Ministry of Land, Transport and Maritime Affairs: Sejong-Si, Republic of Korea, 2009; p. 692. (In Korean)
- Elliott, M.; Quintino, V. The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. Pollut. Bull. 2007, 54, 640–645. [Google Scholar] [CrossRef]
- Levin, L.A.; Boesch, D.F.; Covich, A.; Dahm, C.; Erséus, C.; Ewel, K.C.; Kneib, R.T.; Moldenke, A.; Palmer, M.A.; Snelgrove, P.; et al. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 2001, 4, 430–451. [Google Scholar] [CrossRef]
- Dauvin, J.-C.; Desroy, N. The food web in the lower part of the Seine estuary: A synthesis of existing knowledge. Hydrobiologia 2005, 540, 13–27. [Google Scholar] [CrossRef]
- Long, E.R.; Morgan, L.G. The Potential for Biological Effects of Sediments-Sorbed Contaminants Tested in the National Status and Trends Program; U.S. Dept. of Commerce National Oceanic and Atmospheric Administration National Ocean Service: Seattle, WA, USA, 1990.
- MOF. The General Investigation of Marine Ecosystem—(37° N Asan Bay–38° N the Northernmost Area); Ministry of Oceans and Fisheries: Sejong-Si, Republic of Korea, 2006; p. 692. (In Korean)
- Ryu, J.S.; Khim, J.S.; Kang, S.G.; Kang, D.S.; Lee, C.H.; Koh, C.H. The impact of heavy metal pollution gradients in sediments on benthic macrofauna at population and community levels. Environ. Pollut. 2011, 159, 2622–2629. [Google Scholar] [CrossRef]
- Ra, K.; Kim, E.S.; Kim, K.T.; Kim, J.K.; Lee, J.M.; Choi, J.Y. Assessment of heavy metal contamination and its ecological risk in the surface sediments along the coast of Korea. J. Coast. Res. 2013, 65, 105–110. [Google Scholar] [CrossRef]
- Kröncke, I.; Reiss, H. Influence of macrofauna long-term natural variability on benthic indices used in ecological quality assessment. Mar. Pollut. Bull. 2010, 60, 58–68. [Google Scholar] [CrossRef]
- Dauvin, J.-C. Impact of amoco Cadiz oil spill on the muddy fine sand Abra alba and Melinna palmata community from the Bay of Morlaix. Estuar Coast. Shelf Sci. 1982, 14, 517–531. [Google Scholar] [CrossRef]
- Starczak, V.R.; Fuller, C.M.; Butman, C.A. Effects of barite on aspects of the ecology of the polychaete Mediomastus ambiseta. Mar. Ecol. Prog. Ser. 1992, 85, 269–282. [Google Scholar] [CrossRef]
- Rakocinski, C.F.; Brown, S.S.; Gaston, G.R.; Heard, R.W.; Walker, W.W.; Summers, J.K. Species-abundance-biomass responses by estuarine macrobenthos to sediment chemical contamination. J. Aquat. Ecosyst. Stress Recover. 2000, 7, 201–214. [Google Scholar] [CrossRef]
- Simboura, N.; Zenetos, A. Benthic indicators to use in ecological quality classification of Mediterranean soft bottom marine ecosystems, including a new biotic index. Mediterr. Mar. Sci. 2002, 3, 77–111. [Google Scholar] [CrossRef]
- Van Colen, C.; Thrush, S.F.; Vincx, M.; Ysebaert, T. Conditional responses of benthic communities to interference from an intertidal bivalve. PLoS ONE 2013, 8, e65861. [Google Scholar] [CrossRef]
- Choi, J.W.; Seo, J.Y. Application of biotic indices to assess the health condition of benthic community in Masan Bay, Korea. Ocean Polar Res. 2007, 29, 339–348. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, J.S.; Choi, J.W. Distribution patterns of opportunistic molluscan species in Korean waters. Korean J Environ. Biol. 2013, 31, 1–9, (In Korean with English Abstract). [Google Scholar] [CrossRef]
- Crooks, J.A. Predators of the invasive mussel Musculista senhousia (Mollusca: Mytilidae). Pac. Sci. 2002, 56, 49–56. [Google Scholar] [CrossRef]
- Harriague, A.C.; Misic, C.; Petrillo, M.; Albertelli, G. Stressors affecting the macrobenthic community in Rapallo harbour (Ligurian Sea, Italy). Sci. Mar. 2007, 71, 705–714. [Google Scholar] [CrossRef]
- MLTM. National Investigation of Marine Ecosystem—The Eastern Part of the South Sea (127.8° E Yeosu~129.3° E Busan); Ministry of Land, Transport and Maritime Affairs: Sejong-Si, Republic of Korea, 2011; p. 927. (In Korean)
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; Plymouth Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- Borja, A.; Josefson, A.B.; Miles, A.; Muxika, I.; Olsgard, F.; Phillips, G.; Rodríguez, J.G.; Rygg, B. An approach to the intercalibration of benthic ecological status assessment in the North Atlantic ecoregion, according to the European water Framework Directive. Mar. Pollut. Bull. 2007, 55, 42–52. [Google Scholar] [CrossRef]
- Munari, C.; Mistri, M. Towards the application of the water Framework Directive in Italy: Assessing the potential of benthic tools in Adriatic coastal transitional ecosystems. Mar. Pollut. Bull. 2010, 60, 1040–1050. [Google Scholar] [CrossRef]
- Nilsson, H.C.; Rosenberg, R. Succession in marine benthic habitats and fauna in response to oxygen deficiency: Analysed by sediment profile-imaging and by grab samples. Mar. Ecol. Prog. Ser. Progress. 2000, 197, 139–149. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Thrush, S.F.; Gibbs, M.M. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 2004, 431, 1092–1095. [Google Scholar] [CrossRef]
- Odum, E.P. The strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef]
- Rhoads, D.C.; McCall, P.L.; Yingst, J.Y. Disturbance and production on the estuarine seafloor. Am. Sci. 1978, 66, 577–586. [Google Scholar] [CrossRef]
- Thrush, S.F.; Hewitt, J.E.; Cummings, V.J.; Dayton, P.K.; Cryer, M.; Turner, S.J.; Funnell, G.A.; Budd, R.G.; Milburn, C.J.; Wilkinson, M.R. Disturbance of the marine benthic habitat by commercial fishing: Impacts at the scale of the fishery. Ecol. Appl. 1998, 8, 866–879. [Google Scholar] [CrossRef]
- Jennings, S.; Pinnegar, J.K.; Polunin, N.V.C.; Warr, K.J. Impacts of trawling disturbance on the trophic structure of benthic invertebrate communities. Mar. Ecol. Prog. Ser. 2001, 213, 127–142. [Google Scholar] [CrossRef]
- Ebert, T.A.; Southon, J.R. Red Sea urchins (Strongylocentrotus franciscanus) can live over 100 years: Confirmation with A-bomb 14 carbon. Fish. Bull. 2003, 101, 915–922. [Google Scholar]
- Lubchenco, J. Plant species diversity in a marine intertidal community: Importance of herbivore food preference and algal competitive abilities. Am. Nat. 1978, 112, 23–39. [Google Scholar] [CrossRef]
- Vandermeer, J.; Boucher, D.; Perfecto, I.; de la Cerda, I.G. A theory of disturbance and species diversity: Evidence from Nicaragua after Hurricane Joan. Biotropica 1996, 28, 600–613. [Google Scholar] [CrossRef]

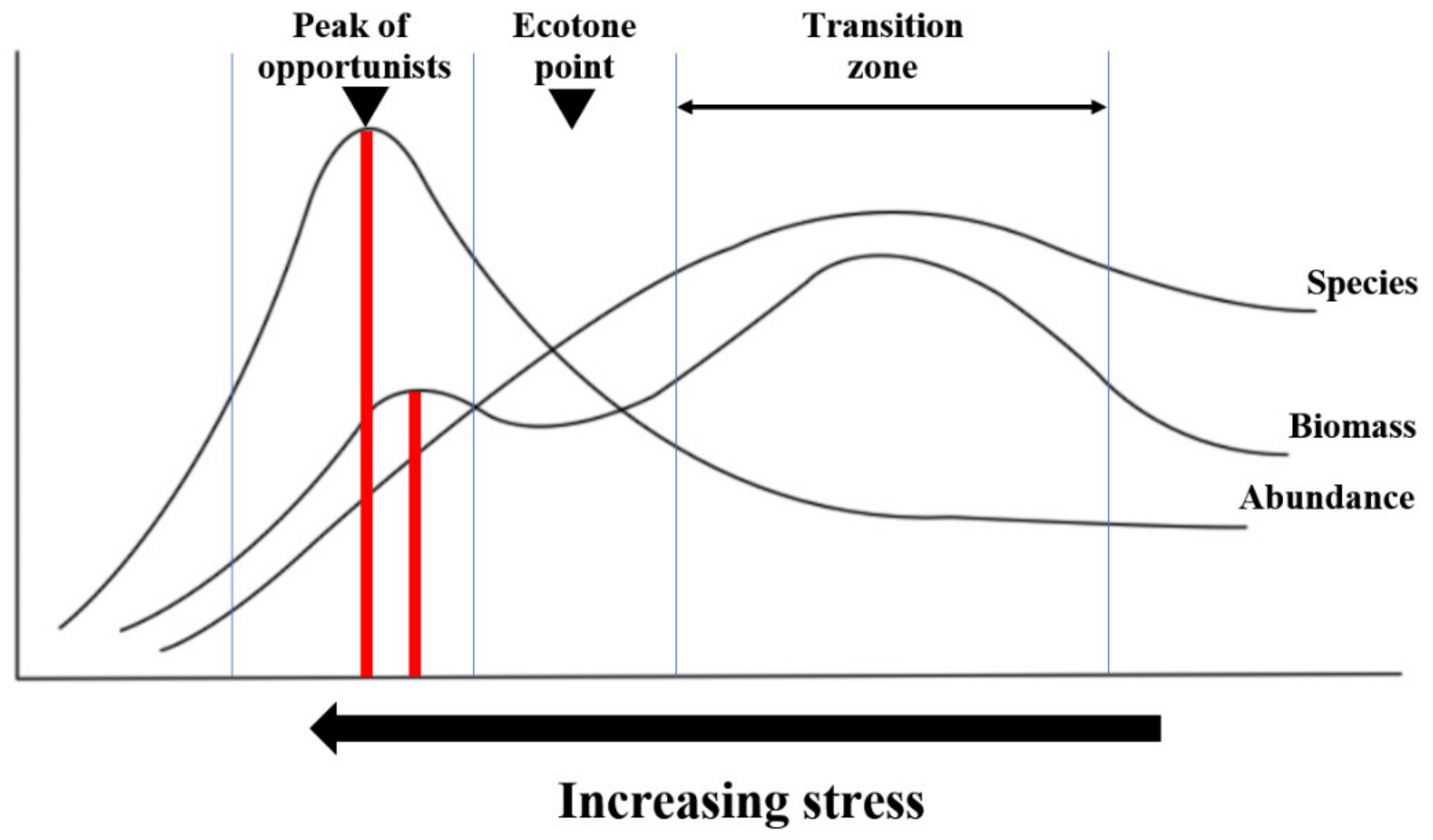
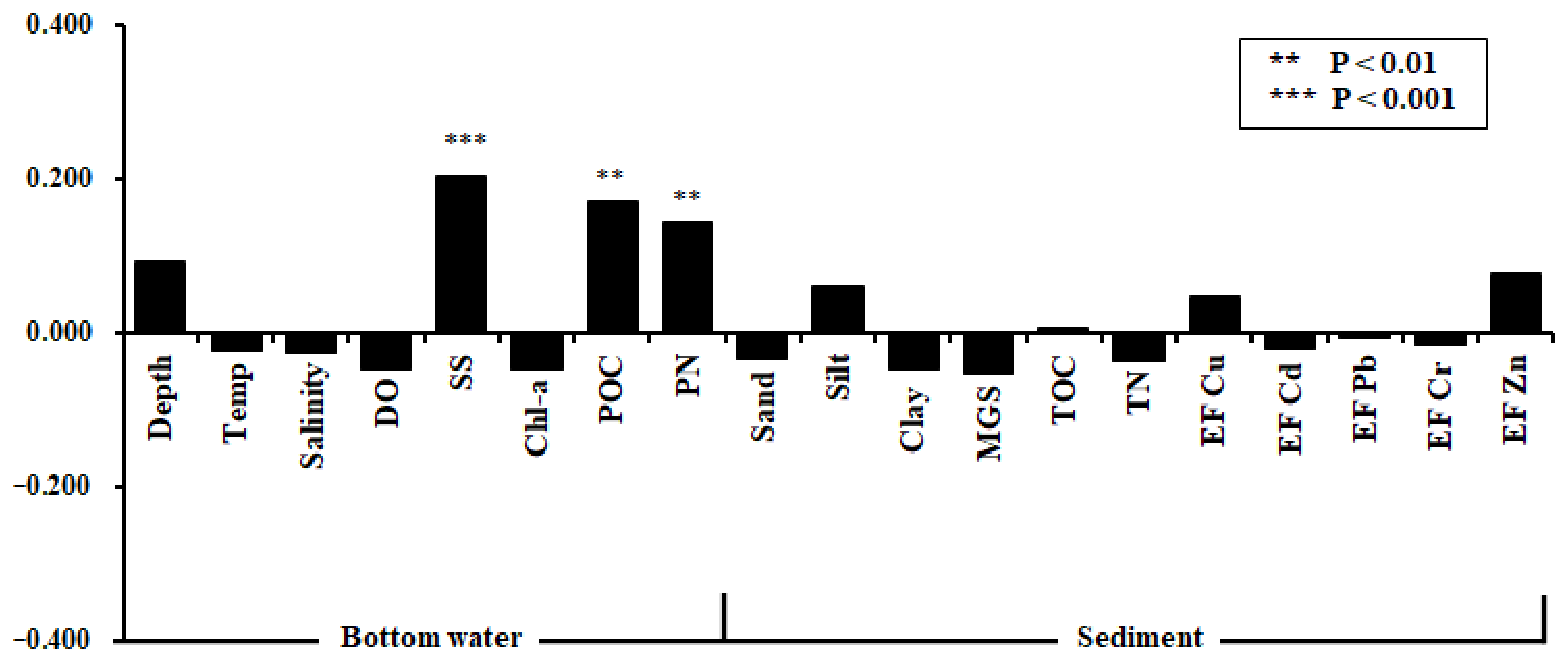

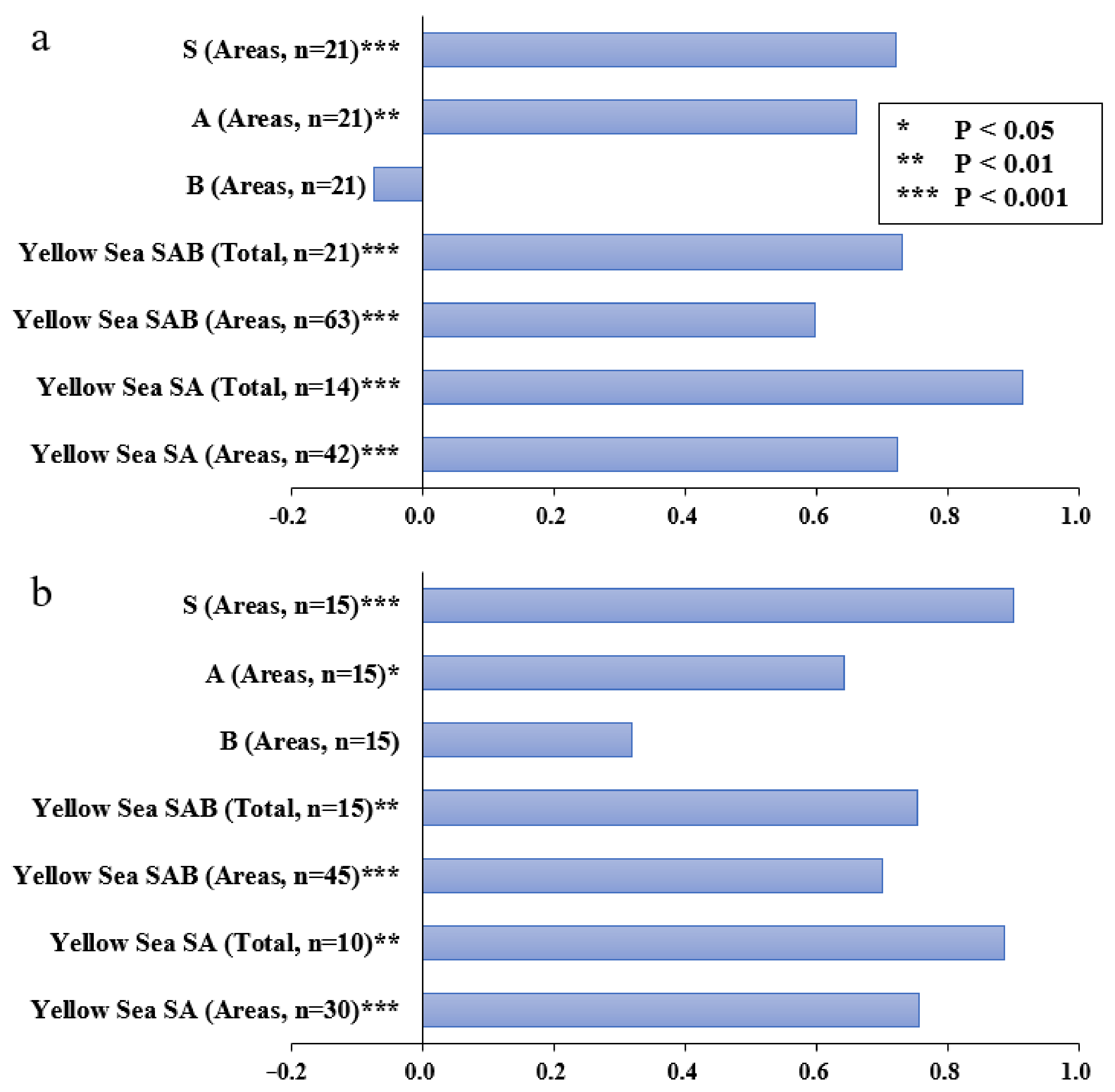

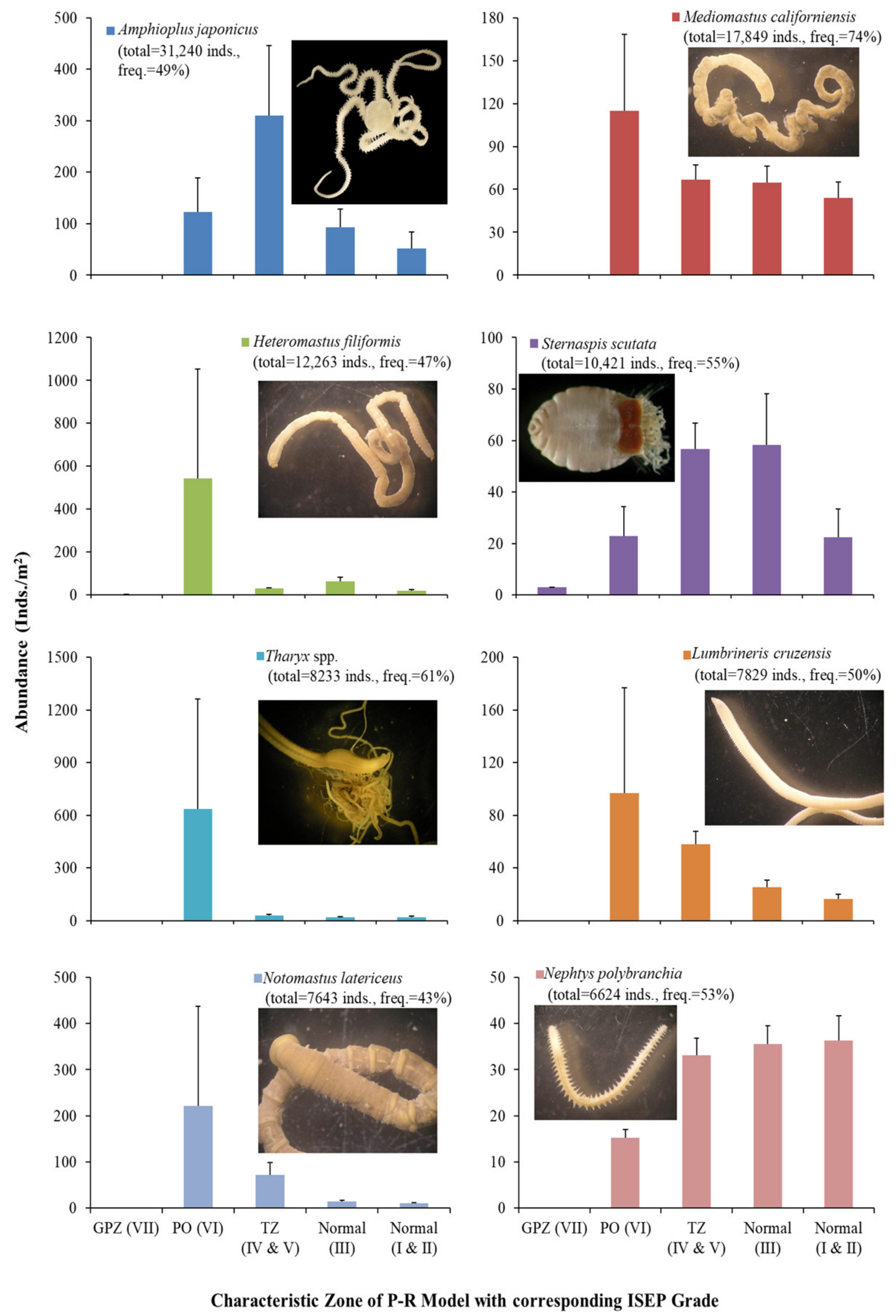
| ISEP Grade | VII | VI | V | IV | III | II | I |
|---|---|---|---|---|---|---|---|
| Percentile | ≤10 | 10< | 20< | 40< | 60< | 80< | 90< |
| Average | 0.144 | 0.291 | 0.359 | 0.426 | 0.61 | 0.912 | |
| SE | 0.047 | 0.007 | 0.003 | 0.005 | 0.014 | 0.043 | |
| 2 SE | 0.094 | 0.013 | 0.005 | 0.011 | 0.028 | 0.085 |
| P-R Model | |||
|---|---|---|---|
| Ecological Status | Abundance | Biomass | Species |
| Bad | 0 | 0 | 0 |
| Poor | 7.6 | 4 | 2.4 |
| Moderate | 4.6 | 3.5 | 4.5 |
| Good | 2.2 | 5.8 | 6.4 |
| High | 2.1 | 3 | 5 |
| Biological Data (Macrobenthic Community) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species (0.3 m2) | Abundance (m2) | Biomass (m2) | ISEP Score Mean (0.1 m2) | ISEP Grade | |||||||
| Min | 1 | 3 | 0.08 | 0.000 | 1 | ||||||
| Max | 141 | 36,791 | 2234.44 | 1.679 | 7 | ||||||
| Average | 47 | 1457 | 92.54 | 0.499 | 3 | ||||||
| SD | 28 | 2965 | 215.22 | 0.208 | 1 | ||||||
| Environmental data (bottom water) | |||||||||||
| Depth (m) | Temperature (°C) | Salinity (psu) | Dissolved oxygen (mg/L) | Suspended solids (mg/L) | Chlorophyll-a (µg/L) | POC (uM) | PN (uM) | ||||
| Min | 3.0 | 2.1 | 14.7 | 3.5 | 0.7 | 0.0 | 9.4 | 1.3 | |||
| Max | 65.0 | 28.7 | 34.0 | 13.1 | 845.1 | 15.0 | 788.0 | 86.0 | |||
| Average | 23.4 | 13.5 | 31.6 | 8.6 | 56.2 | 2.0 | 78.3 | 9.6 | |||
| SD | 14.9 | 6.5 | 1.9 | 1.7 | 103.5 | 2.0 | 93.8 | 10.0 | |||
| Environmental data (sediment) | |||||||||||
| Sand (%) | Silt (%) | Clay (%) | MGS (Φ) | TOC (%) | TN (%) | EF Cu | EF Cd | EF Pb | EF Cr | EF Zn | |
| Min | 0.0 | 0.0 | 0.0 | −0.79 | 0.00 | 0.00 | 0.04 | 0.00 | 1.26 | 0.09 | 0.24 |
| Max | 100.0 | 85.0 | 56.2 | 8.21 | 2.32 | 0.20 | 1.30 | 3.29 | 7.82 | 1.23 | 3.75 |
| Average | 59.6 | 27.1 | 11.7 | 3.86 | 0.30 | 0.04 | 0.24 | 0.53 | 2.75 | 0.58 | 1.01 |
| SD | 36.2 | 25.5 | 14.1 | 2.06 | 0.29 | 0.04 | 0.13 | 0.59 | 0.94 | 0.21 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.-W.; Lee, Y.-W.; Park, M.-R.; Kim, C.-S.; Kim, S.; Lee, C.-L.; Jeong, S.-Y.; Lim, D.; Oh, S.-Y. Application and Validation of an Ecological Quality Index, ISEP, in the Yellow Sea. J. Mar. Sci. Eng. 2022, 10, 1908. https://doi.org/10.3390/jmse10121908
Yoo J-W, Lee Y-W, Park M-R, Kim C-S, Kim S, Lee C-L, Jeong S-Y, Lim D, Oh S-Y. Application and Validation of an Ecological Quality Index, ISEP, in the Yellow Sea. Journal of Marine Science and Engineering. 2022; 10(12):1908. https://doi.org/10.3390/jmse10121908
Chicago/Turabian StyleYoo, Jae-Won, Yong-Woo Lee, Mi-Ra Park, Chang-Soo Kim, Sungtae Kim, Chae-Lin Lee, Su-Young Jeong, Dhongil Lim, and Sung-Yong Oh. 2022. "Application and Validation of an Ecological Quality Index, ISEP, in the Yellow Sea" Journal of Marine Science and Engineering 10, no. 12: 1908. https://doi.org/10.3390/jmse10121908
APA StyleYoo, J.-W., Lee, Y.-W., Park, M.-R., Kim, C.-S., Kim, S., Lee, C.-L., Jeong, S.-Y., Lim, D., & Oh, S.-Y. (2022). Application and Validation of an Ecological Quality Index, ISEP, in the Yellow Sea. Journal of Marine Science and Engineering, 10(12), 1908. https://doi.org/10.3390/jmse10121908






