The Morphological Characteristics of Authigenic Pyrite Formed in Marine Sediments
Abstract
1. Introduction
2. Methods for Morphology Study
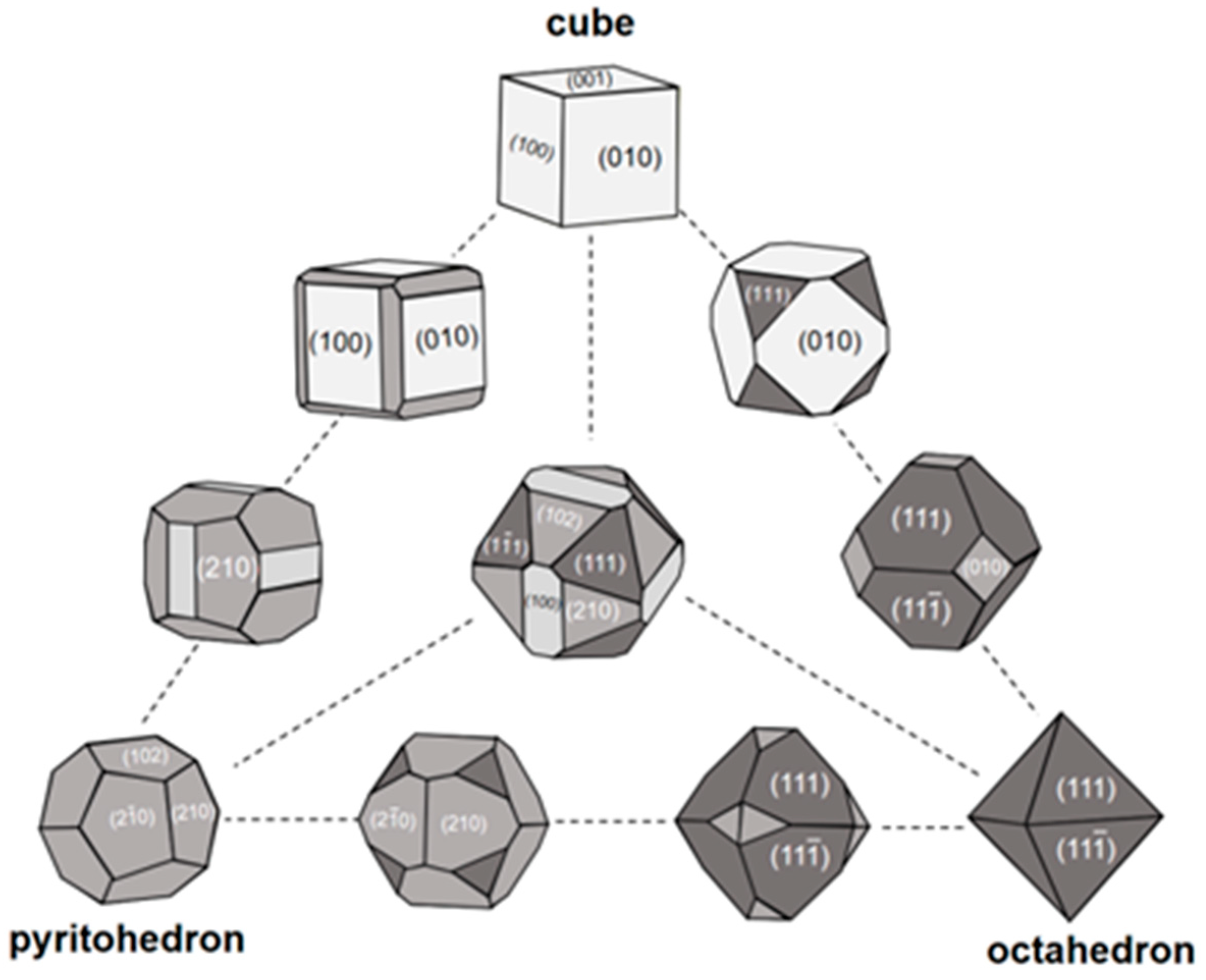
3. Shape of Single Crystal of Pyrite
4. Texture of Pyrite Aggregates
4.1. Framboid
4.2. Sunflower
4.3. Rod-like
5. Textural Evolution
- (1)
- Iron monosulfide (FeS) nucleates;
- (2)
- FeS transforms to greigite (Fe3S4);
- (3)
- Homogeneous greigite microcrystals aggregate to form the framboidal texture;
- (4)
- Some greigite microcrystals grow continuously to form colloidal pyrite;
- (5)
- The pyrites grow radially along the spherical rim of the framboid;
- (6)
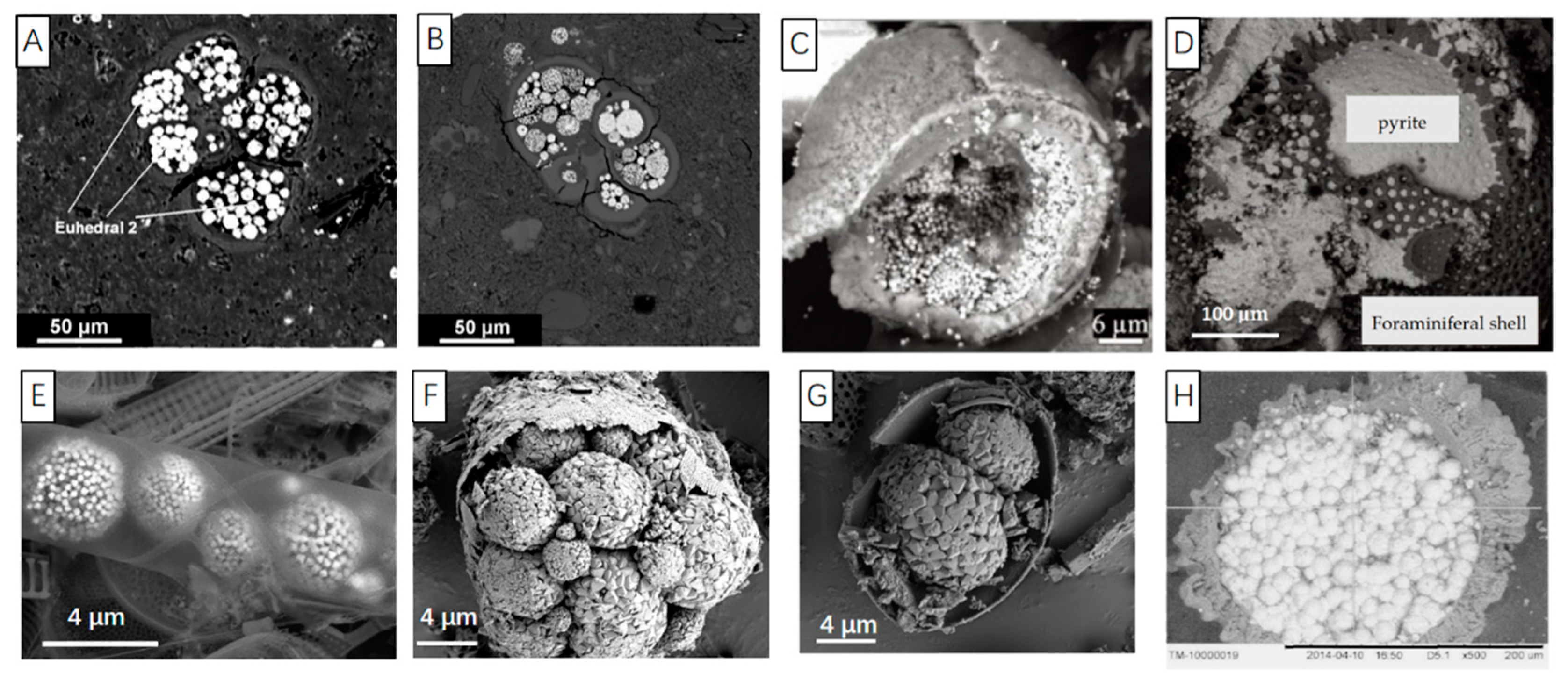
6. The Texture of Pyrite Filling in Organisms
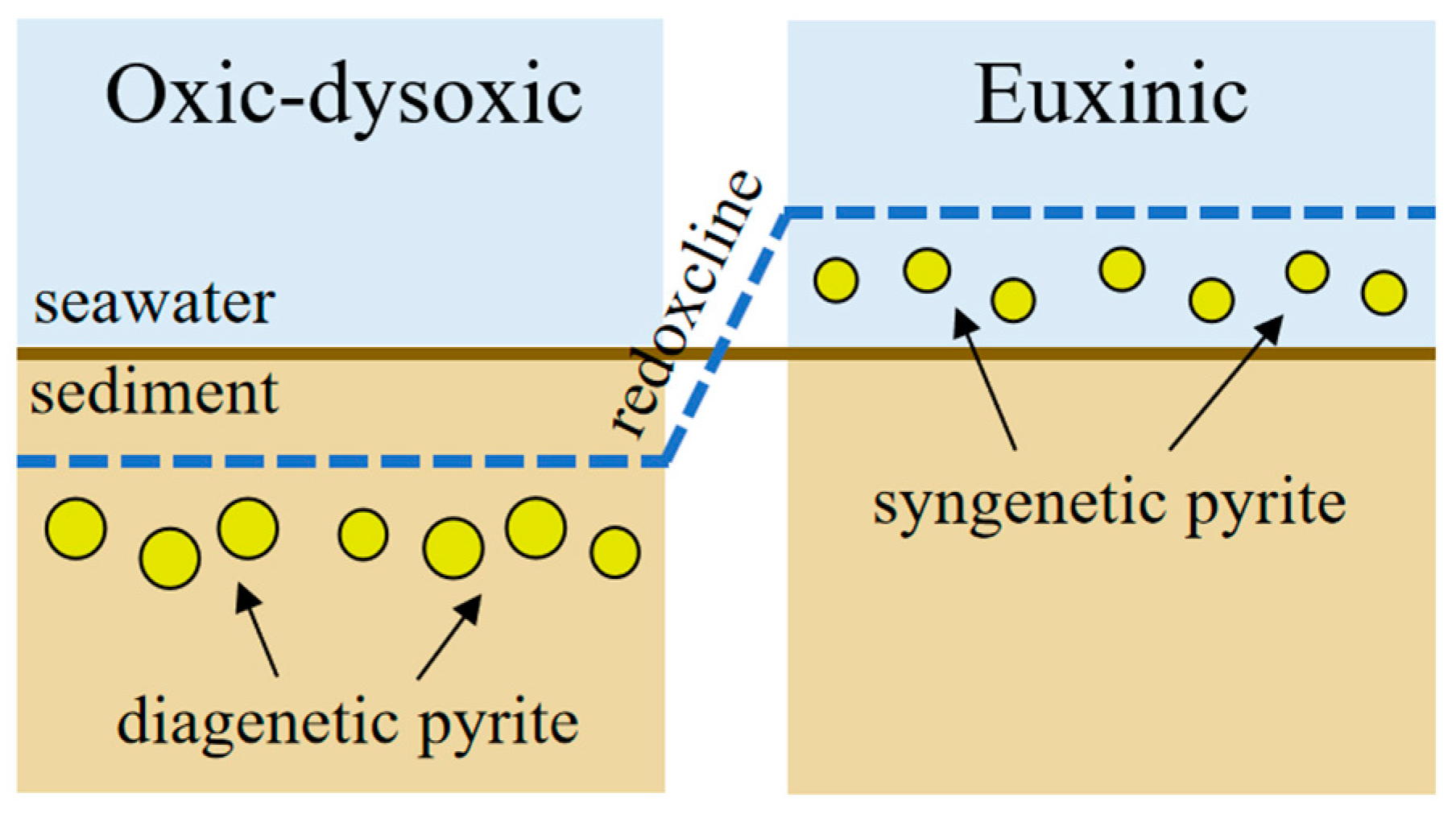
7. Pyrite Morphology in Different Geochemical Zones in Marine Sediments
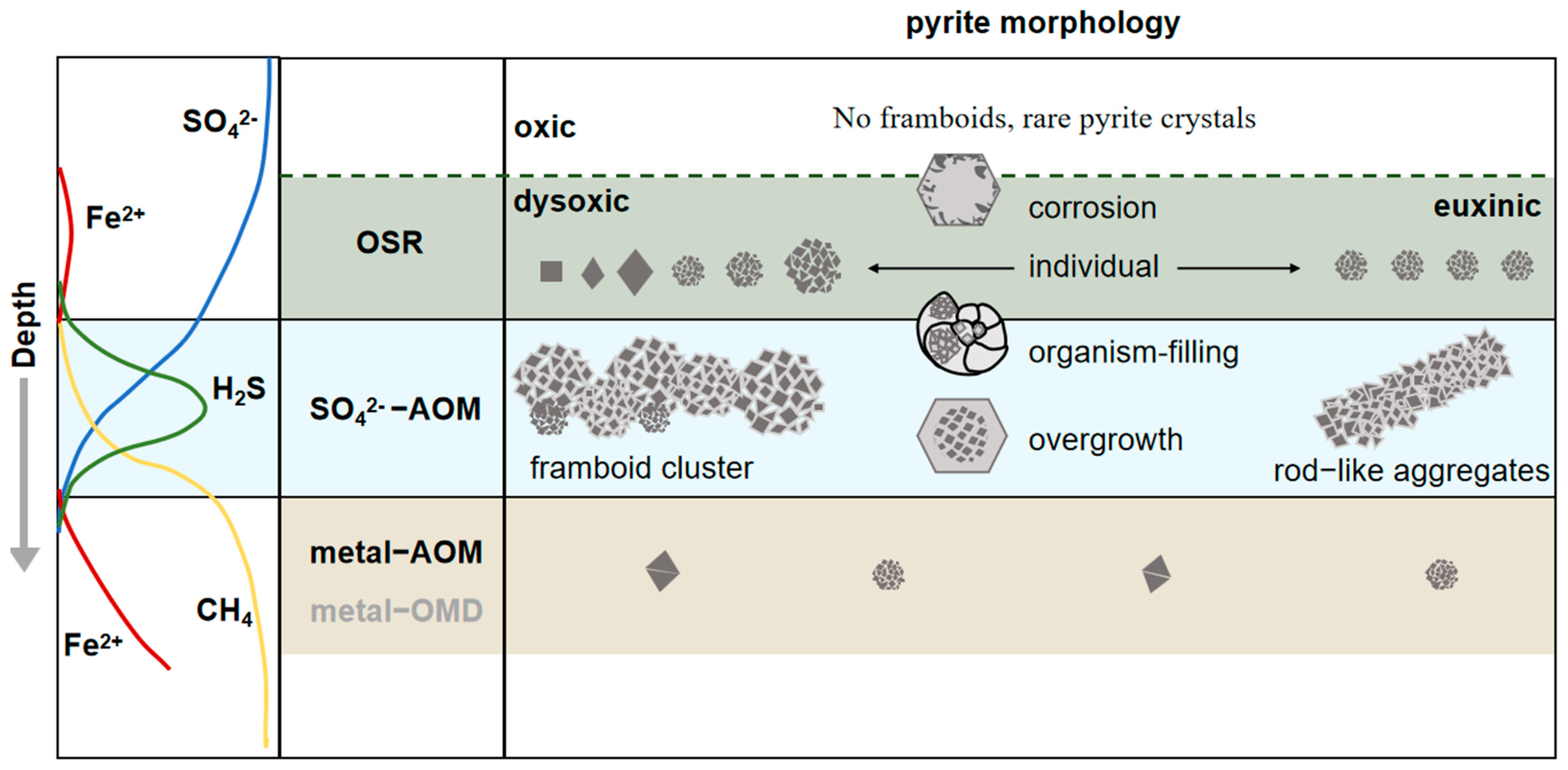
7.1. Organoclastic Sulfate Reduction
- (1)
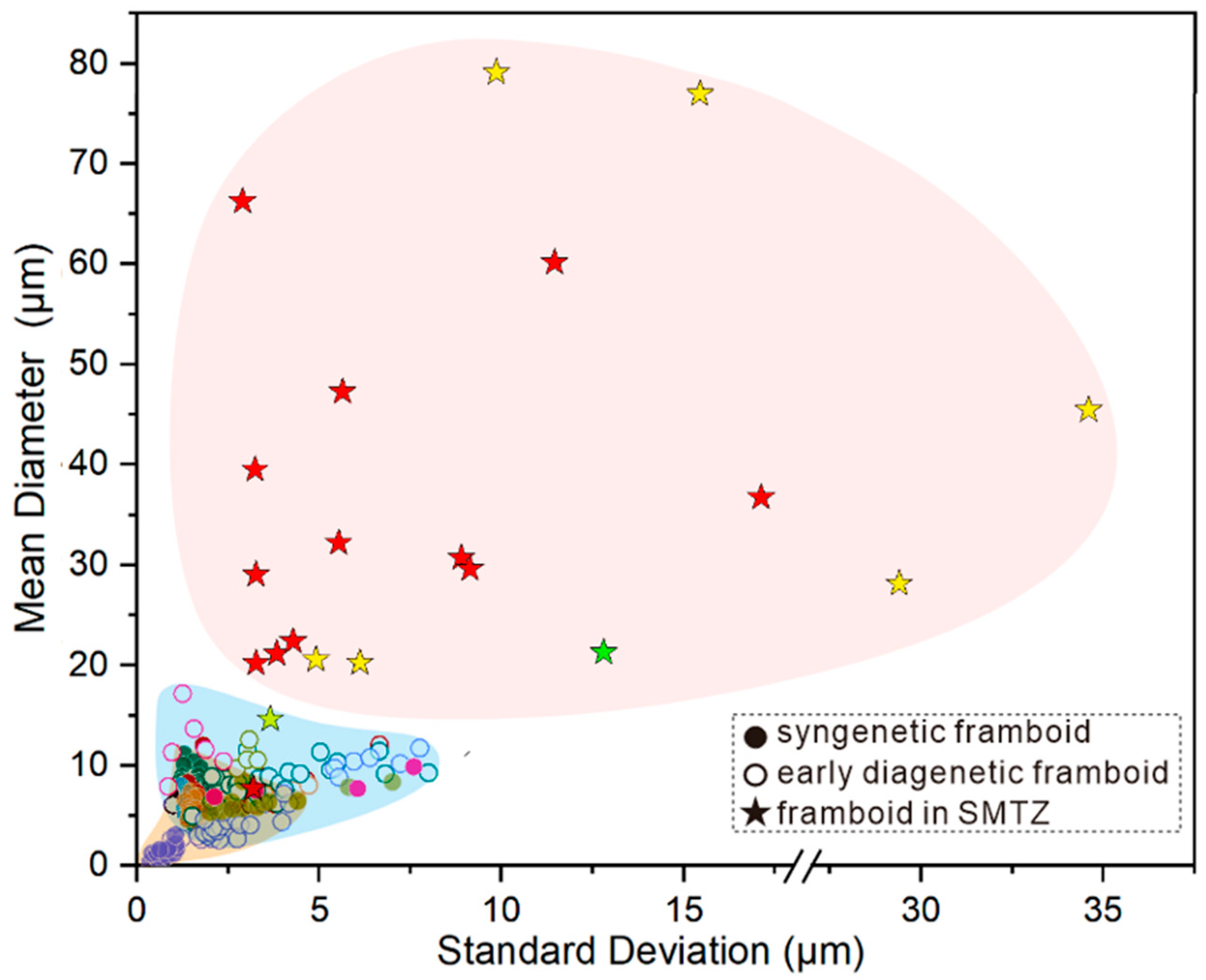
- Under oxic–dysoxic bottom water, the redoxcline is located under the seafloor and pyrite forms in the early diagenetic stage under the redox boundary in marine sediment. In the oxidized environment, rare framboids and pyrite crystals have been found [9,75]. In the dysoxic or anoxic environment, framboids usually with small size (mean diameter, MD < 10 μm); and narrow distribution range (standard deviation, SD < 3 μm).
- (2)
- In the sulfidic–dysoxic bottom water column, a large amount of pyrite are formed in a relatively stable sedimentary environment with sufficient dissolved Fe, H2S and elemental sulfur. Pyrites are formed at a high growth rate and quickly sink to the anoxic sediments. After being settled to the marine sediment, pyrite cannot grow into larger due to the lack of supply of elemental sulfur. Therefore, framboidal pyrite which is formed in sulfide environment is with a small mean diameter (MD < 5 μm) and is homogeneous in grain size [10].
7.2. Sulfate-Driven Anaerobic Oxidation of Methane
- (1)
- A great number of authigenic pyrites are formed in SMTZ, especially framboidal pyrites, because of the abundant organic matter and H2S supply. The content of pyrite is obviously higher in SMTZ of hydrate occurring area than that in sediment without gas hydrate.
- (2)
- The size of the framboid is with a tendency to be larger (mean sizes >20 μm) and more variable in size (standard deviations >3.0 μm).
- (3)
- Most framboids form clusters, and sometimes overgrowth can be observed. Some of the framboids appeared in the shape of vertically oriented rods, which might represent the migration pathways of the strong flux of methane in sediments.
7.3. Metal Driven Anaerobic Oxidation of Methane
8. Alteration in Later Diagenetic Processes
8.1. Oxidation
8.1.1. Abiotic Oxidation
8.1.2. Biological Oxidation
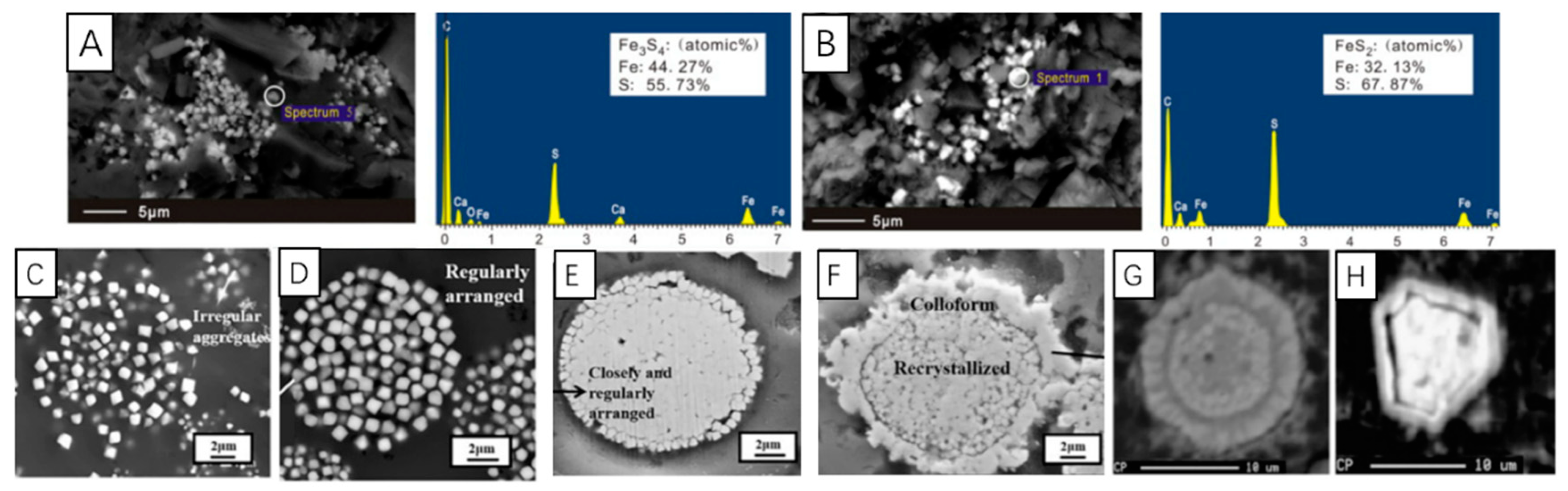
8.2. Recrystallization and Overgrowth
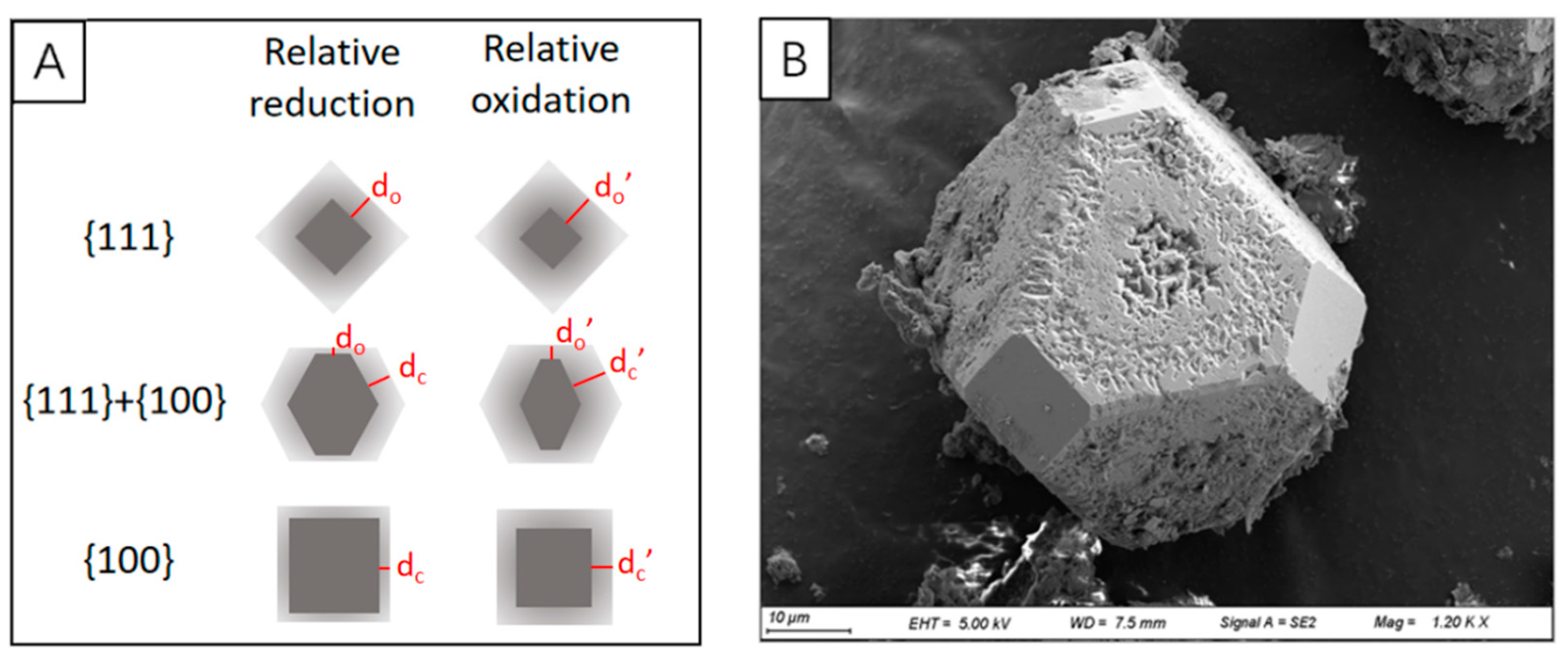
- (1)
- Tabular pyrite regrows outward along the spherical rim of inner framboid, gradually forms a subhedral (polygonal framboid) texture and finally a euhedral texture [79]. Although the framboidal pyrite that has undergone secondary growth will increase its particle size to a certain extent, the diameter of the inner core will not change, which is indicative of redox conditions.
- (2)
- Microcrystals in framboids continuously grow and the internal pores may be filled by newly formed pyrite and colloidal pyrite, the framboid transforms into solid spherical pyrite, which may further evolve into euhedral pyrite.
- (3)
- When the internal material of the framboid is plastic enough to move, the regular euhedral faces directly form via polygonal framboid.
8.3. Redeposition
9. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Berner, R.A.; Leeuw, J.; Spiro, B.; Murchison, D.G.; Eglinton, G. Sedimentary pyrite formation: An update. Geochim. Et Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Gallego-Torres, D.; Reolid, M.; Nieto-Moreno, V.; Martínez-Casado, F.J. Pyrite framboid size distribution as a record for relative variations in sedimentation rate: An example on the Toarcian Oceanic Anoxic Event in Southiberian Palaeomargin. Sediment. Geol. 2015, 330, 59–73. [Google Scholar] [CrossRef]
- Canfield, D.E. Biogeochemistry of Sulfur Isotopes. Stable Isot. Geochem. 2019, 43, 607–636. [Google Scholar] [CrossRef]
- Canfield, D.E.; Habicht, K.S.; Thamdrup, B. The Archean sulfur cycle and the early history of atmospheric oxygen. Science 2000, 288, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Berner, R.A. The carbon and sulfur cycles and atmospheric oxygen from middle Permian to middle Triassic. Geochim. Et Cosmochim. Acta 2005, 69, 3211–3217. [Google Scholar] [CrossRef]
- Berner, R.A. Modeling atmospheric O2 over Phanerozoic time. Geochim. Et Cosmochim. Acta 2001, 65, 685–694. [Google Scholar] [CrossRef]
- Wang, Q.; Morse, J.W. Pyrite formation under conditions approximating those in anoxic sediments I. Pathway and morphology. Mar. Chem. 1996, 52, 99–121. [Google Scholar] [CrossRef]
- Merinero, R.; Cárdenes, V.; Lunar, R.; Boone, M.N.; Cnudde, V. Representative size distributions of framboidal, euhedral, and sunflower pyrite from high-resolution X-ray tomography and scanning electron microscopy analyses. Am. Mineral. 2017, 102, 620–631. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochim. Et Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Wignall, P.B.; Bond, D.P.G.; Kuwahara, K.; Kakuwa, Y.; Newton, R.J.; Poulton, S.W. An 80 million year oceanic redox history from Permian to Jurassic pelagic sediments of the Mino-Tamba terrane, SW Japan, and the origin of four mass extinctions. Glob. Planet. Chang. 2010, 71, 109–123. [Google Scholar] [CrossRef]
- Rickard, D. Sedimentary pyrite framboid size-frequency distributions: A meta-analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 522, 62–75. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, X.; Lu, Y.; Xu, L.; Xu, H.; Lu, H. Mineralogy of authigenic tube pyrite from the Southwest Taiwan Basin of South China Sea and its tracing significance for gas hydrates. Miner. Depos. 2011, 30, 725–734. [Google Scholar] [CrossRef]
- Zhang, M.; Konishi, H.; Xu, H.; Sun, X.; Lu, H.; Wu, D.; Wu, N. Morphology and formation mechanism of pyrite induced by the anaerobic oxidation of methane from the continental slope of the NE South China Sea. J. Asian Earth Sci. 2014, 92, 293–301. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Algeo, T.J.; Sun, F.; Lin, R. Enhanced framboidal pyrite formation related to anaerobic oxidation of methane in the sulfate-methane transition zone of the northern South China Sea. Mar. Geol. 2016, 379, 100–108. [Google Scholar] [CrossRef]
- Ye, Y.; Wu, C.; Zhai, L.; An, Z. Pyrite morphology and episodic euxinia of the Ediacaran Doushantuo Formation in South China. Sci. China Earth Sci. 2017, 60, 102–113. [Google Scholar] [CrossRef]
- Etzel, K.; Huber, H.; Rachel, R.; Schmalz, G.; Thomm, M.; Depmeier, W. Pyrite Surface Alteration of Synthetic Single Crystals as Effect of Microbial Activity and Crystallographic Orientation. Adv. Mater. Res. 2007, 20–21, 350–353. [Google Scholar] [CrossRef]
- Gao, S.H.F.; Wang, Y.; Gao, W. A Review of Research Progress in the Genesis of Colloform Pyrite and Its Environmental Indications. Acta Geol. Sin. 2016, 90, 1353–1369. [Google Scholar] [CrossRef]
- Nozaki, T.; Nagase, T.; Ushikubo, T.; Shimizu, K.; Ishibashi, J.-I. Microbial sulfate reduction plays an important role at the initial stage of subseafloor sulfide mineralization. Geology 2020, 49, 222–227. [Google Scholar] [CrossRef]
- Lim, Y.C.; Lin, S.; Yang, T.F.; Chen, Y.-G.; Liu, C.-S. Variations of methane induced pyrite formation in the accretionary wedge sediments offshore southwestern Taiwan. Mar. Pet. Geol. 2011, 28, 1829–1837. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Böttcher, M.E.; Lüschen, H.; Neretin, L.N.; Volkov, I.I. Anaerobic methane oxidation and a deep H2S sink generate isotopically heavy sulfides in Black Sea sediments. Geochim. Et Cosmochim. Acta 2004, 68, 2095–2118. [Google Scholar] [CrossRef]
- Crémière, A.; Pellerin, A.; Wing, B.A.; Lepland, A. Multiple sulfur isotopes in methane seep carbonates track unsteady sulfur cycling during anaerobic methane oxidation. Earth Planet. Sci. Lett. 2020, 532, 115994. [Google Scholar] [CrossRef]
- Miao, X.; Feng, X.; Liu, X.; Li, J.; Wei, J. Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Mar. Pet. Geol. 2021, 133, 105231. [Google Scholar] [CrossRef]
- Rubanov, S.; Munroe, P.R. Investigation of the structure of damage layers in TEM samples prepared using a focused ion beam. J. Mater. Sci. Lett. 2001, 20, 1181–1183. [Google Scholar] [CrossRef]
- Ye, R.; Zhao, L.; Ma, Z.; Shen, Y.; Zhao, X. STM study on micromorphology of pyrite and dynamic significance of ore-formation. Chin. Sci. Bull. 1999, 1999, 1401–1403. Available online: https://ui.adsabs.harvard.edu/link_gateway/1999ChSBu.44.1401Y (accessed on 29 August 1999). [CrossRef]
- Liu, J.; Pereira, G.G.; Liu, Q.; Regenauer-Lieb, K. Computational challenges in the analyses of petrophysics using microtomography and upscaling: A review. Comput. Geosci. 2016, 89, 107–117. [Google Scholar] [CrossRef]
- Krakowska, P.; Dohnalik, M.; Jarzyna, J.; Wawrzyniak-Guz, K. Computed X-ray microtomography as the useful tool in petrophysics: A case study of tight carbonates Modryn formation from Poland. J. Nat. Gas Sci. Eng. 2016, 31, 67–75. [Google Scholar] [CrossRef]
- Karimpouli, S.; Tahmasebi, P. Conditional reconstruction: An alternative strategy in digital rock physics. Geophysics 2016, 81, D465–D477. [Google Scholar] [CrossRef]
- Deng, S.; LÜ, W.; Liu, Q.; Leng, Z.; Li, T.; Liu, H.; Gu, H.; Xu, C.; Zhang, X.; Lu, X. Research on oil displacement mechanism in conglomerate using CT scanning method. Pet. Explor. Dev. 2014, 41, 365–370. [Google Scholar] [CrossRef]
- Pola, A.; Crosta, G.; Fusi, N.; Barberini, V.; Norini, G. Influence of alteration on physical properties of volcanic rocks. Tectonophysics 2012, 566–567, 67–86. [Google Scholar] [CrossRef]
- Josh, M.; Esteban, L.; Delle Piane, C.; Sarout, J.; Dewhurst, D.N.; Clennell, M.B. Laboratory characterisation of shale properties. J. Pet. Sci. Eng. 2012, 88–89, 107–124. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Che, Y.; Tang, D.; Tang, S.; Huang, W. Non-destructive characterization of coal samples from China using microfocus X-ray computed tomography. Int. J. Coal Geol. 2009, 80, 113–123. [Google Scholar] [CrossRef]
- Karacan, C.; Okandan, E.J.F. Adsorption and gas transport in coal microstructure: Investigation and evaluation by quantitative X-ray CT imaging. Fuel 2001, 80, 509–520. [Google Scholar] [CrossRef]
- Merinero, R.; Lunar, R.; Martinez-Frias, J.; Somoza, L.; Diaz-del-Rio, V. Iron oxyhydroxide and sulphide mineralization in hydrocarbon seep-related carbonate submarine chimneys, Gulf of Cadiz (SW Iberian Peninsula). Mar. Pet. Geol. 2008, 25, 706–713. [Google Scholar] [CrossRef]
- Grigory, G.; Yuri, I.; Sergey, N.; Danil, I.; Evgeny, K.; Konstantin, T. Induced polarization of rocks containing pyrite: Interpretation based on X-ray computed tomography. J. Appl. Geophys. 2018, 154, 50–63. [Google Scholar] [CrossRef]
- Prichard, H.M.; Barnes, S.J.; Dale, C.W.; Godel, B.; Fisher, P.C.; Nowell, G.M. Paragenesis of multiple platinum-group mineral populations in Shetland ophiolite chromitite: 3D X-ray tomography and in situ Os isotopes. Geochim. Et Cosmochim. Acta 2017, 216, 314–334. [Google Scholar] [CrossRef]
- Godel, B. High-Resolution X-Ray Computed Tomography and Its Application to Ore Deposits: From Data Acquisition to Quantitative Three-Dimensional Measurements with Case Studies from Ni-Cu-PGE Deposits. Econ. Geol. 2013, 108, 2005–2019. [Google Scholar] [CrossRef]
- Rust, G.W. Colloidal primary copper ores at Cornwall Mines, southeastern Missouri. J. Geol. 1935, 43, 398–426. [Google Scholar] [CrossRef]
- Liu, J.; Pellerin, A.; Wang, J.; Rickard, D.; Antler, G.; Zhao, J.; Wang, Z.; Jørgensen, B.B.; Ono, S. Multiple sulfur isotopes discriminate organoclastic and methane-based sulfate reduction by sub-seafloor pyrite formation. Geochim. Et Cosmochim. Acta 2022, 316, 309–330. [Google Scholar] [CrossRef]
- Dantas, R.C.; Hassan, M.B.; Cruz, F.W.; Jovane, L. Evidence for methane seepage in South Atlantic from the occurrence of authigenic gypsum and framboidal pyrite in deep-sea sediments. Mar. Pet. Geol. 2022, 142, 105727. [Google Scholar] [CrossRef]
- Chen, H.; Lai, Y.; Lu, H.; Liang, J.; Lu, J.; Fang, Y. Study on authigenic pyrite in sediments of gas hydrate geo-system in the Shenhu area, South China Sea. Haiyang Xuebao 2018, 40, 116–133. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SEAC201807010&DbName=CJFQ2018 (accessed on 15 July 2018).
- Wilkin, R.T.; Arthur, M.A. Variations in pyrite texture, sulfur isotope composition, and iron systematics in the black sea: Evidence for late pleistocene to holocene excursions of the O2-H2S redox transition. Geochim. Et Cosmochim. Acta 2001, 65, 1399–1416. [Google Scholar] [CrossRef]
- Liu, K.; Huang, F.; Gao, S.; Zhang, Z.; Ren, Y.; An, B. Morphology of framboidal pyrite and its textural evolution: Evidence from the Logatchev area, Mid-Atlantic Ridge. Ore Geol. Rev. 2022, 141, 104630. [Google Scholar] [CrossRef]
- Zhang, J. The Coupling of Carbon and Sulfur in Sediments in the Early Diagenesis of Methane Hydrate Potential Area of Northern South China Sea. Xiamen University. 2014. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201402&filename=1014227600.nh (accessed on 1 May 2014).
- Zhang, Q.; Wu, D.; Jin, G.; Xu, X.; Yang, C.; Liu, L. Methane seep in the Shenhu area of the South China sea using geochemical and mineralogical features. Mar. Pet. Geol. 2022, 144, 105829. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.; Yin, X.; Wang, X.; Chen, S.; Qi, H.; Chen, Z.; Zhu, B. Mineralogy, geochemistry, and sulfur isotope characteristics of sediment-hosted hydrothermal sulfide minerals from the southern Okinawa Trough. Acta Oceanol. Sin. 2021, 40, 129–143. [Google Scholar] [CrossRef]
- Shi, S.; Wu, C.; Liang, J.; Wang, Y.; Ye, Y.; Fang, Y.; Ma, J.; Zhai, L. Characterstics and formation mechanism of pyrite tubes in sediments from Xisha Trough in northern South China Sea. J. Palaeogeogr. 2020, 22, 18. [Google Scholar] [CrossRef]
- Li, H.; Lu, X.; Bian, L.; Ma, M.; Zhang, X.; Zhang, Z.; Ding, Z. Geological Significance of Microcrystalline Morphology and Composition of Framboids Pyrite: A Case Study of Marl of Chihsia Formation. Acta Mineral. Sin. 2012, 32, 443–448. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, X.; Lu, Y.; Strauss, H.; Xu, L.; Gong, J.; Teichert, B.M.A.; Lu, R.; Lu, H.; Sun, W.; et al. The enrichment of heavy iron isotopes in authigenic pyrite as a possible indicator of sulfate-driven anaerobic oxidation of methane: Insights from the South China Sea. Chem. Geol. 2017, 449, 15–29. [Google Scholar] [CrossRef]
- Iizasa, K. Petrographic investigations of seafloor sediments from the Kita-Bayonnaise submarine caldera, Shichito-Iwojima Ridge, Izu-Ogasawara Arc, northwestern Pacific. Mar. Geol. 1993, 112, 271–290. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Lepland, A. Biogeochemistry of sulfur in a sediment core from the west-central Baltic Sea: Evidence from stable isotopes and pyrite textures. J. Mar. Syst. 2000, 25, 299–312. [Google Scholar] [CrossRef]
- Chu, F.; Chen, L.; Shen, S.; Shi, X. Morphological Features of authigenic pyrite from South Yellow Sea Sediment. Oceanol. Et Limnol. Sin. 1994, 25, 461–467. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=HYFZ199405000&DbName=CJFQ1994 (accessed on 15 October 1994).
- Lin, Q.; Wang, J.; Fu, S.; Lu, H.; Bu, Q.; Lin, R.; Sun, F. Elemental sulfur in northern South China Sea sediments and its significance. Sci. China Earth Sci. 2015, 58, 2271–2278. [Google Scholar] [CrossRef]
- Sassen, R.; Roberts, H.H.; Carney, R.; Milkov, A.V.; DeFreitas, D.A.; Lanoil, B.; Zhang, C. Free hydrocarbon gas, gas hydrate, and authigenic minerals in chemosynthetic communities of the northern Gulf of Mexico continental slope: Relation to microbial processes. Chem. Geol. 2004, 205, 195–217. [Google Scholar] [CrossRef]
- Meng, L.; Huang, F.; Wang, X.; Gao, W.; Zhang, B.; Song, D.; Li, G.; Zhang, B. An experimental study of the morphological evolution of pyrite under hydrothermal conditions and its implications. J. Geochem. Explor. 2020, 219, 106636. [Google Scholar] [CrossRef]
- Barnard, A.S.; Russo, S.P. Modelling nanoscale FeS2 formation in sulfur rich conditions. J. Mater. Chem. 2009, 19, 3389–3394. [Google Scholar] [CrossRef]
- Rees, C.E. A steady-state model for sulphur isotope fractionation in bacterial reduction processes. Geochim. Et Cosmochim. Acta 1973, 37, 1141–1162. [Google Scholar] [CrossRef]
- Berner, R.A.; Raiswell, R. Burial of organic carbon and pyrite sulfur in sediments over phanerozoic time: A new theory. Geochim. Et Cosmochim. Acta 1983, 47, 855–862. [Google Scholar] [CrossRef]
- Large, R.; Maslennikov, V.; Robert, F.; Danyushevsky, L.; Chang, Z. Multistage Sedimentary and Metamorphic Origin of Pyrite and Gold in the Giant Sukhoi Log Deposit, Lena Gold Province, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Cavalazzi, B.; Barbieri, R.; Cady, S.L.; George, A.D.; Gennaro, S.; Westall, F.; Lui, A.; Canteri, R.; Rossi, A.P.; Ori, G.G.; et al. Iron-framboids in the hydrocarbon-related Middle Devonian Hollard Mound of the Anti-Atlas mountain range in Morocco: Evidence of potential microbial biosignatures. Sediment. Geol. 2012, 263–264, 183–193. [Google Scholar] [CrossRef]
- Zatoń, M.; Marynowski, L.; Szczepanik, P.; Bond, D.P.G.; Wignall, P.B. Redox conditions during sedimentation of the Middle Jurassic (Upper Bajocian–Bathonian) clays of the Polish Jura (south-central Poland). Facies 2008, 55, 103–114. [Google Scholar] [CrossRef]
- Alfonso, D.R. Computational Investigation of FeS2 Surfaces and Prediction of Effects of Sulfur Environment on Stabilities. J. Phys. Chem. C 2010, 114, 8971–8980. [Google Scholar] [CrossRef]
- Rickard, D. Chapter 6—Sedimentary Pyrite. In Developments in Sedimentology; Rickard, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 65, pp. 233–285. [Google Scholar] [CrossRef]
- Ding, H.; Yao, S.; Chen, J. Authigenic pyrite formation and re-oxidation as an indicator of an unsteady-state redox sedimentary environment: Evidence from the intertidal mangrove sediments of Hainan Island, China. Cont. Shelf Res. 2014, 78, 15. [Google Scholar] [CrossRef]
- Huang, F.; Gao, W.; Gao, S.; Meng, L.; Zhang, Z.; Yan, Y.; Ren, Y.; Li, Y.; Liu, K.; Xing, M.; et al. Morphology Evolution of Nano-Micron Pyrite: A Review. J. Nanosci. Nanotechnol. 2017, 17, 5980–5995. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L. Pyrite formation by reactions of iron monosulfides with dissolve dinorganic and organic sulfur species. Geochim. Et Cosmochim Acta 1996, 60, 4167–4179. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Arthur, M.A.; Dean, W.E. History of water-column anoxia in the Black Sea indicated by pyrite framboid size distributions. Earth Planet. Sci. Lett. 1997, 148, 517–525. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Taladay, K.; Lu, H.; Hu, G.; Sun, F.; Lin, R. Coupled pyrite concentration and sulfur isotopic insight into the paleo sulfate–methane transition zone (SMTZ) in the northern South China Sea. J. Asian Earth Sci. 2016, 115, 547–556. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, X.; Peckmann, J.; Lu, Y.; Xu, L.; Strauss, H.; Zhou, H.; Gong, J.; Lu, H.; Teichert, B.M.A. How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chem. Geol. 2016, 440, 26–41. [Google Scholar] [CrossRef]
- Pierre, C. Origin of the authigenic gypsum and pyrite from active methane seeps of the southwest African Margin. Chem. Geol. 2017, 449, 158–164. [Google Scholar] [CrossRef]
- Love, L.G. Microorganisms and the presence of syngenetic pyrite. Q. J. Geol. Soc. Lond. 1957, 113, 429–440. [Google Scholar] [CrossRef]
- Popa, R.; Kinkle, B.K.; Badescu, A. Pyrite Framboids as Biomarkers for Iron-Sulfur Systems. Geomicrobiol. J. 2004, 21, 193–206. [Google Scholar] [CrossRef]
- Butler, I.B.; Rickard, D. Framboidal pyrite formation via the oxidation of iron (II) monosulphide by hydrogen sulphide. Geochim. Et Cosmochim. Acta 2000, 64, 2665–2672. [Google Scholar] [CrossRef]
- Ohfuji, H.; Rickard, D. Experimental syntheses of framboids—A review. Earth-Sci. Rev. 2005, 71, 147–170. [Google Scholar] [CrossRef]
- Rickard, D. How long does it take a pyrite framboid to form? Earth Planet. Sci. Lett. 2019, 513, 64–68. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L. Formation processes of framboidal pyrite. Geochim. Et Cosmochim. Acta 1997, 61, 323–339. [Google Scholar] [CrossRef]
- Wang, P.; Huang, Y.; Wang, C.; Feng, Z.; Huang, Q. Pyrite morphology in the first member of the Late Cretaceous Qingshankou Formation, Songliao Basin, Northeast China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 385, 125–136. [Google Scholar] [CrossRef]
- Large, D.J.; Fortey, N.J.; Milodowski, A.E.; Christy, A.G.; Dodd, J. Petrographic observations of iron, copper, and zinc sulfides in freshwater canal sediment. J. Sediment. Res. 2001, 71, 61–69. [Google Scholar] [CrossRef]
- Scott, R.J.; Meffre, S.; Woodhead, J.; Gilbert, S.E.; Berry, R.F.; Emsbo, P. Development of framboidal pyrite during diagenesis, low-grade regional metamorphism, and hydrothermal alteration. Econ. Geol. 2009, 104, 1143–1168. [Google Scholar] [CrossRef]
- Sawlowicz, Z. Pyrite framboids and their development: A new conceptual mechanism. Geol. Rundsch. 1993, 82, 148–156. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, X.; Strauss, H.; Lu, Y.; Gong, J.; Xu, L.; Lu, H.; Teichert, B.M.A.; Peckmann, J. Multiple sulfur isotope constraints on sulfate-driven anaerobic oxidation of methane: Evidence from authigenic pyrite in seepage areas of the South China Sea. Geochim. Et Cosmochim. Acta 2017, 211, 153–173. [Google Scholar] [CrossRef]
- Xie, L.; Wang, J.; Wu, N.; Wu, D.; Wang, Z.; Zhu, X.; Hu, J.; Chen, H.; Lin, Q. Characteristics of authigenic pyrites in shallow core sediments in the Shenhu area of the northern South China Sea: Implications for a possible mud volcano environment. Sci. China Earth Sci. 2012, 56, 541–548. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, H.; Guan, H.; Liu, L.; Wu, D.; Wu, N. Methane seepage intensities traced by sulfur isotopes of pyrite and gypsum in sediment from the Shenhu area, South China Sea. Acta Oceanol. Sin. 2018, 37, 20–27. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, X.; Lin, Z.; Xu, L.; Gong, J.; Lu, H. Cold seep status archived in authigenic carbonates: Mineralogical and isotopic evidence from Northern South China Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 122, 95–105. [Google Scholar] [CrossRef]
- Rickard, D. The Sedimentary Sulfur System: Biogeochemistry and Evolution through Geologic Time. In Treatise on Geochemistry (Second Edition); Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 267–326. [Google Scholar] [CrossRef]
- Schoonen, M.A.A. Mechanisms of sedimentary pyrite formation. In Sulfur Biogeochemistry—Past and Present; Amend, J.P., Edwards, K.J., Lyons, T.W., Eds.; Geological Society of America: Boulder, CO, USA, 2004; pp. 117–134. [Google Scholar] [CrossRef]
- Kirkeminde, A.; Ren, S. Thermodynamic control of iron pyrite nanocrystal synthesis with high photoactivity and stability. J. Mater. Chem. A 2013, 1, 49–54. [Google Scholar] [CrossRef]
- Hao-Wen, T. Fillings inside foraminiferal tests and their early diagenesis. Acta Micropalaeontologica Sin. 1992, 9, 423–432. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=WSGT199204007&DbName=CJFQ1992 (accessed on 30 December 1992).
- Hudson, J.D. Pyrite in ammonite-bearing shales from the Jurassic of England and Germany. Sedimentology 1982, 29, 639–667. [Google Scholar] [CrossRef]
- Korsunsky, A.M.; Kalinina, O.Y.; Sapozhnikov, P.V.; Everaerts, J.; Salimon, A.I. Pyrite ‘poste restante’ Intra-diatom framboids. Mater. Today 2020, 32, 293–294. [Google Scholar] [CrossRef]
- Nakajima, T.; Volcani, B.E. ϵ-N-Trimethyl-l-δ-hydroxylysine phosphate and its nonphosphorylated compound in diatom cell walls. Biochem. Biophys. Res. Commun. 1970, 39, 28–33. [Google Scholar] [CrossRef]
- Kröger, N.B.C.; Sumper, M. A new calcium binding glycoprotein family constitutes a major diatom cell wall component. EMBO J. 1994, 13, 4676–4683. [Google Scholar] [CrossRef]
- Schieber, J. Iron Sulfide Formation. In Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 486–502. [Google Scholar] [CrossRef]
- Berner, R.A.; Leeuw, J.; Spiro, B.; Murchison, D.G.; Eglinton, G. Sulphate reduction, organic matter decomposition and pyrite formation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1985, 315, 25–38. [Google Scholar] [CrossRef]
- Rickard, D.; Grimes, S.; Butler, I.; Oldroyd, A.; Davies, K.L. Botanical constraints on pyrite formation. Chem. Geol. 2007, 236, 228–246. [Google Scholar] [CrossRef]
- Grimes, S.T.; Davies, K.L.; Butler, I.B.; Brock, F.; Edwards, D.; Rickard, D.; Briggs, D.E.G.; Parkes, R.J. Fossil plants from the Eocene London Clay: The use of pyrite textures to determine the mechanism of pyritization. J. Geol. Soc. 2002, 159, 493–501. [Google Scholar] [CrossRef]
- MacLean, L.C.; Tyliszczak, T.; Gilbert, P.U.; Zhou, D.; Pray, T.J.; Onstott, T.C.; Southam, G. A high-resolution chemical and structural study of framboidal pyrite formed within a low-temperature bacterial biofilm. Geobiology 2008, 6, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Hu, Y.; Li, N.; Feng, D.; Liang, Q.; Tong, H.; Peng, Y.; Tao, J.; Chen, D. Environmental controls on sulfur isotopic compositions of sulfide minerals in seep carbonates from the South China Sea. J. Asian Earth Sci. 2018, 168, 96–105. [Google Scholar] [CrossRef]
- Palomares, R.M.; Hernández, R.L.; González-Sanz, F.J.; Losada, L.S.; Martínez-Frías, J. A Mathematical Algorithm to Simulate the Growth and Transformation of Framboidal Pyrite: Characterization of the Biogenic Influence in Their Size Distributions. In Mathematics of Planet Earth; Eulogio, P., Carolina, G.A., Eds.; Springer: Heidelberg, Germany, 2014; Volume 1, pp. 793–796. [Google Scholar] [CrossRef]
- Ranganath, N.; Padhi, A.K.; Padhi, A.K.; Rai, A.K. Framboidal—Colloform—Recrystallised pyrite in the granitoids of Wahkyn area, West Khasi hills, Meghalaya. J. Geol. Soc. India 2009, 74, 591–596. [Google Scholar] [CrossRef]
- Wei, H.; Algeo, T.J.; Yu, H.; Wang, J.; Guo, C.; Shi, G. Episodic euxinia in the Changhsingian (late Permian) of South China: Evidence from framboidal pyrite and geochemical data. Sediment. Geol. 2015, 319, 78–97. [Google Scholar] [CrossRef]
- Wignall, P.B.; Newton, R.; Brookfield, M.E. Pyrite framboid evidence for oxygen-poor deposition during the Permian–Triassic crisis in Kashmir. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 216, 183–188. [Google Scholar] [CrossRef]
- Wignall, P.B.; Newton, R. Pyrite framboid diameter as a measure of oxygen defi ciency in ancient mudrocks. Am. J. Sci. 1998, 298, 537–552. [Google Scholar] [CrossRef]
- Sweeney, R.E.; Kaplan, I.R. Pyrite framboid formation; laboratory synthesis and marine sediments. Econ. Geol. 1973, 68, 618–634. [Google Scholar] [CrossRef]
- Tian, L.; Tong, J.; Algeo, T.J.; Song, H.; Song, H.; Chu, D.; Shi, L.; Bottjer, D.J. Reconstruction of Early Triassic ocean redox conditions based on framboidal pyrite from the Nanpanjiang Basin, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 412, 68–79. [Google Scholar] [CrossRef]
- Bond, D.P.G.; Wignall, P.B. Pyrite framboid study of marine Permian–Triassic boundary sections: A complex anoxic event and its relationship to contemporaneous mass extinction. Geol. Soc. Am. Bull. 2010, 122, 1265–1279. [Google Scholar] [CrossRef]
- Fan, L.-F.; Lin, S.; Hsu, C.-W.; Tseng, Y.-T.; Yang, T.F.; Huang, K.-M. Formation and preservation of authigenic pyrite in the methane dominated environment. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 138, 60–71. [Google Scholar] [CrossRef]
- Lin, S.; Morse, J.W. Sulfate reduction and iron sulfide mineral formation in Gulf of Mexico anoxic sediments. Am. J. Sci. 1991, 291, 55–89. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Kasten, S. Sulfur Cycling and Methane Oxidation. In Marine Geochemistry; Schulz, H.D., Zabel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 271–309. [Google Scholar] [CrossRef]
- Caridi, F.; Sabbatini, A.; Morigi, C.; Dell’Anno, A.; Negri, A.; Lucchi, R.G. Patterns and environmental drivers of diversity and community composition of macrofauna in the Kveithola Trough (NW Barents Sea). J. Sea Res. 2019, 153, 101780. [Google Scholar] [CrossRef]
- Peckmann, J.; Reimer, A.; Luth, U.; Luth, C.; Reitner, J. Methane-derived carbonates and authigenic pyrite from the northwestern black sea. Mar. Geol. 2001, 177, 129–150. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Hespenheide, B.; Llobet-Brossa, E.; Beardsley, C.; Larsen, O.; Schramm, A.; Wieland, A.; Böttcher, G.; Berninger, U.-G.; Amann, R. The biogeochemistry, stable isotope geochemistry, and microbial community structure of a temperate intertidal mudflat: An integrated study. Cont. Shelf Res. 2000, 20, 1749–1769. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Q.; Feng, Y.; Luo, H.; Pan, X.; Gadd, G.M. Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane. Sci. Total Environ. 2018, 610–611, 759–768. [Google Scholar] [CrossRef]
- Peng, X.; Guo, Z.; Chen, S.; Sun, Z.; Xu, H.; Ta, K.; Zhang, J.; Zhang, L.; Li, J.; Du, M. Formation of carbonate pipes in the northern Okinawa Trough linked to strong sulfate exhaustion and iron supply. Geochim. Et Cosmochim. Acta 2017, 205, 1–13. [Google Scholar] [CrossRef]
- Egger, M.; Rasigraf, O.; Sapart, C.J.; Jilbert, T.; Jetten, M.S.; Rockmann, T.; van der Veen, C.; Banda, N.; Kartal, B.; Ettwig, K.F.; et al. Iron-mediated anaerobic oxidation of methane in brackish coastal sediments. Environ. Sci. Technol. 2015, 49, 277–283. [Google Scholar] [CrossRef]
- Beal, E.J.; House, C.H.; Orphan, V.J. Manganese- and Iron-Dependent Marine Methane Oxidation. Science 2009, 325, 184–187. [Google Scholar] [CrossRef]
- Sivan, O.; Adler, M.; Pearson, A.; Gelman, F.; Bar-Or, I.; John, S.G.; Eckert, W. Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol. Oceanogr. 2011, 56, 1536–1544. [Google Scholar] [CrossRef]
- Du, R.; Xian, H.; Wu, X.; Zhu, J.; Wei, J.; Xing, J.; Tan, W.; He, H. Morphology dominated rapid oxidation of framboidal pyrite. Geochem. Perspect. Lett. 2021, 16, 53–58. [Google Scholar] [CrossRef]
- Jerz, J.K.; Rimstidt, J.D. Pyrite oxidation in moist air. Geochim. Et Cosmochim. Acta 2004, 68, 701–714. [Google Scholar] [CrossRef]
- Zhu, J.; Xian, H.; Lin, X.; Tang, H.; Du, R.; Yang, Y.; Zhu, R.; Liang, X.; Wei, J.; Teng, H.H.; et al. Surface structure-dependent pyrite oxidation in relatively dry and moist air: Implications for the reaction mechanism and sulfur evolution. Geochim. Et Cosmochim. Acta 2018, 228, 259–274. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. Pyrite (FeS2) oxidation: A sub-micron synchrotron investigation of the initial steps. Geochim. Et Cosmochim. Acta 2011, 75, 6239–6254. [Google Scholar] [CrossRef]
- Karavaiko, G.I.; Smolskaja, L.S.; Golyshina, O.K.; Jagovkina, M.A.; Egorova, E.Y. Egorova Bacterial pyrite oxidation: Influence of morphological, physical and chemical properties. Fuel Processing Technol. 1994, 40, 151–165. [Google Scholar] [CrossRef]
- Silverman, M.P.; Ehrlich, H.L. Microbial Formation and Degradation of Minerals. Adv. Appl. Microbiol. 1964, 6, 153–206. [Google Scholar] [CrossRef]
- Bennett, J.C.; Tributsch, H. Bacterial leaching patterns on pyrite crystal surfaces. J. Bacteriol. 1978, 134, 310–317. [Google Scholar] [CrossRef]
- Wacey, D.; Saunders, M.; Brasier, M.D.; Kilburn, M.R. Earliest microbially mediated pyrite oxidation in ~3.4 billion-year-old sediments. Earth Planet. Sci. Lett. 2011, 301, 393–402. [Google Scholar] [CrossRef]
- Schieber, J. Sedimentary pyrite: A window into the microbial past. Geology 2002, 30, 531–534. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.-Q.; Algeo, T.J.; Zhao, L.; Baud, A.; Bhat, G.M.; Zhang, L.; Guo, Z. Two-stage marine anoxia and biotic response during the Permian–Triassic transition in Kashmir, northern India: Pyrite framboid evidence. Glob. Planet. Chang. 2019, 172, 124–139. [Google Scholar] [CrossRef]
- Kershaw, S.; Liu, M. Modern Black Sea oceanography applied to the end-Permian extinction event. J. Palaeogeogr. 2015, 4, 52–62. [Google Scholar] [CrossRef]





| Dissolved Oxygen (mL/L) | Conditions | Framboid Parameters | |||
|---|---|---|---|---|---|
| Mean Diameter (µm) | Texture | ||||
| [105] | [9] | [11] | |||
| 0 | Euxinic | 3–5 | 3–6.7 | 2.9–10.9 | Framboid dominant |
| 0–0.2 | Anoxic | 4–6 | 3.3–11.8 | 3–20.9 | Framboid dominant |
| 0.2–2 | Lower dysoxic | 6–10 | Large framboid and some crystalline pyrite | ||
| Upper dysoxic | <5 | Pyrite crystal dominant | |||
| 2.0–8.0 | Oxic | No framboid, rare pyrite crystals. | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Li, Y.; Lu, H. The Morphological Characteristics of Authigenic Pyrite Formed in Marine Sediments. J. Mar. Sci. Eng. 2022, 10, 1533. https://doi.org/10.3390/jmse10101533
Chang J, Li Y, Lu H. The Morphological Characteristics of Authigenic Pyrite Formed in Marine Sediments. Journal of Marine Science and Engineering. 2022; 10(10):1533. https://doi.org/10.3390/jmse10101533
Chicago/Turabian StyleChang, Jingyi, Yuanyuan Li, and Hailong Lu. 2022. "The Morphological Characteristics of Authigenic Pyrite Formed in Marine Sediments" Journal of Marine Science and Engineering 10, no. 10: 1533. https://doi.org/10.3390/jmse10101533
APA StyleChang, J., Li, Y., & Lu, H. (2022). The Morphological Characteristics of Authigenic Pyrite Formed in Marine Sediments. Journal of Marine Science and Engineering, 10(10), 1533. https://doi.org/10.3390/jmse10101533








