Effect of Saline Irrigation on Accumulation of Na+, K+, Ca2+, and Mg2+ Ions in Rice Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Soil
2.2. Preparation of Pot and Experimental Site
2.3. Irrigation
2.4. Experimental Design, Data Collection and Statistical Analysis
3. Results
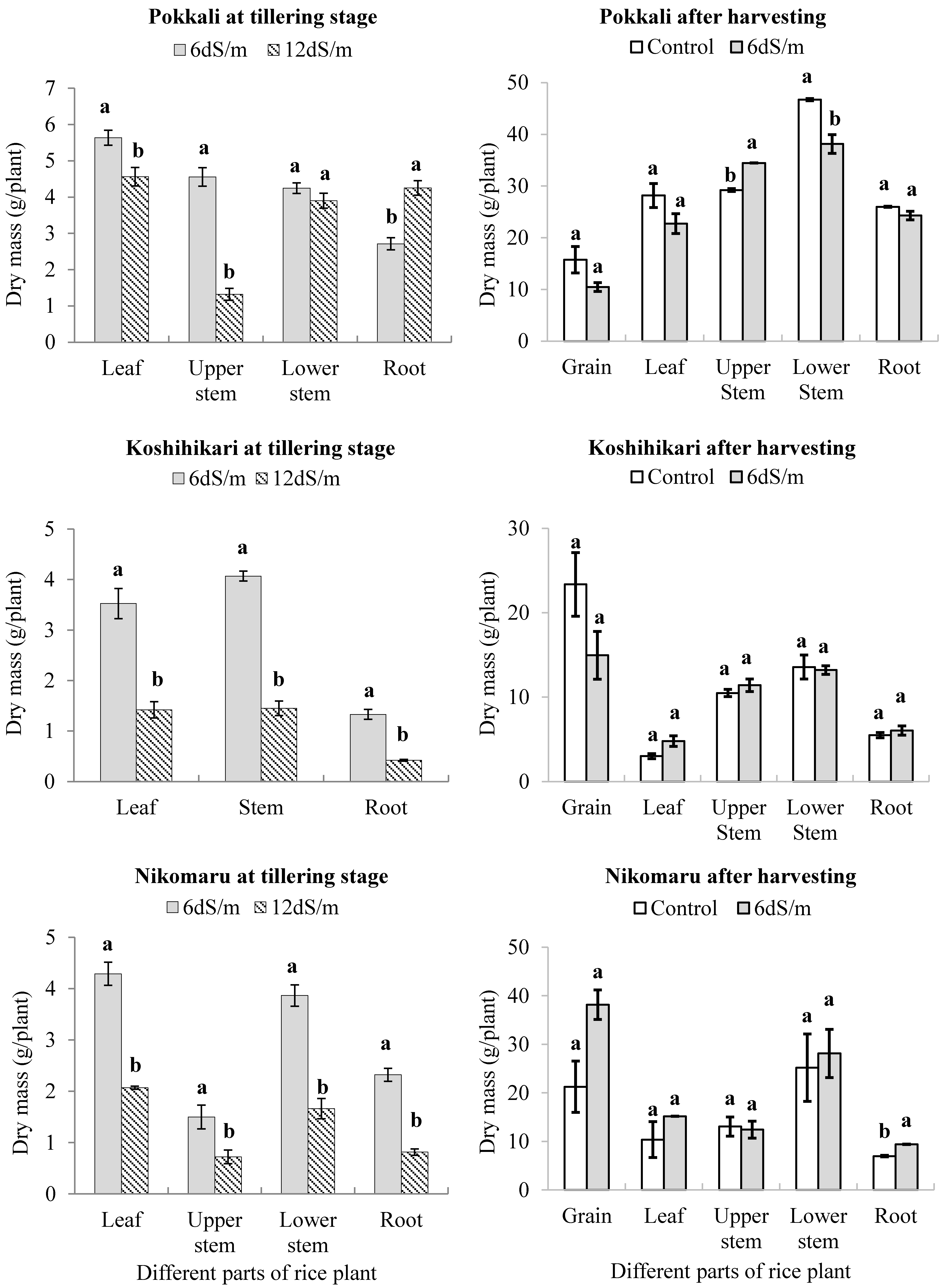
3.1. Effect of Saline Irrigation on the Dry Mass of the Three Rice Cultivars
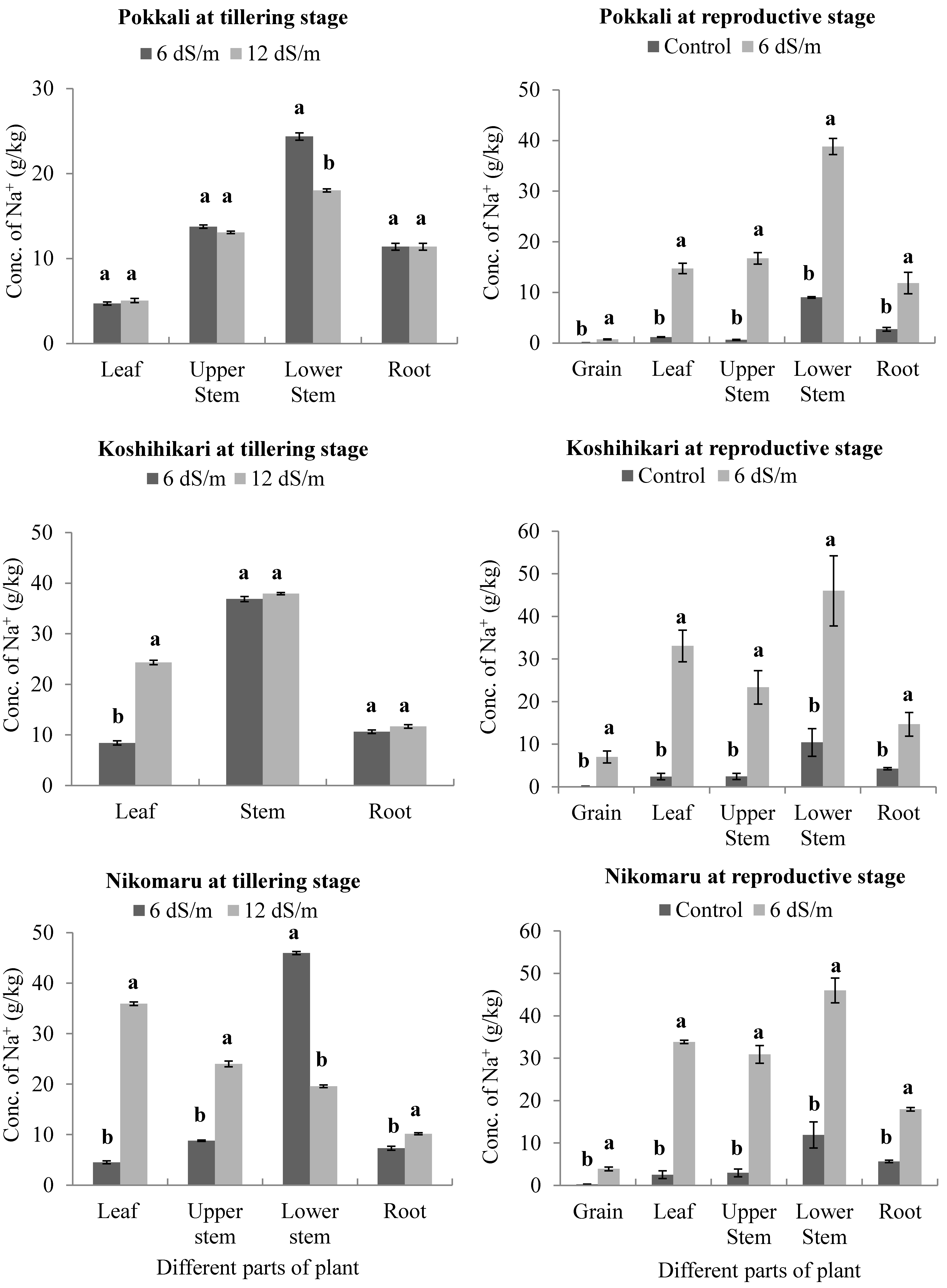
3.2. Effect of Saline Irrigation on the Accumulation of Sodium (Na+) Ions in Different Parts of the Rice Plant Body
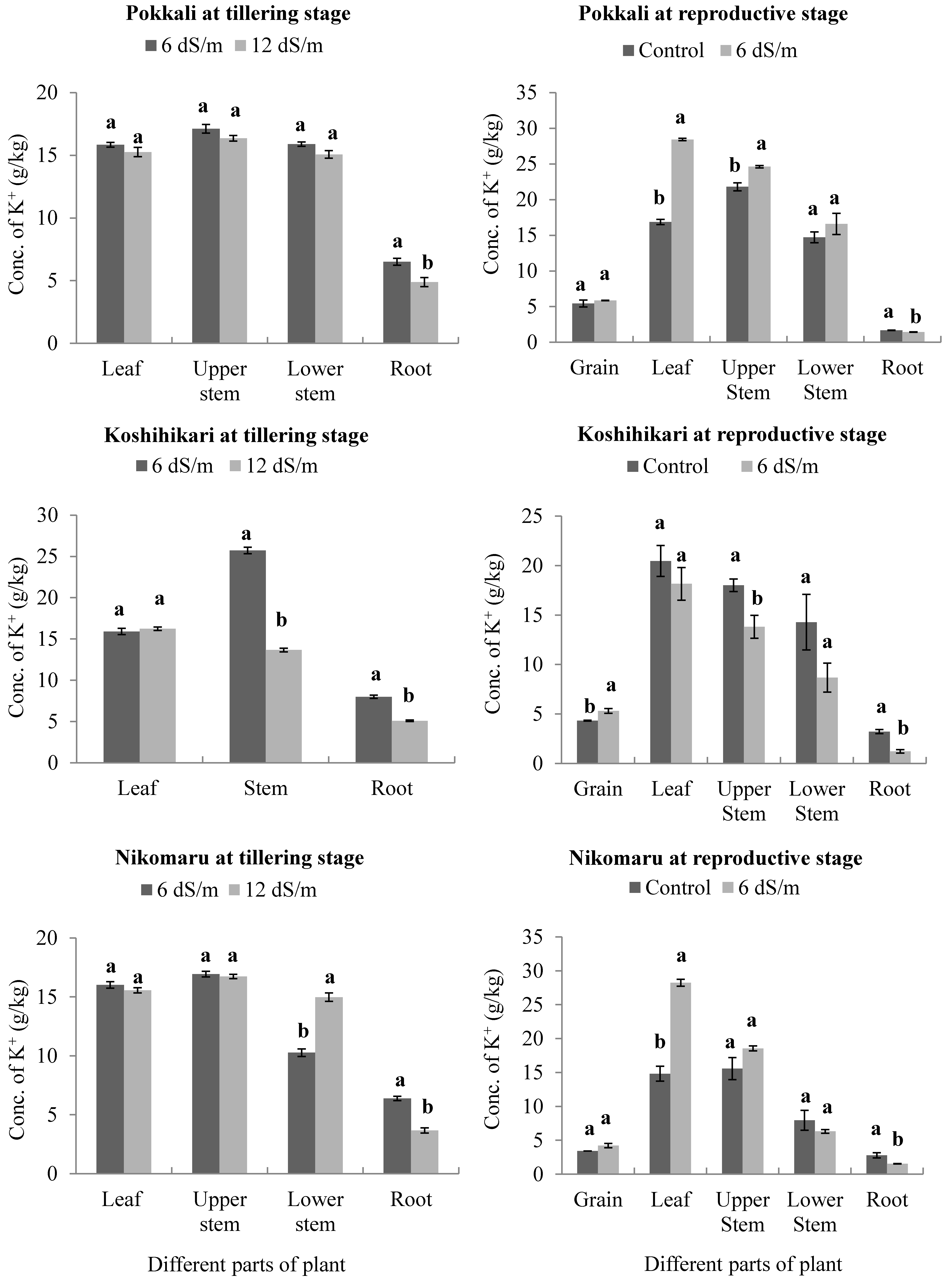
3.3. Effect of Saline Irrigation on the Accumulation of Potassium (K+) Ions in Different Parts of the Rice Plant Body
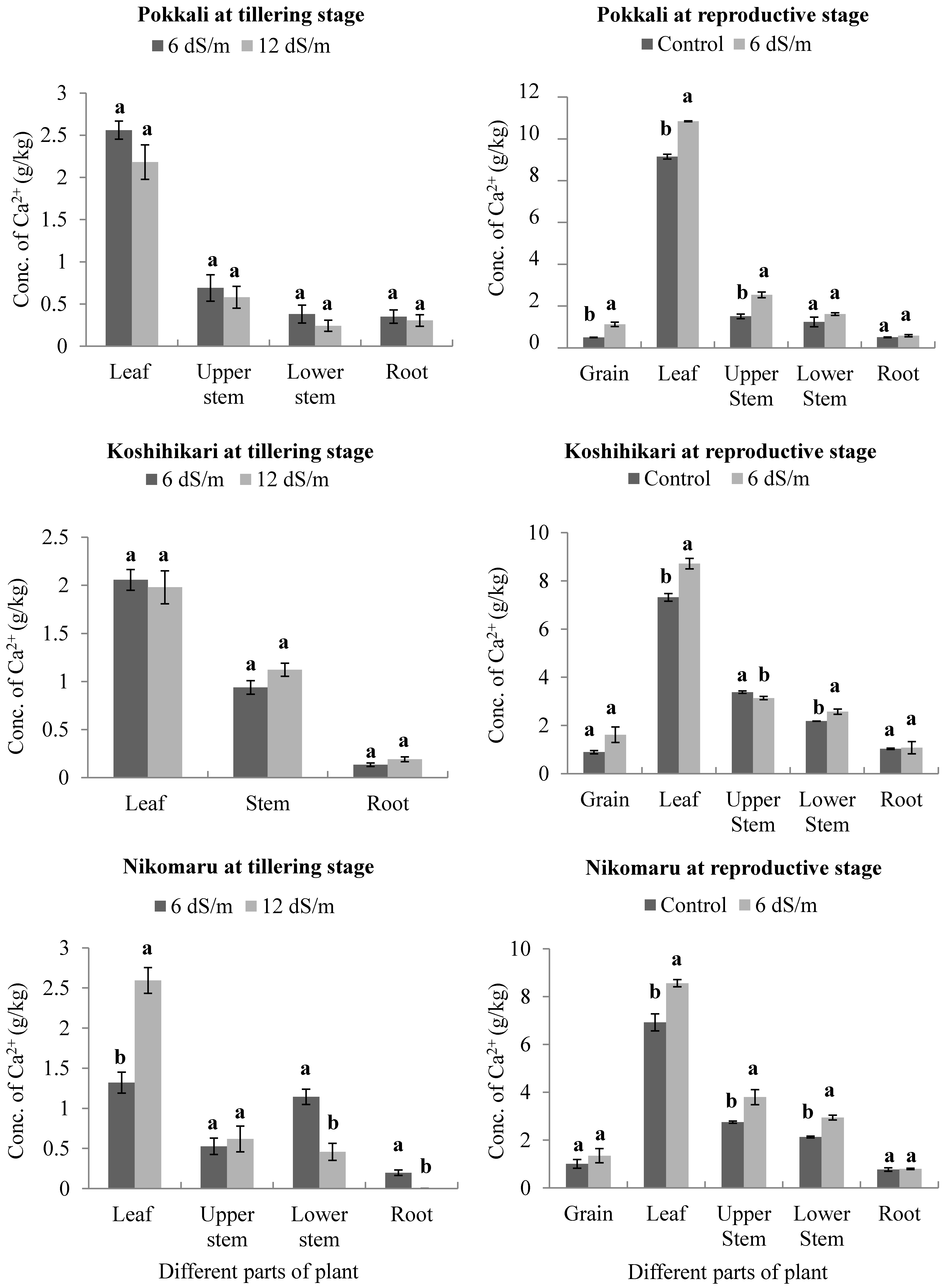
3.4. Effects of Saline Irrigation on the Accumulation of Calcium (Ca2+) Ions in Different Parts of the Rice Plant Body
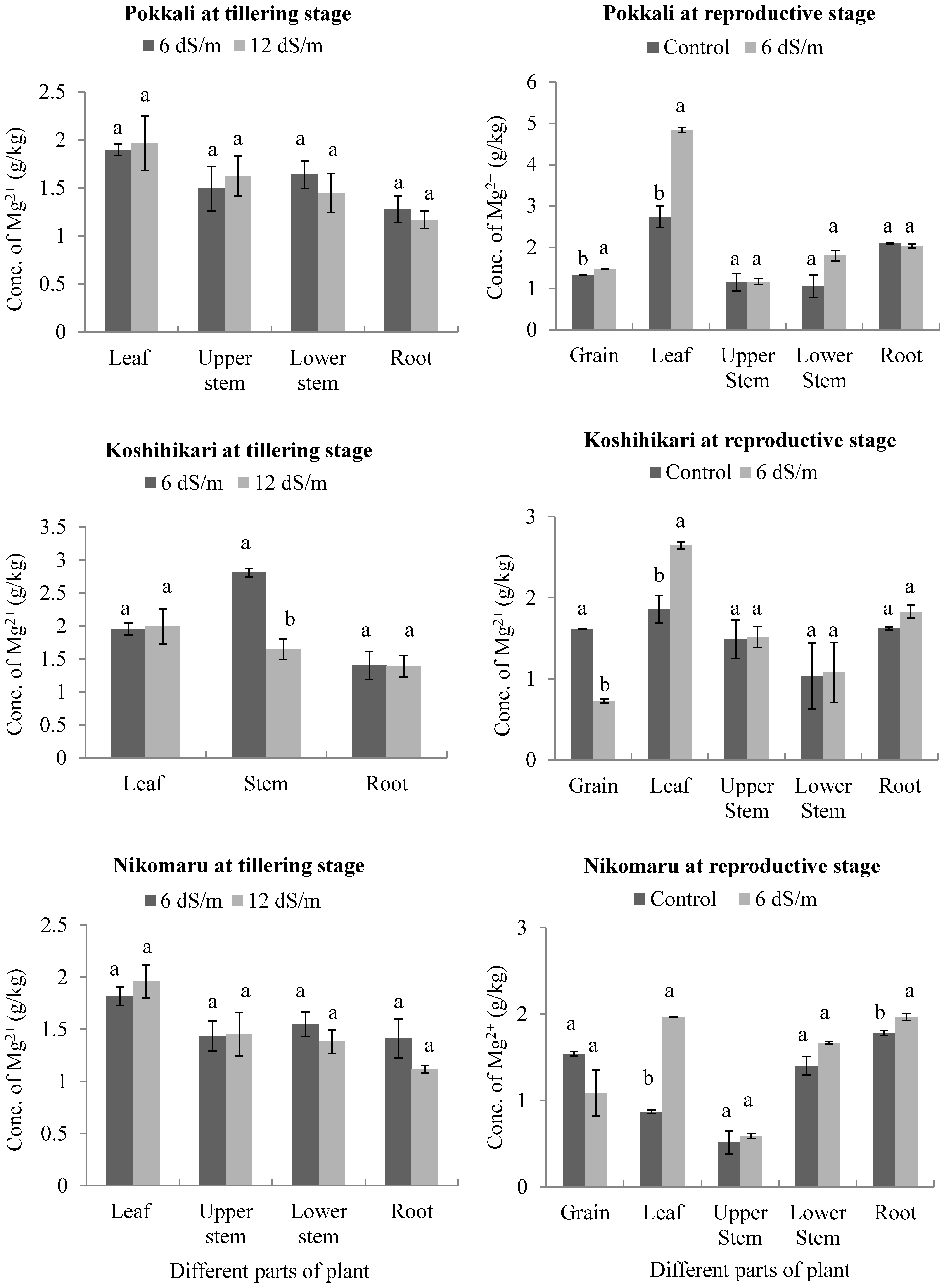
3.5. Effects of Saline Irrigation on the Accumulation of Magnesium (Mg2+) Ions in Different Parts of the Rice Plant Body
3.6. Effects of Saline Irrigation on the Na+/K+ Ratio in Different Parts of the Rice Plant Body
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karki, S.; Rizal, G.; Quick, W.P. Improvement of photosynthesis in rice (Oryza sativa L.) by inserting the C4 pathway. Rice 2013, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO’s Director-General on how to feed the World in 2050. Popul. Dev. Rev. 2009, 35, 837–839. [Google Scholar] [CrossRef]
- Ismail, A.M.; Heuer, S.; Thomson, M.J.; Wissuwa, M. Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Mol. Biol. 2007, 65, 547–570. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Thomson, M.J.; Vergara, G.V.; Rahman, M.A.; Singh, R.K.; Gregorio, G.B.; Mackill, D.J. 12 Designing Resilient Rice Varieties for Coastal Deltas Using Modern Breeding Tools. In Tropical Deltas and Coastal Zones: Food Production, Communities and Environment at the Land-Water Interface; CABI: Wallingford, UK, 2010; pp. 154–165. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Redoña, E.; Refuerzo, L. Varietal improvement for abiotic stress tolerance in crop plants: Special reference to salinity in rice. In Abiotic Stress Adaptation in Plants; Springer: Dordrecht, The Netherlands, 2009; pp. 387–415. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L. Response and correlated response to salt tolerance selection in rice by yield parameters. Cereal Res. Commun. 2004, 32, 477–484. [Google Scholar]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Zeng, L.; Wanamaker, S.I.; Mandal, J.; Xu, J.; Cui, X.; et al. Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol. 2005, 139, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, I.; Stölzle, S.; Ivashikina, N.; Hedrich, R. Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta 2005, 221, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Alemán, F.; Martínez, V.; Rubio, F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant 2010, 3, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.; Shin, R. Transport, signaling, and homeostasis of potassium and sodium in plants. J. Integr. Plant Biol. 2014, 56, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, S.; Wang, R.; Chen, S.; Li, N.; Zhou, X.; Lu, C.; Shen, X.; Zheng, X.; Hu, Z.; et al. Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol. 2009, 29, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007, 59, 173–178. [Google Scholar] [CrossRef]
- Kent, L.M.; Läuchli, A. Germination and seedling growth of cotton: Salinity-calcium interactions. Plant Cell Environ. 2006, 8, 155–159. [Google Scholar] [CrossRef]
- Koksal, N.; Alkan-Torun, A.; Kulahlioglu, I.; Ertargin, E.; Karalar, E. Ion uptake of marigold under saline growth conditions. SpringerPlus 2016, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Plant Salt Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Asch, F.; Dingkuhn, M.; Dörffling, K.; Miezan, K. Leaf K/Na ratio predicts salinity induced yield loss in irrigated rice. Euphytica 2000, 113, 109–118. [Google Scholar] [CrossRef]
- Yeo, A.R.; Yeo, M.E.; Flowers, S.A.; Flowers, T.J. Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theor. Appl. Genet. 1990, 79, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Weimberg, R.; Shannon, M.C. Vigor and salt tolerance in 3 lines of tall wheatgrass. Physiol. Plant 1988, 73, 232–237. [Google Scholar] [CrossRef]
- Asch, F.; Dingkuhn, M.; Dorffling, K. Salinity increases CO2 assimilation but reduces growth in field-grown, irrigated rice. Plant Soil 2000, 218, 1–10. [Google Scholar] [CrossRef]
- Chandramohanan, K.T.; Mohanan, K.V. Rice cultivation in the saline wetlands of Kerala—An overview. In Proceedings of the IInd National Seminar on Genetics, Breeding and Biotechnology (Gregor Mendel Foundation Proceedings 2011), Kerala, India, 16–17 December 2011; pp. 7–12. [Google Scholar]
- Rahman, M.A.; Thomson, M.J.; Shahealam, M.; De, O.M.; Egdane, J.; Ismail, A.M. Exploring novel genetic sources of salinity tolerance in rice through molecular and physiological characterization. Ann. Bot. 2016, 117, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.A.; Juraimi, A.S.; Hanafi, M.M.; Ismail, M.R.; Rafii, M.Y.; Islam, M.M.; Selamat, A. The effect of salinity on growth, ion accumulation and yield of rice varieties. J. Anim. Plant Sci. 2014, 24, 874–885. [Google Scholar]
- Platten, J.D.; Egdane, J.A.; Ismail, A.M. Salinity tolerance, Na+ exclusion and allele mining of HKT1; 5 in Oryza sativa and O. glaberrima: Many sources, many genes, one mechanism? BMC Plant Biol. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Aguilar, L.A.; Grieve, C.M.; Poss, J.; Layfield, D.A. Salinity and alkaline pH in irrigation water affect marigold plants: II. Mineral ion relations. HortScience 2009, 44, 1726–1735. [Google Scholar]
- Carter, C.T.; Grieve, C.M.; Poss, J.A.; Suarez, D.L. Production and ion uptake of Celosia argentea, irrigated with saline wastewaters. Sci. Hortic. 2005, 106, 381–394. [Google Scholar] [CrossRef]
- Razzaque, M.A.; Talukder, N.M.; Islam, M.S.; Bhadra, A.K.; Dutta, R.K. The effect of salinity on morphological characteristics of seven rice (Oryza sativa) genotypes differing in salt tolerance. Pak. J. Biol. Sci. 2009, 12, 406–412. [Google Scholar] [PubMed]
- Momayezi, M.R.; Zaharah, A.R.; Hanafi, M.M.; Mohd Razi, I. Agronomic characteristics and proline accumulation of Iranian rice genotypes at early seedling stage under sodium salts stress. Malays. J. Soil Sci. 2009, 13, 59–75. [Google Scholar]
- Amirjani, M.R. Effect of salinity stress on growth, mineral composition, proline content, antioxidant enzymes of soybean. Am. J. Plant Physiol. 2010, 5, 350–360. [Google Scholar] [CrossRef]
- Summart, J.; Thanonkeo, P.; Panichajakul, S.; Prathepha, P.; McManus, M.T. Effect of salt stress on growth, inorganic ion and proline accumulation in Thai aromatic rice, Khao Dawk Mali 105, callus culture. Afr. J. Biotechnol. 2010, 9, 145–151. [Google Scholar]
- Din, J.; Khan, S.U.; Ali, I. Physiological response of wheat (Triticum aesitivum L.) varieties as influenced by salinity stress. J. Anim. Plant Sci. 2008, 18, 125–129. [Google Scholar]
- Shah, S.H.; Gorham, J.; Forster, B.P.; Wyn Jones, R.G. Salt tolerance in the Triticeae: The contribution of the D genome to cation selectivity in hexaploid wheat. J. Exp. Bot. 1987, 38, 254–269. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 2006. [Google Scholar]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
| Cultivar | Type of Irrigation | Na+/K+ Ratio in Different Plant Parts in the Tillering Stage | |||
|---|---|---|---|---|---|
| Leaf | Upper Stem | Lower Stem | Root | ||
| Pokkali | 6 dS/m | 0.297 ± 0.008 a | 0.803 ± 0.004 a | 1.534 ± 0.044 a | 1.761 ± 0.135 a |
| 12 dS/m | 0.332 ± 0.025 a | 0.799 ± 0.002 a | 1.196 ± 0.012 b | 2.369 ± 0.260 a | |
| p-value (0.05) | 0.2495 | 0.4501 | 0.0018 | 0.1060 | |
| CV * | 10.02 | 0.73 | 4.08 | 17.36 | |
| Koshihikari | 6 dS/m | 0.530 ± 0.038 b | 1.433 ± 0.003 b | 1.329 ± 0.011 b | |
| 12 dS/m | 1.497 ± 0.008 a | 2.775 ± 0.029 a | 2.299 ± 0.112 a | ||
| p-value (0.05) | 0.0001 | 0.0001 | 0.0010 | ||
| CV * | 4.69 | 1.67 | 7.62 | ||
| Nikomaru | 6 dS/m | 0.281 ± 0.013 b | 0.519 ± 0.001 b | 4.488 ± 0.170 a | 1.143 ± 0.031 b |
| 12 dS/m | 2.310 ± 0.010 a | 1.436 ± 0.050 a | 1.310 ± 0.047 b | 2.783 ± 0.112 a | |
| p-value (0.05) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| CV * | 1.53 | 6.22 | 7.46 | 7.25 | |
| Cultivar | Irrigation | Na+/K+ Ratio in Different Plant Parts in the Reproductive Stage | ||||
|---|---|---|---|---|---|---|
| Grain | Leaf | Upper Stem | Lower Stem | Root | ||
| Pokkali | Control | 0.006 ± 0.003 b | 0.070 ± 0.001 b | 0.029 ± 0.005 b | 0.617 ± 0.042 b | 1.626 ± 0.252 b |
| 6 dS/m | 0.122 ± 0.017 a | 0.518 ± 0.033 a | 0.680 ± 0.050 a | 2.396 ± 0.314 a | 8.210 ± 1.250 a | |
| p-value (0.05) | 0.0025 | 0.0002 | 0.0002 | 0.0050 | 0.0067 | |
| CV * | 32.65 | 13.62 | 17.35 | 25.77 | 31.75 | |
| Koshihikari | Control | 0.033 ± 0.008 b | 0.124 ± 0.046 b | 0.138 ± 0.046 b | 0.895 ± 0.428 a | 1.356 ± 0.121 b |
| 6 dS/m | 1.268 ± 0.238 a | 1.814 ± 0.040 a | 1.764 ± 0.437 a | 5.991 ± 2.051 a | 14.102 ± 3.287 a | |
| p-value (0.05) | 0.0004 | 0.0001 | 0.0208 | 0.0718 | 0.0031 | |
| CV * | 63.45 | 7.74 | 56.55 | 74.54 | 73.72 | |
| Nikomaru | Control | 0.069 ± 0.023 b | 0.181 ± 0.075 b | 0.206 ± 0.081 b | 1.773 ± 0.753 b | 2.158 ± 0.425 b |
| 6 dS/m | 0.920 ± 0.032 a | 1.200 ± 0.036 a | 1.663 ± 0.079 a | 7.359 ± 0.783 a | 11.760 ± 0.613 a | |
| p-value (0.05) | 0.0001 | 0.0003 | 0.0002 | 0.0068 | 0.0002 | |
| CV * | 9.78 | 14.77 | 14.79 | 29.14 | 13.13 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, M.; Oue, H. Effect of Saline Irrigation on Accumulation of Na+, K+, Ca2+, and Mg2+ Ions in Rice Plants. Agriculture 2018, 8, 164. https://doi.org/10.3390/agriculture8100164
Akter M, Oue H. Effect of Saline Irrigation on Accumulation of Na+, K+, Ca2+, and Mg2+ Ions in Rice Plants. Agriculture. 2018; 8(10):164. https://doi.org/10.3390/agriculture8100164
Chicago/Turabian StyleAkter, Mahjuba, and Hiroki Oue. 2018. "Effect of Saline Irrigation on Accumulation of Na+, K+, Ca2+, and Mg2+ Ions in Rice Plants" Agriculture 8, no. 10: 164. https://doi.org/10.3390/agriculture8100164
APA StyleAkter, M., & Oue, H. (2018). Effect of Saline Irrigation on Accumulation of Na+, K+, Ca2+, and Mg2+ Ions in Rice Plants. Agriculture, 8(10), 164. https://doi.org/10.3390/agriculture8100164




