Abstract
This paper presents the results of the controlled sedimentation process for deasphalting, caused by targeted formation of the fuel dispersed system components incompatibility (proportion of the paraffins with normal structure increase) experimental investigations. The main purpose was to decrease the contained amount of sulphur in sedentary marine fuel and procure VLSFO. Developed and given account of the laboratorial method of instituting the sediment which modifies standard TSP and allows to control the deasphalting with the take-off of sediment and deasphaltisate for future analysis. In this case, 5 components of marine fuels, their basic physical and chemical properties, and chemical group composition were used as an object of study. Based on the data obtained and via use of worked out software package, 6 compositions of marine fuels were specified. Furthermore, they were then produced and their quality attributes were defined. The results show that the deasphalting caused by the components targeted incompatibility is accompanied by the desulphurization. Sulphur concentration took place in the sediment where its content was 4.5 times higher than in composite fuel. At the same time, sediment content fell from 0.9% to 1.02% by weight according to the fuel composition. The sulphur content in the resulting deasphaltisate declined by approximately 15% in relation to original fuel mix, moreover, other quality indicators improved. In order to find out whether the usage of sediment obtained is possible, its composition and structure were assessed. The results of the interpretation showed, that sediments were inclined to bitumens, which allows them to be mixed with sediments as a way to cut process waste. Targeted deasphalting makes it possible for the expenses on reducing sulphur containment in marine residual fuels to be decreased, which expands the opportunities of fuels application according to ISO 8217:2017.
1. Introduction
Since the 2020 beginning International Maritime Organisation (IMO) sharply tightened the sulphur content requirements in the world’s oceans, according to the new convention in Annex VI MARPOL 73/78. Due to the new directives, the sulphur permissible content in marine residual fuels dropped 7 times, from 3.5% to 0.5% by weight. Three strategies can be identified so that make bunkering fuels conforming to the requirements [1,2]:
- Make use of low-sulphur fuels which contain less than 0.5% sulphur by weight for bunkering (VLSFO);
- Install scrubbers or other exhaust gas treatment systems on bunkered ships;
- Apply competing fuels such as biofuel and liquefied natural gas (LNG).
The presented requirements for the sulphur content reduction are oriented to emission reduction, which is of great current interest in the Arctic region [3,4,5,6,7].
There are some severities which faces the first strategy. These days, downstream sector is on its way to enhance oil refining, as a result, whereas the proportion of light fractions yield rises, the dark fractions proportion decreases. Additionally, the raw materials’ prices growth negatively affects the low-sulphur petroleum products cost, therefore sometimes their production is not profitable. This leads to disability of the modern petroleum refinery to provide consumers with fuels of today with sulphur content under 0.5%, because of the modifications in refinery method to be made. For instance, the technological scheme change of catalytic hydrogenation installation was suggested. The upgrade of high-sulphur fuel oils at the gas processing plant through heat treatment at the elevated hydrogen pressure can carry out an oil product with the sulphur content of less than 1% by weight [8]. Another approach is to purify N-methylpyrrolidone fuel oil in the presence of undecane fraction at mass ratios to raw materials of 3:1 and 0.5:1 with and without preliminary deasphalting with pentane. Consequently, the sulphur content in mazut and its coking properties drop 4 and 2 times, respectively [9]. The developed system of catalysts for demetallization and desulfurization of oil residues with the production of low-sulphur marine fuel results are presented. In this method stationary bed of catalysts was applied. In addition, optimal conditions for the hydrogenation raw materials processing were selected, which made it possible to obtain the required sulphur content of 0.5% by weight. Unfortunately, a lot of solutions proposed in oil refinery greatly affect the final product cost [10]. For that reason, marginality declines significantly which prevents oil refinery from low-sulphur VLSFO fuels production.
Based on the above, these days there are interests in marine residual fuels on global markets. In order to meet the demand for a new type of marine fuel, bunkering companies are actively carrying out processes of mixing different fuel components [11,12]. Hence the required quality indicators of fuels, which are most economically beneficial, are obtained.
Due to the increase in the proportion of mixed fuels, the sedimentation problem becomes more urgent [13,14,15]. Incompatibility is the reason for the fuel system stability loss which causes sedimentation. According to the international standard ISO 8217 fuel quality requirements, the total sediment content must be lower than 0.1% by weight.
The second method also has some weak points, especially expensive installation, maintenance and repair of scrubbers, which decrease shipowners’ profits. According to the emissions in the exhaust gas cleaning technology (EGC) application impact assessment it was determined that burning fuels with a higher sulphur content create more emissions containing SO2 and particulate matter, which should be taken into an account when using high-sulphur fuels (HSFO) [16,17,18,19,20,21].
The third strategy is one of the most relevant in the current environment, when a variety of research is aimed at alternative fuels, such as biofuels, elaboration. Unfortunately, the unfiltered organic complexes found in biofuels from the catalyst cause agglomeration and contribute to fouling and corrosion [22]. LNG makes it effective and eco-friendly to employ marine fuels, although it requires considerable reconstruction expenditure for fuel systems and new ships type construction. The effectiveness of VLSFO and LNG application shows that VLSFO employment is more cost-effective than the LNG strategy in the first 2.5 years [23,24,25]. Notwithstanding, in the long term, LNG application is more economically effective. Based on the evaluation results, the LNG strategy is suitable for ocean container vessels with fixed routes and large ships so that meet IMO requirements. By contrast, VLSFO fuels usage is beneficial for old merchant ships and small vessels [23,26,27].
As to that, the low-sulphur marine fuels (VLSFO) manufacturing with low financial cost is an extremely important issue. One of the proposed methods involves application of emulsified fuels produced by mixing water (5–25%) with an oil product [28]. However, reduction in the fuel emulsion sulphur content and emissions’ environmental friendliness improvement (O2 concentration increased by 4.2% while CO2 concentration decreased by 2.1%) lead to the exhaust gas temperature dwindling by 14.3% and the combustion efficiency of fuels in the boiler decline by approximately 2.6%. In addition, high water content in the fuel strengthens fuel system detriment from corrosion and can be a reason for breakdown frequency growth. Another approach is to reduce sulphur content and cost of fuels produced [29,30] through the biofuels, which are made from wood waste, introduction as a marine fuel component. The investigation shows that the increase in the proportion of wood components in mixed fuels leads to a vigorous sulphur oxides concentration surge from 11 to 95.8% relative to the sulphur oxide formation concentration in homogeneous coal. The wood component in the mixed fuel proportion rise increases calcium sulfate (45.1%) and aluminum sulfate (43.2%) content in the mixture, which is a negative factor. Therefore, such methodology is appropriate only for slight mixing proportions, which in turn will not affect significantly final result. One more mixing type is Hydrothermal Liquefaction Biocrude application as a mixed fuel component. Although, according to the authors’ research [31,32,33], most beneficial supplement proportion is under 10%, which also makes an addition to the ash content in the final product.
When considering alternative sulphur content decline in marine fuels approaches, there is a considerable issue of components compatibility, because of the sedimentation risks due to different components adjustment. This leads to several negative effects:
- fuel’s quality aggravation could be an occasion for fuel systems wear and clogging;
- reservoir efficient volume decreases while tank farms operation;
- processing units’ obstruction during transport;
- downtime and relative expenses.
It should be noted that deposits influence negatively on the metal corrosiveness, especially when operating with produced water and water-oil emulsions [34,35,36,37].
Residual fuels incompatibility manifests itself due to the strong intermolecular interaction occurrences, which are caused by the structural-group individual composition variance, as well as by the residual fuels high-molecular compounds concentrations shifting. This contributes to the formation of associates of molecules, bulk colloidal particles of various shapes and structures. There are risks of incompatibility even when mixing the same brand of fuels because of differences in composition [38,39].
According to colloid-chemical concepts, asphaltenes in the fuel system are suspended in a stable state. The solvation shell prevents the molecules from enlargement; however, when mixing residual fuels which contain paraffin compounds with low C/H ratio and molecular weight, the solvate shell, the so-called protective rings, dissolves. Thus, paraffin compounds play the role of a solvent, which is the reason for asphaltene associates coarsening. The result is the stability loss, in consequence of which sedimentation and deposit accumulation occurs [40,41,42,43]. The industrial methodology of deasphalting is based on this rule; however, its identity with fuels incompatibility performance suggests that it could be possible to use it as a way to upgrade the residual marine fuels [44,45,46].
The purpose of this paper is to educe, whether it is possible to apply targeted deasphalting through waxy components supplementation into the fuel system so as to decline residual marine fuels sulphur content and VLSFO production.
2. Materials and Methods
2.1. Materials
Residual products were taken as objects of study, including 4 high-viscosity black straps obtained from different oils and 1 vacuum gasoil stock with a high content of basic paraffins (about 72%). Residual oil products had been received from various refineries, which provided a variation in the sulphur content from 1.28 to 3.15%, in order to assess the deasphalting effect on the desulfurization degree.
The main physical and chemical properties of oil products examined, namely kinematic viscosity, density, flashpoint and fluidity loss, water content, sulphur content and total sediment were determined in accordance with current standards. Quality indicators are presented in Table 1.

Table 1.
Quality indicators of residual fuels.
2.2. Hydrocarbon Fuels SARA and GCMS
A combined gas chromatography-mass spectrometry method was used to determine the group composition of hydrocarbons. The method was put into practice through the equipment GCMS-QP2010 Shimadzu. In addition, SARA-analysis, which could be observed on the Figure 1, was performed [47,48]. SARA-analysis consists in separating petroleum products into four solubility groups: saturated hydrocarbons, aromatic compounds, resins, and asphaltenes (SARA).
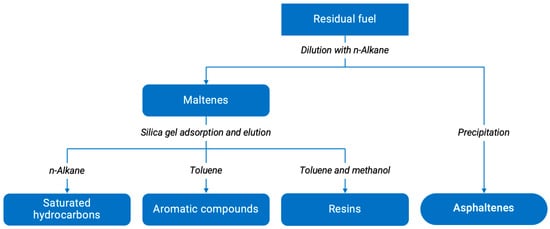
Figure 1.
Residual fuel dilution scheme to determine the group composition of fuels.
The hydrocarbon composition of the resulting liquid-phase mixture (sample dissolved in toluene in ratio 1:100) was determined by the means of the gas chromate-mass spectrometer GCMS-QP2010, concurrently substances that boil off at temperatures up to 300 °C in the chromatograph column were defined [49].
2.3. Sludge Elemental Analysis
Modern methods and laboratory installations employment provided means for fuel sedimentation chemical makeup specification with a high level of reliability, including organic and inorganic micro impurities.
Shimadzu TOC-V instrument with an SSM 5000A attachment for solid samples analysis was applied for determining organic and inorganic carbon occurrence [50,51]. The method’s essence is the complete combustion of a sample and exhaust carbon dioxide content determination through the use of IR cells.
Analyser LECO CHN-628 was also applied during examining fuels sediments elemental composition. Analyser allows carbon, nitrogen and hydrogen presence determination with high precision [52,53]. The analysis durations were carbon—200 s, hydrogen—200 s, and nitrogen—240 s. The limits of determination of C, H and N are from 0 to 100%.
The sulphur containment was defined by the instrumentality of energy-dispersive X-ray fluorescence analyser Spectroscan S. Sediment was previously dissolved in toluene and after the sulphur content exploration, the sulphur contained in fuel sediments was calculated. Based on precipitate–solvent mixture ratio knowledge, the sulphur content in attained sediments was calculated [54,55].
In order to determine fuel sludge ash GOST 1461 methodology was applied. The oxygen content in the sludge was calculated according to ISO 17247:2013.
2.4. Sludge XRD-Analysis
X-ray diffraction analysis of sediment samples was performed on an X-ray diffractometer XRD-7000 Shimadzu (CuKα-radiation, 2.7 kW) by the agency of the polycrystal method. The X-ray pattern was taken at room temperature with long accumulation times (2 s) and a scanning step of 0.02°.
2.5. Sludge FT-IR-Analysis
In order to obtain additional information about fuel deposits structural-group composition, IR spectrometry studies were carried out on an IRAaffinity-1 IR-Fourier spectrometer [49,56]. A precipitate solution was prepared at a concentration of 1% in carbon tetrachloride.
According to the methodology of the studies the absorption bands estimation was performed, additionally, calculations based on obtained data of the deposit IR spectrum were carried out. Unbranched paraffin structures are defined by strong absorption bands corresponding to 720 and 1300 cm−1.
Absorption bands evaluation in the region of 1376 cm−1 and 1464 cm−1 provides an opportunity to define hydrocarbon paraffin chains branching degree. The branching degree characteristic specified with the aid of coefficient β. To determine this coefficient, it is necessary to calculate ratio of the intensities of the most characteristic absorption bands for CH3 and CH2 groups:
The greater the coefficient value, the higher the paraffin structures branching degree in hydrocarbons. The ranges 812–816 cm−1, as well as 1600 cm−1, are absorption bands corresponding to aromatic structures. The aromaticity degree could be characterised intermediately a coefficient, which is determined by the most distinctive absorption bands intensities ratio for aromatic structures relative to the methylene groups of paraffin structures, evaluated through the formula:
For aromatic structures, relative to the methylene groups of paraffin structures, a ratio that shows the fuels aromatisation is determined:
2.6. Sludge SEM
To define the composition of the elements which form inorganic compounds in the precipitate, X-ray fluorescence analysis was carried out by the instrumentality of an X-act detector, equipped with a scanning electron microscope Tescan Vega 3.0 LMH [40]. An electron microscope image of the sample particles was acquired in Secondary Electrons (SE) Resolution scanning mode. The accelerating voltage was 20 kV, and the emission current was 120 µA. The sediment was scanned on the top filter obtained after thermal aging (TSP), filtration and drying. The spectrum processing was operated for all elements based on samples. No peaks were missed and 3 iterations were performed. This indicates a high reliability of the results obtained.
2.7. Modified TSP Analysis
The compatibility and stability of fuels were estimated with the aid of developed laboratory analysis. This method offers sedimentation activity quantitative determination due to a new testing algorithm and the determination of the scoring parameter based on ISO 10307-2. In addition, the methodology allows deasphalted product and the resulting sediment withdrawal for future contemplation.
Modified TSP analysis consists of each component of mixture total sediment content determination; furthermore, components are mixed in the required proportions and the total sediment of the fuel mixture value is evaluated, on the basis of which the compatibility index is calculated.
This approach is basically similar to ISO 10307-2 method from sample preparation and thermal regulation to filtration. During the filtration process, the filters are not washed with a heptane-toluene mixture. In this way, the methodology is as close as possible to industrial process, insomuch as only temperature and filtration conditions are applied.
3. Results and Discussion
In the program developed fuel compositions formulations with a sulphur content in the range from 0.6 to 1.6% by weight were calculated. The resulting calculation models are presented below (Table 2).

Table 2.
Results of fuel blend design model.
Sample mixing in the laboratory was performed via the fuel mixtures calculation model and the recipe proposed.
The obtained compositions were analysed for the main physical and chemical properties, namely, the indicators of kinematic viscosity, density, sulphur content and TSP were determined. The results are presented in Table 3.

Table 3.
Quality indicators of the obtained fuel mixtures.
As expected, the high content of n-paraffins in gasoil (sample No.1) and asphaltenes in the remaining residual fractions caused a process of directional incompatibility through deasphalting.
In the act of TSP evaluation analysis accomplishment, the samples were kept at 100 °C for 24 h. During this time, the possible coagulation reactions of fuels’ heavy components took place and, during subsequent filtration, they sedimented to form a precipitate (sludge). As a way to exclude external factors influence on the sludge variation, it was not washed with a mixture of heptane 85% and toluene 15% according to ISO 10307-2, and the entire procedure was performed pursuant to the method described in Section 2.7.
The deasphaltisate and the resulting sediment (sludge) amount ratio as a consequence of the deasphalting process is shown in the Figure 2. It should be noted that in the laboratory analysis for each fuel composition, the flasks and equipment were chemically cleaned and did not contain other impurities. Images of filters with sediments (sludge) are presented in the Figure 3.
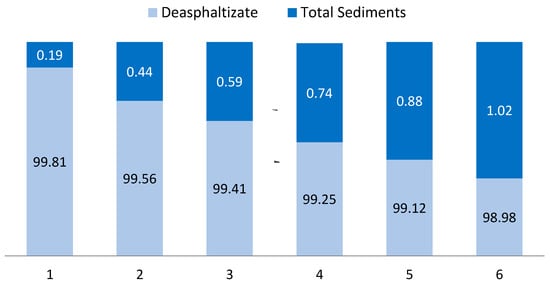
Figure 2.
Ratio of deasphaltisation to total sediments.
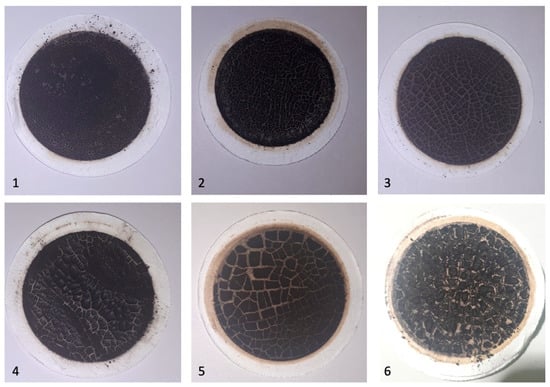
Figure 3.
The obtained sediments (sludge) after deasphaltation and filtration for fuel samples 1–6.
It could be noted that the intensity of sediments (sludges) on the filter varied symbatically with a decrease in the formed TSP sediment content.
3.1. Deasphaltisate Study as VLSFO
At the next stage of research, the deasphaltisate main quality indicators were specified. In the resulting sediment for each fuel mixture, the sulphur content was determined by dissolving the sediment sample in toluene in a ratio of 1 to 10. Onward, the method of X-ray energy dispersive analysis was applied and the sulphur content in the precipitate was defined by calculation. The results of the studies obtained are shown in Table 4.

Table 4.
Deasphaltisate quality and sulphur content in the sludge.
The results of the deasphaltisate laboratory examination show a reduction in the sulphur content in the fuel. Other indicators evaluations make it possible to confirm other qualitative characteristics improvement. There is a decrease in the values of density, kinematic viscosity and pour point due to the heaviest components of the fuel mixture deposition.
Based on the data obtained, an approach for upgrading marine residual fuels is proposed. The methodology is based on residual fractions from primary and secondary refining processes mixing with waxy gasoil fractions. This mixing causes coagulation of asphaltenes, resins, carbenes and carboides with paraffin fractions. This deasphalting method does not require solvents and complex technological solutions application. The sludge contains a significantly higher sulphur content than the fuel mixture, a comparison could be observed in Figure 4.
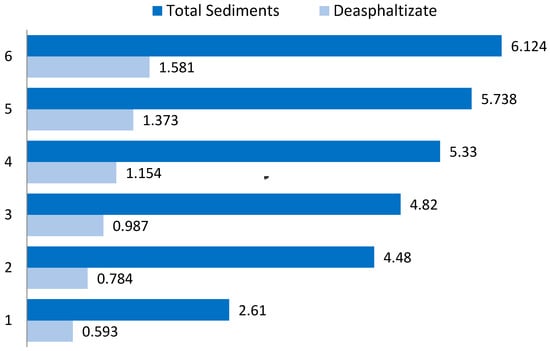
Figure 4.
Sulphur content in total sediments and deasphaltisation.
The enhancement consequences are reductions in the density, kinematic viscosity, and depressant properties. Furthermore, there is a decrease in sulphur content in the resulting fuel by an average of 2% (Figure 5).
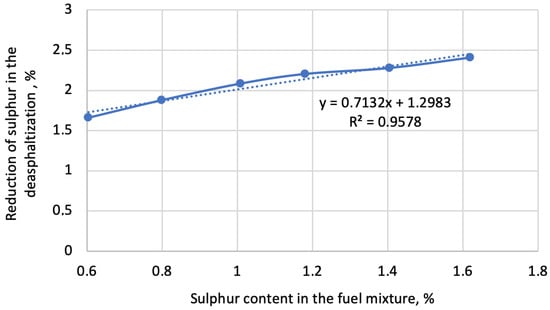
Figure 5.
Reduction of sulphur in the deasphaltisation depending on the sulphur content in the fuel mixture.
The sulphur content in the sediment (sludge) is on average 4.6 times higher than in the fuel composition. However, with this approach, the resulting sediment (sludge) recycling or employment is necessary. There is such demand because while the application of this method on oil refineries a considerable amount of sediment will accumulate. In order to develop a solution, it is necessary to analyse the resulting precipitate (Figure 6).

Figure 6.
Total sediment of the fuel composition.
3.2. Deasphaltisation Sediment (Sludge) Investigation
For a sediment (sludge) detailed study, fuel mixture No.1 was selected (Table 4). To determine the group hydrocarbon composition of samples No.1 and No. 2, the SARA analysis method was used. The results are presented in Table 5.

Table 5.
The group hydrocarbon composition of oil products No.1 and 2 (% wt.).
Based on the marine fuel samples examined hydrocarbon composition definition it can be concluded that sample No.1 contained secondary oil refining residual products. This follows from alkenes presence, moreover, the sample contained asphaltenes and resins, which are prone to precipitation. Sample No.2 has a high content of n-paraffins, which contribute to the precipitation of asphaltene agglomerates.
As a result of gas chromatography-mass spectrometry (Figure 7), the precipitate dissolved in toluene structural-group composition was established (Table 6).
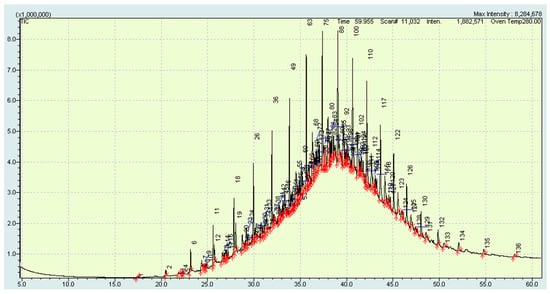
Figure 7.
Chromatogram of sediment from residual fuel mixture No.1.

Table 6.
The group composition of the sediment of the residual fuel mixture No.1.
Thuswise, it was determined that among the identified compounds in the residual fuel, HI-TS fraction main share belonged to n-paraffins. Unidentified components presumably belong to asphaltenes and resins, since the chromatogram (Figure 7) was determined as an undivided peak in the form of a Gaussian. Therefore, the presence of the macromolecular compounds, which boil away at temperatures below 300 °C, complex mixture could be stated. The mixture entered the column, but was not taken into account when calculating the hydrocarbon composition.
The organic carbon content on TOC-V SSM 5000A is 83.50 ± 1.0%, as expected, inorganic carbon was not detected.
The SEM morphology studies outcome is presented in Figure 8. By determining the filter spectrum, the elemental composition of the filter itself was specified (through the agency of the X-act detector) in order to exclude possible influence in the sediment elements spectrum defining (Figure 9).

Figure 8.
Electronic image of filter with sediment particles (sludge).
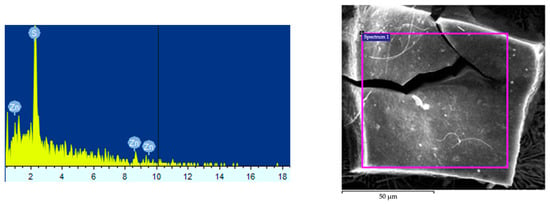
Figure 9.
Spectrum of elements and SEM image of sediment (sludge).
It was recorded that inorganic impurities in the sediment are represented only by sulphur derivatives. The spectrum also contained traces of zinc, which is part of the filter thread component.
The sediment chemical composition defined on LECO CHN-628 analyser is presented in Table 7.

Table 7.
Chemical composition of the sediment of the residual fuel mixture.
The total carbon content, obtained on the LECO CHN 628 (84.94 ± 1.0%) analyser, was higher than the value obtained on the Shimadzu TOC-V (83.50 ± 1.0%). Inasmuch as in the second case the temperature in the oxidising chamber was higher and there was no complete oxidation of carbon, so the values were underestimated. This means that the carbon determination by the two methods results was close in value and made it possible to determine with high accuracy the amount of carbon in the total sediment of residual fuels.
According to the sediment (sludge) chemical composition evaluation results, in terms of carbon to hydrogen ratio (8:1), sediment occupied an intermediate value between liquid petroleum fuels and petroleum carbon materials. The increase in C:H was due to condensation.
The IR spectrum derived as a result of a solution of sediment (sludge) in carbon tetrachloride analysis on an IR-Fourier spectrometer of is shown in Figure 10.
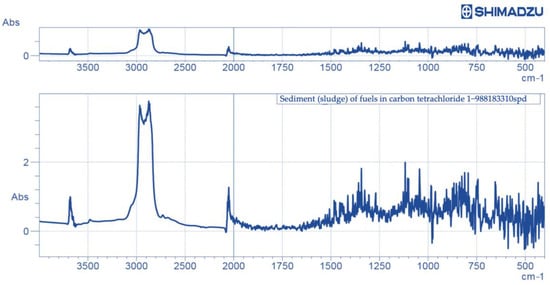
Figure 10.
IR spectrum of sediment (sludge) of fuels in carbon tetrachloride.
Wave numbers of about 3000 cm−1 corresponded to n-paraffins, which confirmed the results obtained by the chromatographic method.
In accordance with IR-Fourier spectroscopy results aromatisation coefficients values were and . Based on these values, it could be concluded that the studied fuel sediment (sludge) was estimated as methane or methane-naphthenic, which was consistent with the results of gas chromatography-mass spectrometry. The coefficient confirms a significant number of branched structures in the explored fuel sediment composition.
Based on the X-ray diffraction method results, it was determined that the total sediment was amorphous carbon, with a degree of crystallinity much lower than in the ideal graphite structure. The amorphous carbon matrix was formed by crystallites that have a turbostratic structure [43]. The crystallites structures main characteristics were the interplanar distances—d002, d110 and the coherent scattering regions sizes in the direction of the crystallographic axes «c» and «a», Lc and La, respectively. For graphite d002 and d110 were 3.354 Å and 1.232 Å, respectively, whereas for turbostatic structure d002 varies from 3.37 Å to 3.60 Å, and d110 varies from 1.215 to 1.230 Å.
Peaks with a maximum of 2θ angels of about 25° and 77° which correspond to 2θ002 and 2θ110 reflections were responsible for the crystal lattice of the deposit (sludge) structure after thermal aging (TSP) [43]. The diffraction analysis results calculated values for the reflections (002) and (110) for the sediment after aging are presented in Table 8.

Table 8.
The results of diffractometric analysis of the total sediment (sludge).
The interplanar distance d002 is 3.5618 Å, while d110 is 1.2307 Å. According to the results of X-ray phase analysis, the carbon materials microstructure nature could be estimated through the ratio of the average height Lc and the average crystallites diameter La. In this case, the ratio of Lc to La was approximately 1 to 16, which showed that structure of crystallites was oblate. This structure indicated the transition beginning to an amorphous solid state.
4. Conclusions
As a result of the research, it was found that the targeted formation of incompatibility leads to deasphalting when mixing highly paraffinic components and oil refining residues. Afterwards the sediment (sludge) filtering it not only improves the quality of residual marine fuel, but also makes it possible to produce VLSFO by reducing the sulphur content 4.6 times.
This initiates the density and kinematic viscosity indicators decrease, and the depressant properties increase. In addition, resulting fuel contains by an average of 2% less sulphur.
In sample No.1, the content of n-paraffins was about 72%, which increased their share in the fuel during mixing and contributed to the active asphaltenes precipitation. It had been recorded that a significant proportion (about 39%) of the total sediment carbon was normal-type alkanes.
X-ray phase analysis made it possible to determine the microstructure of carbon materials. The total sediment was amorphous carbon, with a degree of crystallinity much lower than in the ideal graphite structure. The amorphous carbon matrix was formed by crystallites having a turbostratic structure. The Lc to La ratio was approximately 1 to 16, which indicated an oblate structure of crystallites. Which was to say that the transition from the liquid phase to the amorphous solid state started.
According to the results of chemical composition evaluation the C:H ratio was 8:1. This corresponds to an intermediate value between liquid petroleum fuels and petroleum carbon materials. The growth in C:H was the consequence of a decline in the concentration of H and an increase in C, which could be attributed to the so-called condensation. Polyaromatic hydrocarbons coagulate during the sediment formation, moreover, their molecular weight rose, and they represented amorphous carbon. This equates that sediment (sludge) obtained in the deasphalting procedure could be used as a component for tar and bitumen.
5. Patents
Method of determining compatibility and stability of fuel mixture components. Available online: https://new.fips.ru/ofpstorage/Doc/IZPM/RUNWC1/000/000/002/733/748/%D0%98%D0%97-02733748-00001/document.pdf (accessed on 1 September 2022).
Program for calculating kinematic viscosity, density, sulphur and water content for a mixture of crude oil and petroleum products. Available online: https://new.fips.ru/ofpstorage/Doc/PrEVM/RUNWPR/000/002/020/613/357/2020613357-00001/document.pdf (accessed on 1 September 2022).
Author Contributions
Conceptualization, R.S., S.I. and K.D.; methodology, R.S.; software, R.S. and S.I.; validation, S.I.; formal analysis, A.Z.; investigation, R.S. and S.I.; resources, A.Z. and K.D.; data curation, R.S. and S.I.; writing—original draft preparation, R.S. and S.I.; writing—review and editing, R.S., S.I., A.Z. and K.D.; visualization, S.I. and K.D.; supervision, S.I.; project administration, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was performed at the expense of the subsidy for the state assignment in the field of scientific activity for 2021 No. FSRW-2020-0010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Saint Petersburg Mining University for enabling the laboratory experiments. The investigations were carried out using the equipment of the Scientific Center “Issues of Processing Mineral and Technogenic Resources” and Center for Collective Use of Saint Petersburg Mining University.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| GOST | Russian government standard |
| EGC | Exhaust gas cleaning |
| HSFO | High-sulphur fuel oil |
| IMO | International Maritime Organisation |
| ISO | International organization for standardization |
| LNG | Liquefied natural gas |
| SARA | Saturate, aromatic, resin, and asphaltene |
| TSA | Total sediment accelerated |
| TSP | Total sediment potential |
| VLSFO | Very low sulphur fuel oil |
References
- Vedachalam, S.; Baquerizo, N.; Dalai, A.K. Review on impacts of low sulfur regulations on marine fuels and compliance options. Fuel 2022, 310, 122243. [Google Scholar] [CrossRef]
- Ju, H.-j.; Jeon, S.-k. Analysis of Characteristic Changes of Blended Very Low Sulfur Fuel Oil on Ultrasonic Frequency for Marine Fuel. J. Mar. Sci. Eng. 2022, 10, 1254. [Google Scholar] [CrossRef]
- Lavrik, A.; Zhukovskiy, Y.; Buldysko, A. Features of the Optimal Composition Determination of Energy Sources During Multi-Criterial Search in the Russian Arctic Conditions. In Proceedings of the International Youth Conference on Radio Electronics, Electrical and Power Engineering, Moscow, Russia, 12–14 March 2020; p. 9059215. [Google Scholar] [CrossRef]
- Buslaev, G.; Tsvetkov, P.; Lavrik, A.; Kunshin, A.; Loseva, E.; Sidorov, D. Ensuring the Sustainability of Arctic Industrial Facilities under Conditions of Global Climate Change. Resources 2021, 10, 128. [Google Scholar] [CrossRef]
- Bolshunov, A.V.; Vasilev, D.A.; Ignatiev, S.A.; Dmitriev, A.N.; Vasilev, N.I. Mechanical drilling of glaciers with bottom-hole scavenging with compressed air. Ice Snow 2022, 62, 35–46. [Google Scholar] [CrossRef]
- Turkeev, A.; Vasilev, N.; Lipenkov, V.; Bolshunov, A.; Ekaykin, A.; Dmitriev, A.; Vasilev, D. Drilling the new 5G-5 branch hole at Vostok Station for collecting a replicate core of old meteoric ice. Ann. Glaciol. 2021, 62, 305–310. [Google Scholar] [CrossRef]
- Gendler, S.; Prokhorova, E. Risk-based methodology for determining priority directions for improving occupational safety in the mining industry of the Arctic zone. Resources 2021, 10, 20. [Google Scholar] [CrossRef]
- Nigmetov, R.; Nurahmedova, A.; Popadin, N. On the upgrading of high-sulfur fuel oil of the Astrakhan gas condensate. Bull. Astrakhan State Tech. Univ. 2017, 1, 32–36. [Google Scholar]
- Gaile, A.; Kostenko, A.; Zalischevskii, G.; Semenov, L.; Kaifadzhyan, E. Increasing the quality of furnace residual fuel oils. Extraction treatment with n-methylpyrrolidone. Chem. Technol. Fuels Oils 2005, 41, 249–259. [Google Scholar] [CrossRef]
- Mardashov, D.V.; Bondarenko, A.V.; Raupov, I.R. Design procedure of technological parameters of non-Newtonian fluids injection into an oil well during workover operation. J. Min. Inst. 2022, Online First, 1–14. [Google Scholar]
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M.; Ali Abdelkareem, M.; Kamil, M.; Olabi, A.G. Recent trends for introducing promising fuel components to enhance the anti-knock quality of gasoline: A systematic review. Fuel 2021, 291, 120112. [Google Scholar] [CrossRef]
- Smyshlyaeva, K.I.; Rudko, V.A.; Povarov, V.G.; Shaidulina, A.A.; Efimov, I.; Gabdulkhakov, R.R.; Pyagay, I.N.; Speight, J.G. Influence of Asphaltenes on the Low-Sulphur Residual Marine Fuels’ Stability. J. Mar. Sci. Eng. 2021, 9, 1235. [Google Scholar] [CrossRef]
- Vráblík, A.; Hidalgo-Herrador, J.M.; Černý, R. RGB histograms as a reliable tool for the evaluation of fuel oils stability. Fuel 2018, 216, 16–22. [Google Scholar] [CrossRef]
- Povarov, V.G.; Efimov, I.; Smyshlyaeva, K.I.; Rudko, V.A. Application of the UNIFAC Model for the Low-Sulfur Residue Marine Fuel Asphaltenes Solubility Calculation. J. Mar. Sci. Eng. 2022, 10, 1017. [Google Scholar] [CrossRef]
- Vráblík, A.; Schlehöfer, D.; Dlasková Jaklová, K.; Hidalgo Herrador, J.M.; Černý, R. Comparative Study of Light Cycle Oil and Naphthalene as an Adequate Additive to Improve the Stability of Marine Fuels. ACS Omega 2022, 7, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, S.; Zhu, Y. Characterization of Particle and Gaseous Emissions from Marine Diesel En-gines with Different Fuels and Impact of After-Treatment Technology. Energies 2017, 10, 1110. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Makhova, U.A.; Kapustin, V.M.; Abdellatief, T.M.M.; Karpov, N.V.; Dutlov, E.V.; Borisanov, D.V. Perspective towards a gasoline-property-first approach exhibiting octane hyperboosting based on isoolefinic hydrocarbons. Fuel 2022, 321, 124016. [Google Scholar] [CrossRef]
- Sverchkov, I.; Chukaeva, M.; Matveeva, V. Influence of preparation and combustion parameters of coal-water slurries on gas emission chemistry. Environ. Sci. Pollut. Res. 2022, 29, 44042–44053. [Google Scholar] [CrossRef]
- Corbett, J.J.; Winebrake, J.J. Emissions Tradeoffs among Alternative Marine Fuels: Total Fuel Cycle Analysis of Residual Oil, Marine Gas Oil, and Marine Diesel Oil. J. Air Waste Manage. Assoc. 2008, 58, 538–542. [Google Scholar] [CrossRef]
- Pashkevich, M.; Bykova, M. Methodology for thermal desorption treatment of local soil pollution by oil products at the facilities of the mineral resource industry. J. Min. Inst. 2022, 253, 49–60. [Google Scholar] [CrossRef]
- Gulyaeva, L.; Khavkin, V.; Shmelkova, O.; Vinogradova, N.; Bitiev, G.; Krasilnikova, L.; Yusovskii, A.; Nikulshin, P. Obtaining low-sulphur high-viscosity marine fuel by hydroprocessing of oil residues. Chem. Technol. Fuels Oils 2018, 6, 3–6. [Google Scholar]
- Bolobov, V.; Popov, G. Methodology for testing pipeline steels for resistance to grooving corrosion. J. Min. Inst. 2021, 252, 854–860. [Google Scholar] [CrossRef]
- Wu, P.-C.; Lin, C.-Y. Strategies for the Low Sulfur Policy of IMO—An Example of a Container Vessel Sailing through a European Route. J. Mar. Sci. Eng. 2021, 9, 1383. [Google Scholar] [CrossRef]
- Litvinenko, V. The Role of Hydrocarbons in the Global Energy Agenda: The Focus on Liquefied Natural Gas. Resources 2020, 9, 59. [Google Scholar] [CrossRef]
- Morenov, V.A.; Leusheva, E.L.; Buslaev, G.V.; Gudmestad, O.T. System of Comprehensive Energy-Efficient Utilization of Associated Petroleum Gas with Reduced Carbon Footprint in the Field Conditions. Energies 2020, 13, 4921. [Google Scholar] [CrossRef]
- Fetisov, V.; Tcvetkov, P.; Müller, J. Tariff approach to regulation of the European gas transportation system: Case of Nord Stream. Energy Rep. 2021, 7, 413–425. [Google Scholar] [CrossRef]
- Park, J.; Choi, I.; Oh, J.; Lee, C. Nitrogen Oxides and Particulate Matter from Marine Diesel Oil (MDO), Emulsified MDO, and Dimethyl Ether Fuels in Auxiliary Marine Engines. J. Mar. Sci. Eng. 2020, 8, 322. [Google Scholar] [CrossRef]
- Lee, T.-H.; Lee, S.-H.; Lee, J.-K. Exhaust Gas Emission Improvements of Water/Bunker C Oil-Emulsified Fuel Applied to Marine Boiler. J. Mar. Sci. Eng. 2021, 9, 477. [Google Scholar] [CrossRef]
- Yankovsky, S.; Tolokol’nikov, A.; Gorshkov, A.; Misyukova, A.; Kuznetsov, G. Justification of the Re-duction Possibility of Sulfur Oxides and Fly Ash Emissions during Co-Combustion of Coal and Waste from Woodworking Enterprises. Appl. Sci. 2021, 11, 11719. [Google Scholar] [CrossRef]
- Palyanitsina, A.; Safiullina, E.; Byazrov, R.; Podoprigora, D.; Alekseenko, A. Environmentally Safe Technology to Increase Efficiency of High-Viscosity Oil Production for the Objects with Advanced Water Cut. Energies 2022, 15, 753. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Chiaramonti, D. Blending of Hydrothermal Liquefaction Biocrude with Residual Marine Fuel: An Experimental Assessment. Energies 2022, 15, 450. [Google Scholar] [CrossRef]
- Berdugo Vilches, T.; Maric, J.; Thunman, H.; Seemann, M. Mapping the Effects of Potassium on Fuel Conversion in Industrial-Scale Fluidized Bed Gasifiers and Combustors. Catalysts 2021, 11, 1380. [Google Scholar] [CrossRef]
- Ramirez, J.; Brown, R.; Rainey, T. A Review of Hydrothermal Liquefaction Bio-Crude Properties and Prospects for Upgrading to Transportation Fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef]
- Gulyaeva, L.A.; Lobashova, M.M.; Mitusova, T.N.; Shmel’kova, O.I.; Khavkin, V.A.; Nikul’shin, P.A. Production of Low-Sulfur Marine Fuel. Chem. Technol. Fuels Oils 2020, 55, 704–711. [Google Scholar] [CrossRef]
- Beloglazov, I.; Morenov, V.; Leusheva, E.; Gudmestad, O.T. Modeling of Heavy-Oil Flow with Regard to Their Rheological Properties. Energies 2021, 14, 359. [Google Scholar] [CrossRef]
- Nurgalieva, K.S.; Saychenko, L.A.; Riazi, M. Improving the Efficiency of Oil and Gas Wells Complicated by the Formation of Asphalt–Resin–Paraffin Deposits. Energies 2021, 14, 6673. [Google Scholar] [CrossRef]
- Raupov, I.R.; Burkhanov, R.N.; Lutfullin, A.A.; Maksyutin, A.V.; Lebedev, A.B.; Safiullina, E.U. Experience in the Application of Hydrocarbon Optical Studies in Oil Field Development. Energies 2022, 15, 3626. [Google Scholar] [CrossRef]
- Zvereva, E.R.; Makarova, A.O.; Bakhtiyarova, Y.V.; Korolev, V.I.; Ilyin, N.P.; Turanov, A.N.; Zueva, O.S. Reuse of low sulfur oil residues as a base for boiler and marine fuel. Power Eng. Res. Equip. Technol. 2022, 24, 16–28. [Google Scholar] [CrossRef]
- Ancheyta, J. Hydro-imp technology for heavy oil refining. J. Min. Inst. 2017, 224, 229–234. [Google Scholar] [CrossRef]
- Speight, J.G. Petroleum asphaltenes—Part 2: The effect of asphaltenes and resin constituents on recovery and refining processes. Oil Gas Sci. Technol. 2004, 59, 479–488. [Google Scholar] [CrossRef]
- Kudinova, A.A.; Poltoratckaya, M.E.; Gabdulkhakov, R.R.; Litvinova, T.E.; Rudko, V.A. Parameters influence establishment of the petroleum coke genesis on the structure and properties of a highly porous carbon material obtained by activation of KOH. J. Porous Mater. 2022, 29, 1599–1616. [Google Scholar] [CrossRef]
- Buslaev, G.; Morenov, V.; Konyaev, Y.; Kraslawski, A. Reduction of carbon footprint of the production and field transport of high-viscosity oils in the Arctic region. Chem. Eng. Process. Process Intensif. 2020, 108189, 1–8. [Google Scholar] [CrossRef]
- Smyshlyaeva, K.I.; Rudko, V.A.; Kuzmin, K.A.; Povarov, V.G. Asphaltene genesis influence on the low-sulfur residual marine fuel sedimentation stability. Fuel 2022, 328, 125291. [Google Scholar] [CrossRef]
- Gaile, A.A.; Vereshchagin, A.V.; Klement’ev, V.N. Refining of Diesel and Ship Fuels by Extraction and Combined Methods. Part 1. Use of Ionic Liquids as Extractants. Russ. J. Appl. Chem. 2019, 92, 453–475. [Google Scholar] [CrossRef]
- Gaile, A.A.; Vereshchagin, A.V.; Klement’ev, V.N. Refining of Diesel and Ship Fuels by Extraction and Combined Methods. Part 2. Use of Organic Solvents as Extractants. Russ. J. Appl. Chem. 2019, 92, 583–595. [Google Scholar] [CrossRef]
- Sánchez, R.; Lefevre, J.; Todolí, J.-L. Direct elemental analysis of petroleum heavy fractions by means of ICP-OES equipped with a high temperature torch integrated sample introduction system. J. Anal. At. Spectrom. 2019, 34, 664–673. [Google Scholar] [CrossRef]
- Carter, S.; Clough, R.; Fisher, A.; Gibson, B.; Russell, B.; Waack, J. Atomic spectrometry update: Review of advances in the analysis of metals, chemicals and materials. J. Anal. At. Spectrom. 2019, 34, 2159–2216. [Google Scholar] [CrossRef]
- Popova, A.N.; Sukhomlinov, V.S.; Mustafaev, A.S. Accounting for Interelement Interferences in Atomic Emission Spectroscopy: A Nonlinear Theory. Appl. Sci. 2021, 11, 11237. [Google Scholar] [CrossRef]
- Chukaeva, M.; Matveeva, V.; Sverchkov, I. Complex processing of high-carbon ash and slag waste. J. Min. Inst. 2022, 253, 97–104. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Mironova, M.V.; Vinogradov, M.I.; Bermeshev, M.V.; Berkovich, A.K.; Kulichikhin, V.G. Structural and Morphological Features of Carbon—Silicon-Carbide Fibers Based on Cellulose and Triethoxyvinylsilane. Fibre Chem. 2018, 50, 79–84. [Google Scholar] [CrossRef]
- Savchenkov, S.A. The research of obtaining master alloys magnesium-gadolinium process by the method of metallothermic recovery. Tsvetnye Met. 2019, 5, 33–39. [Google Scholar] [CrossRef]
- Gabdulkhakov, R.R.; Rudko, V.A.; Pyagay, I.N. Methods for modifying needle coke raw materials by introducing additives of various origin (review). Fuel 2022, 310, 122265. [Google Scholar] [CrossRef]
- Savchenkov, S.; Beloglazov, I. Features of the Process Obtaining of Mg-Zn-Y Master Alloy by the Metallothermic Recovery Method of Yttrium Fluoride Melt. Crystals 2022, 12, 771. [Google Scholar] [CrossRef]
- Vasilyeva, N.V.; Boikov, A.V.; Erokhina, O.O.; Trifonov, A.Y. Automated digitization of radial charts. J. Min. Inst. 2021, 247, 82–87. [Google Scholar] [CrossRef]
- Boikov, A.; Payor, V.; Savelev, R.; Kolesnikov, A. Synthetic Data Generation for Steel Defect Detection and Classification Using Deep Learning. Symmetry 2021, 13, 1176. [Google Scholar] [CrossRef]
- Chukaeva, M.; Zaytseva, T.; Matveeva, V.; Sverchkov, I. Purification of Oil-Contaminated Wastewater with a Modified Natural Adsorbent. Ecol. Eng. Environ. Technol. 2021, 22, 46–51. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).