A Review of Modeling Approaches for Understanding and Monitoring the Environmental Effects of Marine Renewable Energy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Changes in Oceanographic Systems
3.1.1. Hydrodynamic Models
3.1.2. Wave Propagation Models
3.1.3. Monitoring and Model Validation
3.2. Underwater Noise
3.2.1. Transmission Loss Models
3.2.2. Nearfield Propagation Models
3.2.3. Farfield Propagation Models
3.2.4. Species-Effects Models
3.2.5. Monitoring and Model Validation
3.3. Electromagnetic Fields
3.3.1. Analytical Models
3.3.2. Numerical Simulations
3.3.3. Monitoring and Model Validation
3.4. Changes in Habitat
3.4.1. Statistical Species Distribution Models
3.4.2. Spatial Ecosystem and Trophic Models
3.4.3. Biophysical Models
3.4.4. Monitoring and Model Validation
3.5. Collision Risk
3.5.1. Encounter Rate/Collision Risk Models
3.5.2. Exposure Time Population Model
3.5.3. Monitoring and Model Validation
3.6. Displacement of Marine Animals
3.6.1. Biophysical and Agent-Based Models
3.6.2. Statistical Species Distribution Models
3.6.3. Monitoring and Model Validation
4. Discussion
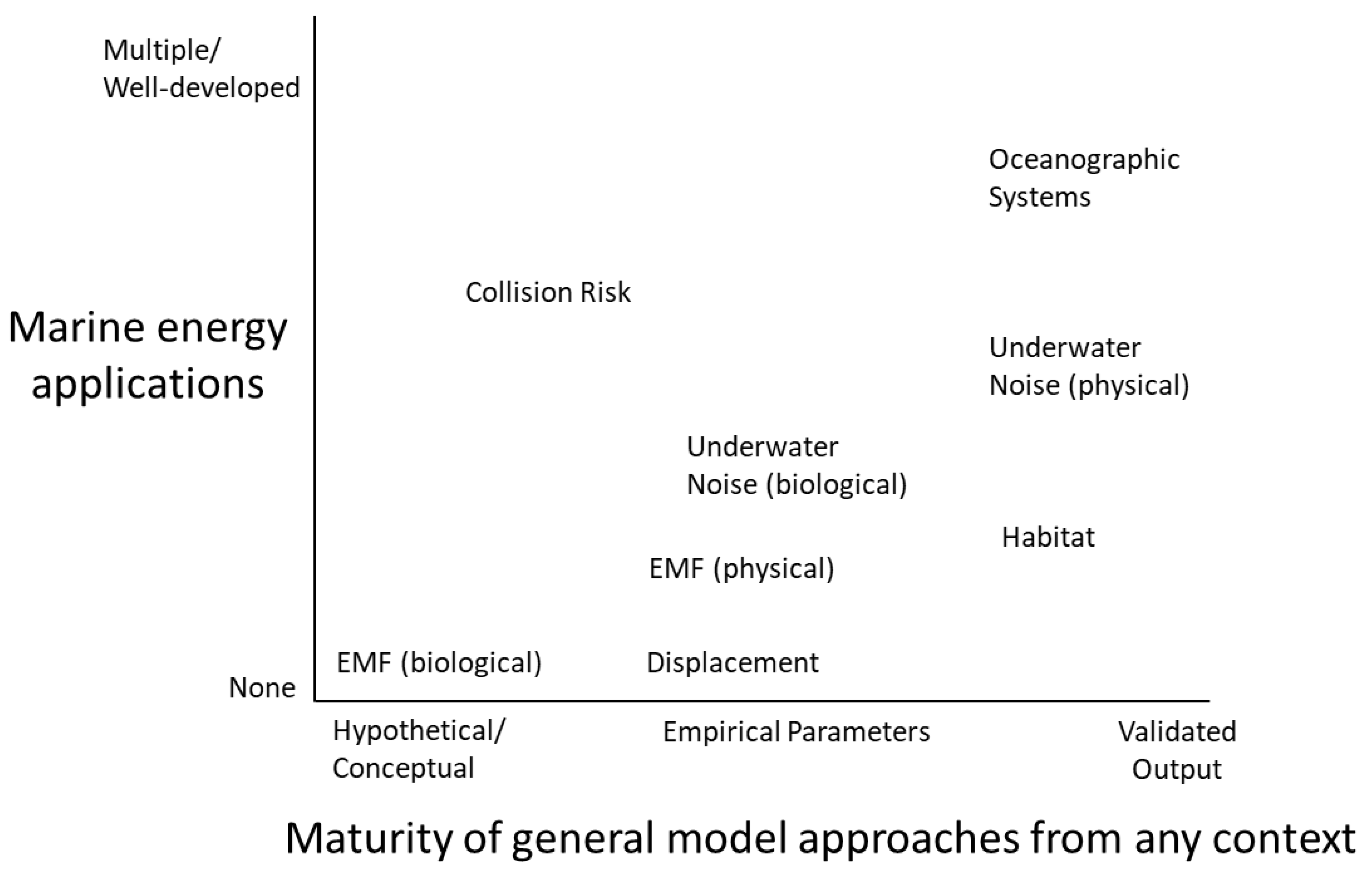
4.1. Availability and Maturity of Models
4.2. Selecting Modeling Approaches
- Device characteristics: Device type, number, and arrangement determine which physical and behavioral processes need to be available in the selected models and what scale and resolution are required.
- Site characteristics: Model functionality needs to be appropriate for the conditions of the site, i.e., water depth, bathymetric complexity, sediment dynamics, and/or biotic interactions.
- Spatiotemporal scales: Computation time is determined by model scale and resolution. It can be improved in some models using simplifications in exchange for specificity. Modeling objectives may require the use of both near- and farfield modeling to estimate both source levels and propagation of effects. The choice of physiological and behavioral functions and parameters may also depend on the scale of the modeling objectives.
- Receptor species: Modeling approaches differ for benthic or pelagic species, mobile or sessile organisms, and different life stages (e.g., adults vs. larvae).
- Existing data: If data of sufficient quality is available to be used in a model analysis, it may constrain the choice of models. This is particularly a consideration if collecting other types of data (in the necessary time frame) is not feasible.
4.3. Model Information Requirements and Uncertainties
4.4. Validation and Feedback between Models and Monitoring
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronyms and Abbreviations
| AC | alternating current |
| CFD | computation fluid dynamic |
| CHD | coastal hydrodynamic |
| CRD | collision risk model |
| DC | direct current |
| EwE | Ecopath with Ecosim |
| FVCOM | finite volume community ocean model |
| GA(M)M | generalized additive (mixed) model |
| GL(M)M | generalized linear (mixed) model |
| IEA | International Energy Agency |
| iPCoD | interim population consequences of disturbance |
| EMF | electromagnetic field |
| ERM | encounter risk model |
| ETPM | exposure time population model |
| FEM | finite element model |
| GPS/GSM | Global Positioning System/Global System for Mobile Communications |
| MaxEnt | maximum entropy |
| ME | marine energy |
| NPZD | nutrient-phytoplankton-zooplankton-detritus |
| OES | Ocean Energy Systems |
| OSW | offshore wind |
| PCoD | population consequences of disturbance |
| PE | parabolic equation |
| PTS | permanent threshold shift |
| RF | random forest |
| TL | transmission loss |
| TTS | temporary threshold shift |
| SWAN | simulating waves nearshore |
| WEC | wave energy converter |
| WSE | water surface elevation |
Appendix A
| Stressor | Device |
|---|---|
| Hydrodynamic model, hydrogeomorphic model, wave model, sediment model Underwater noise model, underwater acoustic model, marine noise model, marine acoustic model, population consequences of disturbance, population model Collision risk model, encounter rate model, collision model, avoidance, behavior, evasion Biophysical model, agent-based model, individual-based model, displacement, migration, barrier effects, statistical models, generalized linear models Change in habitat, habitat change, benthic habitat, pelagic habitat, species distribution, habitat suitability, ecological niche, decision tree, ensemble model, ecosystem model, trophic model | Marine renewable energy, marine hydrokinetic energy, ocean energy, offshore renewable energy Tidal turbine, wave energy converter, tidal kite, tidal energy, wave energy, wake effect of turbines, array |
| Reference | Stressor | Receptor | Device(s) | Model Type | Model Name |
|---|---|---|---|---|---|
| Abanades et al., 2014 | Oceanographic systems | Beach profile | WEC | Wave | SWAN, Xbeach |
| Ahmed et al., 2017 | Oceanographic systems | Nearfield, wake | Tidal turbine | Computational fluid dynamics | Code_Saturne |
| Ashall et al., 2016 | Oceanographic systems | Suspended sediment | Tidal turbine array | Hydrodynamic + wave | Delft-3D-SWAN |
| Balitsky et al., 2019 | Oceanographic systems | Nearfield and farfield wave effects | WEC array | Wave | NEMOH + MILDwave |
| Beels et al., 2010 | Oceanographic systems | Wave heights | WEC array | Wave | MILDwave |
| Bergillos et al., 2018 | Oceanographic systems | Beach profile | WEC | Hydrodynamic, wave | Delft3D-Wave, Xbeach-G |
| Chatzirodou et al., 2019 | Oceanographic systems | Offshore sandbank | Tidal turbine array | Hydrodynamic | Delft3D |
| Churchfield et al., 2013 | Oceanographic systems | Wake propagation | Tidal turbine array | Computational fluid dynamics | OpenFOAM |
| Contardo et al., 2018 | Oceanographic systems | Wave height | WEC | Wave | SNL-SWAN |
| de Dominicis et al., 2017 | Oceanographic systems | Hydrodynamics | Tidal turbine array | Coastal Hydrodynamic | FVCOM |
| Gallego et al., 2017 | Oceanographic systems | Hydrodynamics, suspended sediment, seabed | Tidal turbine array/WEC Array | Hydrodynamic, wave | MIKE3, Delft3D-Flow, MIKE21 |
| Haverson et al., 2018 | Oceanographic systems | Seabed shear stress | Tidal turbine array | Hydrodynamic | Telemac2D |
| Iglesias and Carballo 2014 | Oceanographic systems | Hydrodynamics | WEC Array | Wave | SWAN |
| Jones et al., 2018 | Oceanographic systems | Seabed elevation, near-bed-shear stress | WEC array | Hydrodynamic + wave | Delft3D-FLOW-SNL-SWAN |
| Kang et al., 2012 | Oceanographic systems | Turbine wake | Tidal turbine | Computational fluid dynamics | N/A |
| Li et al., 2019 | Oceanographic systems | Surface waves | Tidal turbine | Computational fluid dynamics | Ansys Fluent, FVCOM |
| Martin-Short et al., 2015 | Oceanographic systems | Flow regime, sediment transport | Tidal turbine array | Hydrodynamic | Fluidity |
| O’Dea et al., 2018 | Oceanographic systems | Nearshore waves and currents | WEC array | Wave | SWAN |
| Robins et al., 2014 | Oceanographic systems | Sediment dynamics | Tidal turbine arrays | Hydrodynamic + morphological, wave | TELEMAC-2D-SISYPHE, SWAN |
| Salunkhe et al., 2019 | Oceanographic systems | Turbine wake | Tidal turbine | Computational fluid dynamics | Ansys Fluent, OpenFOAM |
| Sjökvist et al., 2017 | Oceanographic systems | Device buoy response | WEC | Computational fluid dynamics | WAMIT, COMSOL |
| Sufian et al., 2017 | Oceanographic systems | Wake and wave effects | Tidal turbine | Computation fluid dynamics | Ansys Fluent |
| Stratigaki et al., 2019 | Oceanographic systems | Wave field | WEC | Wave | WAMIT + MILDwave |
| Thiebot et al., 2016, 2020 | Oceanographic systems | Wakes | Tidal turbine | Hydrodynamic | Telemac-3D |
| Verao Fernandez et al., 2019 | Oceanographic systems | Wake and wave effects | WEC | Wave | NEMOH + MILDwave |
| Venugopal et al., 2017 | Oceanographic systems | Wave height | WEC arrays | Wave | MIKE 21 SW, WAMIT |
| Waldman et al., 2017 | Oceanographic systems | Bed stress, current speed | Tidal turbine arrays | Hydrodynamic | MIKE 3, Delft3D |
| Xu et al., 2019 | Oceanographic systems | Nearfield, device effects | WEC | Computation fluid dynamics | OpenFOAM |
| Yang et al., 2013 | Oceanographic systems | Water velocity, volume flux, flushing time | Tidal turbine array | Hydrodynamic | FVCOM |
| Hafla et al., 2018 | Noise | N/A | Generic ME array (3) | Velocity-pressure wave propagation | Paracousti |
| Ikpekha et al., 2014 | Noise | Harbor seal | Wave energy converter | Finite element method | COMSOL Multiphysics |
| Lloyd et al., 2011 | Noise | Atlantic cod | Tidal turbine array (3) | Fast field | SCOOTER in AcTUP |
| Lloyd et al., 2014 | Noise | Nearfield/source | Tidal turbine | Acoustic analogy | OpenFOAM |
| Pine et al., 2014 | Noise | N/A | Tidal turbines (1–2) | Transmission loss | N/A |
| Pine et al., 2019 | Noise | Harbor porpoise, harbor seal | Tidal turbine, tidal kite | Parabolic equation, Gaussian beam trace, listening space reduction | RAMGeo, Bellhop |
| Robertson et al., 2018 | Noise | Harbor porpoise, harbor seal | Tidal turbine | Transmission loss | N/A |
| Adams et al., 2014 | Changes in habitat | Generic species | Generic ME arrays | Biophysical model of larval dispersal | N/A |
| Alexander et al., 2016 | Changes in habitat | 41 functional groups | Generic ME arrays | Spatial ecosystem model | Ecopath with Ecosim and Ecospace |
| Baker et al., 2020 | Changes in habitat | 14 species | Tidal barrage | Hydrodynamic, maximum entropy | Tethys, MaxEnt |
| du Feu et al., 2019 | Changes in habitat | Barnacle, crab | Tidal turbine arrays | Hydrodynamic, maximum entropy | OpenTidalFarm, MaxEnt |
| Lieber et al., 2019 | Changes in habitat | Terns | Tidal turbine | General-additive mixed model | |
| Linder et al., 2017; Linder & Horne 2018 | Changes in habitat | Nekton | Tidal turbine | Generalized regressions, time series, nonparametric models | linear, GLS, GLM, GLMM, GAM, GAMM SSM, Reg-ARMA, Reg-ARMA-GARCH RF, SVR |
| Schuchert et al., 2018 | Changes in habitat | Phytoplankton, zooplankton | Tidal turbine array | Coupled 2D hydrodynamic biogeochemical model | MIKE 21 FM |
| van der Molen et al., 2016 | Changes in habitat | 18 functional groups | Tidal turbine array | 3D hydrodynamics-biogeochemistry model | GETM-ERSEMBFM |
| Band 2016 | Collision | Marine mammals, fish, diving seabirds | Tidal turbine | Collision risk model, encounter rate model, exposure time population model | N/A |
| Bevelhimer et al., 2016 | Collision | Shortnose sturgeon, Atlantic sturgeon | Tidal turbine array | Collision risk model | KFIM (KHPS-Fish interaction model) |
| Copping and Grear 2018 | Collision | Killer whale, harbor seal, harbor porpoise | Tidal turbine array | Collision risk model | N/A |
| Grant et al., 2014 | Collision | Bird | Tidal turbine | Exposure time population model | N/A |
| Hammar et al., 2015 | Collision | Fish | Tidal turbine | Collision risk model | N/A |
| Horne et al., 2021 | Collision | Seal | Tidal kite | Simulation-based approach collision risk model | N/A |
| Joy et al., 2018 | Collision | Harbor seal | Tidal turbine | Encounter rate model | N/A |
| Rossington and Benson 2020 | Collision | Silver eel | Tidal turbine | Agent-based model and collision risk model | N/A |
| Schmitt et al., 2017 | Collision | Seal | Tidal kite | 4D collision risk model | N/A |
| Thompson et al., 2016; Wood et al., 2016 | Collision | Harbor seal | Tidal turbine | Collision risk model | N/A |
| Wilson et al., 2007 | Collision | Herring, harbor porpoise | Tidal turbine | Encounter rate model | N/A |
| Xodus Group 2016 | Collision | Atlantic salmon | Tidal turbine array | Collision risk model | N/A |
| Grippo et al., 2017 | Displacement | Fish | Tidal turbine | Biophysical model | N/A |
| Croft et al., 2013; Lake et al., 2015; Lake et al., 2017; Lake 2017 | Displacement | Harbor porpoise | Tidal turbine | Agent-based model | N/A |
| Waggit et al., 2016 | Displacement | Seabirds | Tidal turbine | Generalized linear mixed models | N/A |
| 1 | Available online: https://www.ocean-energy-systems.org/ocean-energy/what-is-ocean-energy/ (accessed on 24 November 2021). |
| 2 | Available online: https://www.energy.gov/eere/water/marine-and-hydrokinetic-energy-basics (accessed on 24 November 2021). |
References
- Copping, A.E.; Hemery, L.G. (Eds.) Marine Renewable Energy: Environmental Effects and Monitoring Strategies. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development Around the World; Ocean Energy Systems: Seattle, WA, USA, 2020; pp. 18–26. [Google Scholar] [CrossRef]
- Fox, C.J.; Benjamins, S.; Masden, E.A.; Miller, R. Challenges and opportunities in monitoring the impacts of tidal-stream energy devices on marine vertebrates. Renew. Sustain. Energy Rev. 2018, 81, 1926–1938. [Google Scholar] [CrossRef]
- Segura, E.; Morales, R.; Somolinos, J.A. A strategic analysis of tidal current energy conversion systems in the European Union. Appl. Energy 2018, 212, 527–551. [Google Scholar] [CrossRef]
- Boehlert, G.W.; Gill, A.B. Environmental and ecological effects of ocean renewable energy development: A current synthesis. Oceanography 2010, 23, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Wilding, T.A.; Gill, A.B.; Boon, A.; Sheehan, E.; Dauvin, J.C.; Pezy, J.P.; O’Beirn, F.; Janas, U.; Rostin, L.; De Mesel, I. Turning off the DRIP (‘Data-rich, information-poor’)—Rationalising monitoring with a focus on marine renewable energy developments and the benthos. Renew. Sustain. Energy Rev. 2017, 74, 848–859. [Google Scholar] [CrossRef]
- Dannheim, J.; Bergström, L.; Birchenough, S.N.R.; Brzana, R.; Boon, A.R.; Coolen, J.W.P.; Dauvin, J.-C.; De Mesel, I.; Derweduwen, J.; Gill, A.B.; et al. Benthic effects of offshore renewables: Identification of knowledge gaps and urgently needed research. ICES J. Mar. Sci. 2019, 77, 1092–1108. [Google Scholar] [CrossRef]
- Mendoza, E.; Lithgow, D.; Flores, P.; Felix, A.; Simas, T.; Silva, R. A framework to evaluate the environmental impact of OCEAN energy devices. Renew. Sustain. Energy Rev. 2019, 112, 440–449. [Google Scholar] [CrossRef]
- Willsteed, E.; Gill, A.B.; Birchenough, S.N.R.; Jude, S. Assessing the cumulative environmental effects of marine renewable energy developments: Establishing common ground. Sci. Total Environ. 2017, 577, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Isaksson, N.; Masden, E.A.; Williamson, B.J.; Costagliola-Ray, M.M.; Slingsby, J.; Houghton, J.D.R.; Wilson, J. Assessing the effects of tidal stream marine renewable energy on seabirds: A conceptual framework. Mar. Pollut. Bull. 2020, 157, 111314. [Google Scholar] [CrossRef]
- Copping, A.; Gorton, A.; Freeman, M. Data Transferability and Collection Consistency in Marine Renewable Energy; PNNL-27955; U.S. Department of Energy: Richland, WA, USA, 2018.
- Shen, H.; Zydlewski, G.B.; Viehman, H.A.; Staines, G. Estimating the probability of fish encountering a marine hydrokinetic device. Renew. Energy 2016, 97, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Cotter, E.; Polagye, B. Automatic Classification of Biological Targets in a Tidal Channel Using a Multibeam Sonar. J. Atmos. Ocean. Technol. 2020, 37, 1437–1455. [Google Scholar] [CrossRef]
- Isaksson, N.; Cleasby, I.R.; Owen, E.; Williamson, B.J.; Houghton, J.D.R.; Wilson, J.; Masden, E.A. The Use of Animal-Borne Biologging and Telemetry Data to Quantify Spatial Overlap of Wildlife with Marine Renewables. J. Mar. Sci. Eng. 2021, 9, 263. [Google Scholar] [CrossRef]
- Goh, H.-B.; Lai, S.-H.; Jameel, M.; Teh, H.-M. Potential of coastal headlands for tidal energy extraction and the resulting environmental effects along Negeri Sembilan coastlines: A numerical simulation study. Energy 2020, 192, 116656. [Google Scholar] [CrossRef]
- Shabtay, A.; Portman, M.E.; Ofir, E.; Carmel, Y.; Gal, G. Using ecological modelling in marine spatial planning to enhance ecosystem-based management. Mar. Policy 2018, 95, 14–23. [Google Scholar] [CrossRef]
- Ehler, C.; Douvere, F. Marine spatial planning: A step-by-step approach toward ecosystem-based management. In Intergovernmental Oceanographic Commission and Man and the Biosphere Programme; IOC Manual and Guides No. 53, ICAM Dossier No. 6; UNESCO: Paris, France, 2009. [Google Scholar]
- Bender, A.; Francisco, F.; Sundberg, J. A Review of Methods and Models for Environmental Monitoring of Marine Renewable Energy. In Proceedings of the 12th European Wave and Tidal Energy Conference, Cork, Ireland, 27 August–1 September 2017. [Google Scholar]
- Ozkan, C.; Perez, K.; Mayo, T. The impacts of wave energy conversion on coastal morphodynamics. Sci. Total Environ. 2020, 712, 136424. [Google Scholar] [CrossRef]
- Laín, S.; Contreras, L.T.; López, O. A review on computational fluid dynamics modeling and simulation of horizontal axis hydrokinetic turbines. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 375. [Google Scholar] [CrossRef]
- Nachtane, M.; Tarfaoui, M.; Goda, I.; Rouway, M. A review on the technologies, design considerations and numerical models of tidal current turbines. Renew. Energy 2020, 157, 1274–1288. [Google Scholar] [CrossRef]
- Copping, A.; Battey, H.; Brown-Saracino, J.; Massaua, M.; Smith, C. An international assessment of the environmental effects of marine energy development. Ocean Coast. Manag. 2014, 99, 3–13. [Google Scholar] [CrossRef]
- Buenau, K.E.; Garavelli, L.J.; Hemery, L.G.; Garcia Medina, G.; Hibler, L.F. Review of Available Models for Environmental Effects of Marine Renewable Energy; PNNL-29977; Pacific Northwest National Laboratory: Richland, WA, USA, 2020.
- Copping, A.; Sather, N.; Hanna, L.; Whiting, J.; Zydlewski, G.; Staines, G.; Gill, A.; Hutchison, I.; O’Hagan, A.; Simas, T.; et al. Annex IV 2016 State of the Science Report: Environmental Effects of Marine Renewable Energy Development around the World. Report for Ocean Energy Systems (OES); Ocean Energy Systems: Seattle, WA, USA, 2016; pp. 1–199.
- Whiting, J.; Copping, A.; Freeman, M.; Woodbury, A. Tethys knowledge management system: Working to advance the marine renewable energy industry. Int. Mar. Energy J. 2019, 2, 29–38. [Google Scholar] [CrossRef]
- Hipsey, M.R.; Gal, G.; Arhonditsis, G.B.; Carey, C.C.; Elliott, J.A.; Frassl, M.A.; Janse, J.H.; de Mora, L.; Robson, B.J. A system of metrics for the assessment and improvement of aquatic ecosystem models. Environ. Model. Softw. 2020, 128, 04697. [Google Scholar] [CrossRef]
- Kubicek, A.; Jopp, F.; Breckling, B.; Lange, C.; Reuter, H. Context-oriented model validation of individual-based models in ecology: A hierarchically structured approach to validate qualitative, compositional and quantitative characteristics. Ecol. Complex. 2015, 22, 178–191. [Google Scholar] [CrossRef]
- Bennett, N.D.; Croke, B.F.W.; Guariso, G.; Guillaume, J.H.A.; Hamilton, S.H.; Jakeman, A.J.; Marsili-Libelli, S.; Newham, L.T.H.; Norton, J.P.; Perrin, C.; et al. Characterising performance of environmental models. Environ. Model. Softw. 2013, 40, 1–20. [Google Scholar] [CrossRef]
- Williams, J.J.; Esteves, L.S. Guidance on Setup, Calibration, and Validation of Hydrodynamic, Wave, and Sediment Models for Shelf Seas and Estuaries. Adv. Civ. Eng. 2017, 2017, 5251902. [Google Scholar] [CrossRef]
- Gregr, E.J.; Palacios, D.M.; Thompson, A.; Chan, K.M.A. Why less complexity produces better forecasts: An independent data evaluation of kelp habitat models. Ecography 2019, 42, 428–443. [Google Scholar] [CrossRef]
- Stow, C.A.; Jolliff, J.; McGillicuddy, D.J.; Doney, S.C.; Allen, J.I.; Friedrichs, M.A.M.; Rose, K.A.; Wallhead, P. Skill assessment for coupled biological/physical models of marine systems. J. Mar. Syst. 2009, 76, 4–15. [Google Scholar] [CrossRef] [Green Version]
- De Dominicis, M.; O’Hara Murray, R.; Wolf, J. Multi-scale ocean response to a large tidal stream turbine array. Renew. Energy 2017, 114, 1160–1179. [Google Scholar] [CrossRef]
- Haas, K.; Yang, X.; Fritz, H. Modeling impacts of energy extraction from the Gulf Stream system. In Proceedings of the 2nd Annual Marine Energy Technology Symposium (METS), Seattle, WA, USA, 15–17 April 2014. [Google Scholar]
- Bergillos, R.J.; Rodriguez-Delgado, C.; Iglesias, G. Wave farm impacts on coastal flooding under sea-level rise: A case study in southern Spain. Sci. Total Environ. 2019, 653, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Gorbeña, E.; Plomaritis, T.A.; Garel, E.; Gonçalves, J.M.S.; Bentes, L.; Monteiro, P.; Afonso, C.M.L.; Oliveira, F.; Soares, C.; et al. Deployment characterization of a floatable tidal energy converter on a tidal channel, Ria Formosa, Portugal. Energy 2018, 158, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Neary, V.S.; Guala, M.; Sotiropoulos, F. Performance and Wake Characterization of a Model Hydrokinetic Turbine: The Reference Model 1 (RM1) Dual Rotor Tidal Energy Converter. Energies 2020, 13, 5145. [Google Scholar] [CrossRef]
- Bergillos, R.J.; López-Ruiz, A.; Medina-López, E.; Moñino, A.; Ortega-Sánchez, M. The role of wave energy converter farms on coastal protection in eroding deltas, Guadalfeo, southern Spain. J. Clean. Prod. 2018, 12, 356–367. [Google Scholar] [CrossRef]
- Salunkhe, S.; El Fajri, O.; Bhushan, S.; Thompson, D.; O’Doherty, D.; O’Doherty, T.; Mason-Jones, A. Validation of Tidal Stream Turbine Wake Predictions and Analysis of Wake Recovery Mechanism. J. Mar. Sci. Eng. 2019, 7, 362. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, M.L.; Jordanm, L.-B.; McLelland, S.; Parsons, D.R.; Amoudry, L.O.; Song, Q.; Comerford, L. Modelling impacts of tidal stream turbines on surface waves. Renew. Energy 2019, 10, 725–734. [Google Scholar] [CrossRef]
- Soto-Rivas, K.; Richter, D.; Escauriaza, C. A formulation of the thrust coefficient for representing finite-sized farms of tidal energy converters. Energies 2019, 12, 3861. [Google Scholar] [CrossRef] [Green Version]
- Thiebot, J.; Guillou, N.; Guillou, S.; Good, A.; Lewis, M. Wake field study of tidal turbines under realistic flow conditions. Renew. Energy 2020, 151, 1196–1208. [Google Scholar] [CrossRef]
- Ahmed, U.; Apsley, D.D.; Afgan, I.; Stallard, T.; Stansby, P.K. Fluctuating loads on a tidal turbine due to velocity shear and turbulence: Comparison of CFD with field data. Renew. Energy 2017, 112, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, T.P.; Turnock, S.R.; Humphrey, V.F. Assessing the influence of inflow turbulence on noise and performance of a tidal turbine using large eddy simulations. Renew. Energy 2014, 71, 742–754. [Google Scholar] [CrossRef]
- Sufian, S.F.; Li, M.; O’Connor, B.A. 3D modelling of impacts from waves on tidal turbine wake characteristics and energy output. Renew. Energy 2017, 114, 308–322. [Google Scholar] [CrossRef] [Green Version]
- Churchfield, M.J.; Li, Y.; Moriarty, P.J. A large-eddy simulation study of wake propagation and power production in an array of tidal-current turbines. Philos. Trans. R. Soc. A 2013, 371, 20120421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adcock, T.A.; Draper, S.; Nishino, T. Tidal power generation—A review of hydrodynamic modelling. Proc. Inst. Mech. Eng. Part A J. Power Energy 2015, 229, 755–771. [Google Scholar] [CrossRef]
- Thiébot, J.; Guillou, S.; Nguyen, V.T. Modelling the effect of large arrays of tidal turbines with depth-averaged Actuator Disks. Ocean Eng. 2016, 126, 265–275. [Google Scholar] [CrossRef]
- Sjökvist, L.; Göteman, M.; Rahm, M.; Waters, R.; Svensson, O.; Strömstedt, E.; Leijon, M. Calculating buoy response for a wave energy converter—A comparison of two computational methods and experimental results. Theor. Appl. Mech. Lett. 2017, 7, 164–168. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Z. Three-dimensional CFD simulation of a circular OWC with a nonlinear power-takeoff: Model validation and a discussion on resonant sloshing inside the pneumatic chamber. Ocean Eng. 2019, 176, 184–198. [Google Scholar] [CrossRef]
- Ashall, L.M.; Mulligan, R.P.; Law, B.A. Variability in suspended sediment concentration in the Minas Basin, Bay of Fundy, and implications for changes due to tidal power extraction. Coast. Eng. 2016, 107, 102–115. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Copping, A.E. Modeling tidal stream energy extraction and its effects on transport processes in a tidal channel and bay system using a three-dimensional coastal ocean model. Renew. Energy 2013, 50, 605–613. [Google Scholar] [CrossRef]
- Ahmadian, R.; Falconer, R.; Bockelmann-Evans, B. Far-field modelling of the hydro-environmental impact of tidal stream turbines. Renew. Energy 2012, 38, 107–116. [Google Scholar] [CrossRef]
- Waldman, S.; Bastón, S.; Nemalidinne, R.; Chatzirodou, A.; Venugopal, V.; Side, J. Implementation of tidal turbines in MIKE 3 and Delft3D models of Pentland Firth & Orkney Waters. Ocean Coast. Manag. 2017, 147, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Chatzirodou, A.; Karunarathna, H.; Reeve, D.E. 3D modelling of the impacts of in-stream horizontal-axis Tidal Energy Converters (TECs) on offshore sandbank dynamics. Appl. Ocean Res. 2019, 21, 101882. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Beardsley, R.C. An unstructured grid, finite-volume, three-dimensional, primitive equations ocean model: Application to coastal ocean and estuaries. J. Atmos. Ocean. Technol. 2003, 20, 159–186. [Google Scholar] [CrossRef]
- Deltares. Simulation of Multi-Dimensional Hydrodynamic Flows and Transport Phenomena, Including Sediments, User Manual, Version 3.15; Deltares: Delft, The Netherlands, 2021; p. 702. [Google Scholar]
- DHI. Mike 3 Flow Model, Hydrodynamic Module, User Guide; DHI: Hørsholm, Denmark, 2017; p. 128. [Google Scholar]
- Hervouet, J.-M. TELEMAC modelling system: An overview. Hydrol. Processes 2000, 14, 2209–2210. [Google Scholar] [CrossRef]
- Piggott, M.; Gorman, G.; Pain, C.; Allison, P.; Candy, A.; Martin, B.; Wells, M. A new computational framework for multi-scale ocean modelling based on adapting unstructured meshes. Int. J. Numer. Methods Fluids 2008, 56, 1003–1015. [Google Scholar] [CrossRef]
- Gallego, J.; Side, J.; Baston, S.; Waldman, S.; Bell, M.; James, M.; Davies, I.; O’ Hara Murray, R.; Heath, M.; Sabatino, A.; et al. Large scale three-dimensional modeling for wave and tidal energy resource and environmental impact; Methodologies for quantifying acceptable thresholds for sustainable exploitation. Ocean Coast. Manag. 2017, 147, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.; Chang, G.; Raghukumar, K.; McWilliams, S. Spatial Environmental Assessment Tool (SEAT): A modeling tool to evaluate potential environmental risks associated with wave energy converter deployments. Energies 2018, 11, 2036. [Google Scholar] [CrossRef] [Green Version]
- Robins, P.E.; Neill, S.P.; Lewis, M.J. Impact of tidal-stream arrays in relation to the natural variability of sedimentary processes. Renew. Energy 2014, 72, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Haverson, D.; Bacon, J.; Smith, H.C.M.; Venugopal, V. Modelling the hydrodynamic and morphological impacts of a tidal stream development in Ramsey Sound. Renew. Energy 2018, 22, 876–887. [Google Scholar] [CrossRef]
- Martin-Short, R.; Hill, J.; Kramer, S.C.; Avdis, A.; Allison, P.A.; Piggott, M.D. Tidal resource extraction in the Pentland Firth, UK: Potential impacts on flow regime and sediment transport in the Inner Sound of Stroma. Renew. Energy 2015, 76, 596–607. [Google Scholar] [CrossRef] [Green Version]
- Beels, C.; Troch, P.; De Backer, G.; Vantorre, M.; De Rouck, J. Numerical implementation and sensitivity analysis of a wave energy converter in a time-dependent mild-slope equation model. Coast. Eng. 2010, 57, 471–492. [Google Scholar] [CrossRef]
- Stratigaki, V.; Troch, P.; Forehand, D. A fundamental coupling methodology for modeling near-field and far-field wave effects of floating structures and wave energy devices. Renew. Energy 2019, 143, 1608–1627. [Google Scholar] [CrossRef]
- Venugopal, V.; Nemalidinne, R.; Vögler, A. Numerical modelling of wave energy resources and assessment of wave energy extraction by large scale wave farms. Ocean Coast. Manag. 2017, 147, 37–48. [Google Scholar] [CrossRef]
- Penalba, M.; Kelly, T.; Ringwood, J. Using NEMOH for Modelling Wave Energy Converters: A Comparative Study with WAMIT. In Proceedings of the 12th European Wave and Tidal Energy Conference (EWTEC), Cork, Ireland, 1 September 2017. [Google Scholar]
- Verao Fernandez, G.; Stratigaki, V.; Troch, P. Irregular Wave Validation of a Coupling Methodology for Numerical Modelling of Near and Far Field Effects of Wave Energy Converter Arrays. Energies 2019, 12, 538. [Google Scholar] [CrossRef] [Green Version]
- Abanades, J.; Greaves, D.; Iglesias, G. Wave farm impact on the beach profile: A case study. Coast. Eng. 2014, 86, 36–44. [Google Scholar] [CrossRef]
- O’Dea, A.; Haller, M.C.; Özkan-Haller, H.T. The impact of wave energy converter arrays on wave-induced forcing in the surf zone. Ocean Eng. 2018, 161, 322–336. [Google Scholar] [CrossRef]
- Balitsky, P.; Quartier, N.; Stratigaki, V.; Verao Fernandez, G.; Vasarmidis, P.; Troch, P. Analysing the near-field effects and the power production of near-shore WEC array using a new wave-to-wire model. Water 2019, 11, 1137. [Google Scholar] [CrossRef] [Green Version]
- Babarit, A.; Delhommeau, G. Theoretical and numerical aspects of the open source BEM solver {NEMOH}. In Proceedings of the 11th EuropeanWave and Tidal Energy Conference, Nantes, France, 6–11 September 2015. [Google Scholar]
- Guillou, N.; Lavidas, G.; Chapalain, G. Wave Energy Resource Assessment for Exploitation—A Review. J. Mar. Sci. Eng. 2020, 8, 705. [Google Scholar] [CrossRef]
- Cavaleri, L.; Abdalla, S.; Benetazzo, A.; Bertotti, L.; Bidlot, J.R.; Breivik, Ø.; Carniel, S.; Jensen, R.E.; Portilla-Yandun, J.; Rogers, W.E.; et al. Wave modelling in coastal and inner seas. Prog. Oceanogr. 2018, 167, 164–233. [Google Scholar] [CrossRef]
- Kang, S.; Borazjani, I.; Colby, J.A.; Sotiropoulos, F. Numerical simulation of 3D flow past a real-life marine hydrokinetic turbine. Adv. Water Resour. 2012, 39, 33–43. [Google Scholar] [CrossRef]
- Contardo, S.; Hoeke, R.; Hemer, M.; Symonds, G.; McInnes, K.; O’Grady, J. In situ observations and simulations of coastal wave field transformation by wave energy converters. Coast. Eng. 2018, 140, 175–188. [Google Scholar] [CrossRef]
- Popper, A.N.; Hawkins, A. (Eds.) The Effects of Noise on Aquatic Life II; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Hastie, G.D.; Russell, D.J.F.; McConnell, B.; Moss, S.; Thompson, D.; Janik, V.M. Sound exposure in harbour seals during the installation of an offshore wind farm: Predictions of auditory damage. J. Appl. Ecol. 2015, 52, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Palmer, L.; Gillespie, D.; MacAulay, J.D.J.; Sparling, C.E.; Russell, D.J.F.; Hastie, G.D. Harbour porpoise (Phocoena phocoena) presence is reduced during tidal turbine operation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 3543–3553. [Google Scholar] [CrossRef]
- Onoufriou, J.; Russell, D.J.F.; Thompson, D.; Moss, S.E.; Hastie, G.D. Quantifying the effects of tidal turbine array operations on the distribution of marine mammals: Implications for collision risk. Renew. Energy 2021, 180, 157–165. [Google Scholar] [CrossRef]
- Nabe-Nielsen, J.; Sibly, R.M.; Tougaard, J.; Teilmann, J.; Sveegaard, S. Effects of noise and by-catch on a Danish harbour porpoise population. Ecol. Model. 2014, 272, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Pine, M.K.; Schmitt, P.; Culloch, R.M.; Lieber, L.; Kregting, L.T. Providing ecological context to anthropogenic subsea noise: Assessing listening space reductions of marine mammals from tidal energy devices. Renew. Sustain. Energy Rev. 2019, 103, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Etter, P.C. Underwater Acoustic Modeling and Simulation, 5th ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Urick, R. Principles of Underwater Sound, 3rd ed.; Peninsula Publishing: Westport, CT, USA, 1983. [Google Scholar]
- Bailey, H.; Senior, B.; Simmons, D.; Rusin, J.; Picken, G.; Thompson, P.M. Assessing underwater noise levels during pile-driving at an offshore windfarm and its potential effects on marine mammals. Mar. Pollut. Bull. 2010, 60, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Pine, M.K.; Jeffs, A.G.; Radford, C.A. The cumulative effect on sound levels from multiple underwater anthropogenic sound sources in shallow coastal waters. J. Appl. Ecol. 2014, 51, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Lippert, T.; Ainslie, M.A.; von Estorff, O. Pile driving acoustics made simple: Damped cylindrical spreading model. J. Acoust. Soc. Am. 2018, 143, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zampolli, M.; Nijhof, M.J.J.; Jong, C.A.F.d.; Ainslie, M.A.; Jansen, E.H.W.; Quesson, B.A.J. Validation of finite element computations for the quantitative prediction of underwater noise from impact pile driving. J. Acoust. Soc. Am. 2013, 133, 72–81. [Google Scholar] [CrossRef]
- Robertson, F.; Wood, J.; Joslin, J.; Joy, R.; Polagye, B. Marine Mammal Behavioral Response to Tidal Turbine Sound, Final Technical Report for DE-EE0006385; University of Washington: Seattle, WA, USA, 2018. [Google Scholar]
- Talisman. Beatrice Wind Farm Demonstrator Project: Environmental Statement; D/2875/2005; Talisman Energy (UK) Limited: Aberdeen, Scotland, 2005. [Google Scholar]
- Middel, H.; Verones, F. Making marine noise pollution impacts heard: The case of cetaceans in the North Sea within life cycle impact assessment. Sustainability 2017, 9, 1138. [Google Scholar] [CrossRef] [Green Version]
- Ainslie, M.A.; Halvorsen, M.B.; Muller, R.A.J.; Lippert, T. Application of damped cylindrical spreading to assess range to injury threshold for fishes from impact pile driving. J. Acoust. Soc. Am. 2020, 148, 108–121. [Google Scholar] [CrossRef]
- Richardson, W.; Thomson, D. Marine Mammals and Noise; Gulf Professional Publishing: Ontario, QC, Canada, 1995. [Google Scholar]
- Lippert, T.; von Estorff, O. The significance of parameter uncertainties for the prediction of offshore pile driving noise. J. Acoust. Soc. Am. 2014, 136, 2463–2471. [Google Scholar] [CrossRef]
- Farcas, A.; Thompson, P.M.; Merchant, N.D. Underwater noise modelling for environmental impact assessment. Environ. Impact Assess. Rev. 2016, 57, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Marmo, B.; Roberts, I.; Buckingham, M.P.; King, S.; Booth, C. Modelling of Noise Effects of Operational Offshore Wind Turbines Including Noise Transmission through Various Foundation Types; Scottish Government: Edinburgh, UK, 2013.
- Ikpekha, O.; Soberón, F.; Daniels, S. Modelling the propagation of underwater acoustic signals of a marine energy device using finite element method. Renew. Energy Power Qual. J. 2014, 12, 97–102. [Google Scholar] [CrossRef]
- Kim, H.; Miller, J.; Potty, G. Predicting underwater radiated noise levels due to the first offshore wind turbine installation in the United States. J. Acoust. Soc. Am. 2013, 133, 3419. [Google Scholar] [CrossRef]
- Hafla, E.; Johnson, E.; Johnson, C.N.; Preston, L.; Aldridge, D.; Roberts, J.D. Modeling underwater noise propagation from marine hydrokinetic power devices through a time-domain, velocity-pressure solution. J. Acoust. Soc. Am. 2018, 143, 3242–3253. [Google Scholar] [CrossRef] [PubMed]
- Etter, P.C. Review of ocean-acoustic models. In Proceedings of the OCEANS, Biloxi, MS, USA, 26–29 October 2009; pp. 1–6. [Google Scholar]
- Jensen, F.; Kuperman, W.; Porter, M.; Schmidt, H. Computational Ocean Acoustics, 2nd ed.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, T.; Humphrey, V.; Turnock, S. Noise modelling of tidal turbine arrays for environmental impact assessment. In Proceedings of the 9th European Wave and Tidal Energy Conference, Southampton, UK, 5–9 September 2011. [Google Scholar]
- Maggi, A.; Duncan, A. AcTUP v 2.2l Acoustic Toolbox; Center for Marine Science and Technology, Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- van der Molen, J.; Smith, H.C.M.; Lepper, P.; Limpenny, S.; Rees, J. Predicting the large-scale consequences of offshore wind turbine array development on a North Sea ecosystem. Cont. Shelf Res. 2014, 85, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-T.; Newhall, A.E.; Miller, J.H.; Potty, G.R.; Vigness-Raposa, K.J. A three-dimensional underwater sound propagation model for offshore wind farm noise prediction. J. Acoust. Soc. Am. 2019, 145, EL335–EL340. [Google Scholar] [CrossRef]
- Rossington, K.; Benson, T.; Lepper, P.; Jones, D. Eco-hydro-acoustic modeling and its use as an EIA tool. Mar. Pollut. Bull. 2013, 75, 235–243. [Google Scholar] [CrossRef]
- Whyte, K.F.; Russell, D.J.F.; Sparling, C.E.; Binnerts, B.; Hastie, G.D. Estimating the effects of pile driving sounds on seals: Pitfalls and possibilities. J. Acoust. Soc. Am. 2020, 147, 3948–3958. [Google Scholar] [CrossRef]
- Tetra Tech. Underwater Acoustic Modeling Report: Virginia Offshore Wind Technology Advancement Project (VOWTAP); Tetra Tech: Glen Allen, VA, USA, 2013. [Google Scholar]
- Southall, B.; Bowles, A.; Ellison, W.; Finneran, J.; Gentry, R.; Greene, C.J.; Kastak, D.; Ketten, D.; Miller, J.; Nachtigall, P.; et al. Marine mammal noise exposure criteria: Initial scientific recommendations. Aquat. Mamm. 2007, 33, 411–521. [Google Scholar] [CrossRef]
- Southall, B.L.; Finneran, J.J.; Rcichmuth, C.; Nachtigall, P.E.; Ketten, D.R.; Bowles, A.E.; Ellison, W.T.; Nowacek, D.P.; Tyack, P.L. Marine mammal noise exposure criteria: Updated scientific recommendations for residual hearing effects. Aquat. Mamm. 2019, 45, 125–232. [Google Scholar] [CrossRef]
- Nedelec, S.L.; Campbell, J.; Radford, A.N.; Simpson, S.D.; Merchant, N.D. Particle motion: The missing link in underwater acoustic ecology. Methods Ecol. Evol. 2016, 7, 836–842. [Google Scholar] [CrossRef] [Green Version]
- Donovan, C.R.; Harris, C.M.; Milazzo, L.; Harwood, J.; Marshall, L.; Williams, R. A simulation approach to assessing environmental risk of sound exposure to marine mammals. Ecol. Evol. 2017, 7, 2101–2111. [Google Scholar] [CrossRef] [Green Version]
- New, L.F.; Clark, J.S.; Costa, D.P.; Fleishman, E.; Hindell, M.A.; Klanjšček, T.; Lusseau, D.; Kraus, S.; McMahon, C.R.; Robinson, P.W.; et al. Using short-term measures of behaviour to estimate long-term fitness of southern elephant seals. Mar. Ecol. Prog. Ser. 2014, 496, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Harwood, J.; King, S.; Schick, R.S.; Donovan, C.R.; Booth, C. A protocol for implementing the Interim Population Consequences of Disturbance (PCoD) approach: Quantifying and assessing the effects of UK offshore renewable energy developments on marine mammal populations. Scott. Mar. Freshw. Sci. 2014, 5, 97. [Google Scholar]
- Pirotta, E.; Booth, C.G.; Costa, D.P.; Fleishman, E.; Kraus, S.D.; Lusseau, D.; Moretti, D.; New, L.F.; Schick, R.S.; Schwarz, L.K.; et al. Understanding the population consequences of disturbance. Ecol. Evol. 2018, 8, 9934–9946. [Google Scholar] [CrossRef]
- King, S.L.; Schick, R.S.; Donovan, C.; Booth, C.G.; Burgman, M.; Thomas, L.; Harwood, J. An interim framework for assessing the population consequences of disturbance. Methods Ecol. Evol. 2015, 6, 1150–1158. [Google Scholar] [CrossRef]
- Nabe-Nielsen, J.; van Beest, F.M.; Grimm, V.; Sibly, R.M.; Teilmann, J.; Thompson, P.M. Predicting the impacts of anthropogenic disturbances on marine populations. Conserv. Lett. 2018, 11, e12563. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.M.; Hastie, G.D.; Nedwell, J.; Barham, R.; Brookes, K.L.; Cordes, L.S.; Bailey, H.; McLean, N. Framework for assessing impacts of pile-driving noise from offshore wind farm construction on a harbour seal population. Environ. Impact Assess. Rev. 2013, 43, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Harwood, J.; Booth, C.; Sinclair, R.R.; Hague, E. Developing marine mammal Dynamic Energy Budget models and their potential for integration into the iPCoD framework. Scott. Mar. Freshw. Sci. 2020, 11, 74. [Google Scholar] [CrossRef]
- Booth, C.G.; Sinclair, R.R.; Harwood, J. Methods for monitoring for the population consequences of disturbance in marine mammals: A review. Front. Mar. Sci. 2020, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Risch, D.; van Geel, N.; Gillespie, D.; Wilson, B. Characterisation of underwater operational sound of a tidal stream turbine. J. Acoust. Soc. Am. 2020, 147, 2547–2555. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, P.; Pine, M.K.; Culloch, R.M.; Lieber, L.; Kregting, L.T. Noise characterization of a subsea tidal kite. J. Acoust. Soc. Am. 2018, 144, El441. [Google Scholar] [CrossRef]
- Buscaino, G.; Mattiazzo, G.; Sannino, G.; Papale, E.; Bracco, G.; Grammauta, R.; Carillo, A.; Kenny, J.M.; De Cristofaro, N.; Ceraulo, M.; et al. Acoustic impact of a wave energy converter in Mediterranean shallow waters. Sci. Rep. 2019, 9, 9586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippert, S.; Nijhof, M.; Lippert, T.; Wilkes, D.; Gavrilov, A.; Heitmann, K.; Ruhnau, M.; Estorff, O.v.; Schäfke, A.; Schäfer, I.; et al. COMPILE—A generic benchmark case for predictions of marine pile-driving noise. IEEE J. Ocean. Eng. 2016, 41, 1061–1071. [Google Scholar] [CrossRef]
- van Beest, F.M.; Nabe-Nielsen, J.; Carstensen, J.; Teilmann, J.; Tougaard, J. Disturbance Effects on the Harbour Porpoise Population in the North Sea (DEPONS): Status Report on Model Development; Aarhus University, DCE—Danish Centre for Environment and Energy: Aarhus, Denmark, 2015; p. 43. [Google Scholar]
- Alexander, K.A.; Meyjes, S.A.; Heymans, J.J. Spatial ecosystem modelling of marine renewable energy installations: Gauging the utility of Ecospace. Ecol. Model. 2016, 331, 115–128. [Google Scholar] [CrossRef]
- Albert, L.; Deschamps, F.; Jolivet, A.; Olivier, F.; Chauvaud, L.; Chauvaud, S. A current synthesis on the effects of electric and magnetic fields emitted by submarine power cables on invertebrates. Mar. Environ. Res. 2020, 159, 104958. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Yang, K.-L. Using medaka embryos as a model system to study biological effects of the electromagnetic fields on development and behavior. Ecotoxicol. Environ. Saf. 2014, 108, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Harsanyi, P.; Lyndon, A.R. Understanding the effects of electromagnetic field emissions from Marine Renewable Energy Devices (MREDs) on the commercially important edible crab, Cancer pagurus (L.). Front. Mar. Sci. 2018, 131, 580–588. [Google Scholar] [CrossRef]
- Gill, A.; Gloyne-Philips, I.; Kimber, J.; Sigray, P. Marine renewable energy, electromagnetic (EM) fields and EM-sensitive animals. In Marine Renewable Energy Technology and Environmental Interactions; Shields, M., Payne, A., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Slater, M.; Schultz, A.; Jones, R.; Fischer, C. Electromagnetic Field Study; Oregon Wave Energy Trust: Portland, OR, USA, 2010; 346p.
- Lucca, G. Analytical evaluation of sub-sea ELF electromagnetic field generated by submarine power cables. Prog. Electromagn. Res. B 2013, 56, 309–326. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Gloyne-Philips, I. Electromagnetic Simulation of 135 kV Three-Phase Submarine Power Cables; CMACS (Centre for Marine and Coastal Studies Ltd.): Liverpool, England, 2005.
- Dhanak, M.; Coulson, R.; Dibiasio, C.; Frankenfield, J.; Henderson, E.; Pugsley, D.; Valdes, G. Assessment of electromagnetic field emissions from subsea cables. In Proceedings of the 4th Marine Energy Technology Symposium (METS), Washington, DC, USA, 25–27 April 2016. [Google Scholar]
- Kavet, R.; Wyman, M.T.; Klimley, A.P. Modeling magnetic fields from a DC power cable buried beneath San Francisco Bay based on empirical measurements. PLoS ONE 2016, 11, e0148543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, A.B.; Huang, Y.; Spencer, J.; Gloyne-Philips, I. Electromagnetic fields emitted by high voltage alternating current offshore wind power cables and interactions with marine organisms. In Proceedings of the Electromagnetics in Current and Emerging Energy Power Systems Seminar, London, UK, 17–23 June 2013. [Google Scholar]
- Hutchison, Z.L.; Gill, A.B.; Sigray, P.; He, H.; King, J.W. Anthropogenic electromagnetic fields (EMF) influence the behaviour of bottom-dwelling marine species. Sci. Rep. 2020, 10, 4219. [Google Scholar] [CrossRef] [Green Version]
- Dhanak, M.; An, E.; Coulson, R.; Frankenfield, J.; Ravenna, S.; Pugsley, D.; Valdes, G.; Venezia, W. AUV-based characterization of EMF emissions from submerged power cables. In Proceedings of the OCEANS 2015, Genova, Italy, 18–21 May 2015; pp. 1–6. [Google Scholar]
- Thomsen, F.; Gill, A.B.; Kosecka, M.; Andersson, M.; André, M.; Degraer, S.; Folegot, T.; Gabriel, J.; Judd, A.; Neumann, T.; et al. MaRVEN—Environmental Impacts of Noise, Vibrations and Electromagnetic Emissions from Marine Renewable Energy; European Commission: Brussels, Belgium, 2016. [Google Scholar] [CrossRef]
- Norberg, A.; Abrego, N.; Blanchet, F.G.; Adler, F.R.; Anderson, B.J.; Anttila, J.; Araújo, M.B.; Dallas, T.; Dunson, D.; Elith, J.; et al. A comprehensive evaluation of predictive performance of 33 species distribution models at species and community levels. Ecol. Monogr. 2019, 89, e01370. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Scherelis, C.; Penesis, I.; Hemer, M.A.; Cossu, R.; Wright, J.T.; Guihen, D. Investigating biophysical linkages at tidal energy candidate sites: A case study for combining environmental assessment and resource characterisation. Renew. Energy 2020, 159, 399–413. [Google Scholar] [CrossRef]
- Linder, H.L.; Horne, J.K. Evaluating statistical models to measure environmental change: A tidal turbine case study. Ecol. Indic. 2018, 84, 765–792. [Google Scholar] [CrossRef]
- Linder, H.L.; Horne, J.K.; Ward, E.J. Modeling baseline conditions of ecological indicators: Marine renewable energy environmental monitoring. Ecol. Indic. 2017, 83, 178–191. [Google Scholar] [CrossRef]
- Warren, D.L.; Matzke, N.J.; Iglesias, T.L. Evaluating presence-only species distribution models with discrimination accuracy is uninformative for many applications. J. Biogeogr. 2020, 47, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Kingsford, C.; Salzberg, S.L. What are decision trees? Nat. Biotechnol. 2008, 26, 1011–1013. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards , T.C., Jr.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- du Feu, R.J.; Funke, S.W.; Kramer, S.C.; Hill, J.; Piggott, M.D. The trade-off between tidal-turbine array yield and environmental impact: A habitat suitability modelling approach. Renew. Energy 2019, 143, 390––403. [Google Scholar] [CrossRef]
- Baker, A.L.; Craighead, R.M.; Jarvis, E.J.; Stenton, H.C.; Angeloudis, A.; Mackie, L.; Avdis, A.; Piggott, M.D.; Hill, J. Modelling the impact of tidal range energy on species communities. Ocean Coast. Manag. 2020, 193, 105221. [Google Scholar] [CrossRef]
- Lieber, L.; Nimmo-Smith, W.A.M.; Waggitt, J.J.; Kregting, L. Localised anthropogenic wake generates a predictable foraging hotspot for top predators. Commun. Biol. 2019, 2, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymans, J.J.; Coll, M.; Link, J.S.; Mackinson, S.; Steenbeek, J.; Walters, C.; Christensen, V. Best practice in Ecopath with Ecosim food-web models for ecosystem-based management. Ecol. Model. 2016, 331, 173–184. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Raoux, A.; Tecchio, S.; Pezy, J.-P.; Lassalle, G.; Degraer, S.; Wilhelmsson, D.; Cachera, M.; Ernande, B.; Le Guen, C.; Haraldsson, M.; et al. Benthic and fish aggregation inside an offshore wind farm: Which effects on the trophic web functioning? Ecol. Indic. 2017, 72, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Schuchert, P.; Kregting, L.; Pritchard, D.; Savidge, G.; Elsäßer, B. Using coupled hydrodynamic biogeochemical models to predict the effects of tidal turbine arrays on phytoplankton dynamics. J. Mar. Sci. Eng. 2018, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- van der Molen, J.; Garcia-Garcia, L.M.; Whomersley, P.; Callaway, A.; Posen, P.E.; Hyder, K. Connectivity of larval stages of sedentary marine communities between hard substrates and offshore structures in the North Sea. Sci. Rep. 2018, 8, 14772. [Google Scholar] [CrossRef] [Green Version]
- Bray, L.; Kassis, D.; Hall-Spencer, J.M. Assessing larval connectivity for marine spatial planning in the Adriatic. Mar. Environ. Res. 2017, 125, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Adams, T.P.; Miller, R.G.; Aleynik, D.; Burrows, M.T.; Frederiksen, M. Offshore marine renewable energy devices as stepping stones across biogeographical boundaries. J. Appl. Ecol. 2014, 51, 330–338. [Google Scholar] [CrossRef]
- Ross, R.E.; Nimmo-Smith, W.A.M.; Torres, R.; Howell, K.L. Comparing deep-sea larval dispersal models: A cautionary tale for ecology and conservation. Front. Mar. Sci. 2020, 7, 431. [Google Scholar] [CrossRef]
- Kuhn, M.; Johnson, K. Applied Predictive Modeling; Springer: New York, NY, USA, 2013. [Google Scholar]
- Winship, A.J.; Thorson, J.T.; Clarke, M.E.; Coleman, H.M.; Costa, B.; Georgian, S.E.; Gillett, D.; Grüss, A.; Henderson, M.J.; Hourigan, T.F.; et al. Good practices for species distribution modeling of deep-sea corals and sponges for resource management: Data collection, analysis, validation, and communication. Front. Mar. Sci. 2020, 7, 303. [Google Scholar] [CrossRef]
- Guillaumot, C.; Artois, J.; Saucde, T.; Demoustier, L.; Moreau, C.; Elaume, M.; Agera, A.; Danis, B. Broad-scale species distribution models applied to data-poor areas. Prog. Oceanogr. 2019, 175, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Sparling, C.E.; Seitz, A.C.; Masden, E.; Smith, K. Collision risk for animals around turbines. In OES-Environmental 2020 State of the Science Report: Environmental Effects of Marine Renewable Energy Development Around the World; Copping, A.E., Hemery, L.G., Eds.; Ocean Energy Systems: Seattle, WA, USA, 2020; pp. 29–65. [Google Scholar] [CrossRef]
- Wilson, B.; Batty, R.S.; Daunt, F.; Carter, C. Collision Risks between Marine Renewable Energy Devices and Mammals, Fish and Diving Birds; Report to the Scottish Executive; Scottish Association for Marine Science: Oban, Scotland, 2007. [Google Scholar]
- ABP Marine Environmental Research Ltd. Collision Risk of Fish with Wave and Tidal Devices (R.1516); Report by ABP Marine Environmental Research Ltd. for Welsh Assembly Government: Southampton, Hampshire, 2010.
- Chamberlain, D.; Rehfisch, M.; Fox, A.; Desholm, M.; Anthony, S. The effect of avoidance rates on bird mortality predictions made by wind turbine collision risk models. Ibis 2006, 148, 198–202. [Google Scholar] [CrossRef]
- Masden, E.A.; Cook, A.S.C.P. Avian collision risk models for wind energy impact assessments. Environ. Impact Assess. Rev. 2016, 56, 43–49. [Google Scholar] [CrossRef]
- Horne, N.; Culloch, R.M.; Schmitt, P.; Lieber, L.; Wilson, B.; Dale, A.C.; Houghton, J.D.R.; Kregting, L.T. Collision risk modelling for tidal energy devices: A flexible simulation-based approach. J. Environ. Manag. 2021, 278, 111484. [Google Scholar] [CrossRef]
- Band, B. Using a Collison Risk Model to Assess Bird Collision Risks for Offshore Windfarms; The Crown Estate Strategic Ornithological Support Services (SOSS) Report SOSS-02: Thetford, UK, 2012.
- Band, B. Assessing Collision Risk between Underwater Turbines and Marine Wildlife; Scottish Natural Heritage guidance note; Scottish National Heritage: Inverness, Scotland, 2016.
- Thompson, D.; Onoufriou, J.; Brownlow, A.; Morris, C. Data Based Estimates of Collision Risk: An Example Based on Harbour Seal Tracking Data Around a Proposed Tidal Turbine Array in Pentland Firth; Scottish Natural Heritage Commissioned Report No. 900: Inverness, Scotland, 2016.
- Joy, R.; Wood, J.D.; Sparling, C.E.; Tollit, D.J.; Copping, A.E.; McConnell, B.J. Empirical measures of harbor seal behavior and avoidance of an operational tidal turbine. Mar. Pollut. Bull. 2018, 136, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Joy, R.; Sparling, C. Harbor Seal—Tidal Turbine Collision Risk Models. An Assessment of Sensitivities; SMRU Consulting: Friday Harbor, WA, USA, 2016. [Google Scholar]
- Copping, A.; Grear, M. Applying a simple model for estimating the likelihood of colllision of marine mammals with tidal turbines. Int. Mar. Energy J. 2018, 1, 27–33. [Google Scholar] [CrossRef]
- Bevelhimer, M.; Colby, J.A.; Adonizio, M.A.; Tomichek, C.; Scherelis, C. Informing a Tidal Turbine Strike Probability Model through Characterization of Fish Behavioral Response Using Multibeam Sonar Output; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016.
- Hammar, L.; Eggertsen, L.; Andersson, S.; Ehnberg, J.; Arvidsson, R.; Gullstrom, M.; Molander, S. A probabilistic model for hydrokinetic turbine collision risks: Exploring impacts on fish. PLoS ONE 2015, 10, e0117756. [Google Scholar] [CrossRef] [Green Version]
- Xodus Group. Collision Risk Modelling—Atlantic Salmon; Brims Tidal Array Ltd.: London, UK, 2016. [Google Scholar]
- Schmitt, P.; Culloch, R.; Lieber, L.; Molander, S.; Hammar, L.; Kregting, L. A tool for simulating collision probabilities of animals with marine renewable energy devices. PLoS ONE 2017, 12, e0188780. [Google Scholar] [CrossRef] [Green Version]
- Rossington, K.; Benson, T. An agent-based model to predict fish collisions with tidal stream turbines. Renew. Energy 2020, 151, 1220–1229. [Google Scholar] [CrossRef]
- Grant, M.C.; Trinder, M.; Harding, N.J. A Diving Bird Collision Risk Assessment Framework for Tidal Turbines. Scottish Natural Heritage Commissioned Report No. 773: Inverness, Scotland, 2014. [Google Scholar]
- Onoufriou, J.; Brownlow, A.; Moss, S.; Hastie, G.; Thompson, D. Empirical determination of severe trauma in seals from collisions with tidal turbine blades. J. Appl. Ecol. 2019, 56, 1712–1724. [Google Scholar] [CrossRef]
- Sparling, C.; Lonergan, M.; McConnell, B. Harbour seals (Phoca vitulina) around an operational tidal turbine in Strangford Narrows: No barrier effect but small changes in transit behaviour. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Rothermel, E.R.; Balazik, M.T.; Best, J.E.; Breece, M.W.; Fox, D.A.; Gahagan, B.I.; Haulsee, D.E.; Higgs, A.L.; O’Brien, M.H.P.; Oliver, M.J.; et al. Comparative migration ecology of striped bass and Atlantic sturgeon in the US Southern mid-Atlantic bight flyway. PLoS ONE 2020, 15, e0234442. [Google Scholar] [CrossRef]
- Braithwaite, J.E.; Meeuwig, J.J.; Hipsey, M.R. Optimal migration energetics of humpback whales and the implications of disturbance. Conserv. Physiol. 2015, 3, cov001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hin, V.; Harwood, J.; de Roos, A.M. Bio-energetic modeling of medium-sized cetaceans shows high sensitivity to disturbance in seasons of low resource supply. Ecol. Appl. 2019, 29, e01903. [Google Scholar] [CrossRef]
- Grippo, M.; Shen, H.; Zydlewski, G.; Rao, S.; Goodwin, A. Behavioral Responses of Fish to a Current-Based Hydrokinetic Turbine under Multiple Operational Conditions: Final Report; ANL/EVS-17/6; Argonne National Laboratory: Argonne, IL, USA, 2017.
- Croft, T.N.; Masters, I.; Lake, T. Methods for individual based modelling of harbour porpoise. In Proceedings of the 10th European Wave and Tidal Energy Conference, Aalborg, Denmark, 5 September 2013. [Google Scholar]
- Lake, T. Computational Modelling of Interactions of Marine Mammals and Tidal Stream Turbines. Ph.D. Thesis, Swansea University, Swansea, UK, 2017. [Google Scholar]
- Lake, T.; Masters, I.; Croft, T.N. Simulating harbour porpoise habitat use in a 3D tidal environment. In Proceedings of the 11th European Wave and Tidal Energy Conference, Nantes, France, 6–11 September–1 September 2015. [Google Scholar]
- Lake, T.; Masters, I.; Croft, T.N. Algorithms for marine mammal modelling and an application in Ramsey Sound. In Proceedings of the 12th European Wave and Tidal Energy Conference, Cork, Ireland, 27 August 2017. [Google Scholar]
- Waggitt, J.J.; Cazenave, P.W.; Torres, R.; Williamson, B.J.; Scott, B.E.; Siriwardena, G. Quantifying pursuit-diving seabirds’ associations with fine-scale physical features in tidal stream environments. J. Appl. Ecol. 2016, 53, 1653–1666. [Google Scholar] [CrossRef] [Green Version]
- Gilles, A.; Viquerat, S.; Becker, E.A.; Forney, K.A.; Geelhoed, S.C.V.; Haelters, J.; Nabe-Nielsen, J.; Scheidat, M.; Siebert, U.; Sveegaard, S.; et al. Seasonal habitat-based density models for a marine top predator, the harbor porpoise, in a dynamic environment. Ecosphere 2016, 7, e01367. [Google Scholar] [CrossRef]
- Copping, A.E.; Hemery, L.G.; Viehman, H.; Seitz, A.C.; Staines, G.J.; Hasselman, D.J. Are fish in danger? A review of environmental effects of marine renewable energy on fishes. Biol. Conserv. 2021, 262, 109297. [Google Scholar] [CrossRef]
- Jolliff, J.K.; Kindle, J.C.; Shulman, I.; Penta, B.; Friedrichs, M.A.M.; Helber, R.; Arnone, R.A. Summary diagrams for coupled hydrodynamic-ecosystem model skill assessment. J. Mar. Syst. 2009, 76, 64–82. [Google Scholar] [CrossRef]
- Rose, K.A.; Roth, B.M.; Smith, E.P. Skill assessment of spatial maps for oceanographic modeling. J. Mar. Syst. 2009, 76, 34–48. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Page, H.; Simons, R.; Zaleski, S.; Miller, R.; Dugan, J.; Schroeder, D.; Doheny, B.; Goddard, J. Distribution and potential larval connectivity of the non-native Watersipora (Bryozoa) among harbors, offshore oil platforms, and natural reefs. Aquat. Invasions 2019, 14, 615–637. [Google Scholar] [CrossRef]
- Yates, K.L.; Bouchet, P.J.; Caley, M.J.; Mengersen, K.; Randin, C.F.; Parnell, S.; Fielding, A.H.; Bamford, A.J.; Ban, S.; Barbosa, A.M.; et al. Outstanding Challenges in the Transferability of Ecological Models. Trends Ecol. Evol. 2018, 33, 790–802. [Google Scholar] [CrossRef] [Green Version]
- Mannocci, L.; Roberts, J.J.; Pedersen, E.J.; Halpin, P.N. Geographical differences in habitat relationships of cetaceans across an ocean basin. Ecography 2020, 43, 1250–1259. [Google Scholar] [CrossRef]
- Peron, C.; Authier, M.; Gremillet, D. Testing the transferability of track-based habitat models for sound marine spatial planning. Divers. Distrib. 2018, 24, 1772–1787. [Google Scholar] [CrossRef]
- Goodwin, R.A.; Nestler, J.M.; Anderson, J.J.; Weber, L.J.; Loucks, D.P. Forecasting 3-D fish movement behavior using a Eulerian–Lagrangian–agent method (ELAM). Ecol. Model. 2006, 192, 197–223. [Google Scholar] [CrossRef]
- Posen, P.E.; Hyder, K.; Alves, M.T.; Taylor, N.G.H.; Lynam, C.P. Evaluating differences in marine spatial data resolution and robustness: A North Sea case study. Ocean Coast. Manag. 2020, 192, 105206. [Google Scholar] [CrossRef]
- Scales, K.L.; Hazen, E.L.; Jacox, M.G.; Edwards, C.A.; Boustany, A.M.; Oliver, M.J.; Bograd, S.J. Scale of inference: On the sensitivity of habitat models for wide-ranging marine predators to the resolution of environmental data. Ecography 2017, 40, 210–220. [Google Scholar] [CrossRef]
- Williamson, B.J.; Fraser, S.; Blondel, P.; Bell, P.S.; Waggitt, J.J.; Scott, B.E. Multisensor Acoustic Tracking of Fish and Seabird Behavior Around Tidal Turbine Structures in Scotland. IEEE J. Ocean. Eng. 2017, 42, 948–965. [Google Scholar] [CrossRef] [Green Version]
- Fraser, S.; Nikora, V.; Williamson, B.J.; Scott, B.E. Automatic active acoustic target detection in turbulent aquatic environments. Limnol. Oceanogr. Methods 2017, 15, 184–199. [Google Scholar] [CrossRef] [Green Version]
- Brownscombe, J.W.; Lédée, E.J.I.; Raby, G.D.; Struthers, D.P.; Gutowsky, L.F.G.; Nguyen, V.M.; Young, N.; Stokesbury, M.J.W.; Holbrook, C.M.; Brenden, T.O.; et al. Conducting and interpreting fish telemetry studies: Considerations for researchers and resource managers. Rev. Fish Biol. Fish. 2019, 29, 369–400. [Google Scholar] [CrossRef]
- Staines, G.; Deng, Z.; Li, X.; Martinez, J.; Kohn, N.; Harker-Klimeŝ, G. Using acoustic telemetry for high-resolution sablefish movement informing potential interactions with a tidal turbine. In Proceedings of the OCEANS 2019 MTS/IEEE SEATTLE, Seattle, WA, USA, 27–31 October 2019; pp. 1–5. [Google Scholar]
- Lennox, R.J.; Engler-Palma, C.; Kowarski, K.; Filous, A.; Whitlock, R.; Cooke, S.J.; Auger-Méthé, M. Optimizing marine spatial plans with animal tracking data. Aquat. Sci. 2019, 76, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Pendleton, D.E.; Holmes, E.E.; Redfern, J.; Zhang, J.L. Using modelled prey to predict the distribution of a highly mobile marine mammal. Divers. Distrib. 2020, 26, 1612–1626. [Google Scholar] [CrossRef]
- Carter, M.I.D.; Russell, D.J.F.; Embling, C.B.; Blight, C.J.; Thompson, D.; Hosegood, P.J.; Bennett, K.A. Intrinsic and extrinsic factors drive ontogeny of early-life at-sea behaviour in a marine top predator. Sci. Rep. 2017, 7, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.A.; Lewis, S.; Gonzalez-Solis, J.; Daunt, F. Causes and consequences of individual variability and specialization in foraging and migration strategies of seabirds. Mar. Ecol. Prog. Ser. 2017, 578, 117–150. [Google Scholar] [CrossRef] [Green Version]
- Wearmouth, V.J.; Sims, D.W. Sexual segregation in marine fish, reptiles, birds and mammals: Behaviour patterns, mechanisms and conservation applications. In Advances in Marine Biology; Sims, D.W., Ed.; Academic Press: Cambridge, MA, USA, 2008; Volume 54, pp. 107–170. [Google Scholar]
- Thomas, L.; Russell, D.J.F.; Duck, C.D.; Morris, C.D.; Lonergan, M.; Empacher, F.; Thompson, D.; Harwood, J. Modelling the population size and dynamics of the British grey seal. Aquat. Conserv. -Mar. Freshw. Ecosyst. 2019, 29, 6–23. [Google Scholar] [CrossRef]
- Robinson, N.M.; Nelson, W.A.; Costello, M.J.; Sutherland, J.E.; Lundquist, C.J. A systematic review of marine-based species distribution models (SDMs) with recommendations for best practice. Front. Mar. Sci. 2017, 4, 421. [Google Scholar] [CrossRef] [Green Version]
- Zucchetta, M.; Venier, C.; Taji, M.A.; Mangin, A.; Pastres, R. Modelling the spatial distribution of the seagrass Posidonia oceanica along the North African coast: Implications for the assessment of Good Environmental Status. Ecol. Indic. 2016, 61, 1011–1023. [Google Scholar] [CrossRef]
- Lynch, D.R.; McGillicuddy, D.J.; Werner, F.E. Skill assessment for coupled biological/physical models of marine systems. J. Mar. Syst. 2009, 76, 1–3. [Google Scholar] [CrossRef]
| Name | Type | Scale | Depth | Diffraction | Explicit Model of Device | Coupled with (in Reviewed Studies) |
|---|---|---|---|---|---|---|
| WAMIT | Boundary element method | Nearfield | Constant | Yes | Yes | MILDwave |
| NEMOH | Boundary element method | Nearfield | Constant | Yes | Yes | MILDwave |
| MILDwave | Wave propagation, time domain | Farfield | Deep to shallow, mild slope | Yes | Yes | WAMIT, NEMOH |
| SWAN | Spectral wave action | Farfield | Deep to shallow | Approximated | No | Delft3D |
| MIKE21 SW | Spectral wave action | Farfield | Deep to shallow | Approximated | No | MIKE 3 |
| Model Type | Shallow Water | Deep Water | Published Marine Energy Applications and Software Used | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low Frequency | High Frequency | Low Frequency | High Frequency | ||||||
| RI | RD | RI | RD | RI | RD | RI | RD | ||
| Fast-field/wavenumber integration | ++ | + | ++ | + | ++ | + | + | + | Lloyd et al. [102] SCOOTER |
| Parabolic equation | + | ++ | + | ++ | + | + | Pine et al. [82] RAMGeo | ||
| Ray/Gaussian beam tracing | + | ++ | + | + | ++ | ++ | Pine et al. [82] Bellhop | ||
| Normal mode | ++ | + | ++ | + | ++ | + | + | ||
| Multipath expansion | + | + | + | + | ++ | + | |||
| Device | Morphology/Sediment | Water | Organism Abundance/Distribution | Animal Behavior | Physiology and Vital Rates | Other | |
|---|---|---|---|---|---|---|---|
| Changes in oceanographic systems | |||||||
| Hydrodynamic models | Device geometry or parameters for approximation | Bathymetry, sediment type, and material properties, bottom friction | Current velocity, tides, WSE, temperature, salinity, river discharge | Wind, (precipitation, air temperature) | |||
| Wave propagation | Device geometry or parameters for approximation | Bathymetry | WSE, incoming waves, current velocity, tides | Wind, air-sea temperature difference | |||
| Underwater noise | |||||||
| Transmission loss | Source sound level | Depth, (material properties of sediment) | Temperature, salinity | (Recorded sound at distance from the source) | |||
| Nearfield propagation | Device geometry | Bathymetry, sediment type, and material properties, bottom roughness | WSE, temperature, salinity, surface roughness | ||||
| Farfield propagation | Source sound level | Bathymetry; sediment type (by layer), roughness, material properties | WSE, temperature, salinity, surface roughness | ||||
| Species effects | Sound level maps | Species distribution, prey distribution | Swimming, diving, noise response, dispersal, migration | Audiograms, TTS/PTS thresholds, vital rates | |||
| EMF | |||||||
| Physical EMF (analytical or numerical) | Cable configuration, burial depth | Sediment type and resistivity | Water resistivity | ||||
| EMF behavioral response * | Species distribution | Movement, dispersal, behavioral response to EMF | Physiological response to EMF, feeding:growth, vital rates | ||||
| Changes in habitat | |||||||
| Statistical species distribution | Bathymetry, slope, roughness, sediment type | Current velocity, shear stress, temperature, salinity, chlorophyll, nutrients, dissolved gases | Presence, presence/absence, abundance | ||||
| Spatial ecosystem and trophic | Abundance, biomass | Dispersal | Feeding, growth, production:biomass, vital rates | Habitat type | |||
| Biophysical | Current velocity, (chlorophyll, nutrients, dissolved gases) | Swimming, diving, (foraging, response to devices) | Larval stage duration, larval survival, feeding, growth, production:biomass | ||||
| Collision risk | |||||||
| Encounter/collision risk | Device geometry | Channel width and depth | Current velocity | Distribution in the water column | Swimming, diving, foraging, avoidance, evasion | Shape, size | |
| Exposure time population model | Device geometry | Distribution in the water column | Diving | Reproduction, survival | |||
| Displacement | |||||||
| Biophysical/agent-based | Current velocity, temperature, salinity | Species distribution, prey distribution | Swimming, diving | ||||
| Statistical species distribution | Current velocity, shear stress, temperature, salinity | Species distribution | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buenau, K.E.; Garavelli, L.; Hemery, L.G.; García Medina, G. A Review of Modeling Approaches for Understanding and Monitoring the Environmental Effects of Marine Renewable Energy. J. Mar. Sci. Eng. 2022, 10, 94. https://doi.org/10.3390/jmse10010094
Buenau KE, Garavelli L, Hemery LG, García Medina G. A Review of Modeling Approaches for Understanding and Monitoring the Environmental Effects of Marine Renewable Energy. Journal of Marine Science and Engineering. 2022; 10(1):94. https://doi.org/10.3390/jmse10010094
Chicago/Turabian StyleBuenau, Kate E., Lysel Garavelli, Lenaïg G. Hemery, and Gabriel García Medina. 2022. "A Review of Modeling Approaches for Understanding and Monitoring the Environmental Effects of Marine Renewable Energy" Journal of Marine Science and Engineering 10, no. 1: 94. https://doi.org/10.3390/jmse10010094






