Abstract
The sediment microbial community plays a key role in the cycling of organic matter in marine ecosystems. The characteristics of the sediment microbial community are significantly related to changes in the environment. In this study, we analyzed the diversity and distribution of microbial communities in sediments from different geographical regions (the Liao River estuary, Yellow River estuary, hypoxic zone, and offshore zone) of the Bohai Sea using high-throughput sequencing. The results showed that the community richness and diversity (especially the unique diversity) of the Liao River estuary were larger than those of the Yellow River estuary, hypoxic zone, and offshore zone. The phylum Proteobacteria dominated in the Liao River estuary (46.26%), hypoxic zone (76.19%), and offshore zone (69.79%), while the dominant phylum in the Yellow River estuary was the Bacteroidetes phylum. The genus Gillisia was the dominant genus in both the Liao River estuary and Yellow River estuary. The offshore zone and hypoxic zone shared the same dominant Photobacterium genus. The results of Pearson correlation and redundancy analysis showed that environmental parameters such as nitrite, silicate, nitrate, phosphate, ammonia, dissolved oxygen, pH, and salinity interfered significantly with the structure and diversity of the microbial community. The results of this study will provide support for future research on the interaction mechanism of pollutants and microorganisms in the estuaries and a typical hypoxic zone of the Bohai Sea.
1. Introduction
The Bohai Sea, the only semi-enclosed marginal sea surrounded by land on three sides in northeast China, is rich in resources. Its five most prominent resources include fishing, ports, oil, tourism, and sea salt. However, with this economic development, the ecological environment of Bohai Sea is experiencing great pressure. The functions of the marine ecosystem in some offshore areas have become damaged. The need for marine environmental protection becomes greater every year [1,2]. Due to the strong sealing, Bohai Sea has a long seawater exchange period. Therefore, the environment of the Bohai Sea is strongly affected by pollutants coming in from its estuaries [3]. The Liao River estuary and Yellow River estuary are the two largest estuaries in the Bohai Sea. The degree of pollution in these two estuaries has a significant effect on the environment of the Bohai Sea and may interfere with bacterial communities. Oxygen-deficient environments are known to affect the structure of bacterial communities [4]. Bacterial communities in the sediment of Bohai, China, have been affected [5] by a hypoxic zone found at the bottom of the Bohai Sea [6]. According to previous research, the growth, abundance, and distribution of microbes were significantly influenced by environmental factors [7,8,9,10]. For instance, microbial growth, abundance, and activity were restricted by nutrients including organic carbon, nitrogen, and phosphorus [11]. Total phosphorus concentration was significantly correlated with the distribution of bacterial communities in the sediments of a shallow, eutrophic, and temperate freshwater lake [11]. Temperature and salinity were key factors controlling bacterial and archaeal community structure, respectively [12]. The low pH values observed in the area have limited its biodiversity and ecosystem balance [13,14]. Significant changes in community structure and lower diversity were seen in response to pH being reduced from 8.7 to 6.7 [15]. Lower biodiversity was found in a hypoxic zone where the concentration of dissolved oxygen (DO) was 2 mg/L lower than in the general sea area [16,17]. Environmental parameters are key factors affecting the marine microbial community. However, little information has been reported concerning the correlation of environment parameters and microbial communities in estuarine and hypoxic zone sediments of the Bohai Sea.
Microorganisms in sediments play a pivotal role in the material exchange and energy flow of different marine ecosystems, including marine hypoxic zones and estuarine ecosystems [18]. Sediment microorganisms contribute significantly to completing the degradation and biogeochemical cycling of nitrogen, sulfur, phosphorus, and carbon in aquatic ecosystems through metabolic processes such as uptake and decomposition. Sediment microorganisms become the executors of various biogenic elements in the biogeochemical cycle and have a profound impact on the global biogeochemical cycle [18,19,20]. As microorganisms are very sensitive to changes in aquatic ecosystem environments, the characteristics of microbial diversity and community structure are closely related to environmental changes. Abundance and diversity are important parameters for evaluating the function of marine microbial communities in sedimentary environments [18,19]. Therefore, to determine how to maintain the stability of marine ecosystems and cope with global climate change, it is necessary to comparatively explore the abundance and diversity of microbial communities in marine hypoxic zones and estuary sediments and evaluate the potential impact of environmental factors on microbial communities.
In this study, four typical regions in the Bohai Sea were selected: the Liao River estuary (station BHHZ77), Yellow River estuary (station BHHZ68), the hypoxic zone (station BHHZ83), and the offshore zone (station BHHZ64). The offshore zone was used for comparison with the other zones. Each zone has distinct characteristics. The Yellow River estuary and Liao River estuary are the two largest estuaries in the Bohai Sea. The BHHZ83 station is the only hypoxic zone reported in the Bohai Sea. High-throughput sequencing was used to analyze the composition, diversity, and differences among microbial communities in sediments at different stations. In addition, aquatic environmental parameters from the four regions were measured. The relationship between water characteristics and the dominant microbial population was analyzed to determine the key parameters affecting the diversity and distribution characteristics of microbial communities. This work provides a punctual description of microbial communities in estuarine and hypoxic zone sediments of the Bohai Sea in association with environmental parameters.
2. Materials and Methods
2.1. Nutrient Sampling and Preparation
The locations of BHHZ77, BHHZ68, BHHZ83, and BHHZ64 stations are shown in Figure 1. Samples from four stations were collected in the summer of 2018. The location, depth, and other information are shown in Table 1. Nutrient samples were filtered through a syringe with a 0.25 um membrane. Ammonia was analyzed immediately after sampling or after refrigeration at 4 °C and analyzed within 24 h of sampling. Samples for silicate analysis were refrigerated at 4 °C and analyzed after returning to the lab. Samples for nitrate, nitrite, and phosphate analysis were refrigerated at −20 °C and analyzed after returning to the lab.

Figure 1.
Study areas and sampling locations in Bohai Sea, China.

Table 1.
The information of stations to collect different samples.
Analysis for nutrients including nitrate, nitrite, phosphate, and silicate were run with a Technicon AA3 Auto-Analyzer according to national standard methods [21]. Accuracy was maintained through the use of internal standards during sampling analysis (every 10 samples). The RSD (relative standard deviation) of the concentrations was no more than 5%, and the RSD of the slope of the standard working curve per day per parameter was no more than 5%.
2.2. High-Throughput Sequencing Analysis
Samples collected from different station were stored at −80 °C. The DNA extraction and high-throughput sequencing analysis were performed by Microanaly Technology Co., Ltd. (Taichung City, Taiwan) DNA was extracted using a QIAGEN extraction kit, and total DNA was examined using Thermo Qubit.
The V3–V4 region was selected for 16S rDNA amplification. A universal primer was used. The index sequence and connector sequence suitable for MiSeq PE300 sequencing were added to the 5′ end of the universal primer to complete the design of the specific fusion primer. The forward primer (5′–3′) and reverse primer (5′–3′) were CCTACGGGNGGCWGCAG (F) and GACTACHVGGGTWTCTAAT (R), respectively. The diluted genomic DNA acted as a template. NOvizan’s Taq DNA Polymerase was used for PCR to ensure the accuracy and efficiency of amplification. A Fragment Analyzer was used to inspect the PCR product library.
After the library was qualified, the corresponding ratio column was mixed according to the data volume requirements of each sample. A QIAquick gel recovery kit (QIAGEN) was used to purify the mixed library by gel cutting (range: 500–750 bp). After the collection, the Fragment Analyzer and Applied Biosystems QuantStudio 6 real-time fluorescence quantitative PCR instrument were used for quality inspection and quantification. An Illumina MiSeq PE300 was used for sequencing. The 16S specific primers were designed to amplify specific regions, and 425 bp amplified fragments were obtained. Using the MiSeq platform, 2 × 300 bp paired-end data were obtained via sequencing. Through splicing, long sequences could be obtained for 16S analysis.
2.3. Analysis Software, Method, and Gene Sequence Process
The long reads in hypervariable regions were demultiplexed and quality-filtered using fastp software version of 0.20.0 [22] and merged using FLASH software version of 1.2.7 [23]. We followed these criteria: (i) reads with average quality values below 20 were removed; (ii) reads with more than three bases containing N were removed; and (iii) the length of reads was kept at ranges from 220 to 500 nt.
Operational taxonomic units (OTUs) with a 97% similarity cutoff [24,25] were clustered using Usearch software [24], and chimeric sequences were identified and removed. The taxonomy of each OTU representative sequence was analyzed using RDP Classifier version 2.2 [26] against the 16S rRNA database using a confidence threshold of 0.7.
A Venn diagram can reflect the number of OTUs shared between groups and within groups. The VennDiagram function was implemented using the VennDiagram package in R. QIIME software was used to obtain the dilution curve of the alpha diversity index, and flattening parameters were selected according to the dilution curve. QIIME software was then used to analyze the flattened OTUs [27]. One read was extracted from the OTUs as a representative sequence using the RDP method. This representative sequence was aligned with the 16S database, and species classification was performed for each OTU [26,28]. After classification, an OTU abundance table was constructed according to the number of sequences in each out. Subsequent analysis was conducted according to the OTU abundance table.
The sequence numbers of species or OTUs on each annotation from different samples were arranged by phylum, class, order, family, and genus to form a profiling bar chart and statistical table.
3. Results and Discussion
3.1. Environmental Factor Analysis
Environmental factors were investigated to characterize the differences in the environment at the four stations shown in Table 2. The regions with high average salinity (31.1454‰ and 31.5976‰, respectively) were BHHZ 64 and BHHZ83. These stations were located in the middle part of the Bohai Sea far away from the beach. Therefore, the salinity at these stations was not significantly affected by freshwater input from rivers. As shown in Table 2, the average DO (4.265 mg/L) and average pH (7.78) in the sediment of BHHZ83 were lower than in BHHZ64 (6.12 mg/L and 7.925, respectively), BHHZ77 (6.6 mg/L and 7.98, respectively), and BHHZ68 (6.685 mg/L and 7.915, respectively), indicating that a low oxygen zone formed in this region (confirmed by another study) [6]. The lowest average pH (7.78) was observed at Station BHHZ83. In BHHZ77, the average amount of phosphate (0.365 μM), nitrite (4.675 μM), and nitrate (6.71 μM) was the highest of all regions, especially the latter two nutrients. Ammonia appeared only in the Yellow River estuary, indicating that the level of phosphate, nitrite, and nitrate, which are pollutants that come from land-based sources, are highest in the Yellow River estuary area. The sediment silicate average concentration was highest in sediments at station BHHZ83 (10.81 μM), followed by BHHZ64 (6.27 μM), BHHZ77 (5.28 μM), and BHHZ68 (3.07 μM). Previous literature has reported that an increase in salinity promotes the formation of aluminum hydroxide and iron hydroxide colloids, which can absorb the active silicon in seawater and convert it into iron and aluminosilicate compounds [29]. For this reason, the silicate concentration in the estuary area was lower than in other regions. The above information suggests that environmental factors were quite different at each sampling site in the Bohai Sea.

Table 2.
Nutrient concentration at different areas of Bohai Sea, China (August, 2018).
3.2. Microbial Community Abundance and Diversity Analysis of Sediments from Different Areas of the Bohai Sea
Sludge samples collected from sediments at four sites were analyzed using high-throughput sequencing. The high-throughput sequencing results were used to evaluate the microbial community and the dominant microorganism under different conditions using alpha diversity analysis and beta diversity analysis.
3.2.1. Alpha Diversity Analysis
In community ecology, alpha diversity focuses on single-sample diversity analysis, which reflects the number of species in the microbial community. This method estimates species abundance and diversity by analyzing a series of statistical indexes. The large Chao1 index and observed species index represent high microbial community richness [30], and a high Shannon index represents high diversity [31]. A rank abundance curve can be used to explain species abundance and evenness of sample diversity. The abundance of species is reflected by the length of the curve along the horizontal axis. A wide curve represents rich species composition. The uniformity of species composition is reflected by the shape of the curve; a flatter curve represents more uniformity of species composition. As shown in Table 3, the largest CHAO1 index, observed species index, and largest Shannon index were detected in BHHZ77, indicating that community richness and diversity were highest in the Liao River estuary. Figure 2 shows that the sediment collected in the Liao River estuary (BHHZ 77) had the most uniformity and the richest species. Most of the nutrient concentrations around the Liao River estuary, except silicate and ammonia, were higher than in the other areas (Table 2), suggesting that the degree of pollution in the Liao River estuary was the highest of all the study areas. The community richness and diversity in the hypoxic zone (BHHZ83) were the lowest of all areas, even though high concentrations of nitrate and silicate were detected. Overall, the obvious differences in environmental factors might contribute significantly to variations in the microbial community, which will be discussed in detail later.

Table 3.
Average level of the alpha diversity index of the microbial community.
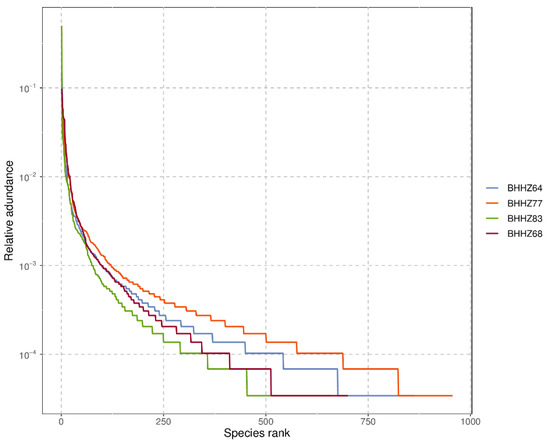
Figure 2.
Rank abundance curve.
3.2.2. Beta Diversity Analysis
In community ecology, the beta diversity index focuses on the comparison of diversity between different habitats. Downstream analyses were conducted for each sample. A total of 956 (BHHZ77), 919 (BHHZ64), 741 (BHHZ68), and 722 (BHHZ83) OTUs were obtained. A Venn diagram of microbial communities in different sediments is shown in Figure 3. Other studies [32,33] have reported that a significant regional difference with a random distribution or environmental selection will result in different microbial structures, which was also true in this study. Due to the different environmental conditions, the number of shared OTUs sequences was small, and the diversities of sedimentary microorganisms were obvious different. The unique sequence number of OTUs was: BHHZ77 > BHHZ64 > BHH68 > BHHZ83, indicating that the amount of unique diversity in the Liao River estuary was the largest among the other zones or estuaries.
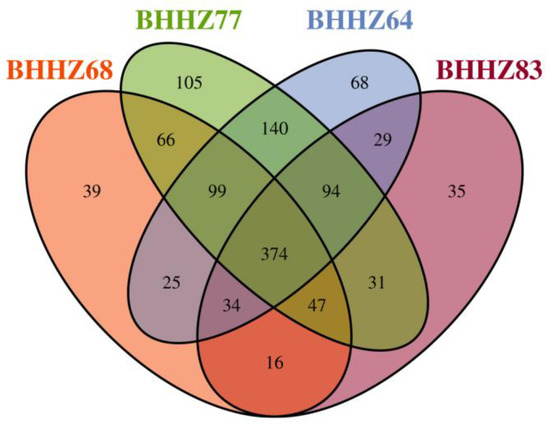
Figure 3.
Venn diagram of microbial communities in different sediments.
3.2.3. Microbial Community Characteristics in the Sediments of Estuaries and a Hypoxic Zone
The sequence with the highest abundance was selected as the representative sequence from each group of OTUs. The representative sequence was compared with the 16S rRNA database of known species to classify each OTU. Using the species comparison annotation results, we constructed a histogram of species profiles for each group at the phylum and genus levels, as shown in Figure 4.
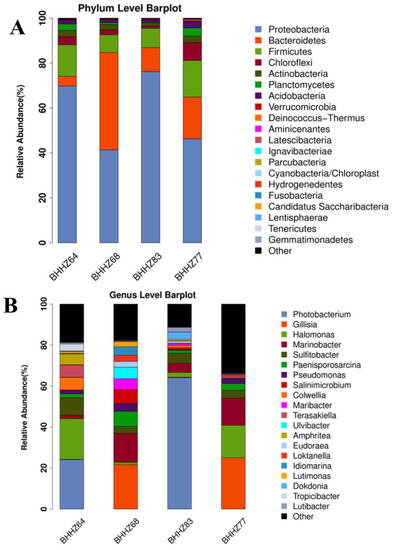
Figure 4.
Profiling histogram of species at the phylum and genus levels for different stations: (A) phylum levels and (B) genus levels.
Twenty-four phyla of bacteria were detected in four groups of samples from different stations in the Bohai Sea. The dominant bacterial phyla in each group of samples were similar, but the relative abundance varied. The analysis results showed that the phyla Proteobacteria, Bacteroidetes, Firmicutes, Chloroflexi, Actinobacteria, Planctomycetes, Acidobacteria, and Verrucomicrobia were dominant in all samples. According to the literature, Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes are the main phyla of denitrifying bacteria [34,35]. Bacteroidetes and Actinobacteria are able to degrade organic pollutants [36,37]. Actinobacteria can promote the decomposition of the remains of animals and plants to complete the mineralization of organics in the sediment [38]. Bacteroidetes, which are found mainly in anoxic environments, can not only hydrolyze macromolecules but also promote the transformation of nitrogenous substances [39]. In addition to the above phyla, Planctomycetes, Acidobacteria, and Verrucomicrobia were also commonly found in the Bohai Sea [33]. Chloroflexi plays an important role in the hydrolysis of proteins and polysaccharides [40]. From the above functional analysis results, we demonstrated that organics were the main pollutants in the Bohai sea. As shown in Figure 4, Proteobacteria was the dominant phylum type in three areas, namely, the Liao River estuary (station BHHZ77, 46.26%), the hypoxic zone (station BHHZ83, 76.19%), and the offshore zone (station BHHZ64, 69.79%), while the dominant phylum in the Yellow River estuary (station BHHZ68 station) was Bacteroidetes. Bacteroidetes had the largest relative abundance (43.31%), followed by Proteobacteria (41.37%). All of the relative abundances of Firmicutes, Chloroflexi, Actinobacteria, Planctomycetes, and Acidobacteria in the Liao River estuary (station BHHZ77) were higher than in the Yellow River estuary, hypoxic zone, or offshore zone. Lentisphaerae, Candidatus, and Saccharibacteria were detected only at stations BHHZ77 and BHHZ64. Armatimonadetes and Deferribacteres were detected only at station BHHZ77, and Spirochaetes and Chlamydiae were found only at station BHHZ64. Gemmatimonadetes and Parcubacteria were not detected in the BHHZ68 station, and Tenericutes and Fusobacteria were not found in the sediments of BHHZ64 and BHHZ83. In summary, we demonstrated that the microbial community was different at each of the four stations, which could be the result of different environmental parameters. The effects of environmental parameters on the microbial community will be analyzed in the next section.
The dominant bacterial genera in four groups of samples were different from each other (Figure 4B and Figure 5). The genus Gillisia was dominant in the estuaries of the Bohai Sea, with a relative abundance of 16.34% in the Liao River estuary and 19.21% in the Yellow River estuary. Halomonas (10.53%), Marinobacter (8.8%), Paenisporosarcina (3.04%), and Pseudomonas (1.47%) were the other dominant genera in the Liao River estuary. Marinobacter (12.62%), Sulfitobacter (3.62%), Paenisporosarcina (6.51%), Salinimicrobium (6.00%), Maribacter (4.69%), and Pseudomonas (3.43%) were the other dominant genera in the Yellow River estuary. The offshore zone and hypoxic zone were both dominated by Photobacterium (18.02% and 51.78%, respectively). In the hypoxic zone, the dominant genera also included Sulfitobacter (4.52%), Marinobacter (3.57%), Halomonas (1.91%), Colwellia (1.11%), Maribacter (0.80%), and Salinimicrobium (0.79%). Halomonas (14.77%), Sulfitobacter (6.49%), Colwellia (4.57%), and Pseudomonas (1.41%) were the other dominant genera in the offshore zone. The dark column in Figure 4 represents the relative abundance of sequences that did not belong to any known classification hierarchy, indicating the presence of unidentified microorganisms in the area. The relative abundance of unknown genera was highest in BHZZ77, followed by BHZZ64, BHZZ68, and BHZZ83 (Figure 5). This suggests that the environmental parameters significantly affected the structure and diversity of microbial communities, which has been confirmed by other reports [41,42].
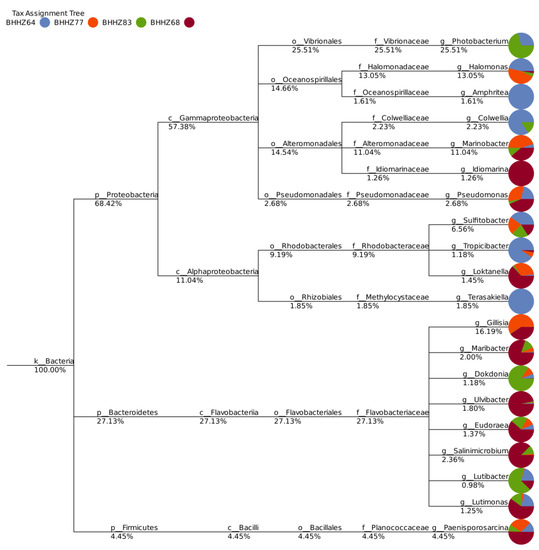
Figure 5.
The proportion of main genera in different sediments (k, p, c, o, f, and g represent kingdom, phylum, class, order, family, and genus, respectively).
3.3. Effects of Environmental Parameters on Microbial Communities
Microorganisms are sensitive to changes in the aquatic environment, so microbial diversity and community structure are closely related to environmental changes. For this reason, it is important to study the association between environmental factors and microbial community diversity. In this study, the environmental parameters pH, DO, salinity, silicate, nitrite, nitrate, ammonia, and phosphate were assessed at four stations. Pearson correlation coefficient analysis (Originlab origin 2021 software) and redundancy analysis (Canoco 5.0 software) of environmental factors and dominant microorganisms were performed at the phylum and genus levels (Figure 6 and Figure 7).

Figure 6.
Pearson correlation between environmental parameters and microbial diversity.

Figure 7.
RDA analysis of dominant bacteria at the phylum and genus levels: (A) phylum level and (B) genus level.
As shown in Figure 6, the nitrite concentration was related to observed species index, Shannon index, CHAO 1, and Simpson indices. The first two indices were particularly strong, indicating that a high concentration of nitrite was conducive to the proliferation of microorganisms. DO, pH, and salinity were positively related to the CHAO 1, observed species, Simpson, and Shannon indices. Notably, DO and pH were most closely related to the Simpson and Shannon indices, while the relationship of salinity with the Simpson and Shannon indices showed an opposite trend. The silicate concentration showed a significant negative correlation with the Shannon and Simpson indices. However, no obvious relationship between nitrate concentration and any of the indices was found, indicating that nitrate concentration had little impact on microbial abundance and diversity. As has been reported, microbial diversity declines within hypoxic zones [43,44] and low pH destroys biodiversity and ecosystem balance [13,14]. For instance, a larval carry-over effect occurred with Ostrea lurida when pH < 7.8 [45], and communities showed significant changes in structure and diversity in response to pH declining from 8.7 to 7.7, 7.3, and 6.7 [15]. Therefore, it is reasonable to conclude that (i) the high microbial abundance and diversity in BHHZ77 was correlated with relatively high values of pH, DO, and nitrite, and (ii) the hypoxic zone that formed in the sediment of BHHZ83 and relatively low pH was not conducive to the reproduction and function of microorganisms.
To the best of our knowledge, reports on the direct or inverse correlation between silicate and the microbial community in ocean systems are rare. Some researchers have confirmed that low-heat Portland cement, which contains silicate as the main component, had strong resistance to seawater erosion [46,47]. Others have demonstrated that modified clay flocculating materials prepared from silicate clay minerals and modifiers could improve the removal of Microcystis aeruginosa and eutrophic water pollutants by a flocculation reaction [48]. Therefore, we proposed that a relatively high concentration of silicate might be one of the environmental parameters causing the differences in microbial communities at station BHHZ83 and the other stations in this study. Further study should be conducted to support this opinion.
Redundancy analysis with Canoco 5.0 software was used to analyze the correlation between environmental parameters and dominant microorganisms at the phylum and genus levels (Figure 7). The length of the response arrow in Figure 7 represents the intensity of the impact of the environmental parameters on the microbial community. A long arrow corresponds to a strong correlation. An acute angle between the arrow lines indicates a positive correlation, while an obtuse angle indicates a negative correlation. DO, pH, nitrite, salinity, ammonia, and silicate had obvious impacts on the microbial phyla. However, the composition in microbial genera appeared to be obviously associated with nitrate, phosphate, ammonia, and silicate. Specifically, pH, nitrite, salinity, and DO were positively related to Deinococcus-Thermus, Acidobacteria, Chloroflexi, Aminicenantes, Actinobacteria Planctomycetes, and Firmicutes. Ammonia concentration had a positive association with Bacteroidetes but had a inverse correlation with other phyla. The proportion of Planctomycetes and Firmicutes was positively associated with silicate concentration and negatively related to other environment factors. At the genus level, nitrate concentration was negatively related to all dominant genera except for Photobacterium. Photobacterium was positively correlated with silicate and phosphate concentrations but negatively correlated with ammonia concentration. Marinobacter, Gillisia, Loktanella, Maribacter, Idiomarina, Paenisporosarcina, Pseudomonas, and Lutimonas were more positively correlated with ammonia concentration than other environmental variables.
As has been reported, microbial communities change with environment parameters. For instance, a low pH changed the biogeochemical cycles of carbon, nitrogen, and sulfur by interfering with microbial and phytoplankton communities such as Trichodesmium, Nitrosomonas, Nitrobacterium, E. huxleyi, and Phaeocystis globosa [15,49]. In addition, it was confirmed that salinity was a key factor controlling bacterial and archaeal community structure along a 7000-mile latitudinal transect from the Mediterranean Sea across the Atlantic Ocean to the Brazilian coastal sea. The same study showed that N, P, and Si contributed to eukaryotic variation [12]. It has also been reported that salinity and pH influenced microorganism distribution and community structure in Dongzhai port mangrove sediments and in Paranaguá Bay, Brazil [50,51]. The salt tolerance of microorganisms varies under different environmental conditions, especially for Betaproteobacteriales [52,53]. Moreover, high concentrations of ammonia nitrogen inhibit the autotrophic nitrification activity of β-proteobacteria, resulting in a decrease in the richness and diversity of proteobacteria [54], while the abundance of Deltaproteobacteria increased with the concentration of organic pollutants [55]. In summary, the environment parameters discussed above interfered with microbial communities. In this study we found that the diversity and distribution characteristics of microbial communities in the estuary sediments of the Bohai Sea differed from those of the hypoxic zone. DO, pH, nitrite, salinity, ammonia, and silicate were key environmental parameters that contributed greatly to differences in microbial communities. These factors should be investigated further in future studies of pollution in Bohai Bay. Other environmental factors, such as heavy metal and temperature, might also be influencing the microbial community in Bohai Bay [1,12,56]. Therefore, further study is needed to determine the correlation between the above environmental parameters and microbial communities in the Bohai Sea.
4. Conclusions
Environmental parameters significantly affected the microbial community in sediments from the Bohai Sea. In terms of alpha diversity, microbial communities in the sediment of four different stations were ranked as Liao River estuary > offshore zone > hypoxic zone > Yellow River estuary. In terms of community abundance and diversity, the ranking was Liao River estuary > Yellow River estuary > offshore zone > hypoxic zone. In terms of beta diversity, the Liao River estuary had the greatest unique diversity. Nitrite concentration, silicate concentration, DO, pH, and salinity were obviously associated with microbial abundance and diversity. The main phyla in sediment samples were similar in all four stations in Bohai Bay, while the relative abundance and the dominant bacterial genera were significantly different from each other. Gillisia was the dominant genus in the Liao River estuary and Yellow River estuary. The known dominant genera differed substantially between the four stations. The offshore zone and hypoxic zone had the same dominant genus (Photobacterium). The largest relative abundance of unknown genera was detected in the Liao River estuary.
Author Contributions
Methodology, F.G.; software, P.Z.; validation, G.X.; investigation, H.Z.; data curation, C.Z. and G.L.; data analysis, P.Z.; writing—original draft preparation, C.Z.; writing—review and editing, F.G. and G.X.; visualization, H.Z.; project administration, F.G.; supervision, C.Z. and G.L.; reference editing, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the National Key Research and Development Program of China, grant number [2018YFC1407604], State Environmental Protection Key Laboratory of Coastal Ecosystem fund, grant number [202105] and Dalian Young Star of Science and Technology Project, grant number [2018RQ77].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The work was supported by the National Key Research and Development Program of China (No. 2018YFC1407604), State Environmental Protection Key Laboratory of Coastal Ecosystem fund (No. 202105), and Dalian Young Star of Science and Technology Project (No. 2018RQ77). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, M.; Yanwen, Q.; Binghui, Z.; Lei, Z. Heavy metal pollution in Tianjin Bohai Bay, China. J. Environ. Sci. 2008, 7, 6. [Google Scholar]
- Liu, X.; Zhang, H.; Li, L.; Fu, C.; Tu, C.; Huang, Y.; Wu, L.; Tang, J.; Luo, Y.; Christie, P. Levels, distributions and sources of veterinary antibiotics in the sediments of the Bohai Sea in China and surrounding estuaries. Mar. Pollut. Bull. 2016, 109, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gang, L.; Gao, C.; Sun, W. Analysis of Comparative Advantage of the Main Aquaculture Products in Bohai Rim. Chin. Agric. Sci. Bull. 2014, 57, 1093–1096. [Google Scholar]
- Sørensen, K.; Teske, A. Stratified Communities of Active Archaea in Deep Marine Subsurface Sediments. Appl. Environ. Microbiol. 2006, 72, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.; Zheng, B.; Zhu, Y.; Wang, X. Analysis of the bacterial community in the two typical intertidal sediments of Bohai Bay, China by pyrosequencing. Mar. Pollut. Bull. 2013, 72, 181–187. [Google Scholar] [CrossRef]
- Zhao, H.D.; Kao, S.J.; Zhai, W.D.; Zang, K.P.; Zheng, N.; Xu, X.M.; Huo, C.; Wang, J.Y. Effects of stratification, organic matter remineralization and bathymetry on summertime oxygen distribution in the Bohai Sea, China. Cont. Shelf Res. 2017, 134, 15–25. [Google Scholar] [CrossRef]
- Bell, T.H.; Yergeau, E.; Maynard, C.; Juck, D.; Whyte, L.G.; Greer, C.W. Predictable bacterial composition and hydrocarbon degradation in Arctic soils following diesel and nutrient disturbance. ISME J. 2013, 7, 1200–1210. [Google Scholar] [CrossRef]
- Ren, G.; Ren, W.; Teng, Y.; Li, Z. Evident bacterial community changes but only slight degradation when polluted with pyrene in a red soil. Front. Microbiol. 2015, 6, 00022. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; He, T.; Dai, Y.; Xie, S. Sediment Bacterial Communities Associated with Anaerobic Biodegradation of Bisphenol A. Microb. Ecol. 2015, 70, 97–104. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, R.; Lv, Z.; Shao, Y.; Zhao, X.; Li, C. Analysis of gut microbiota revealed Lactococcus garviaeae could be an indicative of skin ulceration syndrome in farmed sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2018, 80, 148–154. [Google Scholar] [CrossRef]
- Song, H.; Li, Z.; Du, B.; Wang, G.; Ding, Y. Bacterial communities in sediments of the shallow Lake Dongping in China. J. Appl. Microbiol. 2011, 112, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, X.; Zhang, C.Y.; Chen, G.F.; Lao, Y.M.; Jin, H.; Cai, Z.H. Distribution Patterns of Microbial Community Structure Along a 7000-Mile Latitudinal Transect from the Mediterranean Sea Across the Atlantic Ocean to the Brazilian Coastal Sea. Microb. Ecol. 2018, 76, 592–609. [Google Scholar] [CrossRef] [PubMed]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef]
- Hale, R.; Calosi, P.; Mcneill, L.; Mieszkowska, N.; Widdicombe, S. Predicted levels of future ocean acidification and temperature rise could alter community structure and biodiversity in marine benthic communities. Oikos 2011, 120, 661–674. [Google Scholar] [CrossRef]
- Campbell, L.G.; Thrash, J.C.; Rabalais, N.N.; Mason, O.U. Extent of the annual Gulf of Mexico hypoxic zone influences microbial community structure. Public Libr. Sci. 2019, 14, 0209055. [Google Scholar] [CrossRef]
- Ekau, W.; Auel, H.; Pörtner, H.O.; Gilbert, D. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 2010, 7, 1669–1699. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 27. [Google Scholar] [CrossRef]
- Perkins, T.L.; Katie, C.; Baas, J.H.; Jago, C.F.; Jones, D.L.; Malham, S.K.; Mcdonald, J.E.; Gomes, N. Sediment Composition Influences Spatial Variation in the Abundance of Human Pathogen Indicator Bacteria within an Estuarine Environment. PLoS ONE 2014, 9, 112951. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Li, Q.; Han, T.; Xie, J.; Hu, Y.; Chai, L. Phylogenetic analysis of bacterial community composition in sediment contaminated with multiple heavy metals from the Xiangjiang River in China. Mar. Pollut. Bull. 2013, 70, 134–139. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Publishers Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucl. Acids Res. 2014, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Paul, F.K.; Josephine, Y.A. Bacterial diversity in aquatic and other environments: What 16S rDNA libraries can tell us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Shannan, X.; Yong, L.; Fan, J.; Yayuan, X.; Zhanhui, Q.; Manogaran, L. Impact of salinity variation and silicate distribution on phytoplankton community composition in Pearl River estuary, China. Ecohydrol. Hydrobiol. 2022, in press. [Google Scholar]
- Zhang, C.; Gao, F.; Wu, Y.; Xu, G.; Liu, H.; Zhang, H.; Yang, F.; Xu, Y. Small-sized salt-tolerant denitrifying and phosphorus removal aerobic granular sludge cultivated with mariculture waste solids to treat synthetic mariculture wastewater. Biochem. Engin. J. 2022, 181, 108396. [Google Scholar] [CrossRef]
- Dong, Q.; Li, W.; Li, W.; Wu, X.; Wang, Y.; Liu, Y. Salt-tolerance aerobic granular sludge: Formation and microbial community characteristics. Bioresour. Technol. 2018, 249, 132–138. [Google Scholar]
- Jin, X.; Kou, W.; Yu, H.; Liu, Y.; Wu, L. Environmental Factors Influencing the Spatial Distribution of Sediment Bacterial Community Structure and Function in Poyang Lake. Res. Environ. Sci. 2017, 30, 529–536. [Google Scholar]
- Xu, H.; Wang, L.; Bao, X.; Jiang, N.; Yang, X.; Hao, Z.; Chang, Y.; Ding, J. Microbial communities in sea cucumber (Apostichopus japonicus) culture pond and the effects of environmental factors. Aquac. Res. 2019, 10, 14002. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Abayneh, B.; Ding, Y.; Yan, D.; Bai, J. Microbial community structure of different electrode materials in constructed wetland incorporating microbial fuel cell. Bioresour. Technol. 2016, 221, 697–702. [Google Scholar] [CrossRef]
- Kizito, S.; Tao, L.; Wu, S.; Ajmal, Z.; Luo, H.; Dong, R. Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: Role of media and tidal operation. Sci. Total Environ. 2017, 592, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshiguchi, K.; Ariesyady, H.D.; Okabe, S. Identification and quantification of key microbial trophic groups of methanogenic glucose degradation in an anaerobic digester sludge. Bioresour. Technol. 2012, 123, 599–607. [Google Scholar] [CrossRef]
- Mayer, R.E.; Bofillmas, S.; Egle, L.; Reischer, G.H.; Schade, M.; Fernandez-Cassi, X. Occurrence of human-associated Bacteroidetes genetic source tracking markers in raw and treated wastewater of municipal and domestic origin and comparison to standardand alternative indicators of faecal pollution. Water Res. 2016, 90, 265–276. [Google Scholar] [CrossRef]
- Lee, I.S.; Parameswaran, P.; Rittmann, B.E. Effects of solids retention time on methanogenesis in anaerobic digestion of thickened mixed sludge. Bioresour Technol. 2011, 102, 10266–10272. [Google Scholar] [CrossRef]
- Skariyachan, S.; Garka, S.; Puttaswamy, S.; Shanbhogue, S.; Devaraju, R.; Narayanappa, R. Environmental monitoring and assessment of antibacterial metabolite producing actinobacteria screened from marine sediments in south coastal regions of Karnataka, India. Environ. Monit. Assessment. 2017, 189, 283. [Google Scholar] [CrossRef]
- Weissbrodt, D.G.; Neu, T.R.; Kuhlicke, U.; Rappaz, Y.; Holliger, C. Assessment of bacterial and structural dynamics in aerobic granular biofilms. Front. Microbiol. 2013, 4, 00175. [Google Scholar] [CrossRef]
- Zhu, T.; Tian, C. Analysis of microbial diversity and its correlation with environmental factors in reservoir sediment. Acta Sci. Nat. Univ. Pekinesis 2018, 54, 625–632. [Google Scholar]
- Liu, X.B.; Yang, Y.; Zhong-Yuan, L.I.; Lin, Y.Q. Analysis of microbial community diversity in the water from Changli coastal area of Bohai Sea, Hebei. J. Saf. Environ. 2019, 19, 1817–1823. [Google Scholar]
- Beman, J.M.; Carolan, M.T. Deoxygenation alters bacterial diversity and community composition in the ocean’s largest oxygen minimum zone. Nat. Commun. 2013, 4, 3075. [Google Scholar] [CrossRef] [PubMed]
- Jessica, A.B.; Frank, J.S.; John, M.E.; Edward, F.D. Microbial community phylogenetic and trait diversity declines with depth in a marine oxygen minimum zone. Ecology 2012, 93, 1659–1673. [Google Scholar]
- Hettinger, A.; Sanford, E.; Hill, T.M.; Lenz, E.A.; Russell, A.D.; Gaylord, B. Larval carry-over effects from ocean acidification persist in the natural environment. Global Chang. Biol. 2013, 19, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Wen, H. Research of Low Heat Portland Cement for Marine Engineering. Master’s Thesis, China Building Materials Academy, Beijing, China, 2018. [Google Scholar]
- Liu, H. Application of Low-Heat Portland Cement in Marine Engineering Concrete. Master’s Thesis, Guangzhou University, Guangzhou, China, 2019. [Google Scholar]
- Yan, Q. Researchon on New Composite Flocculant Material of Modified Layer Silieates and Microorganism. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2009. [Google Scholar]
- Yu, J.; Zhang, Z.; Tian, J.; Yang, G.; Jia, H. Effects of ocean acidification on cycles of carbon, nitrogen and sulfur. T. Oceanol. Limn. 2018, 3, 79–87. [Google Scholar]
- Zhang, P.; Xie, X.; Li, Q.; Gan, Z.; Hu, T.; Yang, J.; Deng, Y.; Gan, Y.; Zhang, Y. Microbial community structure and its Response to environment in mangrove sediments of Dongzhai Port. Earth Sci. 2022, 3, 1122–1135. [Google Scholar]
- Ceccon, D.M.; Faoro, H.; da Cunha Lana, P. Metataxonomic and Metagenomic Analysis of Mangrove Microbiomes Reveals Community Patterns Driven by Salinity and pH Gradients in Paranaguá Bay, Brazil. Sci. Total Environ. 2019, 694, 133609. [Google Scholar] [CrossRef]
- Bai, Y.N.; Ren, P.; Feng, P.Y. Shift in Rhizospheric and Endophytic Bacterial Communities of Tomato Caused by Salinity and Grafting. Sci. Total Environ. 2020, 734, 139388. [Google Scholar] [CrossRef]
- Behera, P.; Mahapatra, S.; Mohapatra, M. Salinity and Macrophyte Drive the Biogeography of the Sedimentary Bacterial Communities in a Brackish Water Tropical Coastal Lagoon. Sci. Total Environ. 2017, 595, 472–485. [Google Scholar] [CrossRef]
- Wang, K.; Ke, S.; Yuan, H.; Zhu, J.; Li, W. Effect of ammonia-nitrogen concentration on microbial community structure in a MBBR process. Environ. Eng. 2020, 38, 119–125. [Google Scholar]
- Liu, M.; Huang, H.; Bao, S. Microbial Community Structure of Soils in Bamenwan Mangrove Wetland. Sci. Rep. 2019, 9, 8406. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, C.T.A. Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res. 2012, 46, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).