Miscanthus Biochar had Limited Effects on Soil Physical Properties, Microbial Biomass, and Grain Yield in a Four-Year Field Experiment in Norway
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Description and Experimental Design
2.2. Soil and Biochar Analysis (Table 1)
2.3. Sampling and Analysis Methods
2.3.1. Soil Sampling
2.3.2. Soil Water Content in the Field
2.3.3. Soil Water Retention and Pore Size Distribution from Intact Cores
2.3.4. Aggregate Stability
2.3.5. Soil Penetration Resistance
2.3.6. Soil Microbial Biomass C and Respiration Potential
2.3.7. Plant Grain and Straw Yields
2.4. Statistical Analysis
3. Results
3.1. Effects of Biochar on Bulk Density
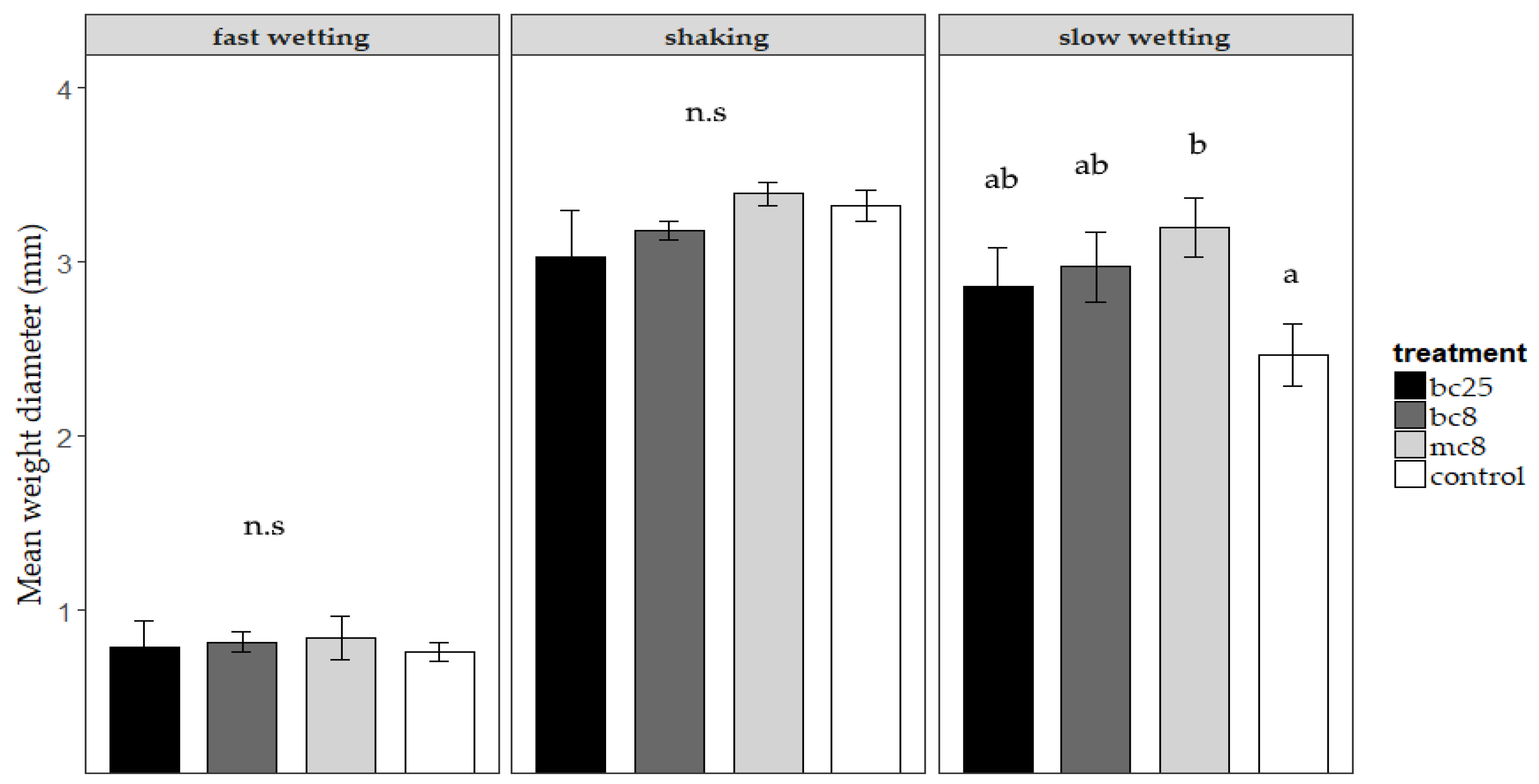
3.2. Soil Aggregate Distribution and Stability
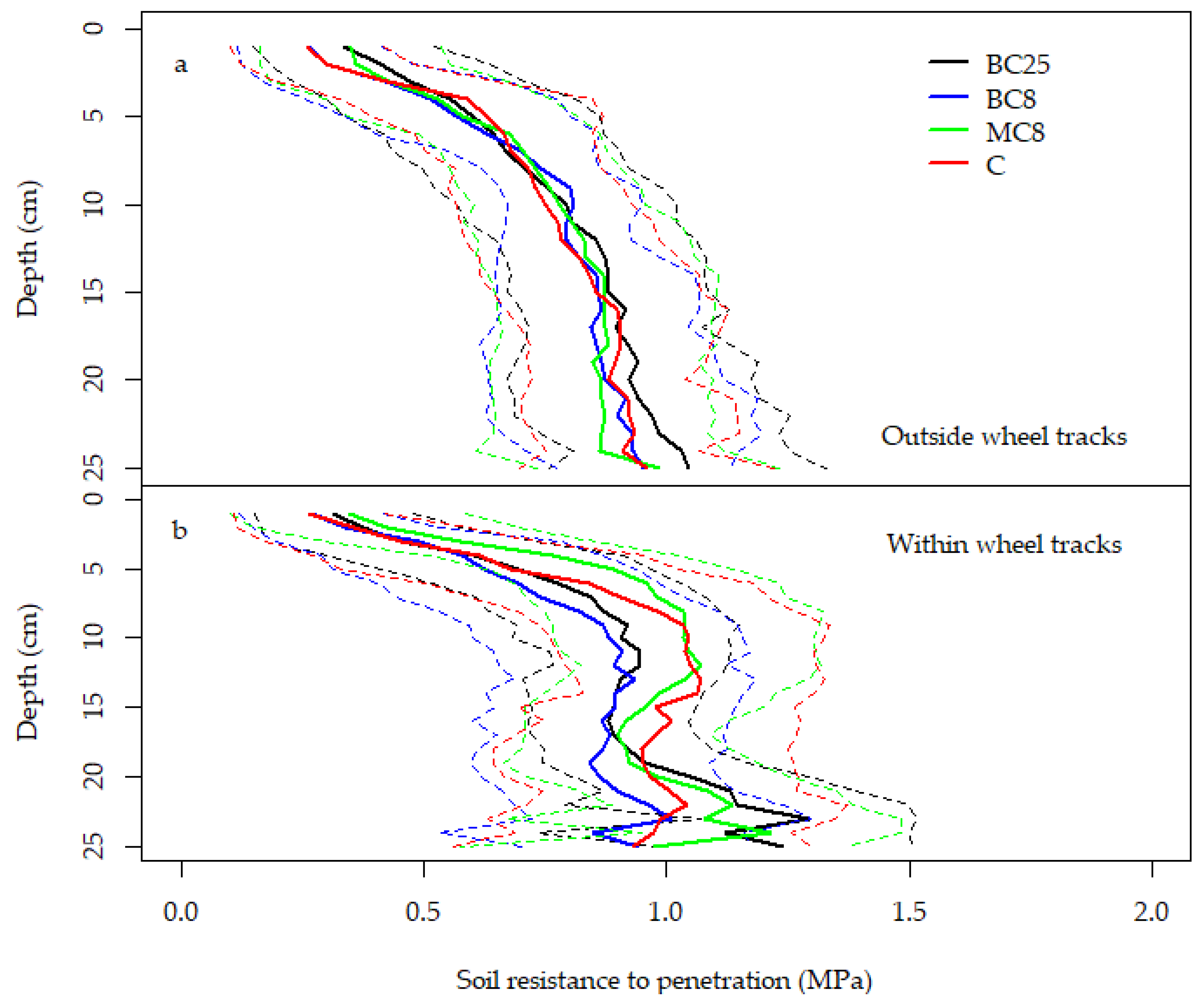
3.3. Soil Resistance to Penetration
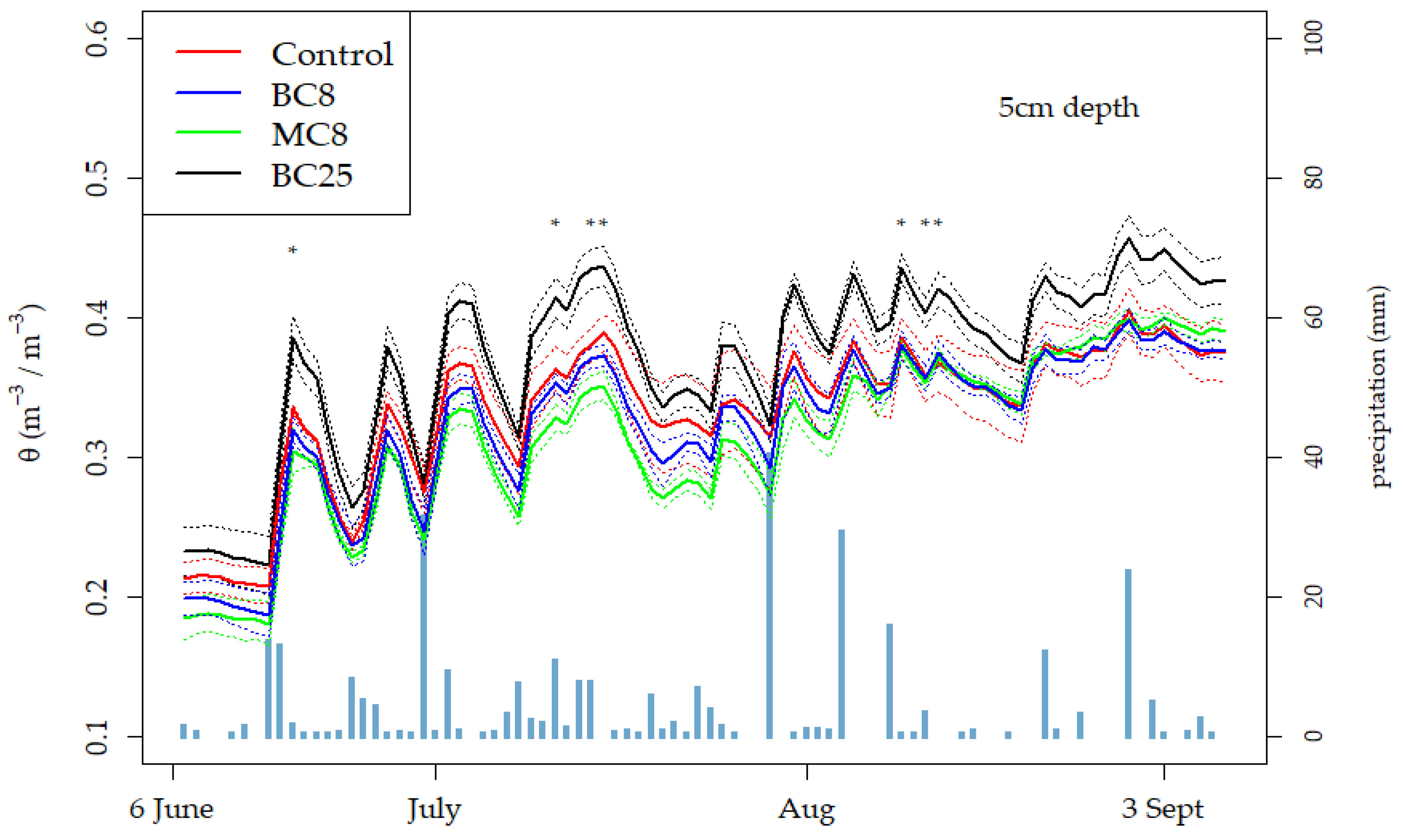
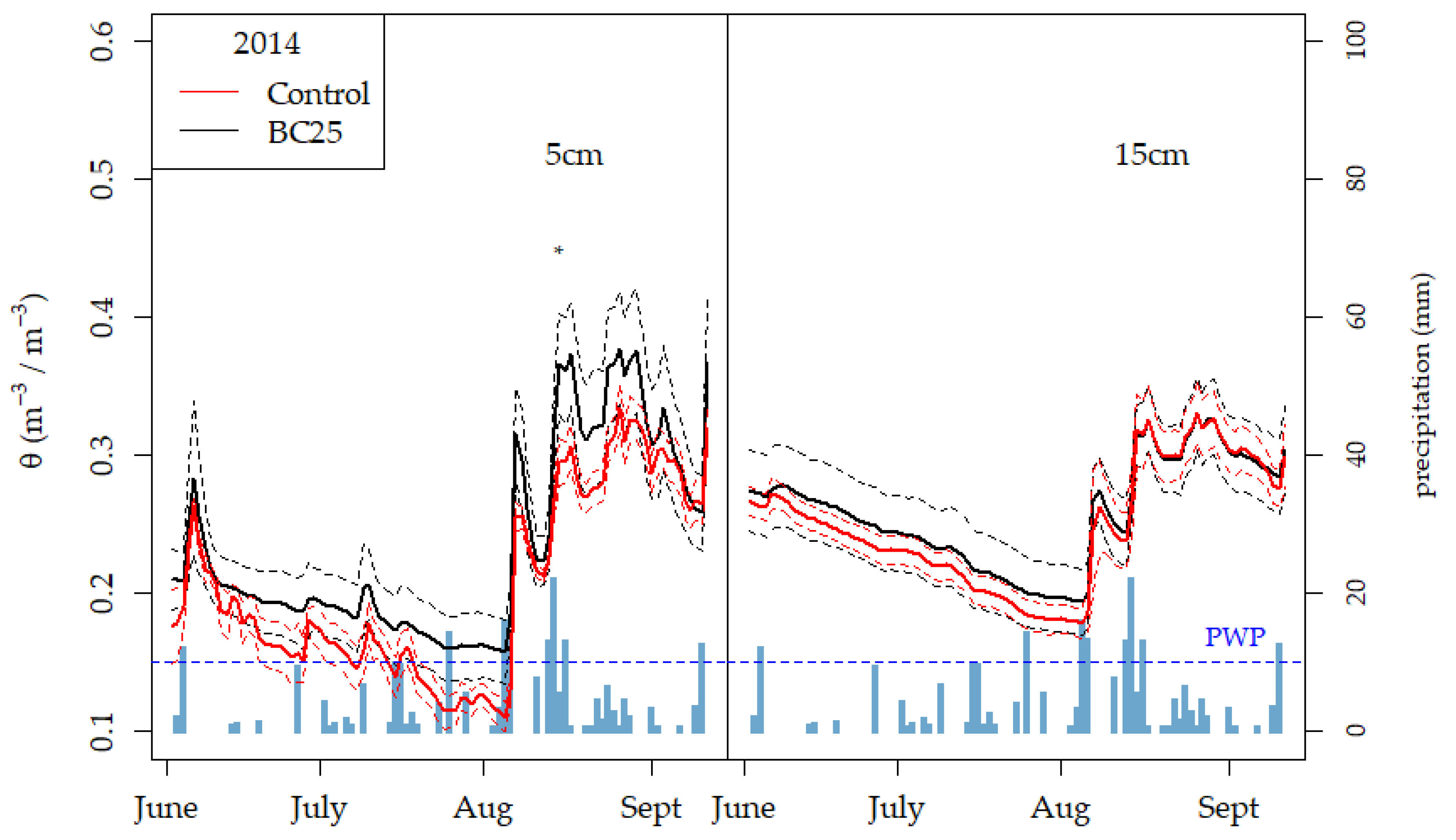
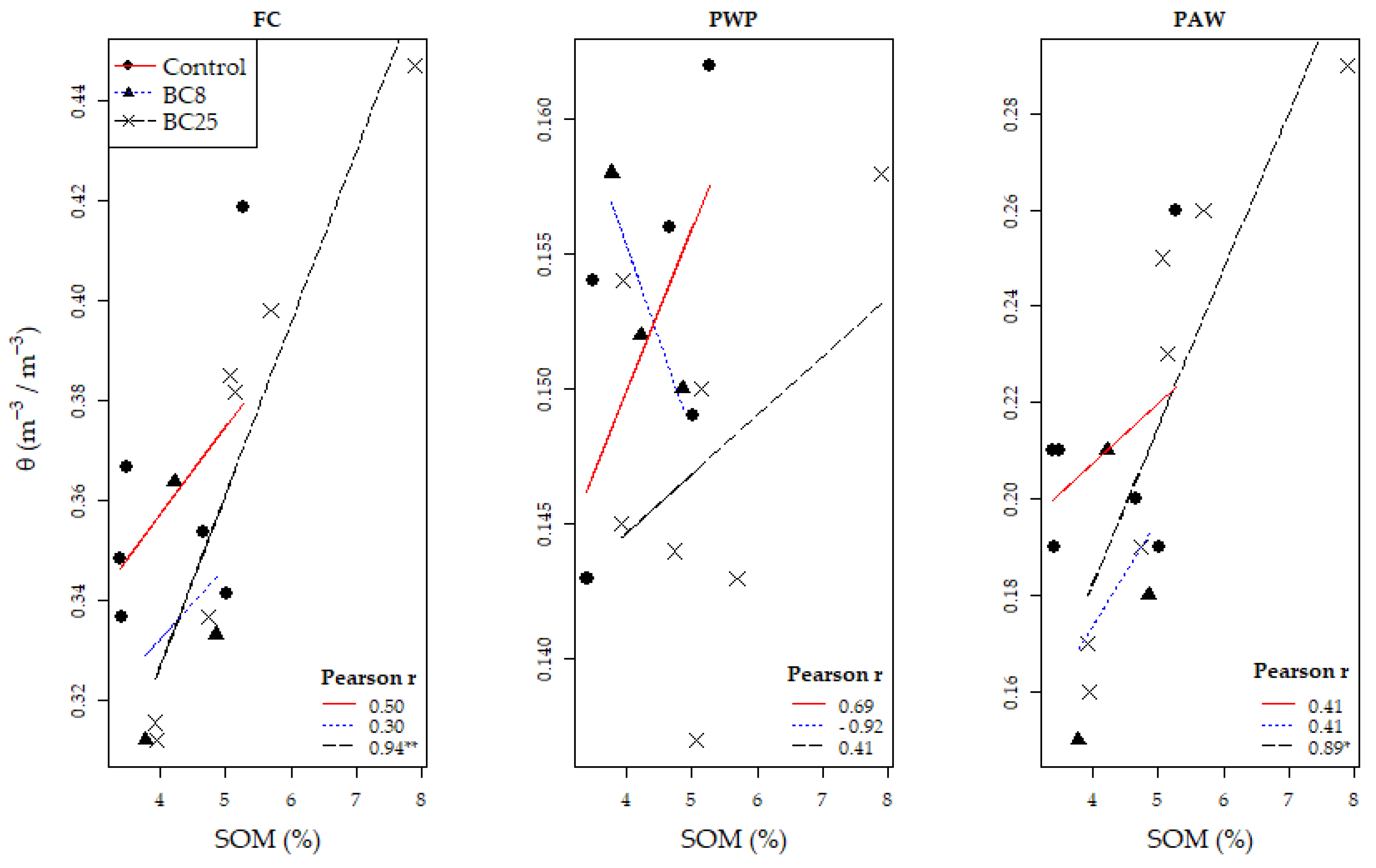
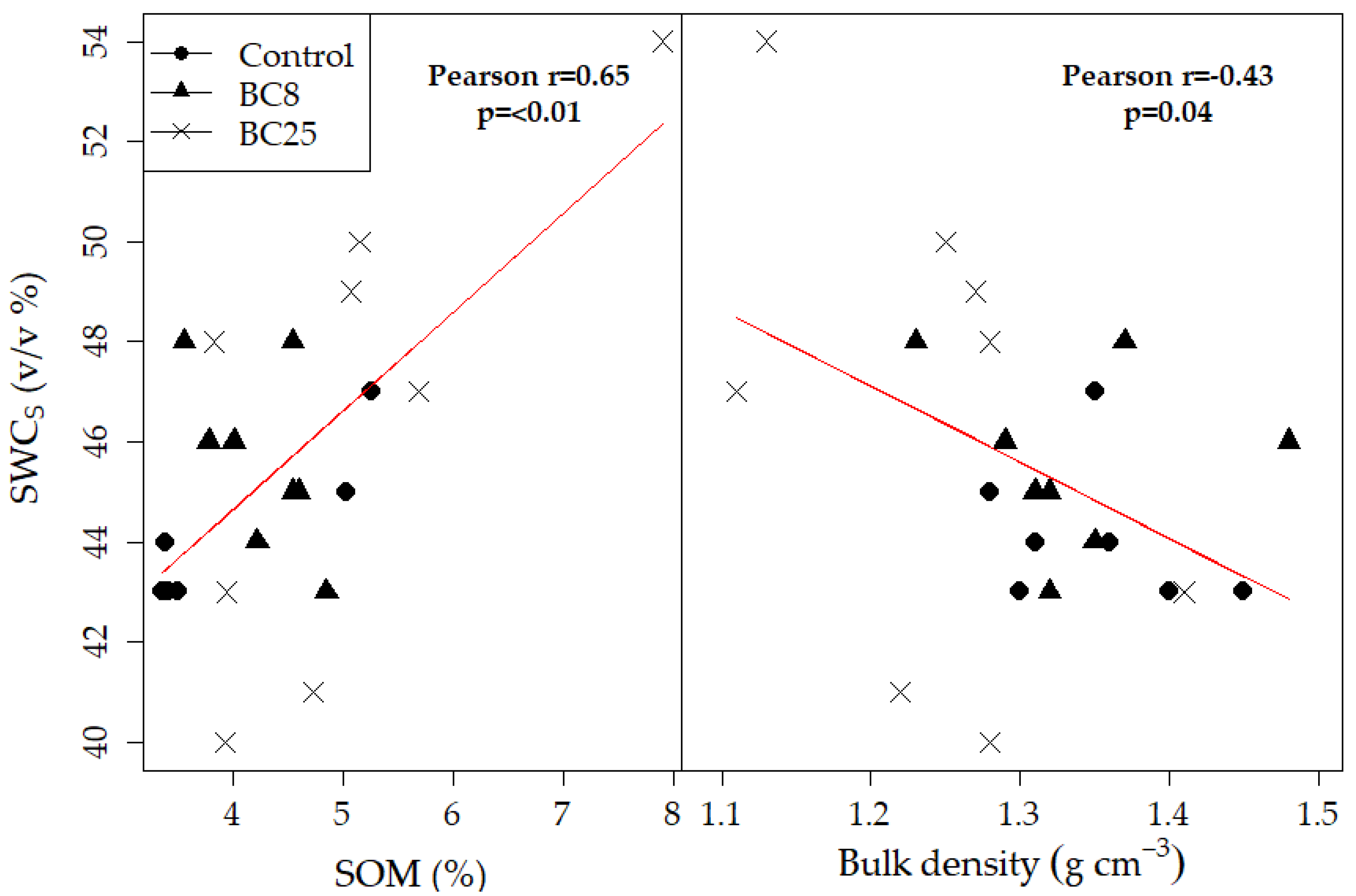
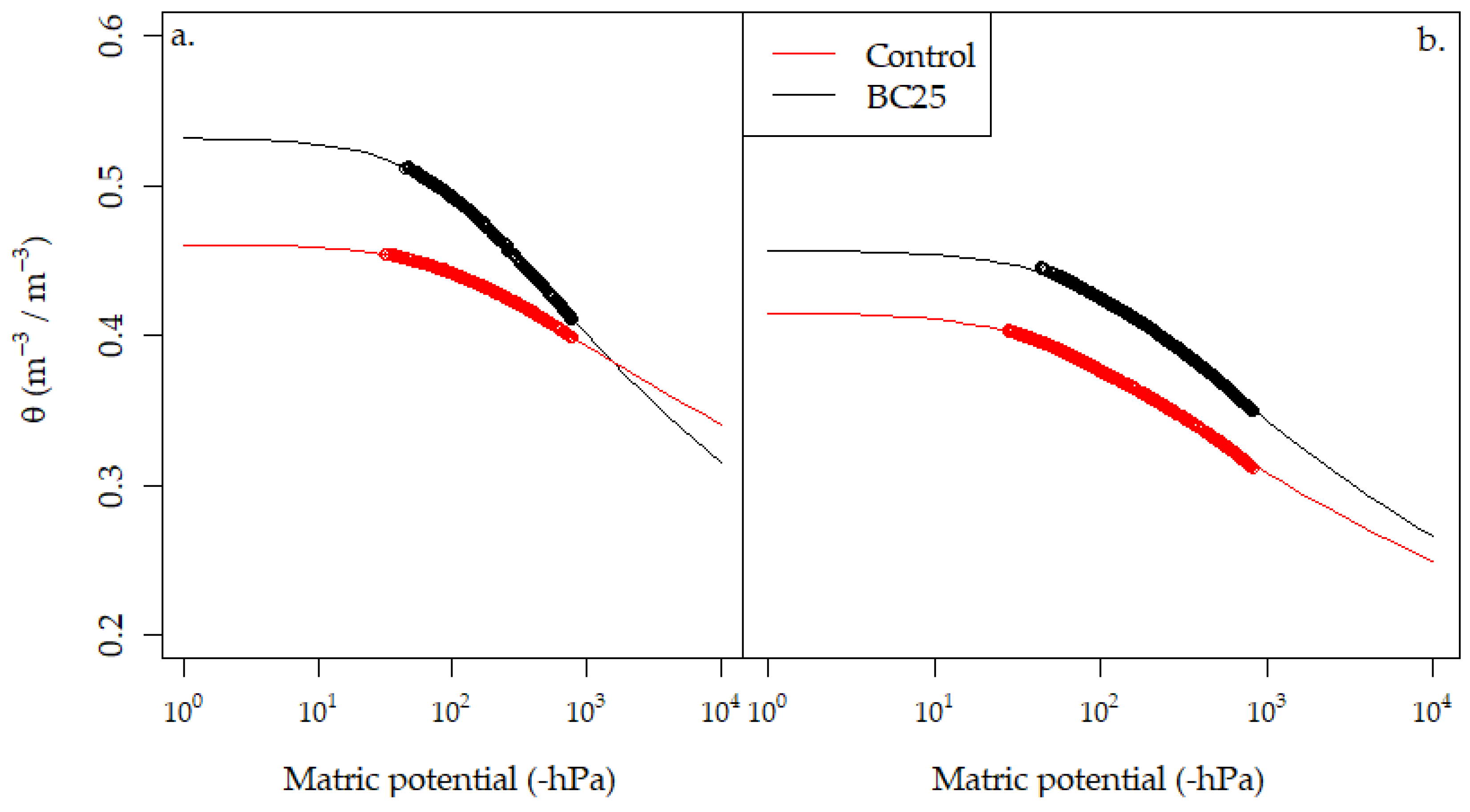
3.4. Soil Water Content and Retention
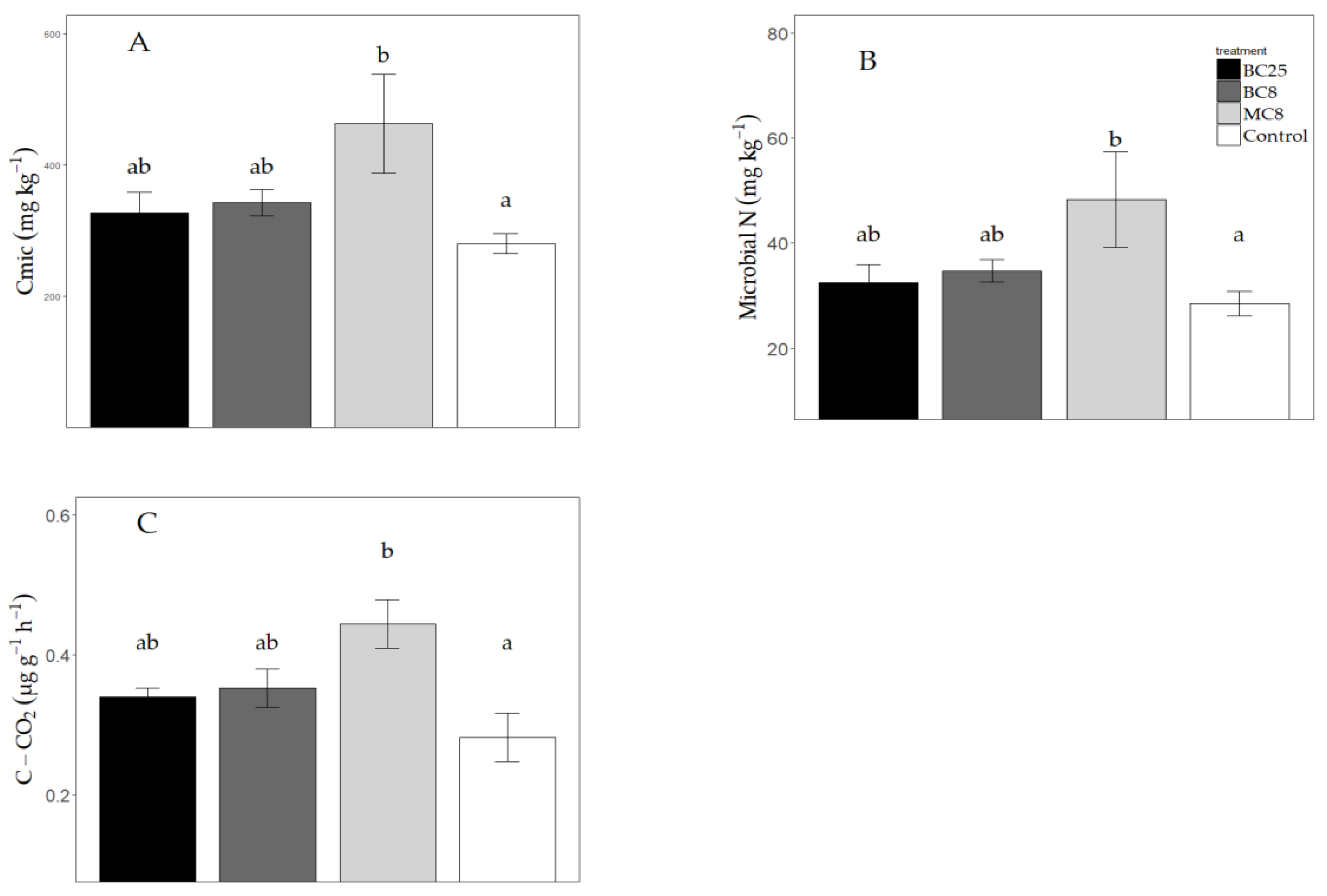
3.5. Microbial Biomass C and N and Basal Respiration
3.6. Grain and Straw Yield
4. Discussion
4.1. Biochar Suitability as a Soil C Amendment in Reference to Regulations and Standards
4.2. Biochar Effects on Soil Physical Properties
4.3. Effects on Microbial C and N and Basal Respiration
4.4. Grain Yield and Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, P.; Gregory, P.J. Climate change and sustainable food production. Proc. Nutr. Soc. 2013, 72, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strat. Glob. Chang. 2006, 11, 395–419. [Google Scholar] [CrossRef]
- O’Toole, A.; Rasse, D. Biochar: Soil carbon and fertility. In Encyclopedia of Soil Science, 3rd ed.; Rattan, L., Ed.; Taylor and Francis: New York, NY, USA, 2016; pp. 193–197. [Google Scholar]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Kammann, C.; Abalos, D. Biochar effects on methane emissions from soils: A meta-analysis. Soil Biol. Biochem. 2016, 101, 251–258. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef]
- Strategy and Incentives for Reducing Emissions of GHGs from the Agricultural Sector; Norwegian Environment Department: Oslo, Norway, 2010.
- Rasse, D.P.; Budai, A.; O’Toole, A.; Ma, X.; Rumpel, C.; Abiven, S. Persistence in soil of Miscanthus biochar in laboratory and field conditions. PLoS ONE 2017, 12, e0184383. [Google Scholar] [CrossRef] [PubMed]
- Peake, L.R.; Reid, B.J.; Tang, X. Quantifying the influence of biochar on the physical and hydrological properties of dissimilar soils. Geoderma 2014, 235, 182–190. [Google Scholar] [CrossRef]
- Sun, F.; Lu, S. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Gonnermann, H.M. Biochar particle size, shape, and porosity act together to influence soil water properties. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.T.; Gallagher, M.E.; Masiello, C.A.; Liu, A.; Dugan, B. Biochar-induced changes in soil hydraulic conductivity and dissolved nutrient fluxes constrained by laboratory experiments. PLoS ONE 2014, 9, e108340. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.J.; Spokas, K.A.; Feyereisen, G.; Novak, J.M. Predicting the impact of biochar additions on soil hydraulic properties. Chemosphere 2016, 142, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Masiello, C.M.; Dugan, B.; Brewer, C.; Spokas, K.A.; Novak, J.M.; Liu, Z.; Sorrenti, G. Biochar effects on soil hydrology. In Biochar for Environmental Management, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 543–562. [Google Scholar]
- Obia, A.; Mulder, J.; Martinsen, V.; Cornelissen, G.; Børresen, T. In situ effects of biochar on aggregation, water retention and porosity in light-textured tropical soils. Soil Till. Res. 2016, 155, 35–44. [Google Scholar] [CrossRef]
- Herath, H.; Camps-Arbestain, M.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209, 188–197. [Google Scholar] [CrossRef]
- Busscher, W.J.; Novak, J.M.; Evans, D.E.; Watts, D.W.; Niandou, M.A.S.; Ahmedna, M. Influence of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci. 2010, 175, 10–14. [Google Scholar] [CrossRef]
- Obia, A.; Børresen, T.; Martinsen, V.; Cornelissen, G.; Mulder, J. Effect of biochar on crust formation, penetration resistance and hydraulic properties of two coarse-textured tropical soils. Soil Till. Res. 2017, 170, 114–121. [Google Scholar] [CrossRef]
- Ahmed, A.; Gariepy, Y.; Raghavan, V. Influence of wood-derived biochar on the compactibility and strength of silt loam soil. Int. Agrophys. 2017, 31, 149–155. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Zong, Y.; Chen, D.; Lu, S. Impact of biochars on swell-shrinkage behavior, mechanical strength, and surface cracking of clayey soil. J. Plant Nutr. Soil Sci. 2014. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Hanley, K.; Enders, A.; Lehmann, J. Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol. Biochem. 2014, 72, 152–162. [Google Scholar] [CrossRef]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Yu, J.; Deem, L.M.; Crow, S.E.; Deenik, J.L.; Penton, C.R. Biochar application Influences microbial assemblage complexity and composition due to soil and bioenergy crop type interactions. Soil Biol. Biochem. 2018, 117, 97–107. [Google Scholar] [CrossRef]
- Nielsen, S.; Minchin, T.; Kimber, S.; van Zwieten, L.; Gilbert, J.; Munroe, P.; Joseph, S.; Thomas, T. Comparative analysis of the microbial communities in agricultural soil amended with enhanced biochars or traditional fertilisers. Agric. Ecosyst. Environ. 2014, 191, 73–82. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2011, 48, 271–284. [Google Scholar] [CrossRef]
- Krogstad, T. Methods for Soil Analysis (in Norwegian); Institute for Soil Science, Norwegian Agricultural College: Ås, Norway, 1992. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenerg. 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Brown, R.A.; Kerche, A.K.; Nguyen, T.H.; Nagle, D.C.; Ball, W.P. Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Org. Geochem. 2006, 37, 321–333. [Google Scholar] [CrossRef]
- Schindler, U. A rapid method for measuring the hydraulic conductivity in cylinder core samples from unsaturated soil. Archiv fur Acker-und Pflanzenbau und Bodenkunde 1980, 24, 1–7. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Riley, H. Estimation of physical properties of cultivated soils in southeast Norway from readily available soil information. Norway from readily available soil information. Norw. J. Agric. Sci. 1996, 25, 51. [Google Scholar]
- Schjønning, P. Size distribution of dispersed and aggregated particles and of soil pores in 12 Danish soils. Acta Agric. Scand. B Plant Soil Sci. 1992, 42, 26–33. [Google Scholar]
- Njøs, A. Dry sieving. In West-European Methods for Soil Structure Determination; De Boodt, M., Ed.; State Faculty of Agricultural Sciences: Ghent, Belgium, 1967. [Google Scholar]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distributlon. In Methods of Soil Analysis; USDA: Washington, DC, USA, 1986. [Google Scholar]
- Bissonnais, Y.L. Aggregate stability and assessment of soil crustability and erodibility: I. Theory and methodology. Eur. J. Soil Sci. 1996, 47, 425–437. [Google Scholar] [CrossRef]
- Perfect, E.; Groenevelt, P.H.; Kay, B.D.; Grant, C.D. Spatial variability of soil penetrometer measurements at the mesoscopic scale. Soil Till. Res. 1990, 16, 257–271. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: The use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol. Biochem. 1987, 19, 697–702. [Google Scholar] [CrossRef]
- Sparling, G.P.; West, A.M. Modifications to the Flmigation-extraction technique to permit simultaneous extraction and estimation of soil microbial C and N. Commun. Soil Sci. Plant Anal. 1988, 19, 327–344. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landmann, A.; Prugen, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Robertson, G.P.; Wedin, D.; Groffmann, P.M.; Blair, J.M.; Holland, E.A.; Nadelhoffer, K.J. Soil carbon and nitrogen availability: Nitrogen mineralization, nitrification, and soil respiration potentials. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Coleman, D.C., Bledsoe, C.S., Sollins, P, Eds.; Oxford University Press: New York, NY, USA, 1999; pp. 258–271. [Google Scholar]
- R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 15 April 2018).
- Budai, A.; Zimmerman, A.R.; Cowie, A.L.; Webber, J.B.W.; Singh, B.P.; Glaser, B.; Masiello, C.A; Andersson, D.; Shields, F.; Lehmann, J.; et al. Biochar Carbon Stability Test Method: An Assessment of Methods to Determine Biochar Carbon Stability; International Biochar Initiative: Canandaigua, NY, USA, 2013. [Google Scholar]
- Schimmelpfennig, S.; Glaser, B. One step forward toward characterization: Some important material properties to distinguish biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for a Sustainable Production of Biochar. Version 6.2E. Available online: http://www.european-biochar.org/biochar/media/doc/ebc-guidelines.pdf (accessed on 15 April 2018).
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. 2010. Biochar impact on nutrient leaching from a midwestern agricultural soil. Geoderma 2018, 158, 436–442. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. Elements of the Nature and Properties of Soils, 3 ed.; Prentice Hall: New Jersey, NJ, USA, 2010. [Google Scholar]
- Droogers, P.; Fermont, A.; Bouma, J. Effects of ecological soil management on workability and trafficability of a loamy soil in the Netherlands. Geoderma 1996, 73, 131–145. [Google Scholar] [CrossRef]
- Kjersti, U.A.; Trond, B.; Johannes, D.; Wendy, W.; Einar, S.; Azzaroli, B.M.; Till, S.; Sigrun, K.; Tove, S.; Morten, L.; et al. Økt Kornproduksjon Gjennom Forbedret Agronomisk Praksis. En Vurdering Av Agronomiske Tiltak Som Kan Bidra Til Avlingsøkninger i Kornproduksjonen; NIBIO: Ås, Norway, 2017. [Google Scholar]
- Hohle, E.; Appointed Working Group. Agriculture and Climate Change; Norwegian Agriculture and Food Department: Oslo, Norway, 2016. [Google Scholar]
- Seehusen, T.; Børresen, T.; Rostad, B.I.; Fleige, H.; Zink, A.; Riley, H. Verification of traffic-induced soil compaction after long-term ploughing and 10 years minimum tillage on clay loam soil in South-East Norway. Acta Agric. Scand. B Soil Plant Sci. 2014, 64, 312–328. [Google Scholar] [CrossRef]
- Martino, D.L.; Shaykewich, C.F. Root penetration profiles of wheat and barley as affected by soil penetration resistance in field conditions. Can. J. Soil Sci. 1994, 74, 193–200. [Google Scholar] [CrossRef]
- Hseu, Z.-Y.; Jien, S.-H.; Chien, W.-H.; Liou, R.-C. Impacts of biochar on physical properties and erosion potential of a mudstone slopeland soil. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.; Holm, J.K. Hauggaard-Nielsen, H. Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Rasse, D.P.; Smucker, A.J.M.; Santos, D. Alfalfa root and shoot mulching effects on soil hydraulic properties and aggregation. Soil Sci. Soc. Am. J. 2000, 64, 725. [Google Scholar] [CrossRef]
- Bamminger, C.; Poll, C.; Sixt, C.; Högy, P.; Wüst, D.; Kandeler, E.; Marhan, S. Short-term response of soil microorganisms to biochar addition in a temperate agroecosystem under soil warming. Agric. Ecosyst. Environ. 2016, 233, 308–317. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Joseph, S.; Graber, E.R.; Chia, C.; Munroe, P.; Donne, S.; Thomas, T.; Nielsen, S.; Marjo, C.; Rutlidge, H.; Pan, G.X.; et al. Shifting paradigms: Development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag. 2013, 4, 323–343. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- O’Toole, A.; de Zarruk, K.K.; Steffens, M.; Rasse, D.P. Characterization, Stability, and Plant Effects of Kiln-Produced Wheat Straw Biochar. J. Environ. Qual. 2013, 42, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Biochar application to a fertile sandy clay loam in boreal conditions: Effects on soil properties and yield formation of wheat, turnip rape and faba bean. Plant Soil 2014, 374, 89–107. [Google Scholar] [CrossRef]
- Ruysschaert, G.; Nelissen, V.; Postma, R.; Bruun, E.; O’Toole, A.; Hammond, J.; Rödger, J.M.; Hylander, L.; Kihlberg, T.; Zwart, K.; et al. Field applications of pure biochar in the North Sea region and across Europe. In Biochar in European Soils and Agriculture: Science and Practice; Shackley, S., Ruysschaert, G., Zwart, K., Glaser, B., Eds.; Routledge: London, UK, 2016; pp. 99–136. [Google Scholar]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Klakegg, O. Soil Statistics for Oslo and Akerhus Counties; Norwegian Institute for Soil and Forestry Mapping: Ås, Norway, 2002. [Google Scholar]

| Unit | Miscanthus Biochar | Miscanthus Straw (Biochar Feedstock) | Soil (Spring 2011) | |
|---|---|---|---|---|
| Fixed C | %DM | 81.10 | - | - |
| Volatile matter | %DM | 7.40 | - | - |
| Ash | %DM | 11.50 | 3.50 | - |
| Total C | %DM | 80.00 | 46.73 | 2.45 |
| H | %DM | 1.2 | - | - |
| N | %DM | 0.6 | 0.20 | 0.23 |
| O | %DM | 6.6 | - | - |
| S | %DM | 0.10 | 0.05 | - |
| C:N | Ratio | 256.77 | 233.65 | 17.45 |
| Total P | mg kg−1 | 1300 | 80 | 2900 |
| P-AL | mg kg−1 | 1100 | - | 106 |
| K-AL | mg kg−1 | 7500 | - | 86 |
| Ca-AL | mg kg−1 | 4600 | - | 2058 |
| Mg-AL | mg kg−1 | 640 | - | 116 |
| Na-AL | mg kg−1 | 360 | - | 28 |
| Si | mg kg−1 | - | 3.40 | - |
| NO3 | mg kg−1 | 3.32 | - | 12.10 |
| NH4 | mg kg−1 | - | - | 1.50 |
| Fe | mg kg−1 | 1100 | - | - |
| Mn | mg kg−1 | 160 | - | - |
| Mo | mg kg−1 | <1.1 | - | - |
| Zn | mg kg−1 | 39 | - | - |
| Cl | mg kg−1 | 477 | - | - |
| B | mg kg−1 | 5.10 | - | - |
| BET-N2 | m2 g−1 | 348 | - | - |
| pH (±SD, n = 9) | (H2O) | 7.86 ± 0.05 (n = 3) | - | 6.39 ± 0.2 |
| EC | mS/m | 130 | - | 4.10 |
| Δ13C (±SD, n = 3) | ‰ | −13.60 ± 0.2 | −12.38 ± 0.1 | −27.13 ± 0.1 |
| H:C (atomic) | 0.18 | - | - | |
| O:C (atomic) | 0.06 | - | - |
| BD (1–8 cm) | BD (12–19 cm) | Total Porosity (%) | |
|---|---|---|---|
| 2012 | |||
| Control | 1.30 ± 0.02 a | - | 50.87 ± 0.84 a |
| MC8 | 1.19 ± 0.04 a | - | 54.99 ± 1.32 a |
| BC8 | 1.16 ± 0.05 a | - | 56.33 ± 2.03 a |
| BC25 | 1.22 ± 0.07 a | - | 53.87± 2.56 a |
| 2014 | |||
| Control | 1.30 ± 0.02 a | 1.36 ± 0.02 a | 49.93 ± 0.66 a |
| BC8 | 1.29 ± 0.02 a | 1.38 ± 0.03 a | 49.67 ± 1.04 a |
| BC25 | 1.21 ± 0.03 b | 1.26 ± 0.02 b | 53.27 ± 0.81 b |
| 2011—Oat | 2012—Barley | 2013—Oat | 2014—Oat | |
|---|---|---|---|---|
| Grain | t ha−1 | t ha−1 | t ha−1 | t ha−1 |
| Control | 5.33 ± 0.36 | 3.76 ± 0.94 | 4.84 ± 0.16 | 1.92 ± 0.10 |
| MC8 | 5.24 ± 0.24 | 3.77 ± 0.45 | 4.58 ± 0.17 | 1.93 ± 0.14 |
| BC8 | 5.13 ± 0.09 | 4.07 ± 0.65 | 4.92 ± 0.24 | 1.88 ± 0.25 |
| BC25 | 5.64 ± 0.27 | 3.96 ± 0.73 | 4.84 ± 0.11 | 2.04 ± 0.50 |
| Straw | t ha−1 | t ha−1 | t ha−1 | t ha−1 |
| Control | 3.10 ± 0.20 | 1.38 ± 0.19 | - | 0.99 ± 0.12 |
| MC8 | 2.65 ± 0.22 | 1.66 ± 0.05 | - | 1.01 ± 0.86 |
| BC8 | 2.96 ± 0.98 | 1.47 ± 0.25 | - | 0.94 ± 0.14 |
| BC25 | 3.06 ± 0.18 | 1.68 ± 0.12 | - | 1.03 ± 0.09 |
| 1000 grain weight | g | g | ||
| Control | - | 37.04 ± 0.37 | - | 32.56 ± 0.54 |
| BC25 | - | 37.74 ± 0.16 | - | 33.11 ± 0.31 |
| Grain protein | % | % | ||
| Control | - | 9.85 ± 0.23 | - | 9.80 ± 0.44 |
| BC25 | - | 10.33 ± 0.25 | - | 9.02 ± 0.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Toole, A.; Moni, C.; Weldon, S.; Schols, A.; Carnol, M.; Bosman, B.; Rasse, D.P. Miscanthus Biochar had Limited Effects on Soil Physical Properties, Microbial Biomass, and Grain Yield in a Four-Year Field Experiment in Norway. Agriculture 2018, 8, 171. https://doi.org/10.3390/agriculture8110171
O’Toole A, Moni C, Weldon S, Schols A, Carnol M, Bosman B, Rasse DP. Miscanthus Biochar had Limited Effects on Soil Physical Properties, Microbial Biomass, and Grain Yield in a Four-Year Field Experiment in Norway. Agriculture. 2018; 8(11):171. https://doi.org/10.3390/agriculture8110171
Chicago/Turabian StyleO’Toole, Adam, Christophe Moni, Simon Weldon, Anne Schols, Monique Carnol, Bernard Bosman, and Daniel P. Rasse. 2018. "Miscanthus Biochar had Limited Effects on Soil Physical Properties, Microbial Biomass, and Grain Yield in a Four-Year Field Experiment in Norway" Agriculture 8, no. 11: 171. https://doi.org/10.3390/agriculture8110171
APA StyleO’Toole, A., Moni, C., Weldon, S., Schols, A., Carnol, M., Bosman, B., & Rasse, D. P. (2018). Miscanthus Biochar had Limited Effects on Soil Physical Properties, Microbial Biomass, and Grain Yield in a Four-Year Field Experiment in Norway. Agriculture, 8(11), 171. https://doi.org/10.3390/agriculture8110171





