Durum Wheat Breeding: In the Heat of the Senegal River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Panel Structure
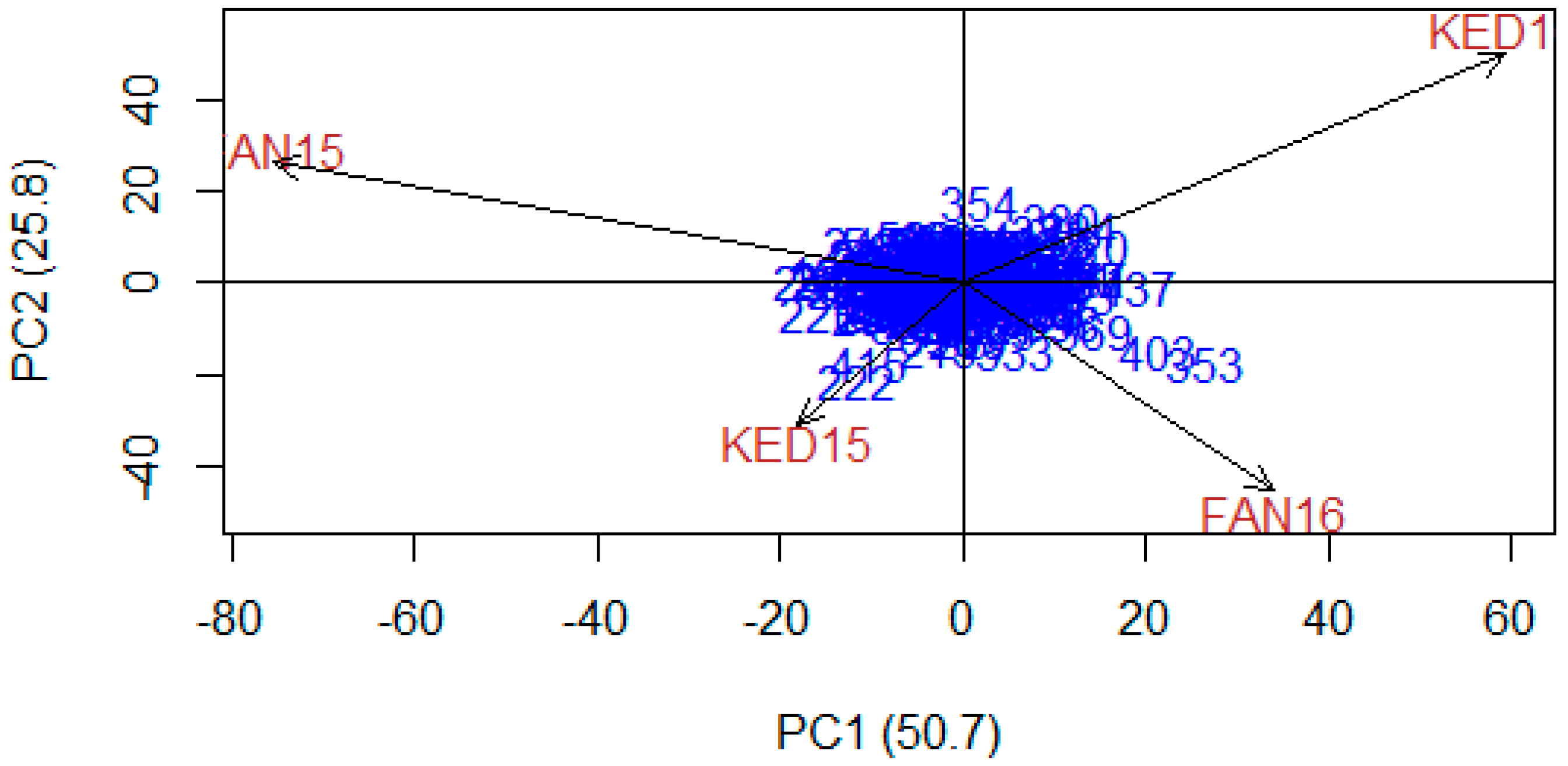
2.2. Population Sub-Structure
2.3. Field Trials and Phenotypic Characterization
2.4. Statistical Analyses
3. Results
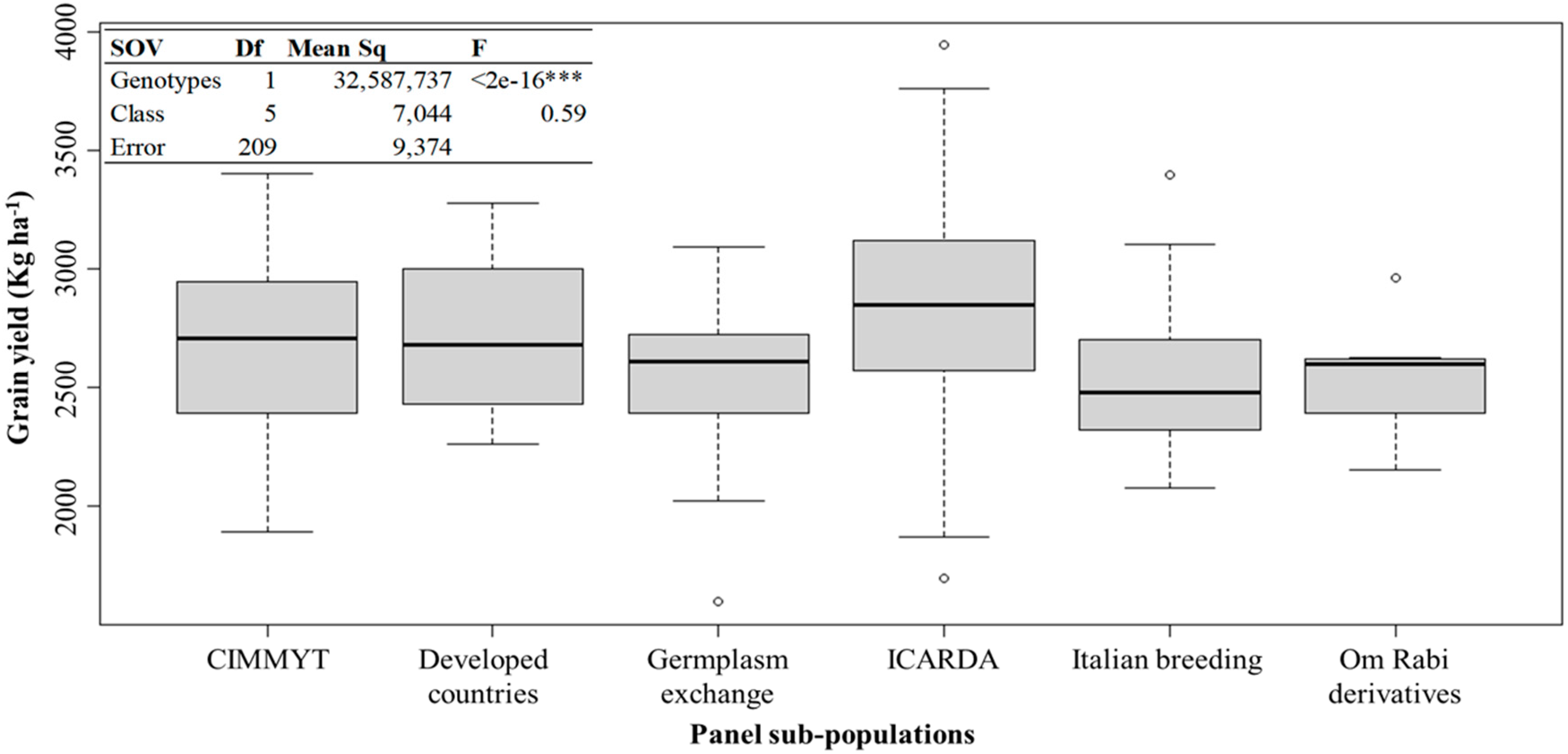
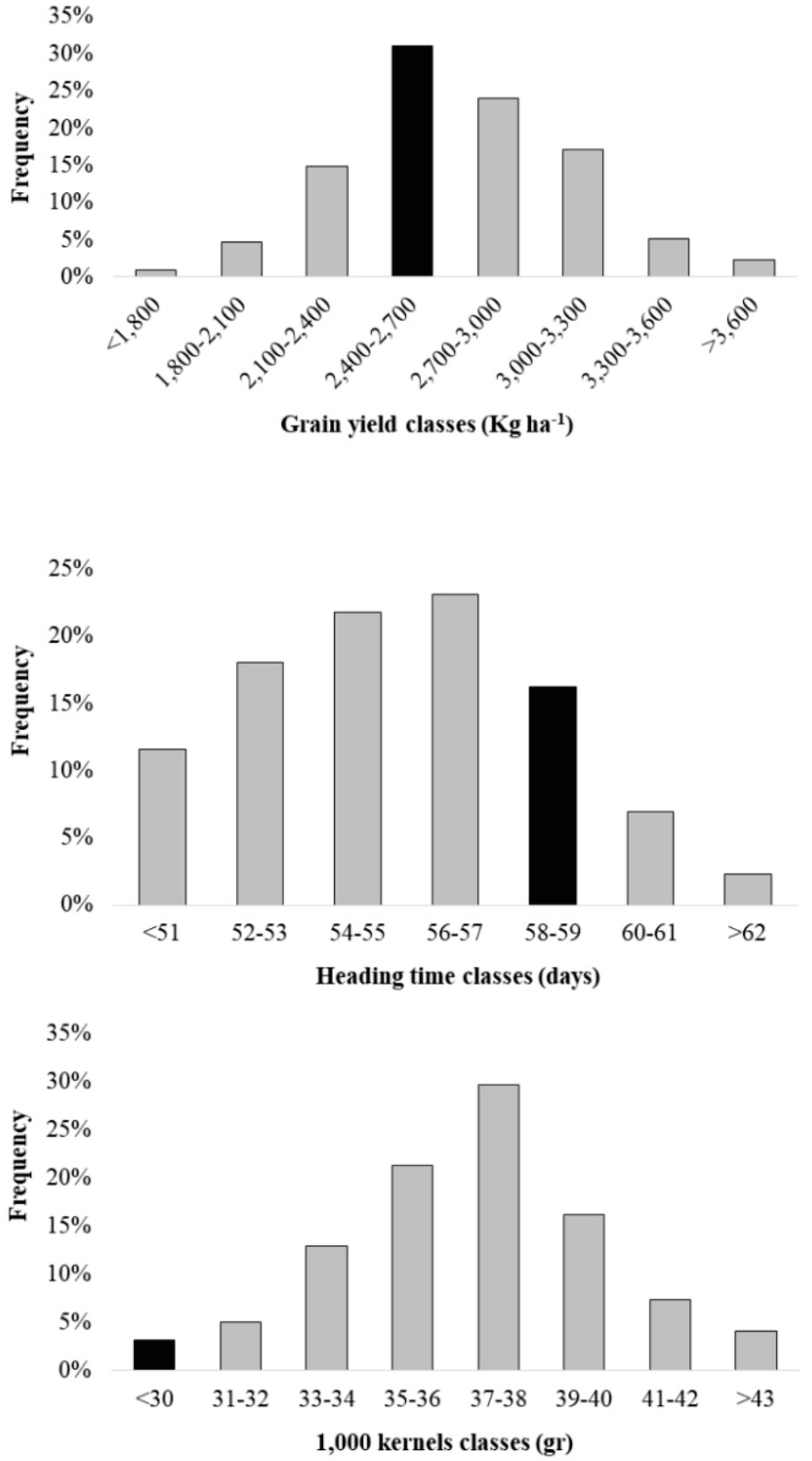
3.1. Phenotypic Variation of the Agronomical Traits
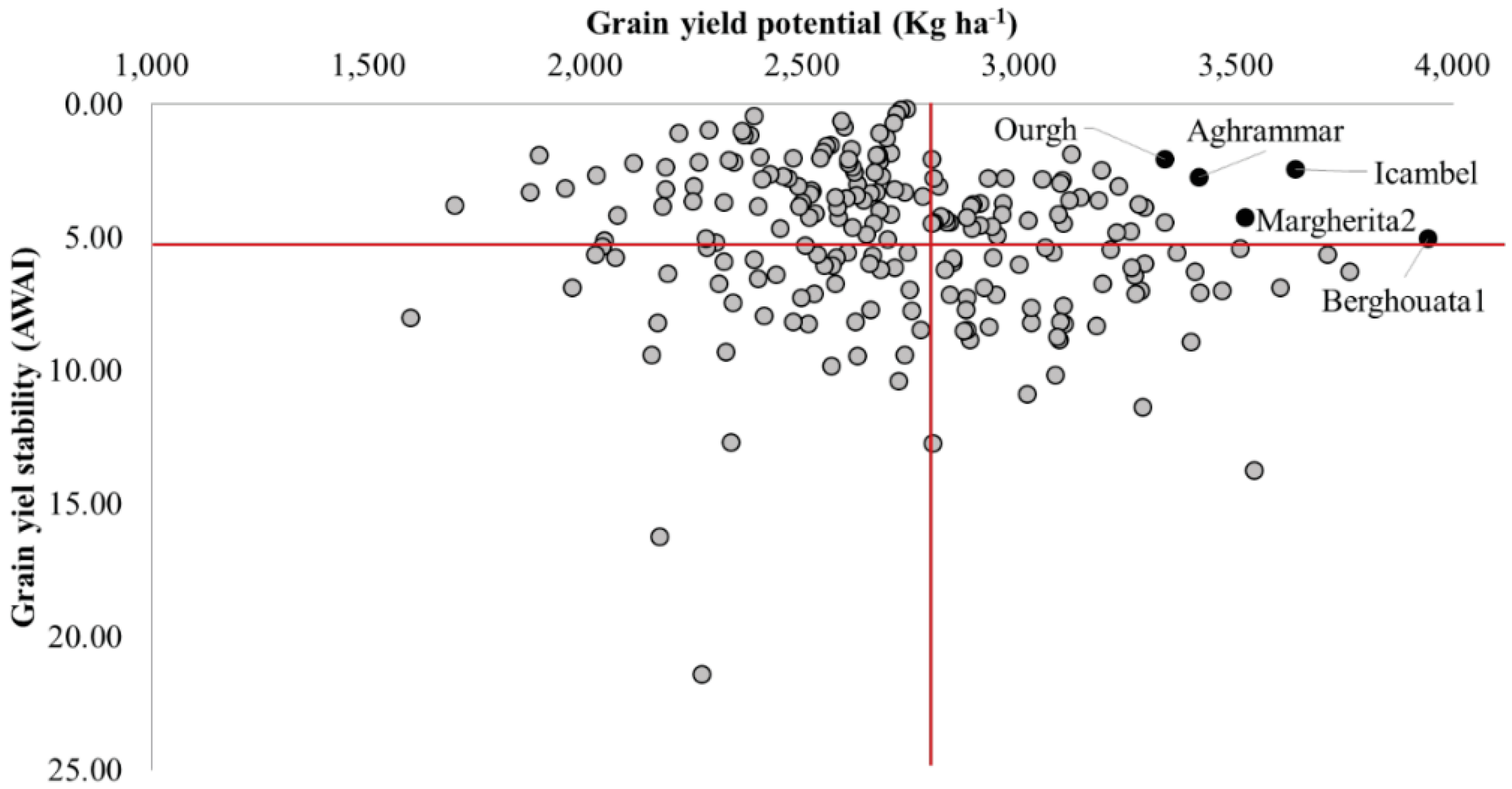
3.2. Yield Stability of Specific Durum Lines Against Heat
3.3. Correlation between Phenology, Yield and Its Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, L. Climate change impacts on agriculture across Africa. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Von Grebmer, K.; Bernstein, J.; Hossain, N.; Brown, T.; Prasai, N.; Yohannes, Y.; Patterson, F.; Sonttag, A.; Zimmermnan, S.-M.; Towey, O. Global Hunger Index: The Inequalities of Hunger; International Food Policy Research Institute: Washington, DC, USA; Bonn, Germany; Dublin, Ireland, 2017. [Google Scholar]
- FAO (Food and Agriculture Organization). Senegal: Irrigation Market Brief; Report No. 26; FAO: Rome, Italy, 2016; Available online: http://www.fao.org/3/a-i5365e.pdf (accessed on 11 May 2017).
- Wiebe, K.; Sulser, T.B.; Mason-D’Croz, D.; Rosegrant, M.W. The effects of climate change on agriculture and food security in Africa. In A Thriving Agricultural Sector in a Changing Climate: Meeting Malabo Declaration Goals Through Climate-Smart Agriculture; De Pinto, A., Ulimwengu, J.M., Eds.; International Food Policy Research Institute: Washington, DC, USA, 2017; pp. 5–21. [Google Scholar]
- Sall, A.T.; Kabbaj, H.; Cisse, M.; Gueye, H.; Ndoye, I.; Maltouf, A.F.; El-Mourid, M.; Ortiz, R.; Bassi, F.M. Heat tolerance of durum wheat (tritcum durum desf.) elite germplasm tested along the senegal river. J. Agric. Sci. 2018, 10. [Google Scholar] [CrossRef]
- Ama. Le Soleil, 18 April 2013; 10.
- Ashraf, M.; Quresh, A.S.; Ghafoor, I.A.; Khan, N.A. Genotype environment interaction in wheat. Pakistan J. Biol. Sci. 2001, 1, 356–357. [Google Scholar]
- Najafian, G.; Kaffashi, A.K.; Jafar-Nezhad, A. Analysis of grain yield stability in hexaploid wheat genotypes grown in temperate regions of Iran using additive main effects and multiplicative interaction. J. Agric. Sci. Technol. 2010, 12, 213–222. [Google Scholar]
- Zali, H.; Farshadfar, E.; Sabaghpour, S.H. Non-parametric analysis of phenotypic stability in chickpea (Cicer arietinum L.) genotypes in Iran. Crop Breed. J. 2011, 1, 89–100. [Google Scholar]
- Yaghotipoor, A.; Farshadfar, E. Non-parametric estimation and component analysis of phenotypic stability in chickpea (Cicer arietinum L.). Pak. J. Biol. Sci. 2007, 10, 26–46. [Google Scholar]
- Zobel, R.W.; Wright, M.J.; Gauch, H.G. Statistical analysis of a yield trial. Agron. J. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Malosetti, M.; Ribaut, J.; Van, E.F.A. The statistical analysis of multi-environment data: Modeling genotype-by-environment interaction and its genetic basis. Front. Physiol. 2013, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bassi, F.M.; Sanchez-Garcia, M. Adaptation and stability analysis of ICARDA durum wheat (Triticum durum Desf.) elites across 18 countries. Crop Sci. 2017, 57, 1–12. [Google Scholar] [CrossRef]
- Ortiz, R. Plant Breeding in the Omics Era; Springer International: New York, NY, USA, 2017. [Google Scholar]
- Kabbaj, H.; Sall, A.T.; Al-Abdallat, A.; Geleta, M.; Amri, A.; Filali-Maltouf, A.; Belkadi, B.; Ortiz, R.; Bassi, F.M. Genetic diversity within a global panel of durum wheat (Triticum durum) landraces and modern germplasm reveals the history of alleles exchange. Front. Plant Sci. 2017, 8, 1277. [Google Scholar] [CrossRef] [PubMed]
- Winfield, M.O.; Allen, A.M.; Burridge, A.J.; Barker, G.L.; Benbow, H.R.; Wilkinson, P.A. High-density SNP genotyping array for hexaploid wheat and its secondary and tertiary gene pool. Plant Biotechnol. J. 2016, 14, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Maccaferri, M.; Corinna, M.; Demontis, S.A.; El-Ahmed, A.; Garcia del Moral, L.; Maalouf, F.; Nachit, M.; Nserallah, N.; Ouabbou, H.; Rhouma, S.; et al. Association mapping in durum wheat grown across a broad range of water regimes. J. Exp. Bot. 2011, 62, 409–438. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Dreisigacker, S.; Peña, R.J.; Sukumaran, S.; Reynolds, M.P. Genetic characterization of the wheat association mapping initiative (WAMI) panel for dissection of complex traits in spring wheat. Theor. Appl. Genet. 2014, 128, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Baril, C.P. Factor regression for interpreting genotype-environment interaction in bread-wheat trials. Theor. Appl. Genet. 1992, 83, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Sharifi, P.; Karimizadeh, R.; Shefazadeh, M.K. Relationships between grain yield and yield components in bread wheat under different water availability (dryland and supplemental irrigation conditions). Not. Bot. Horti Agrobo. 2012, 40, 195–200. [Google Scholar] [CrossRef]
- Gupta, N.K.; Gupta, S.; Kumar, A. Effect of water stress on physiological attributes and their relationship with growth and yield in wheat cullivars at different growth stages. J. Agron. 2001, 86, 1437–1439. [Google Scholar]
- Tyagi, P.K.; Pannu, R.K.; Sharma, K.D.; Chaudhary, B.D.; Singh, D.P. Response of different wheat (Triticum aestivum L.) cultivars to terminal heat stress. Tests Agrochem. Cultiv. 2003, 24, 20–21. [Google Scholar]
- Singha, P.; Bhowmick, J.; Chaudhury, B.K. Effect of temperature on yield and yield components of fourteen wheat (Triticum aestivum L.) genotypes. Environ. Ecol. 2006, 24, 550–554. [Google Scholar]
- Atefeh, N.A.; Etminan, A.; Teixeira da Silva, J.A.; Mohammadi, R. Assessment of yield, yield-related traits and drought tolerance of durum wheat genotypes (Triticum turjidum var. durum Desf.). Aus. J. Crop Sci. 2011, 5, 8–16. [Google Scholar]
- Dias, A.S.; Lidon, F.C. Evaluation of grain filling rate and duration in bread and durum wheat, under heat stress after anthesis. J. Agron. Crop Sci. 2009, 195, 137–147. [Google Scholar] [CrossRef]
- Abbassenne, F.; Bouzerzour, H.; Hachemi, L. Phénologie et production du blé dur (Triticum durum Desf.) en zone semi-aride d’altitude. Ann. Inst. National Agron. Harrach 1997, 18, 24–36. [Google Scholar]
- Masoni, A.; Ercoli, L.; Mariotti, M.; Arduini, I. Post-anthesis accumulation and remobilization of dry matter, nitrogen and phosphorus in durum wheat as affected by soil type. Eur. J. Agron. 2007, 26, 179–186. [Google Scholar] [CrossRef]
- Bahlouli, F.; Bouzerzour, H.; Benmahammed, A. Effets de la vitesse et de la durée du remplissage du grain ainsi que de l’accumulation des assimilats de la tige dans l’élaboration du rendement du blé dur (Triticum durum Desf.) dans les conditions de culture des hautes plaines orientales d’Algérie. Biotechnol. Agron. Soc. Environ. 2008, 12, 31–39. [Google Scholar]

| Traits z | MSG | MSE | MSG×E | H2 |
|---|---|---|---|---|
| GY | 1,263,893 * | 344,471,841 * | 673,841 * | 0.47 |
| DtH | 76 * | 1794 * | 14 * | 0.82 |
| PLH | 105 * | 9915 * | 60 * | 0.43 |
| TKW | 77 * | 3659 * | 37 * | 0.52 |
| Biom | 20,246,949 * | 1,469,491,352 * | 16,734,589 * | 0.17 |
| HI | 87 * | 39,829 * | 55 * | 0.37 |
| Name | Cluster | Pedigree | Grain Yield (kg ha−1) | Yield Stability (AWAI) | Heading (days) | 1000-Kernels (gr) |
|---|---|---|---|---|---|---|
| Berghouata1 | ICARDA | Ter1//Mrf1/Stj2 | 3943 | 5.00 | 50 | 38 |
| Icambel | ICARDA | Mrb3/Mna1//Ter1/3/IcamorTA0459/Ammar7/4/Beltagy2 | 3637 | 2.46 | 53 | 40 |
| Margherita2 | ICARDA | Terbol975/Geruftel2 | 3522 | 4.24 | 52 | 35 |
| Aghrammar | ICARDA | Mgnl3/Ainzen1//Ammar1 | 3415 | 2.75 | 52 | 37 |
| Ourgh | ICARDA | D67GTA/2/BOYERO/BIT//MEXICALI | 3336 | 2.08 | 51 | 37 |
| Icavicre | ICARDA | IcamorTA0468/6/21563/AA//Fg/3/D68102A2A1A/4/Vitron/5/Bcr | 3336 | 4.42 | 50 | 39 |
| IDuWUE-024 | CIMMYT | GS/CRA//SBA81/3/HO/MEXI1/5/MEMO/6/2 * | 3288 | 3.87 | 53 | 36 |
| Italo | Developed | Cross Italian × Turkish | 3275 | 3.74 | 55 | 38 |
| Kunmiki | ICARDA | MorlF38//Bcrch1/Kund1149/3/Bicrederaa1/Miki | 3257 | 4.78 | 50 | 39 |
| Ouassara1 | ICARDA | Ouasloukos1/5/Azn1/4/BEZAIZSHF//SD19539/Waha/3/Gdr2 | 3228 | 3.07 | 51 | 38 |
| FAN15 | KED15 | FAN16 | KED16 | Across Environments | |
|---|---|---|---|---|---|
| DtH z | −0.26 | −0.27 | −0.36 * | −0.19 | −0.47 * |
| DtM | −0.47 * | 0.08 | −0.17 | ||
| GFP | 0.29 * | 0.19 | 0.31 * | ||
| PLH | −0.02 | 0.17 | 0.15 | 0.23 | 0.19 |
| TKW | 0.34 * | 0.22 | 0.33 * | 0.11 | 0.30 * |
| Spk.m−2 | 0.46 * | 0.49 * | 0.28 | ||
| Gr.spk−1 | 0.63 * | 0.51 * | 0.30 * | ||
| Biom | 0.51 * | 0.68 * | 0.14 | 0.55 * | 0.45 * |
| HI | 0.57 * | 0.77 * | 0.61 * | 0.48 * | 0.62 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sall, A.T.; Bassi, F.M.; Cisse, M.; Gueye, H.; Ndoye, I.; Filali-Maltouf, A.; Ortiz, R. Durum Wheat Breeding: In the Heat of the Senegal River. Agriculture 2018, 8, 99. https://doi.org/10.3390/agriculture8070099
Sall AT, Bassi FM, Cisse M, Gueye H, Ndoye I, Filali-Maltouf A, Ortiz R. Durum Wheat Breeding: In the Heat of the Senegal River. Agriculture. 2018; 8(7):99. https://doi.org/10.3390/agriculture8070099
Chicago/Turabian StyleSall, Amadou T., Filippo M. Bassi, Madiama Cisse, Habibou Gueye, Ibrahima Ndoye, Abdelkarim Filali-Maltouf, and Rodomiro Ortiz. 2018. "Durum Wheat Breeding: In the Heat of the Senegal River" Agriculture 8, no. 7: 99. https://doi.org/10.3390/agriculture8070099
APA StyleSall, A. T., Bassi, F. M., Cisse, M., Gueye, H., Ndoye, I., Filali-Maltouf, A., & Ortiz, R. (2018). Durum Wheat Breeding: In the Heat of the Senegal River. Agriculture, 8(7), 99. https://doi.org/10.3390/agriculture8070099







