Spinach (Spinacea oleracea L.) Response to Salinity: Nutritional Value, Physiological Parameters, Antioxidant Capacity, and Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Irrigation and Treatments
2.2. Plant and Soil Mineral Analysis
2.3. Oxygen Radical Absorbance Capacity (ORAC), Total Phenolics and Oxalate analyses
2.4. Leaf Photosynthetic Performance
2.5. Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Salt Increase in Spinach Tissues and Mineral Tissue Composition
3.2. Shoot Biomass, Antioxidant Capacity, and Oxalic Acid
3.3. Leaf Photosynthetic Performance
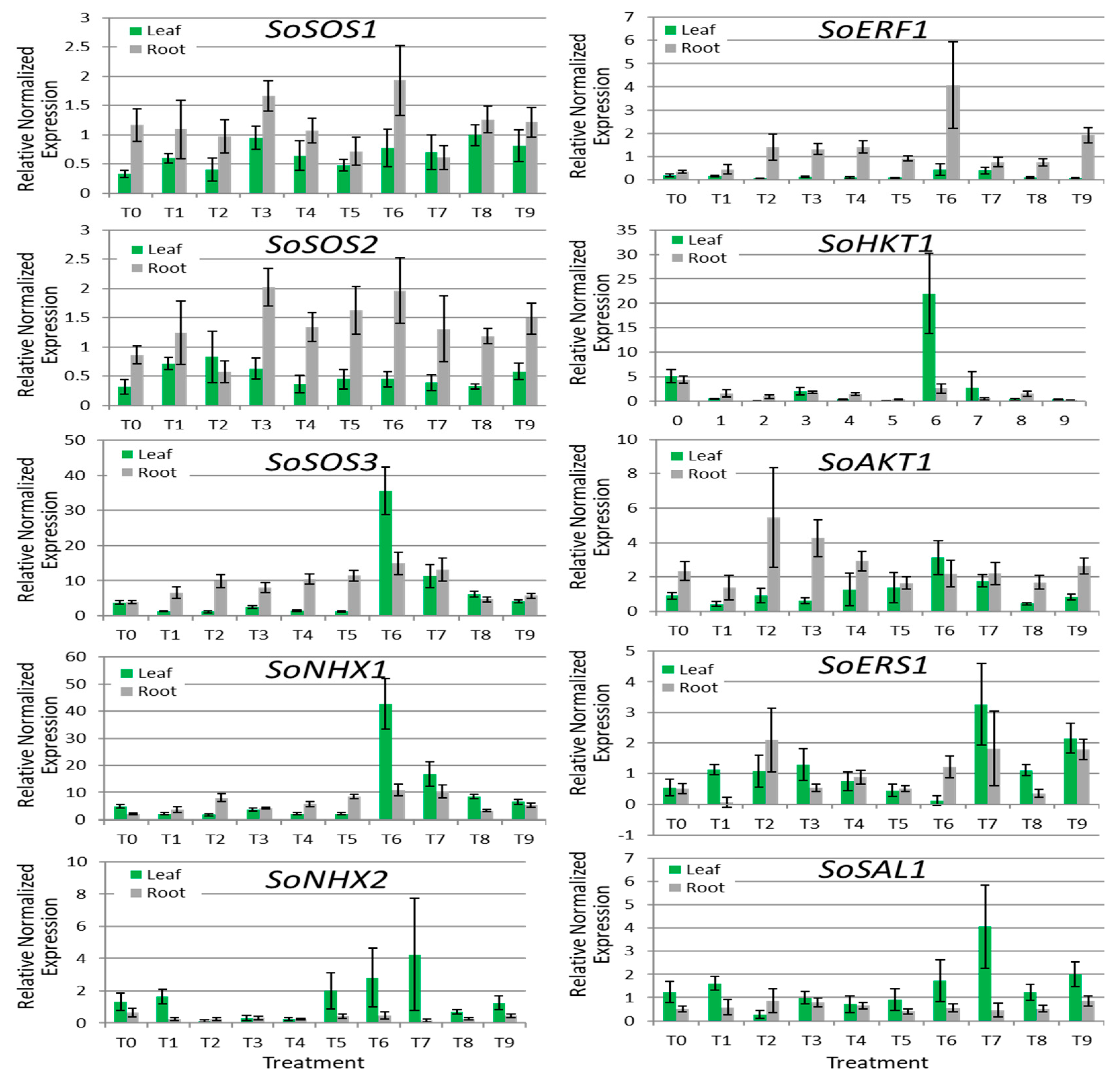
3.4. Gene Expression Analyses
4. Discussion
4.1. Salt Increase in Spinach Tissues and Mineral Tissue Composition
4.2. Shoot Biomass, Antioxidant Capacity, and Oxalic Acid
4.3. Leaf Photosynthetic Performance
4.4. Gene Expression Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heaney, R.P.; Weaver, C.M.; Recker, R.R. Calcium absorbability from spinach. Am. J. Clin. Nutr. 1988, 47, 707–709. [Google Scholar] [CrossRef] [PubMed]
- McConn, M.M.; Nakata, P.A. Oxalate reduces calcium availability in the pads of the prickly pear cactus through formation of calcium oxalate crystals. J. Agric. Food Chem. 2004, 52, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Ors, S.; Suarez, D.L. Spinach biomass yield and physiological response to interactive salinity and water stress. Agric. Water Manag. 2017, 190, 31–41. [Google Scholar] [CrossRef]
- Scudiero, E.; Corwin, D.L.; Anderson, R.G.; Yemoto, K.; Clary, W.; Wang, Z.; Skaggs, T.H. Remote sensing is a viable tool for mapping soil salinity in agricultural lands. Calif. Agric. 2017, 71, 231–238. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity–mineral nutrient relations in horticultural crops. Sci. Hort. 1999, 78, 127–157. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Marschner, H.; Possingham, J.V. Effect of K+ and Na+ on growth of leaf discs of sugar beet and spinach. Z. Pflanzenphysiol. 1975, 75, 6–16. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A functional plant nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar] [CrossRef]
- Fisarakis, I.; Chartzoulakis, K.; Stavrakas, D. Response of Sultana vines (V. vinifera L.) on six rootstocks to NaCl salinity exposure and recovery. Agric. Water Manag. 2001, 51, 13–27. [Google Scholar] [CrossRef]
- Chow, W.S.; Ball, M.C.; Anderson, J.M. Growth and photosynthetic responses of spinach to salinity: Implications of K+ nutrition for salt tolerance. Func. Plant Biol. 1990, 17, 563–578. [Google Scholar] [CrossRef]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Alam, M.Z. Effects of salinity and salinity-induced augmented bioactive compounds in purslane (Portulaca oleracea L.) for possible economical use. Food Chem. 2015, 169, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, D.; Cornacchione, M.V.; Ferreira, J.F.; Suarez, D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 2017, 7, 42958. [Google Scholar] [CrossRef] [PubMed]
- Nemadodzi, L.E.; Araya, H.; Nkomo, M.; Ngezimana, W.; Mudau, N.F. Nitrogen, phosphorus, and potassium effects on the physiology and biomass yield of baby spinach (Spinacia oleracea L.). J. Plant Nutr. 2017, 40, 2033–2044. [Google Scholar] [CrossRef]
- Ors, S.; Suarez, D.L. Salt tolerance of spinach as related to seasonal climate. Hort. Sci. (Prague) 2016, 43, 33–41. [Google Scholar] [CrossRef]
- Koike, S.T.; Cahn, M.; Cantwell, M.; Fennimore, S.; Lestrange, M.; Natwick, E.; Smith, R.F.; Takele, E. Spinach Production in California; UC Agriculture and Natural Resources: Davis, CA, USA, 2011; pp. 1–6. [Google Scholar]
- Simunek, J.; Suarez, D.L. Sodic soil reclamation using multicomponent transport modeling. J. Irrig. Drain. Eng. 1997, 123, 367–376. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity [oxygen radical absorbance capacity (ORAC)] of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total Phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Xu, C.; Jiao, C.; Sun, H.; Cai, X.; Wang, X.; Ge, C.; Zheng, Y.; Liu, W.; Sun, X.; Xu, Y.; et al. Draft genome of spinach and transcriptome diversity of 120 Spinacia accessions. Nat. Commun. 2017, 8, 15275. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, S.; Kasuga, A.; Aoyagi, Y. Nitrogen-to-protein conversion factors for common vegetables in Japan. J. Food Sci. 2001, 66, 412–415. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional value. J. Am. Soc. Hort. Sci. 2016, 141, 12–21. [Google Scholar]
- Sheikhi, J.; Ronaghi, A. Growth and macro and micronutrients concentration in spinach (Spinacia oleracea L.) as influenced by salinity and nitrogen rates. Int. Res. J. Appl. Basic Sci. 2012, 3, 770–777. [Google Scholar]
- Gülser, F. Effects of ammonium sulphate and urea on NO3− and NO2− accumulation, nutrient contents and yield criteria in spinach. Sci. Hortic. 2005, 106, 330–340. [Google Scholar] [CrossRef]
- Aisha, H.A.; Hafez, M.M.; Asmaa, R.M.; Shafeek, M.R. Effect of bio and chemical fertilizers on growth, yield and chemical properties of spinach plant (Spinacia oleracea L.). Middle East J. 2013, 2, 16–20. [Google Scholar]
- Howard, L.R.; Pandjaitan, N.; Morelock, T.; Gil, M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J. Agric. Food Chem. 2002, 50, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- Pandjaitan, N.; Howard, L.R.; Morelock, T.; Gil, M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and maturation. J. Agric. Food Chem. 2005, 53, 8618–8623. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Kevers, C.; Tabart, J.; Defraigne, J.O.; Dommes, J. Cultivars, culture conditions, and harvest time influence phenolic and ascorbic acid contents and antioxidant capacity of strawberry (Fragaria × ananassa). J. Food Sci. 2012, 77, C205–C210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trend. Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.R.; Horner, H.T. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Mou, B. Evaluation of oxalate concentration in the U.S. spinach germplasm collection. HortScience 2008, 43, 1690–1693. [Google Scholar]
- Zhang, Y.; Li, Y.; Wei, J.; Sun, M.; Tian, Y.; Li, Z. Effects of nitrogen and calcium nutrition on oxalate contents, forms, and distribution in spinach. J. Plant Nutr. 2009, 32, 2123–2139. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the Move. Trend. Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.L.; Grieve, C.M. Growth, yield, and ion relations of strawberry in response to irrigation with chloride-dominated waters. J. Plant Nutr. 2013, 36, 1963–1981. [Google Scholar] [CrossRef]
- Tregeagle, J.M.; Tisdall, J.M.; Tester, M.; Walker, R.R. Cl− uptake, transport and accumulation in grapevine rootstocks of differing capacity for Cl− exclusion. Funct. Plant Biol. 2010, 37, 665–673. [Google Scholar] [CrossRef]

| Treatment | [Na+] | [Cl−] | [K+] | ECiw | NO3− | PO4−3 | HCO3− | Ca+2 | Mg+2 | SO4−2 |
|---|---|---|---|---|---|---|---|---|---|---|
| (meq L−1) | (dS m−1) | (meq L−1) | ||||||||
| City water | 2 | 1 | 2 | 0.6 | 0.38 | 0.001 | 3.3 | 3.2 | 0.78 | 1.4 |
| T0 + (control) | 2 | 1 | 3 | 1.4 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T1 | 20 | 19 | 3 | 3.4 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T2 | 20 | 21 | 5 | 3.7 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T3 | 20 | 23 | 7 | 3.8 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T4 | 40 | 39 | 3 | 5.6 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T5 | 40 | 41 | 5 | 6.0 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T6 | 40 | 43 | 7 | 6.0 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T7 | 80 | 79 | 3 | 9.3 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T8 | 80 | 81 | 5 | 9.3 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| T9 | 80 | 83 | 7 | 9.8 | 7.50 | 1.500 | 3.3 | 7.0 | 2.00 | 2.0 |
| Treatment | [NaCl] | [K+] | EC | Na | Cl | K | N | Ca | Mg | S | P | Plant DW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (meq L−1) | (dS m−1) | (g kg−1 DW) | (g plant−1) | |||||||||
| Roots | ||||||||||||
| T0 (control) | 2 | 3 | 1.4 | 2.9cγ | 6.9cδ | 41.0bα | 26.1aα | 2.9bα | 5.3aαβ | 2.0aα | 5.7aα | 0.45aαβ |
| T1 | 20 | 3 | 3.4 | 4.1cA | 22.4bA | 49.9aA | 25.3aA | 2.7cA | 5.0aA | 1.7aA | 6.5aA | 0.45aA |
| T2 * | 20 | 5 | 3.7 | 2.6 | 14.0 | 23.8 | - | 2.4 | 4.1 | 1.1 | 0.7 | 0.14 |
| T3 | 20 | 7 | 3.8 | 2.3bB | 26.2aA | 41.1aA | - | 2.1aA | 3.5aB | 1.4aB | 4.7aB | 0.30bB |
| Avg (T1–T3) | 3.2γ | 24.3γ | 45.5α | 25.3α | 2.4α | 4.2β | 1.5α | 5.6α | 0.38αβ | |||
| T4 | 40 | 3 | 5.6 | 6.9bA | 29.9abA | 46.4abA | 27.8aA | 3.2aA | 5.5aA | 1.9aA | 7.1aA | 0.35aA |
| T5 | 40 | 5 | 6.0 | 6.0bA | 32.0aA | 49.1aA | - | 2.5aA | 5.0aA | 1.8aA | 6.3aA | 0.31aA |
| T6 | 40 | 7 | 6.0 | 5.6abA | 35.5aA | 51.4aA | - | 2.2aA | 5.0aA | 1.8aA | 7.0aA | 0.29bA |
| Avg (T4–T6) | 6.2β | 32.5β | 49.0α | 27.8α | 2.6α | 5.2αβ | 1.8α | 6.8α | 0.32β | |||
| T7 | 80 | 3 | 9.3 | 9.9aA | 38.5aA | 51.0aA | 29.0aA | 2.6cA | 5.9aA | 2.0aA | 7.7aA | 0.40aA |
| T8 | 80 | 5 | 9.3 | 9.4aA | 38.3aA | 50.8aA | 25.1 | 2.3aA | 5.6aA | 1.9aA | 7.3aA | 0.46aA |
| T9 | 80 | 7 | 9.8 | 8.4aA | 37.4aA | 51.7aA | 25.8 | 2.5aA | 5.2aA | 1.7aA | 6.2aA | 0.5aA |
| Avg (T7–T9) | 9.3α | 38.1α | 51.2α | 26.6α | 2.5α | 5.6α | 1.9α | 7.1α | 0.46α | |||
| Shoots | ||||||||||||
| T0 (control) | 2 | 3 | 1.4 | 4.0dδ | 10.1dδ | 59.2aβγ | 40.7aα | 7.7aα | 11.8aα | 3.9aα | 3.6aβ | 4.7aα |
| T1 | 20 | 3 | 3.4 | 11.1cA | 21.9cA | 59.9aB | 41.8aA | 6.7aAB | 13.3aA | 4.0aA | 3.8aA | 4.7aA |
| T2* | 20 | 5 | 3.7 | 7.3 | - | 59.2 | 39.0 | 8.2 | 13.7 | 3.4 | 0.9 | 1.6 |
| T3 | 20 | 7 | 3.8 | 7.5cB | 22.8bA | 74.8aA | 43.7aA | 4.9aB | 10.4aB | 3.9aA | 4.4aA | 4.0aA |
| Avg (T1–T3) | 9.3γ | 22.4γ | 67.4αβ | 42.8α | 5.8α | 11.9α | 3.9α | 4.1αβ | 4.4α | |||
| T4 | 40 | 3 | 5.6 | 19.5bA | 29.7bA | 61.5aB | 44.2aA | 5.9aA | 12.8aAB | 3.8aA | 4.4aA | 4.1aA |
| T5 | 40 | 5 | 6.0 | 17.3bA | 31.4bA | 70.2aAB | 43.4aA | 6.5aA | 13.7aA | 4.3aA | 4.7aA | 4.1aA |
| T6 | 40 | 7 | 6.0 | 15.3bA | 28.9bA | 78.2aA | 45.2aA | 5.8aA | 11.5aB | 4.2aA | 4.6aA | 3.7aA |
| Avg (T4–T6) | 17.4β | 30.0β | 70.0α | 44.3α | 6.1α | 12.7α | 4.1α | 4.6α | 4.0α | |||
| T7 | 80 | 3 | 9.3 | 26.7aA | 39.9aA | 55.9aA | 46.1aA | 5.3aA | 12.5aA | 4.2aA | 3.9aA | 4.1aA |
| T8 | 80 | 5 | 9.3 | 27.7aA | 39.5aA | 53.7aA | 46.1aA | 5.3aA | 13.8aA | 4.1abA | 4.3aA | 3.9aA |
| T9 | 80 | 7 | 9.8 | 29.4aA | 54.3aA | 53.4bA | 39.8aA | 7.9aA | 11.9aA | 3.9aA | 3.3aA | 4.8aA |
| Avg (T7–T9) | 27.9α | 44.6α | 54.3γ | 44.0α | 6.2α | 12.7α | 4.1α | 3.8β | 4.3α | |||
| Treatment | [NaCl] | [K+] | EC | ORACHydro | ORACLipo | TPHydro | Oxalate |
|---|---|---|---|---|---|---|---|
| (meq L−1) | (dS m−1) | (µmol TE g−1 DW) | (mg GAE g−1 DW) | (mg g−1 DW) | |||
| T0 (control) | 2 | 3 | 1.4 | 188.1aα | 26.2aαβ | 4.0aα | 76.1aα |
| T1 | 20 | 3 | 3.4 | 171.2aA | 34.6aA | 3.6abA | 95.2aA |
| T2 | 20 | 5 | 3.7 | 234.0aA | 13.6bB | 4.9aA | 76.5aA |
| T3 | 20 | 7 | 3.8 | 144.9aA | 32.8aAB | 3.8aA | 80.7aA |
| Avg (T1–T3) | 158.0αβ | 33.7αβ | 3.7αβ | 88.0α | |||
| T4 | 40 | 3 | 5.6 | 135.9aA | 36.6aA | 3.3bA | 93.4aA |
| T5 | 40 | 5 | 6.0 | 165.5aA | 33.3aA | 3.4aA | 95.8aA |
| T6 | 40 | 7 | 6.0 | 113.3aA | 34.6aA | 3.0aA | 103.0aA |
| Avg (T4–T6) | 138.2αβ | 34.8α | 3.2β | 97.4α | |||
| T7 | 80 | 3 | 9.3 | 129.9aA | 26.7aA | 3.3bA | 92.4aA |
| T8 | 80 | 5 | 9.3 | 108.6aA | 23.5abA | 3.0aA | 98.8aA |
| T9 | 80 | 7 | 9.8 | 131.9aA | 22.6aA | 3.2aA | 84.2aA |
| Avg (T7–T9) | 123.5β | 24.3β | 3.2β | 91.8α | |||
| Treatment | NaCl | K+ | ECiw | SPAD | Pn | gs | Tr | WUE |
|---|---|---|---|---|---|---|---|---|
| (meq L−1) | (dS m−1) | (µmol CO2 m−2 s−1) | (mol H2O m−2 s−1) | (mmol H2O m−2 s−1) | (µmol CO2 mmol−1 H2O) | |||
| 19 days after treatment | ||||||||
| T0 (control) | 2 | 3 | 1.4 | 66.9aα | 20.1abα | 0.37aα | 5.8aα | 3.4cβ |
| T1 | 20 | 3 | 3.4 | 67.9aA | 20.7aA | 0.32aA | 4.9bA | 4.2abA |
| T2 | 20 | 5 | 3.7 | 64.6aA | 12.9bC | 0.20bB | 3.1aC | 4.2aA |
| T3 | 20 | 7 | 3.8 | 66.6aA | 18.0aAB | 0.32aA | 4.0aB | 4.5aA |
| Avg (T1–T3) | 67.3α | 19.4α | 0.32α | 4.5β | 4.4α | |||
| T4 | 40 | 3 | 5.6 | 68.5aA | 19.0abA | 0.34aA | 4.5bcA | 4.2abA |
| T5 | 40 | 5 | 6.0 | 66.9aA | 17.3aA | 0.32aA | 3.9aAB | 4.4aA |
| T6 | 40 | 7 | 6.0 | 67.5aA | 16.9aA | 0.36aA | 3.9aB | 4.4aA |
| Avg (T4–T6) | 67.6α | 17.7αβ | 0.34α | 4.1βγ | 4.3α | |||
| T7 | 80 | 3 | 9.3 | 69.3aA | 17.6bA | 0.26aA | 3.9cAB | 4.5aA |
| T8 | 80 | 5 | 9.3 | 67.9aA | 17.5aA | 0.20bAB | 4.1aA | 4.3aA |
| T9 | 80 | 7 | 9.8 | 66.7aA | 16.0aA | 0.19bB | 3.3bB | 4.9aA |
| Avg (T7–T9) | 68.0α | 17.0β | 0.22β | 3.8γ | 4.6α | |||
| Gene | Tissue Expression | Correlation with ECiw | p Value |
|---|---|---|---|
| SoSOS1 | Leaf | 0.62 | 0.06 |
| Root | −0.15 | 0.67 | |
| SoSOS2 | Leaf | −0.31 | 0.39 |
| Root | 0.22 | 0.53 | |
| SoSOS3 | Leaf | 0.20 | 0.57 |
| Root | 0.13 | 0.72 | |
| SoNHX1 | Leaf | 0.26 | 0.48 |
| Root | 0.30 | 0.40 | |
| SoNHX2 | Leaf | 0.35 | 0.31 |
| Root | −0.35 | 0.33 | |
| SoERF1 | Leaf | 0.43 | 0.21 |
| Root | 0.18 | 0.62 | |
| SoHKT1 | Leaf | −0.07 | 0.85 |
| Root | −0.62 | 0.06 | |
| SoAKT1 | Leaf | 0.13 | 0.72 |
| Root | −0.31 | 0.39 | |
| SoERS1 | Leaf | 0.58 | 0.08 |
| Root | 0.36 | 0.31 | |
| SoSAL1 | Leaf | 0.55 | 0.10 |
| Root | −0.08 | 0.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.F.S.; Sandhu, D.; Liu, X.; Halvorson, J.J. Spinach (Spinacea oleracea L.) Response to Salinity: Nutritional Value, Physiological Parameters, Antioxidant Capacity, and Gene Expression. Agriculture 2018, 8, 163. https://doi.org/10.3390/agriculture8100163
Ferreira JFS, Sandhu D, Liu X, Halvorson JJ. Spinach (Spinacea oleracea L.) Response to Salinity: Nutritional Value, Physiological Parameters, Antioxidant Capacity, and Gene Expression. Agriculture. 2018; 8(10):163. https://doi.org/10.3390/agriculture8100163
Chicago/Turabian StyleFerreira, Jorge F. S., Devinder Sandhu, Xuan Liu, and Jonathan J. Halvorson. 2018. "Spinach (Spinacea oleracea L.) Response to Salinity: Nutritional Value, Physiological Parameters, Antioxidant Capacity, and Gene Expression" Agriculture 8, no. 10: 163. https://doi.org/10.3390/agriculture8100163
APA StyleFerreira, J. F. S., Sandhu, D., Liu, X., & Halvorson, J. J. (2018). Spinach (Spinacea oleracea L.) Response to Salinity: Nutritional Value, Physiological Parameters, Antioxidant Capacity, and Gene Expression. Agriculture, 8(10), 163. https://doi.org/10.3390/agriculture8100163








