Assessment of Heavy Metals Eluted from Materials Utilized in Artificial Reefs Implemented in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Concentrations of Heavy Metals in Seawater and Marine Organisms
2.2. In-Door Elution Experiment
3. Results
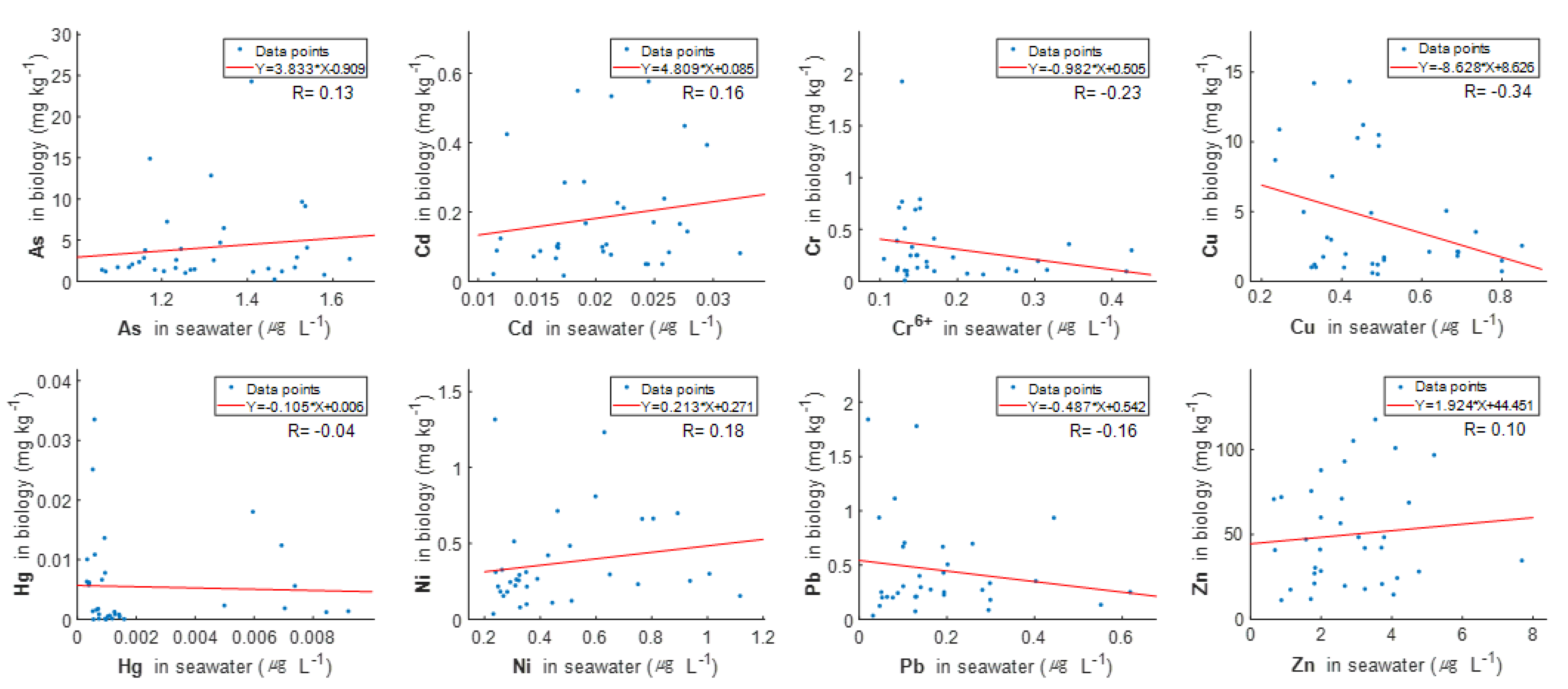
3.1. Concentrations of Heavy Metals in Seawater
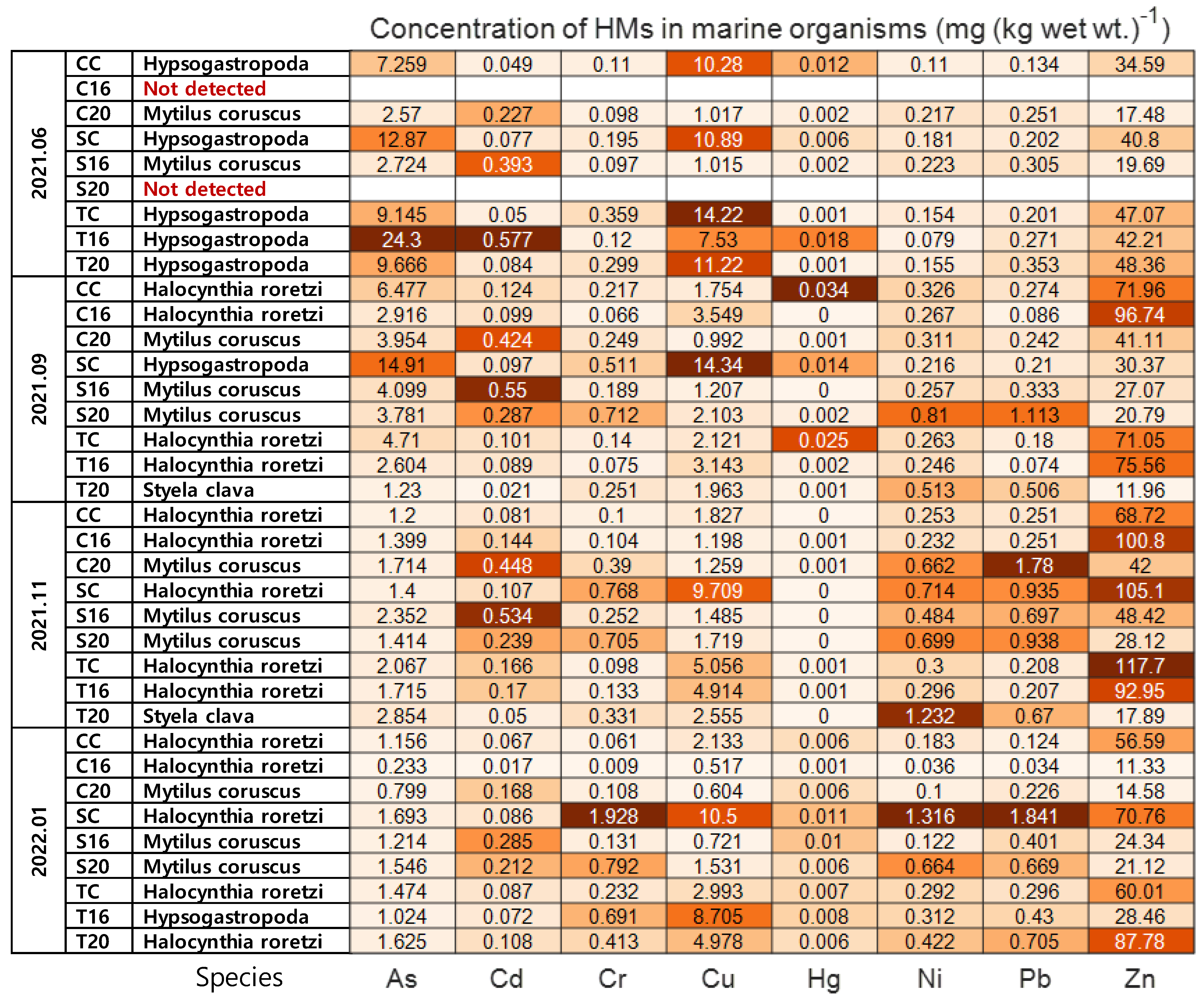
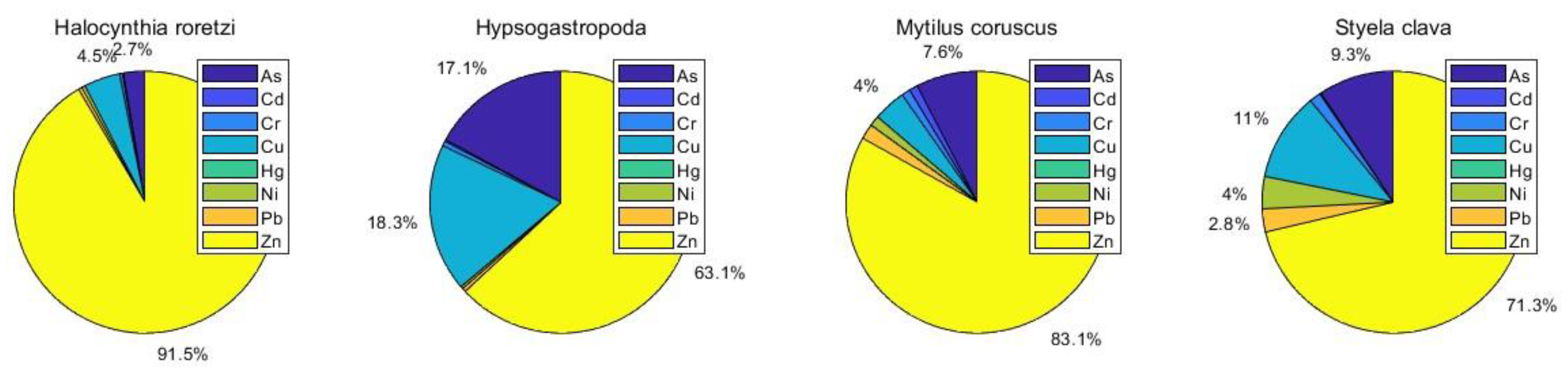
3.2. Concentrations of Heavy Metals in Marine Organisms
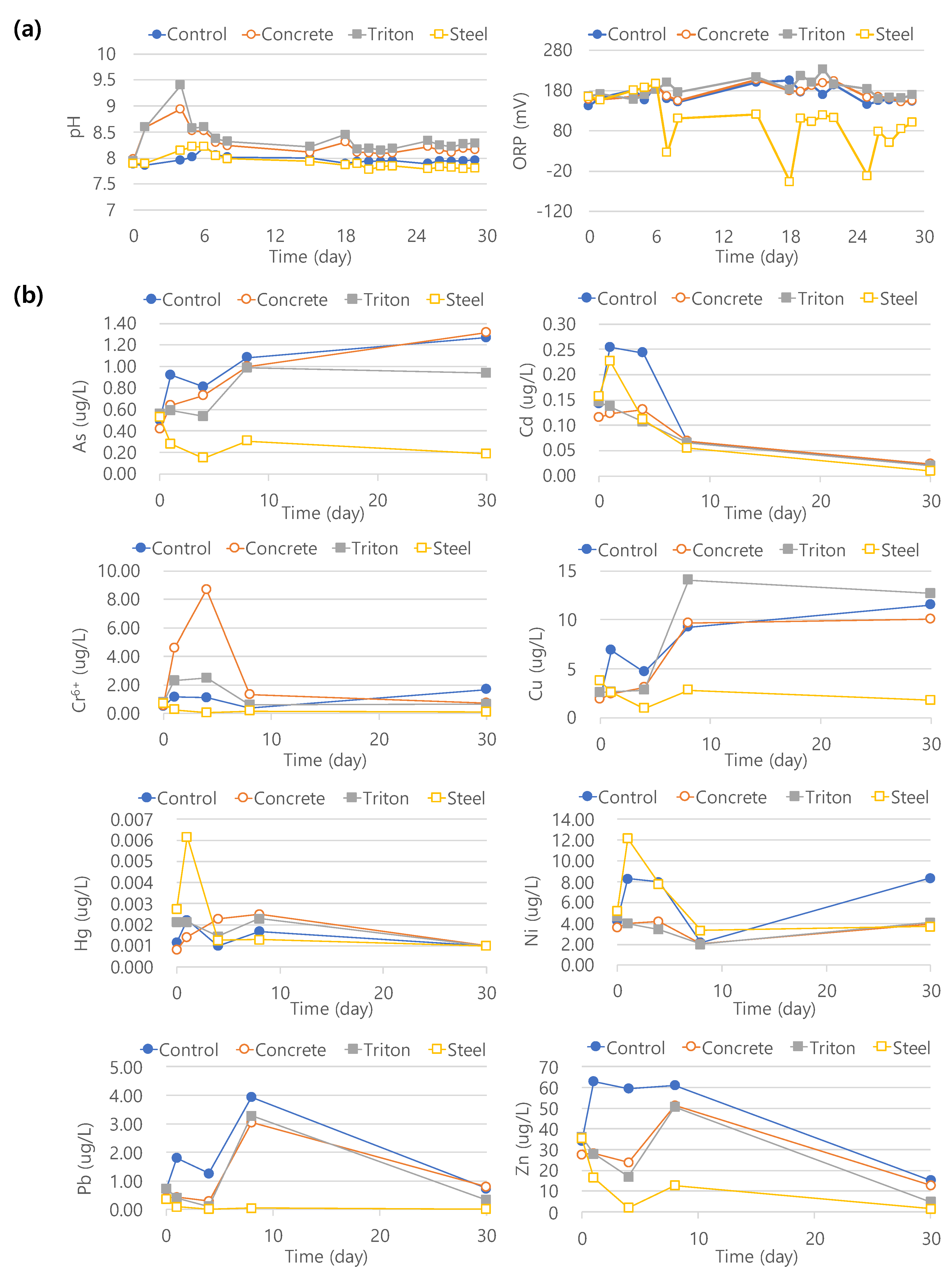
3.3. Indoor Elution Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sutton, S.G.; Bushnell, S.L. Socio-economic aspects of artificial reefs: Considerations for the Great Barrier Reef Marine Park. Ocean. Coast. Manag. 2007, 50, 829–846. [Google Scholar] [CrossRef]
- Antoine de Ramon, N.Y.; Chynoweth, D.P.; Capron, M.E.; Stewart, J.R.; Hasan, M.A. Negative carbon via ocean afforestation. Process Saf. Environ. Prot. 2012, 90, 467–474. [Google Scholar]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Glob. Change Biol. 2018, 24, 4919–4928. [Google Scholar] [CrossRef] [PubMed]
- Harvell, C.D.; Kim, K.; Burkholder, J.; Colwell, R.R.; Epstein, P.R.; Grimes, D.J.; Hofmann, E.E.; Lipp, E.; Osterhaus, A.; Overstreet, R.M. Emerging marine diseases—Climate links and anthropogenic factors. Science 1999, 285, 1505–1510. [Google Scholar] [CrossRef]
- Jung, S.; Chau, T.V.; Kim, M.; Na, W.-B. Artificial Seaweed Reefs That Support the Establishment of Submerged Aquatic Vegetation Beds and Facilitate Ocean Macroalgal Afforestation: A Review. J. Mar. Sci. Eng. 2022, 10, 1184. [Google Scholar] [CrossRef]
- Tsiamis, K.; Salomidi, M.; Gerakaris, V.; Mogg, A.O.; Porter, E.S.; Sayer, M.D.; Küpper, F.C. Macroalgal vegetation on a north European artificial reef (Loch Linnhe, Scotland): Biodiversity, community types and role of abiotic factors. J. Appl. Phycol. 2020, 32, 1353–1363. [Google Scholar] [CrossRef]
- Watanuki, A.; Yamamoto, A. Settlement of seaweeds on coastal structures. Hydrobiologia 1990, 204, 275–280. [Google Scholar] [CrossRef]
- Harris, L.E.; Mostkoff, B.; Zadikoff, G. Artificial reefs: From waste to resources. In Proceedings of the OCEANS 96 MTS/IEEE Conference Proceedings. The Coastal Ocean-Prospects for the 21st Century, Fort Lauderdale, FL, USA, 23–26 September 1996; pp. 754–759. [Google Scholar]
- Seaman, W.; Jensen, A.C. Purposes and Practices of Artificial Reef Evaluation; CRC Press LLC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Yu, J.; Chen, P.; Tang, D.; Qin, C. Ecological effects of artificial reefs in Daya Bay of China observed from satellite and in situ measurements. Adv. Space Res. 2015, 55, 2315–2324. [Google Scholar] [CrossRef]
- Lee, I.-C.; Park, S.; Woo, H.-E.; Jeong, I.; Choi, C.G.; Kim, K. A Study on Macroalgae Establishment on Concrete Substratum Covered by Oyster Shells. J. Korean Soc. Mar. Environ. Saf. 2021, 27, 639–646. [Google Scholar] [CrossRef]
- FIRA (Korea Fisheries Resources Agency). Artificial Reef Installation Statistics (1971~2019); Korea Fisheries Resources Agency: Busan, Korea, 2020. [Google Scholar]
- MOF (Ministry of Oceans and Fisheries). Implementation Guidelines for Fisheries Resources Development Projects; Ministry of Oceans and Fisheries: Sejong-si, Korea, 2021.
- Baine, M. Artificial reefs: A review of their design, application, management and performance. Ocean. Coast. Manag. 2001, 44, 241–259. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Liu, Y.; Hu, W.; Ni, W. On the use of blast furnace slag and steel slag in the preparation of green artificial reef concrete. Constr. Build. Mater. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- AQUABIO. Artificial Reefs Made from Steel:Potential Benefits and Issues. Available online: http://www.aquabio.com/artificial-reefs-made-from-steel.html. (accessed on 18 July 2022).
- POSCO. Construction of Sea Forest. Korean Patent 1020110135308, 15 December 2011.
- POSCO. POSCO Uses Steel Slag to Create a Sea Forest and Save the Marine Ecosystem. Available online: https://newsroom.posco.com/en/posco-uses-steel-slag-to-create-a-sea-forest-and-save-the-marine-ecosystem/ (accessed on 10 September 2022).
- Clark, S.; Edwards, A. An evaluation of artificial reef structures as tools for marine habitat rehabilitation in the Maldives. Aquat.Conserv. Mar. Freshw. Ecosyst. 1999, 9, 5–21. [Google Scholar] [CrossRef]
- Lan, C.-H.; Hsui, C.-Y. The deployment of artificial reef ecosystem: Modelling, simulation and application. Simul. Model. Pract. Theory 2006, 14, 663–675. [Google Scholar] [CrossRef]
- Layman, C.A.; Allgeier, J.E. An ecosystem ecology perspective on artificial reef production. J. Appl. Ecol. 2020, 57, 2139–2148. [Google Scholar] [CrossRef]
- Maanan, M. Heavy metal concentrations in marine molluscs from the Moroccan coastal region. Environ. Pollut. 2008, 153, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ruilian, Y.; Xing, Y.; Yuanhui, Z.; Gongren, H.; Xianglin, T. Heavy metal pollution in intertidal sediments from Quanzhou Bay, China. J. Environ. Sci. 2008, 20, 664–669. [Google Scholar]
- Hosono, T.; Su, C.-C.; Delinom, R.; Umezawa, Y.; Toyota, T.; Kaneko, S.; Taniguchi, M. Decline in heavy metal contamination in marine sediments in Jakarta Bay, Indonesia due to increasing environmental regulations. Estuar. Coast. Shelf Sci. 2011, 92, 297–306. [Google Scholar] [CrossRef]
- Naser, H.A. Assessment and management of heavy metal pollution in the marine environment of the Arabian Gulf: A review. Mar. Pollut. Bull. 2013, 72, 6–13. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Fowler, S.W. Critical review of selected heavy metal and chlorinated hydrocarbon concentrations in the marine environment. Mar. Environ. Res. 1990, 29, 1–64. [Google Scholar] [CrossRef]
- Kwon, H. Manual of Seaweed Restoration in Whitening Coastal Area. Master’s Thesis, Department of Environmental Engineering, Pukyong National University, Busan, Korea, 2008. [Google Scholar]
- No. 2018-10; Marine Environmental Standards. MOF (Ministry of Oceans and Fisheries): Sejong-si, Korea, 2018.
- FSK. A Comparative Study on Domestic and Foreign Food Standards: Establishing Criteria for Selecting Candidates for the Food Safety InformationService’s Foreign Food Standards Investigation; Food Safety Korea: Cheongju, Korea, 2016. [Google Scholar]
- Shi, C. Steel slag—Its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Yadav, S.; Mehra, A. Experimental study of dissolution of minerals and CO2 sequestration in steel slag. Waste Manag. 2017, 64, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Barca, C.; Gérente, C.; Meyer, D.; Chazarenc, F.; Andrès, Y. Phosphate removal from synthetic and real wastewater using steel slags produced in Europe. Water Res. 2012, 46, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. Principles and Prevention of Corrosion; Macmillan Publishing Company (USA): New York, NY, USA, 1992; 568p. [Google Scholar]
- Pyle, G.; Swanson, S.; Lehmkuhl, D. The influence of water hardness, pH, and suspended solids on nickel toxicity to larval fathead minnows (Pimephales promelas). Water Air Soil Pollut. 2002, 133, 215–226. [Google Scholar] [CrossRef]
- Jo, Y.-J.; Choi, S.-H. Engineering Performance and Applicability of Eco-Friendly Concrete for Artificial Reefs Using Electric Arc Furnace Slags. KSCE J. Civ. Environ. Eng. Res. 2015, 35, 533–544. [Google Scholar] [CrossRef]
- Lim, M.; Han, G.-C.; Ahn, J.-W.; You, K.-S. Environmental remediation and conversion of carbon dioxide (CO2) into useful green products by accelerated carbonation technology. Int. J. Environ. Res. Public Health 2010, 7, 203–228. [Google Scholar] [CrossRef]
- Mok, J.-S.; Lee, K.-J.; Shim, K.-B.; Lee, T.-S.; Song, K.-C.; Kim, J.-H. Contents of heavy metals in marine invertebrates from the Korean coast. J. Korean Soc. Food Sci. Nutr. 2010, 39, 894–901. [Google Scholar] [CrossRef]
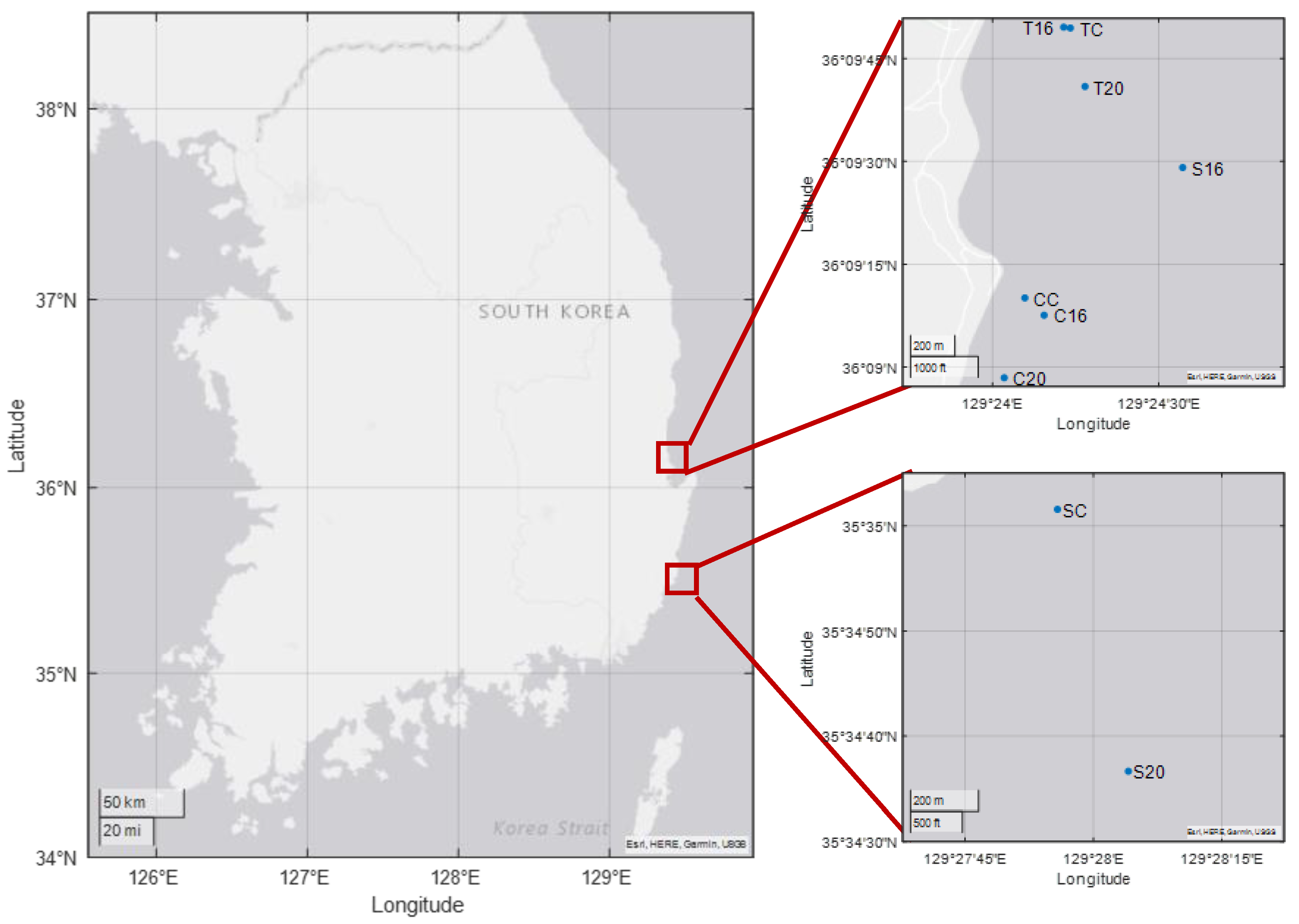

| Cases | Latitude (N) | Longitude (E) | Depth (m) | |
|---|---|---|---|---|
| Concrete AR | CC | 36°09.168′ | 129°24.097′ | 5 |
| C16 | 36°09.126′ | 129°24.155′ | 10 | |
| C20 | 36°08.974′ | 129°24.035′ | 10 | |
| Steel AR | SC | 35°35.026′ | 129°27.930′ | 10 |
| S16 | 36°09.485′ | 129°24.572′ | 19 | |
| S20 | 35°34.611′ | 129°28.068′ | 24 | |
| Triton AR | TC | 36°09.824′ | 129°24.234′ | 11 |
| T16 | 36°09.826′ | 129°24.214′ | 9 | |
| T20 | 36°09.682′ | 129°24.278′ | 13 | |
| Standards for Concentration of HMs | As | Cd | Cr6+ | Cu | Hg | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| MEPS for HMs in seawater (μg L−1) | Short-term 1 | 9.4 | 19 | 200 | 3.0 | 1.8 | 11 | 7.6 | 34 |
| Long-term 2 | 3.4 | 2.2 | 2.8 | 1.2 | 1.0 | 1.8 | 1.6 | 11 | |
| SACHM for HMs in marine organisms (mg kg−1) | - | 1.0–5.5 | - | 30–100 | 0.5–1.0 | - | 2–10 | 1000 | |
| HPS for in-door elution experiment (μg L−1) | 50 | 10 | 50 | 20 | 0.5 | - | 50 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, J.R.; Kim, Y.R.; Yoon, S.; Kim, K. Assessment of Heavy Metals Eluted from Materials Utilized in Artificial Reefs Implemented in South Korea. J. Mar. Sci. Eng. 2022, 10, 1720. https://doi.org/10.3390/jmse10111720
Park S, Kim JR, Kim YR, Yoon S, Kim K. Assessment of Heavy Metals Eluted from Materials Utilized in Artificial Reefs Implemented in South Korea. Journal of Marine Science and Engineering. 2022; 10(11):1720. https://doi.org/10.3390/jmse10111720
Chicago/Turabian StylePark, Seongsik, Jong Ryol Kim, Young Ryun Kim, Seokjin Yoon, and Kyunghoi Kim. 2022. "Assessment of Heavy Metals Eluted from Materials Utilized in Artificial Reefs Implemented in South Korea" Journal of Marine Science and Engineering 10, no. 11: 1720. https://doi.org/10.3390/jmse10111720
APA StylePark, S., Kim, J. R., Kim, Y. R., Yoon, S., & Kim, K. (2022). Assessment of Heavy Metals Eluted from Materials Utilized in Artificial Reefs Implemented in South Korea. Journal of Marine Science and Engineering, 10(11), 1720. https://doi.org/10.3390/jmse10111720





