The Role of Exogenously Applied Polyamines to Improve Heat Tolerance in Tomatoes: A Review
Abstract
1. Introduction
2. Tomato Responses to HS
2.1. Agronomic Traits
| Name | Exposure Temperature | Effects |
|---|---|---|
| Tomato (Solanum lycopersicum) | (42/37 °C) Day/night | Reduction in roots more than shoots and slow to recover [38,39] |
| Tomato (Solanum lycopersicum) 43 varieties | (34/24 °C) Day/night | Reduction in plant height and stem diameter [42] |
| Tomato (Solanum lycopersicum) cultivars ‘Dafnis’ and ’Minichal | (40 °C) Daytime | Reduction in plant height, shoot fresh weight, root fresh weight, fruit yield, fruit length, fruit diameter [50] |
| Tomato (Solanum lycopersicum) cultivar Aromata | (36/28 °C) Day/night | Reduction in the shoot fresh weight [67] |
| (Solanum lycopersicum Mill.) cultivar (FL7156) | (32/28 °C) Day/night | Flower abortion [68] |
| Tomato (Solanum lycopersicum) | (37.8/26.7 °C) Day/night | Reduction in flower production [69] |
| Tomato (Solanum lycopersicum) genotypes (Binatomato-6, Binatomato-5, CLN-2413, D6 12 and D6 18) | (32 °C) Daytime | Significant reduction in the number of fruits, individual fruit weight and fruit yield/plant [66] |
| Tomato (Solanum lycopersicum) cultivars ’Minichal | (40 °C) Daytime | Decline in fruit length (7.1%) and fruit diameter (12%) [50] |
| Tomato (Solanum lycopersicum) cultivars ‘Dafnis’ | (40 °C) Daytime | Decline in fruit weight (31.9%), fruit length (14.1%), fruit diameter (19.1%), and fruit hardness (19.1%) [50] |
| Tomato (Solanum lycopersicum) genotypes NC8288 | (29 °C) Daytime | Reduction in fruit number, fruit weight per plant, and seed number per fruit [59] |
| Tomato (Solanum lycopersicum) | (32/28 °C) Day/night | Fruit abortion [49] |
| Tomato (Solanum lycopersicum) | (35/23 °C) Day/night | No fruit set [70] |
| Forty-four diverse tomatoes (Solanum lycopersicum) lines | (44/37 °C) Day/night | Reduction in fruit set [41,54,55,71,72] |
| Tomato (Solanum lycopersicum) | (above 30 °C) Daytime | Low pollen viability, slow pollen tube elongation, and fruit abortion [46,47] |
| Tomato (Solanum lycopersicum) | (35/23 °C) Day/night | Blossom end rot [65] |
| Tomato (Solanum lycopersicum) genotypes (Binatomato-6, Binatomato-5, CLN-2413, D6 12 and D6 18) | (32 °C) Daytime | Cracked and streaked ripening [66] |
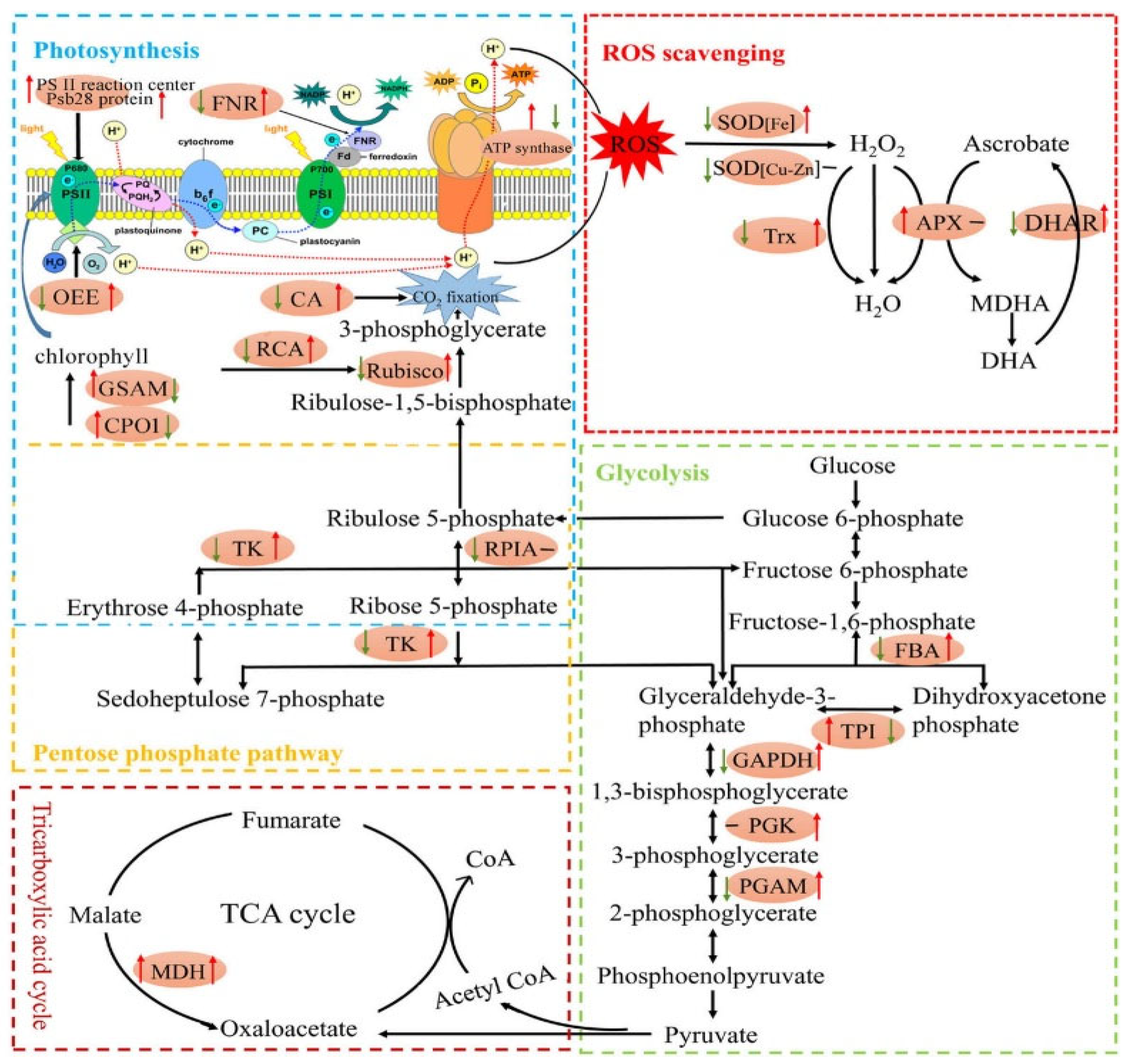
2.2. Physiological Traits
2.3. Biochemical Traits
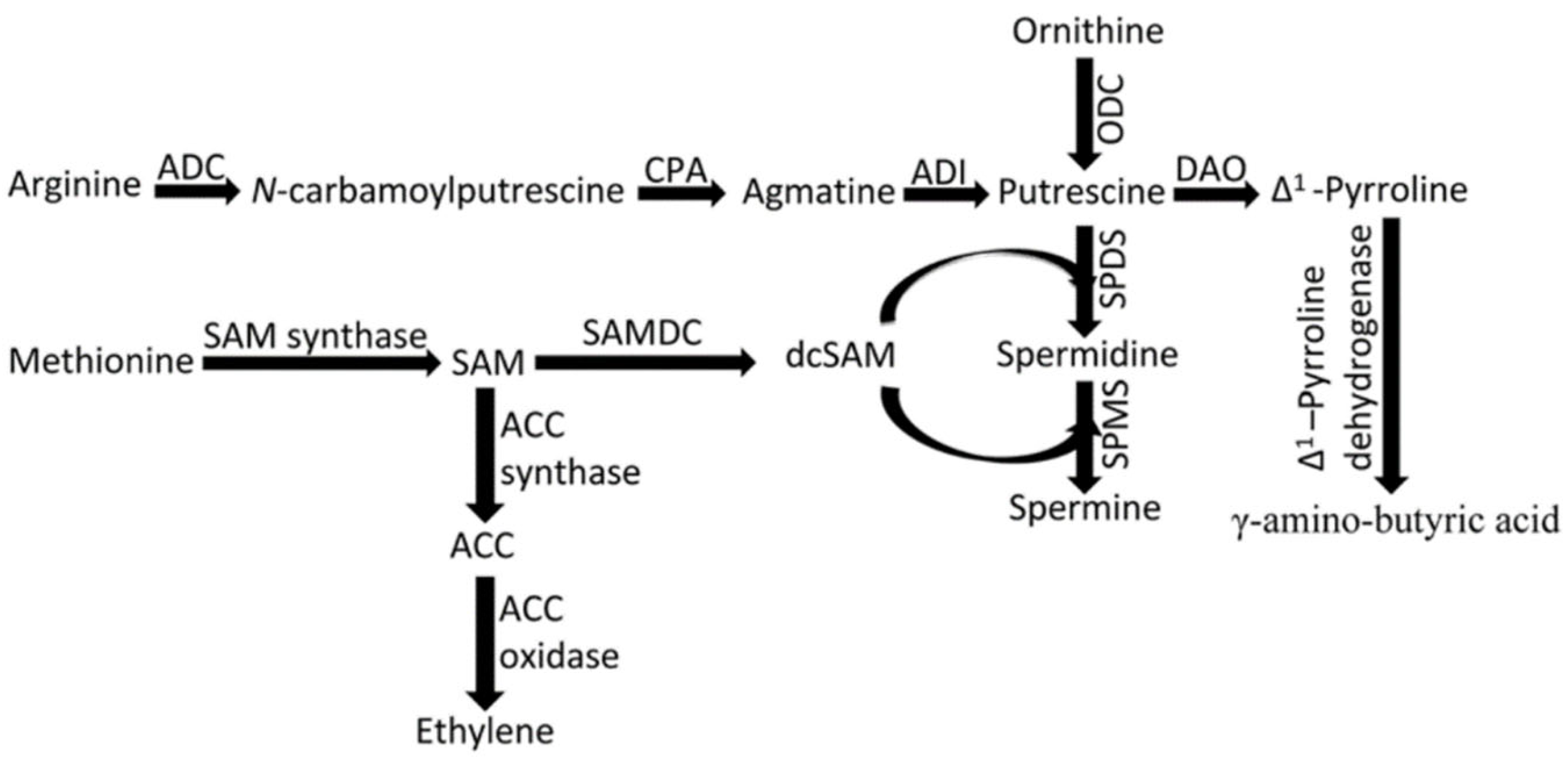
3. The Biosynthetic Pathways of PAs and Their Role
3.1. Improve Resistance to Abiotic Stresses by Exogenous Application of PAs
| PAs (Type, Application Concentration, Durations) | Plant | Type of Stress | Effectiveness and Plant Response |
|---|---|---|---|
| Spd Foliar sprayed (0.1 mM) (3, 6, 12, 24, and 48 h) | Tomato (Solanum lycopersicum) | Salinity | Enhanced ion homeostasis (Na+/K+ ratio) Photosynthetic performance Enhanced expression of stress-responsive genes (RBOH1) Improved ROS scavenging [120] |
| Put, Spd, Spm Irrigation (1 mM) (5, 12, and 19 days) | Rice (Oryza sativa L.) | Salinity | Higher K+/Na+ ratio in the shoots Put induced a decrease in shoot water content [121] |
| Put Soaking seeds (0.01 mM) (8 h) | Belladonna (Atropa belladonna) | Salinity | Reduction of accumulation of (Na+/K+) ions Better germination Early seedling growth [122] |
| Put Foliar sprayed (0.1 mM) (Every 2 days) | Rice (Oryza sativa L.) | Salinity | Increased ROS scavenging enzyme activity Reduction of the EL [123] |
| Spd, Spm, Put Foliar sprayed (100 mg/L Spd, 150 mg/L Spm, and 150 mg/L Put) | Soybean (Glycine max L.) | Salinity | Spd enhanced the taproot Spm enhanced POD 39.66% and CAT 57.94% activity Put increased plant height, relative growth rate by 42.86% [124] |
| Put Irrigation (0.1 mM) (7 days) | Indian mustard (Brassica juncea) | Salinity | Induction of enzymes in leaf tissues APX > GR > CAT > SOD > POD Preventing membrane peroxidation Improving seedling growth [125] |
| Spd Soaking seeds (0.25 mM) (10 h) | Tomato (Solanum lycopersicum) | Salinity and Alkalinity | Enhanced root dry weights Enhance antioxidant capacity [126] |
| Spm Irrigation (0.5 mM) (Once a day) | Tomato (Solanum lycopersicum) | Salinity and Paraquat | Increased growth, photosynthesis, and PSII function, membrane stability Enhanced gene expression for stress tolerance Reduction of oxidative stress [127] |
| Put Foliar sprayed (0.20 and 40 mg/L) (Before flowering initiation phase, for three continuous weeks) | Thyme (Thymus vulgaris L.) | Drought | Improved leaf water content Upregulated antioxidant enzyme activities Increased essential oil content by 23.07% [137] |
| Put Soaking seed (0.1, 0.01, and 0.001 mM) (10 h) | Hybrid maize (Zea mays L.) | Drought | Improved plant biomass components, leaf water status, leaf area, germination rates, and antioxidant enzyme activities [138] |
| Spm Foliar sprayed (25 mg/L) (25 days) | Maize (Zea mays L.) | Drought | Increased total phenol and flavonoid concentration Improved water use efficiency, osmotic adjustment, and antioxidant enzyme activities [128] |
| Spd, Spm, Put Foliar sprayed (1 mM) | Grafted Tomato | Drought | Lower ROS and higher CAT, SOD activities Improve water use efficiency, osmotic adjustment, and antioxidant enzyme activities [129] |
| Spm, Spd Foliar sprayed (0.1 mM Spm, Put 0.2 mM) (15, 30, and 45 days after sowing) | Sesame (Sesamum indicum L.) | Drought | Enhancement in photosynthetic pigments stomatal conductance, water relations, relative water content, membrane stability index, excised leaf water retention, plant height, leaf area, number of capsules per plant, 1000-seed weight, seed yield, oil content, plant nutrient content (N, P, K) Highly significant amelioration in osmo-protectants (free proline, soluble sugars), and antioxidant enzyme activities (CAT, POD, SOD, APX) Highly significant reduction in oxidative stress markers (MDA, EL, O2−, H2O2) [130] |
| Put Prior flooding (2 mM) | Welsh onion (Allium fstulosum) | Drought | Alleviation of relative water content, plant growth and chlorophyll fluorescence Reduction of (O2−), (H2O2) contents [133] |
| Spd, Spm, Put Foliar sprayed (1 mM Spd, 1 mM Spm, and 2 mM Put) (Daily for 6 days) | Wheat (Triticum aestivum L.) | Drought | Improved grain weight, size, starch, and protein content in grains Activation of enzymes involved in starch and protein metabolism Improved physiological responses, water use efficiency, and osmotic adjustment. Enhanced antioxidant enzyme activities and oxidative stress markers. Improved impact on drought stress [131] |
| Spd Foliar sprayed before chilling (0.5 mM) (12 h before chilling) | Cucumber (Cucumis sativus L. cv Jinchun No. 3 and cv Suyo) | Chilling | Reduction of H2O2, ROS, and EL in leaves Alleviation of chilling injury Activities of antioxidant enzymes Improved membrane stability, plant growth [134] |
| Put, Spd Foliar sprayed (1 mM put, 0.5 mM Spd) (24 h before chilling) | Cucumber (Cucumis sativas L.) | Chilling | Reduction of EL and MDA content Activation of antioxidant enzymes [135] |
| Spd, Spm, Put Foliar sprayed before chilling (1 mM) (For 12 h daily) | Tomato (Solanum lycopersicum Mill.) | Chilling | Activation of antioxidant enzyme Gene expression (ornithine decarboxylase (ODC), arginine decarboxylase (ADC), and S-adenosylmethionine decarboxylase (SAMDC) Put plays an important role in tomato chilling tolerance [136] |
3.2. PAs and Their Roles in Molecular Level
3.3. Effects of PAs in Mitigating HS in Tomatoes
| Effectiveness and Plant Response | PAs (Type, Concentration, Application) |
|---|---|
| Increased plant biomass and growth Alleviated photosynthetic pigments, the levels of Chl a, Chl b, and total Chl (a + b) by 20.1, 21.8, and 20.6%, respectively | (Spd, 1 mM, foliar spray) [143] |
| Improved chlorophyll fluorescence properties, hardening and the activity of PSII | (Spd, 4 mM, foliar spray) [161] |
| Enhanced photosynthetic rate | (Put, 1 mM, foliar spray) [121] (Spd, 0.5 mM, root drench) [135] (Spm, 0.25 mM, foliar spray) [149] (Mixture of PAs, 0.5 mM) [150] |
| Improved the gene expression and activity of key enzymes for N metabolism | (Spd, 1 mM, foliar spray) [164] |
| Opening of stomatal pores and enhanced transpiration | (Spd, 1 mM, foliar spray) [134,151] (Spd, Put, Spm 1 mM, foliar spray) [152] (Spm, 0.5 mM, foliar spray) [177] |
| Reduced H2O2 and MDA accumulation, alleviated oxidative damage Increased antioxidant enzymes’ activities, protection of membrane lipid peroxidation ROS scavengers’ osmotic balance | (Spd, Put, Spm 1 mM, foliar spray) [101] (Spm, 1 mM, foliar spray) [121] (Spd, 1 mM, foliar spray) [134,151] |
| Improved the accumulation of lycopene as an anti-senescence agent in fruit ripening/senescence processes | (Increase polyamine levels by genetic modification) [176] |
| Increased fruit shelf-life and enhanced fruit juice quality Delayed ripening | (Increase polyamine levels by genetic modification) [94,172,173,174,175] |
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HS | Heat stress |
| PAs | Polyamines |
| Spd | Spermidine |
| Put | Putrescine |
| Spm | Spermine |
| EL | Electrolyte leakage |
| REC | Relative electrical conductivity |
| RCA | Rubisco activase |
| CC | Chlorophyll content |
| CWIN | Cell wall invertase |
| ROS | Reactive Oxygen Species |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| ASA | Ascorbic acid |
| H2O2 | Hydrogen peroxide |
| O2− | Superoxide radical |
| Chl | Chlorophyll |
References
- Costa, J.; Heuvelink, E. Introduction: The Tomato Crop and Industry. In Tomatoes; CABI Publishing: Wallingford, UK, 2005; pp. 1–19. [Google Scholar] [CrossRef]
- FAOSTAT. Global Tomato Production in 2021; FAO: Rome, Italy, 2021. Available online: http://www.fao.org/faostat/en/#data/TP (accessed on 2 October 2021).
- Ganguly, D.R.; Crisp, P.A.; Eichten, S.R.; Pogson, B.J. Maintenance of Pre-existing DNA Methylation States Through Recurring Excess-light Stress. Plant Cell Environ. 2018, 41, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular Mechanisms Governing Plant Responses to High Temperatures. J. Integr. Plant Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef] [PubMed]
- Hideg, É.; Jansen, M.A.; Strid, Å. UV-B Exposure, ROS, and Stress: Inseparable Companions or Loosely Linked Associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Easterling, W.E.; Aggarwal, P.K.; Batima, P.; Brander, K.M.; Erda, L.; Howden, S.M.; Tubiello, F.N. Food, Fibre and Forest Products. Clim. Change 2007, 2007, 273–313. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_SPM_FINAL.pdf (accessed on 25 September 2024).
- Ortiz, R.; Braun, H.J.; Crossa, J.; Crouch, J.H.; Davenport, G.; Dixon, J.; Dreisigacker, S.; Duveiller, E.; He, Z.; Iwanaga, M.; et al. Wheat Genetic Resources Enhancement by the International Maize and Wheat Improvement Center (CIMMYT). Genet. Resour. Crop Evol. 2008, 55, 1095–1140. [Google Scholar] [CrossRef]
- Cardell, M.F.; Amengual, A.; Romero, R.; Ramis, C. Future Extremes of Temperature and Precipitation in Europe Derived from a Combination of Dynamical and Statistical Approaches. Int. J. Climatol. 2020, 40, 4800–4827. [Google Scholar] [CrossRef]
- Carvalho, D.; Cardoso Pereira, S.; Rocha, A. Future Surface Temperature Changes for the Iberian Peninsula According to EURO-CORDEX Climate Projections. Clim. Dyn. 2021, 56, 123–138. [Google Scholar] [CrossRef]
- Pinke, Z.; Lövei, G.L. Increasing temperature cuts back crop yields in Hungary over the last 90 years. Glob. Change Biol. 2017, 23, 5426–5435. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zhang, S.W.; Ai, G.; Li, M.; Ye, Z.B.; Zhang, J.H. Tomato LrgB Regulates Heat Tolerance and the Assimilation and Partitioning of Carbon. Plant Sci. 2018, 274, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.-Y.; Na Lee, Y.; Kim, S.-G.; Lee, Y.-H.; Park, W.J.; Park, C.-M. The MYB96 Transcription Factor Mediates Abscisic Acid Signaling During Drought Stress Response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High Temperature Effects on Photosynthetic Activity of Two Tomato Cultivars with Different Heat Susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.; Li, J.; Guo, S.R.; Kang, Y.Y. Exogenous Spermidine Affects Polyamine Metabolism in Salinity-Stressed Cucumis sativus Roots and Enhances Short-Term Salinity Tolerance. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef]
- Yadav, P.; Ansari, M.W.; Kaula, B.C.; Rao, Y.R.; Meselmani, M.A.; Siddiqui, Z.H.; Brajendra; Kumar, S.B.; Rani, V.; Sarkar, A.; et al. Regulation of Ethylene Metabolism in Tomato Under Salinity Stress Involving Linkages with Important Physiological Signaling Pathways. Plant Sci. 2023, 334, 111736. [Google Scholar] [CrossRef]
- Killiny, N.; Nehela, Y. Citrus Polyamines: Structure, Biosynthesis, and Physiological Functions. Plants 2020, 9, 426. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Responses of Plants to Climate Change: Metabolic Changes During Abiotic Stress Combination in Plants. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Paschalidis, K.; Tsaniklidis, G.; Wang, B.Q.; Delis, C.; Trantas, E.; Loulakakis, K.; Makky, M.; Sarris, P.F.; Ververidis, F.; Liu, J.-H. The Interplay Among Polyamines and Nitrogen in Plant Stress Responses. Plants 2019, 8, 315. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites Against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Tyagi, A.; Ali, S.; Ramakrishna, G.; Singh, A.; Park, S.; Mahmoudi, H.; Bae, H. Revisiting the Role of Polyamines in Plant Growth and Abiotic Stress Resilience: Mechanisms, Crosstalk, and Future Perspectives. J. Plant Growth Regul. 2023, 42, 5074–5098. [Google Scholar] [CrossRef]
- Jahan, M.S.; Hasan, M.M.; Alotaibi, F.S.; Alabdallah, N.M.; Alharbi, B.M.; Ramadan, K.M.A.; Bendary, E.S.A.; Alshehri, D.; Jabborova, D.; Al-Balawi, D.A.; et al. Exogenous putrescine increases heat tolerance in tomato seedlings by regulating chlorophyll metabolism and enhancing antioxidant defense efficiency. Plants 2022, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Karwa, S.; Taunk, J.; Maurya, S.; Das, A.; Krishna, G.K.; Arya, S.S.; Kumar, A.; Kumar, S.; Kumar, P.; Chinnusamy, V.; et al. Spermidine exogenous application mollifies reproductive stage heat stress ramifications in rice. Front. Plant Sci. 2022, 13, 1027662. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Yuqing, T.; Xincheng, L.; Huidong, Y.; Yuting, W.; Zhongdong, H.; Xinlong, H.; Buchun, L.; Jing, S. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of citrus seedlings under high temperature. Plant Signal Behav. 2022, 17, 2086372. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Shu, S.; Wang, Y.; Du, J.; Shi, L.; Jahan, M.S.; Guo, S. Transcriptome Analysis of the Regulatory Mechanism of Exogenous Spermidine in High Temperature Stress Resistance of Tomato Seedlings. Agronomy 2023, 13, 285. [Google Scholar] [CrossRef]
- Luo, L.; Li, Z.; Tang, M.Y.; Cheng, B.Z.; Zeng, W.H.; Peng, Y.; Nie, G.; Zhang, X.Q. Metabolic regulation of polyamines and γ-aminobutyric acid in relation to spermidine-induced heat tolerance in white clover. Plant Biol. 2020, 22, 794–804. [Google Scholar] [CrossRef]
- Yang, X.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine enhances the photosynthesis and ultrastructure of lettuce seedlings under high-temperature stress. Sci. Hortic. 2022, 291, 110570. [Google Scholar] [CrossRef]
- Panthee, D.R.; Gotame, T.P. Improving Heat Stress Tolerance in Tomato; CABI Reviews: Online, 2020. [Google Scholar] [CrossRef]
- Harel, D.; Fadida, H.; Slepoy, A.; Gantz, S.; Shilo, K. The Effect of Mean Daily Temperature and Relative Humidity on Pollen, Fruit Set, and Yield of Tomato Grown in Commercial Protected Cultivation. Agronomy 2014, 4, 167–177. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.O.; Wu, Z. Screening and Validation of Tomato Genotypes under Heat Stress Using Fv/Fm to Reveal the Physiological Mechanism of Heat Tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Lu, T.; Meng, Z.; Zhang, G.; Qi, M.; Sun, Z.; Liu, Y.; Li, T. Sub-high Temperature and High Light Intensity Induced Irreversible Inhibition on Photosynthesis System of Tomato Plant (Solanum lycopersicum L.). Front. Plant Sci. 2017, 8, 365. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, M.K.; Singh, V.; Singh, R.; Raghuvanshi, T.; Singh, C. Debilitation in Tomato (Solanum lycopersicum L.) as a Result of Heat Stress. J. Pharmacogn. Phytochem. 2017, 6, 1917–1922. [Google Scholar]
- Shaheen, M.R.; Ayyub, C.M.; Amjad, M.; Waraich, E.A. Morpho-physiological Evaluation of Tomato Genotypes under High Temperature Stress Conditions. J. Sci. Food Agric. 2016, 96, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Laxman, R.H.; John, S.V.S.; Biradar, G.; Pavithra, G.B.; Manasa, K.M.; Sadashiva, A.T.; Bhatt, R.M. Growth, Reproductive Development, and Yield of Tomato (Solanum lycopersicum L.) Genotypes under Mild Temperature Elevation. Asian J. Bot. 2018, 1, 1–10. [Google Scholar]
- Yu, W.; Wang, L.; Zhao, R.; Sheng, J.; Zhang, S.; Li, R.; Shen, L. Knockout of SlMAPK3 Enhances Tolerance to Heat Stress Involving ROS Homeostasis in Tomato Plants. BMC Plant Biol. 2019, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat Stress Decreases Levels of Nutrient-Uptake and Assimilation Proteins in Tomato Roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef]
- Shorobi, F.M.; Vyavahare, G.D.; Seok, Y.J.; Park, J.H. Effect of Polypropylene Microplastics on Seed Germination and Nutrient Uptake of Tomato and Cherry Tomato Plants. Chemosphere 2023, 329, 138679. [Google Scholar] [CrossRef]
- Driedonks, N.J.W. From Flower to Fruit in the Heat—Reproductive Thermotolerance in Tomato and Its Wild Relatives. Ph.D. Thesis, The Radboud University, Nijmegen, The Netherlands, 2018. [Google Scholar]
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Bhattarai, S.; Harvey, J.T.; Djidonou, D.; Leskovar, D.I. Exploring Morphophysiological Variation for Heat Stress Tolerance in Tomato. Plants 2021, 10, 347. [Google Scholar] [CrossRef]
- Alsamir, M.; Ahmad, N.M.; Mahmood, T.; Trethowan, R. Morpho-Physiological Traits Linked to High Temperature Stress Tolerance in Tomato (S. lycopersicum L.). Am. J. Plant Sci. 2017, 8, 2681. [Google Scholar] [CrossRef]
- Sato, S.; Kamiyama, M.; Iwata, T.; Makita, N.; Furukawa, H.; Ikeda, H. Moderate Increase of Mean Daily Temperature Adversely Affects Fruit Set of Lycopersicon esculentum by Disrupting Specific Physiological Processes in Male Reproductive Development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef]
- Song, J.; Nada, K.; Tachibana, S. Suppression of S-Adenosylmethionine Decarboxylase Activity is a Major Cause for High-Temperature Inhibition of Pollen Germination and Tube Growth in Tomato (Lycopersicon esculentum Mill.). Plant Cell Physiol. 2002, 43, 619–627. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Jin, Y.; Yang, Y.-J.; Li, G.-J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Rieu, I.; Twell, D.; Firon, N. Pollen Development at High Temperature: From Acclimation to Collapse. Plant Physiol. 2017, 173, 1967–1976. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature Stress and Plant Sexual Reproduction: Uncovering the Weakest Links. J. Exp. Bot. 2010, 61, erq053. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Peet, M.M.; Thomas, J.F. Physiological Factors Limit Fruit Set of Tomato (Lycopersicon esculentum Mill.) under Chronic, Mild Heat Stress. Plant Cell Environ. 2000, 23, 719–726. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Yang, E.Y.; Jeong, H.B.; Cho, M.C.; Chae, S.Y.; Paudel, N. Heat Treatment in Two Tomato Cultivars: A Study of the Effect on Physiological and Growth Recovery. Horticulturae 2021, 7, 119. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave, C.R.J. Effect of Temperature on the Growth and Development of Tomato Fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Yang, T.; He, Y.; Niu, S.; Zhang, Y. A YABBY Gene CRABS CLAW (CRCa) Negatively Regulates Flower and Fruit Sizes in Tomato. Plant Sci. 2022, 320, 111285. [Google Scholar] [CrossRef]
- Wang, D.; Seymour, G.B. Molecular and Biochemical Basis of Softening in Tomato. Mol. Horticult. 2022, 2, 5. [Google Scholar] [CrossRef]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An Overview of Heat Stress in Tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef]
- Stokes, M.; Geitmann, A. Screening Methods for Thermotolerance in Pollen. Ann. Bot. 2025, 135, 71–88. [Google Scholar] [CrossRef]
- Johri, B.M.; Vasil, I.K. Physiology of Pollen. Bot. Rev. 1961, 27, 325–381. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, P.; Kumar, S.; Srivastava, K. Role of Pollen Starch and Soluble Sugar Content on Fruit Set in Tomato under Heat Stress. Sabrao J. Breed Genet. 2015, 47, 406–412. Available online: https://www.researchgate.net/publication/292160041 (accessed on 20 September 2024).
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Ampomah-Dwamena, C.; Sodedji, F.A.K.; Asante, I.K.; Danquah, E.Y. Accelerating Breeding for Heat Tolerance in Tomato (Solanum lycopersicum L.): An Integrated Approach. Agronomy 2019, 9, 720. [Google Scholar] [CrossRef]
- Peet, M.M.; Sato, S.; Gardner, R.G. Comparing Heat Stress Effects on Male-Fertile and Male-Sterile Tomatoes. Plant Cell Environ. 1998, 21, 225–231. [Google Scholar] [CrossRef]
- Paupière, M.J.; van Haperen, P.; Rieu, I.; Visser, R.G.F.; Tikunov, Y.M.; Bovy, A.G. Screening for Pollen Tolerance to High Temperatures in Tomato. Euphytica 2017, 213, 130. [Google Scholar] [CrossRef]
- Xu, J.; Wolters-Arts, M.; Mariani, C.; Huber, H.; Rieu, I. Heat Stress Affects Vegetative and Reproductive Performance and Trait Correlations in Tomato (Solanum lycopersicum). Euphytica 2017, 213, 156. [Google Scholar] [CrossRef]
- Sato, S.; Peet, M.M. The Effects of Moderately Elevated Temperature Stress on the Timing of Pollen Release and Germination in Tomato (Lycopersicon esculentum Mill.). J. Hortic. Sci. Biotechnol. 2005, 80, 23–28. [Google Scholar] [CrossRef]
- Raja, M.M.; Vijayalakshmi, G.; Naik, M.L.; Basha, P.O.; Sergeant, K.; Hausman, J.F.; Khan, P.S.S.V. Pollen development and function under heat stress: From effects to responses. Acta Physiol. Plant. 2019, 41, 47. [Google Scholar] [CrossRef]
- Müller, F.; Rieu, I. Acclimation to high temperature during pollen development. Plant Reprod. 2016, 29, 107–118. [Google Scholar] [CrossRef]
- Spaldon, S.; Samnotra, R.K.; Chopra, S. Climate resilient technologies to meet the challenges in vegetable production. Int. J. Curr. Microbiol. App. Sci. 2015, 3, 28–47. Available online: https://www.researchgate.net/publication/277664533 (accessed on 15 September 2024).
- Islam, M.T. Effect of temperature on photosynthesis, yield attributes and yield of tomato genotypes. Int. J. Expt. Agric. 2011, 2, 8–11. Available online: https://www.researchgate.net/publication/267705727 (accessed on 12 September 2024).
- Zhou, R.; Kjær, K.H.; Rosenqvist, E.; Yu, X.; Wu, Z.; Ottosen, C.-O. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J. Agron. Crop Sci. 2017, 203, 68–80. [Google Scholar] [CrossRef]
- Sato, S.; Peet, M.M.; Gardner, R.G. Altered flower retention and developmental patterns in nine tomato cultivars under elevated temperature. Sci. Hortic. 2004, 101, 95–101. [Google Scholar] [CrossRef]
- El Ahmadi, A.B.; Stevens, M.A. Reproductive responses of heat-tolerant tomatoes to high temperatures. J. Am. Soc. Hortic. Sci. 1979, 104, 686–691. [Google Scholar] [CrossRef]
- Khan, Q.; Wang, Y.; Xia, G.; Yang, H.; Luo, Z.; Zhang, Y. Deleterious Effects of Heat Stress on the Tomato, Its Innate Responses, and Potential Preventive Strategies in the Realm of Emerging Technologies. Metabolites 2024, 14, 283. [Google Scholar] [CrossRef]
- Abdelmageed, A.H.A.; Gruda, N. Influence of grafting on growth, development and some physiological parameters of tomatoes under controlled heat stress conditions. Eur. J. Hortic. Sci. 2009, 74, 16–20. [Google Scholar] [CrossRef]
- EL-Saka, Z.I. Tomato breeding for heat stress conditions. Eur. J. Acad. Essays 2016, 3, 87–93. Available online: https://www.researchgate.net/publication/368876692 (accessed on 15 September 2024).
- Alsamir, M.; Ahmad, N.M.; Keitel, C.; Mahmood, T.; Trethowan, R. Identification of high-temperature tolerant and agronomically viable tomato (S. lycopersicum) genotypes from a diverse germplasm collection. Adv. Crop Sci. Technol. 2017, 5, 299. [Google Scholar] [CrossRef]
- Berova, M.; Stoeva, N.; Zlatko, Z.; Ganeva, D. Physiological response of some tomato genotypes (Lycopersicon esculentum L.) to high-temperature stress. J. Cent. Eur. Agric. 2008, 9, 723–732. [Google Scholar]
- Zhang, J.; Jiang, X.; Li, T.; Chang, T. Effect of Elevated Temperature Stress on the Production and Metabolism of Photosynthate in Tomato (Lycopersicon esculentum L.) Leaves. J. Hortic. Sci. Biotechnol. 2012, 87, 367–373. [Google Scholar] [CrossRef]
- Hu, W.J.; Wu, Q.; Liu, X.; Shen, Z.J.; Chen, J.; Zhu, C.Q.; Zhang, Y.L.; Wang, Y.L.; Zhang, L.L.; Zhang, J.L.; et al. Comparative Proteomic Analysis Reveals the Effects of Exogenous Calcium Against Acid Rain Stress in Liquidambar formosana Hance Leaves. J. Proteome Res. 2015, 15, 216–228. [Google Scholar] [CrossRef]
- Sharkey, T.D. Effects of Moderate Heat Stress on Photosynthesis: Importance of Thylakoid Reactions, Rubisco Deactivation, Reactive Oxygen Species, and Thermotolerance Provided by Isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to High Temperature Stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat Cultivars Selected for High Fv/Fm under Heat Stress Maintain High Photosynthesis, Total Chlorophyll, Stomatal Conductance, Transpiration, and Dry Matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, X.Q.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.P.; Wang, Y.L.; Yu, W.G.; Zhao, T.M.; Wu, Z. Drought Stress Had a Predominant Effect over Heat Stress on Three Tomato Cultivars Subjected to Combined Stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Karkute, S.G.; Ansari, W.A.; Singh, A.K.; Singh, P.M.; Rai, N.; Bahadur, A.; Singh, J. Characterization of High-Temperature Stress-Tolerant Tomato (Solanum lycopersicum L.) Genotypes by Biochemical Analysis and Expression Profiling of Heat-Responsive Genes. 3 Biotech 2021, 11, 45. [Google Scholar] [CrossRef]
- Nankishore, A.; Farrell, A.D. The Response of Contrasting Tomato Genotypes to Combined Heat and Drought Stress. J. Plant Physiol. 2016, 202, 75–82. [Google Scholar] [CrossRef]
- Dai, Y.; Yuan, L.; Zhang, S.; Wang, J.; Xie, S.; Zhao, M.; Zhang, Y.; Zhang, L.; Zhang, J.; Zhang, H.; et al. Comprehensive Evaluation for Cold Tolerance in Wucai (Brassica campestris L.) by the Performance Index on an Absorption Basis (PIabs). Agronomy 2019, 9, 61. [Google Scholar] [CrossRef]
- Qi, B.; Wu, C. Potential roles of stigma exsertion on spikelet fertility in rice (Oryza sativa L.) under heat stress. Front. Plant Sci. 2022, 13, 123. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Offler, C.E.; Ruan, Y.-L. Cell Wall Invertase Promotes Fruit Set under Heat Stress by Suppressing ROS-Independent Cell Death. Plant Physiol. 2016, 172, 163–180. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.D.; Li, T.L.; Cao, X.J. Photosynthesis and ultrastructure of photosynthetic apparatus in tomato leaves under elevated temperature. Photosynthetica 2014, 52, 430–436. [Google Scholar] [CrossRef]
- Ilahy, R.; Siddiqui, M.W.; Piro, G.; Lenucci, M.S.; Hdider, C. Year-to-year variations in antioxidant components of high-lycopene tomato (Solanum lycopersicum L.) breeding lines. Turk. J. Agric. Food Sci. Technol. 2016, 4, 486–492. [Google Scholar] [CrossRef]
- Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S.; Guerriero, G.; Quinet, M. Comparison of Drought and Heat Resistance Strategies among Six Populations of Solanum chilense and Two Cultivars of Solanum lycopersicum. Plants 2021, 10, 1720. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.J.; Hu, H.; Zhang, S.B. Moderate Photoinhibition of Photosystem II Protects Photosystem I from Photodamage at Chilling Stress in Tobacco Leaves. Front. Plant Sci. 2016, 7, 182. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Silva, J.A.T.D.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar] [CrossRef]
- De Pinto, M.C.; Locato, V.; Paradiso, A.; De Gara, L. Role of Redox Homeostasis in Thermo-Tolerance under a Climate Change Scenario. Ann. Bot. 2015, 116, 487–496. [Google Scholar] [CrossRef]
- Gujjar, R.S.; Karkute, S.G.; Rai, A.; Singh, M.; Singh, B. Proline-Rich Proteins May Regulate Free Cellular Proline Levels during Drought Stress in Tomato. Curr. Sci. 2018, 114, 915–920. [Google Scholar] [CrossRef]
- Aldubai, A.A.; Alsadon, A.A.; Migdadi, H.H.; Alghamdi, S.S.; Al-Faifi, S.A.; Afzal, M. Response of Tomato (Solanum lycopersicum L.) Genotypes to Heat Stress Using Morphological and Expression Study. Plants 2022, 11, 615. [Google Scholar] [CrossRef]
- Cheng, L.; Zou, Y.; Ding, S.; Zhang, J.; Yu, X.; Cao, J.; Lu, G. Polyamine Accumulation in Transgenic Tomato Enhances the Tolerance to High Temperature Stress. J. Integr. Plant Biol. 2009, 51, 489–499. [Google Scholar] [CrossRef]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Amorós, A.; Botella, M.A. Polyamines and Ethylene Changes during Germination of Different Plant Species under Salinity. Plant Sci. 2004, 167, 781–788. [Google Scholar] [CrossRef]
- Liu, Q.; Nishibori, N.; Imai, I.; Hollibaugh, J.T. Response of Polyamine Pools in Marine Phytoplankton to Nutrient Limitation and Variation in Temperature and Salinity. Mar. Ecol. Prog. Ser. 2016, 544, 93–105. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous Applications of Polyamines Modulate Drought Responses in Wheat through Osmolytes Accumulation, Increasing Free Polyamine Levels and Regulation of Polyamine Biosynthetic Genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Q.; Pan, Y.; Che, F.; Wang, Q.; Meng, X.; Rao, J. Changes of Polyamines and CBFs Expressions of Two Hami Melon (Cucumis melo L.) Cultivars during Low Temperature Storage. Sci. Hortic. 2017, 224, 8–16. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Sobieszczuk-Nowicka, E. Polyamine as Signaling Molecules and Leaf Senescence. In Senescence Signalling and Control in Plants; Sarwat, M., Tuteja, N., Eds.; Academic Press: Cambridge, UK, 2018; pp. 125–138. [Google Scholar] [CrossRef]
- Pál, M.; Ivanovska, B.; Oláh, T.; Tajti, J.; Hamow, K.Á.; Szalai, G.; Khalil, R.; Vanková, R.; Dobrev, P.; Misheva, S.P.; et al. Role of polyamines in plant growth regulation of Rht wheat mutants. Plant Physiol. Biochem. 2019, 137, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Slocum, R.D.; Kaur-Sawhney, R.; Galston, A.W. The physiology and biochemistry of polyamines in plants. Arch. Biochem. Biophys. 1984, 235, 283–303. [Google Scholar] [CrossRef]
- Childs, C.; Holdsworth, R.E.; Christopher, A.L.; Jackson, M.; Anzocchi, T.; Walsh, J.J.; Yielding, G. Introduction to the geometry and growth of normal faults. Geol. Soc. Spec. Publ. 2017, 439, 1–9. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, X.; Jahan, M.S.; Guo, S.; Tian, M.; Zhou, R.; Liu, H.; Feng, B.; Shu, S. Putrescine regulates stomatal opening of cucumber leaves under salt stress via the H2O2-mediated signaling pathway. Plant Physil. Bioch 2022, 170, 87–97. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, Z.; Wang, B.; Zheng, L.; Tan, J.; Chen, F. Physiological and transcriptome changes induced by exogenous putrescine in anthurium under chilling stress. Bot. Stud. 2020, 61, 28. [Google Scholar] [CrossRef]
- El-Badri, A.M.A.; Batool, M.; Mohamed, I.A.A.; Khatab, A.; Sherif, A.; Wang, Z.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J.; et al. Modulation of salinity impact on early seedling stage via nano-priming application of zinc oxide on rapeseed (Brassica napus L.). Plant Physil. Bioch. 2021, 166, 376–392. [Google Scholar] [CrossRef]
- Sen, S.; Ghosh, D.; Mohapatra, S. Modulation of polyamine biosynthesis in Arabidopsis thaliana by a drought mitigating Pseudomonas putida strain. Plant Physil. Bioch. 2018, 129, 180–188. [Google Scholar] [CrossRef]
- Shi, Y.; Lv, M.; Liu, Z.; Yang, X.; Yang, L.; Dong, L.; Lei, F.; Xie, A.; Zhang, D.; Bao, M.; et al. The Polyamine Signaling Pathway in Response to Waterlogging Stress of Paeonia lactiflora. Horticulturae 2024, 10, 928. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Tiwari, B.S.; Chattopadhyay, G.; Bose, A.; Sengupta, D.N.; Ghosh, B. Protective role of exogenous polyamines on salinity-stressed rice (Oryza sativa) plants. Physiol. Plant. 2002, 116, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.G.; Guo, S.Y.; Li, Y.F.; Li, W.H. Effects of polyamines on agronomic traits and photosynthetic physiology of wheat under high temperature stress. Photosynthetica 2019, 57, 912–920. [Google Scholar] [CrossRef]
- Choudhary, S.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Cellular responses, osmotic adjustments, and role of osmolytes in providing salt stress resilience in higher plants: Polyamines and nitric oxide crosstalk. J. Plant Growth Regul. 2022, 7, 347. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Scalschi, L.; Vicedo, B.; Marcos-Barbero, E.L.; Morcuende, R.; Camañes, G. Putrescine: A key metabolite involved in plant development, tolerance and resistance responses to stress. Int. J. Mol. Sci. 2022, 23, 2971. [Google Scholar] [CrossRef]
- Islam, M.J.; Uddin, M.J.; Hossain, M.A.; Henry, R.; Begum, M.K.; Sohel, M.A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.S. Exogenous putrescine attenuates the negative impact of drought stress by modulating physio-biochemical traits and gene expression in sugar beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef]
- Farooq, M.S.; Uzair, M.; Raza, A.; Habib, M.; Xu, Y.; Yousuf, M.; Yang, S.H.; Ramzan Khan, M. Uncovering the research gaps to alleviate the negative impacts of climate change on food security: A review. Front. Plant Sci. 2022, 13, 927535. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ. Sci. Pollut. Res. 2021, 28, 44768–44779. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef]
- Fortes, A.M.; Agudelo-Romero, P.; Pimentel, D.; Alkan, N. Transcriptional modulation of polyamine metabolism in fruit species under abiotic and biotic stress. Front. Plant Sci. 2019, 10, 107. [Google Scholar] [CrossRef]
- Shao, J.; Huang, K.; Batool, M.; Idrees, F.; Afzal, R.; Haroon, M.; Noushahi, H.A.; Wu, W.; Hu, Q.; Lu, X.; et al. Versatile roles of polyamines in improving abiotic stress tolerance of plants. Front. Plant Sci. 2022, 13, 1003155. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef] [PubMed]
- Raziq, A.; Din, A.M.U.; Anwar, S.; Wang, Y.; Jahan, M.S.; He, M.; Ling, C.G.; Sun, J.; Shu, S.; Guo, S. Exogenous spermidine modulates polyamine metabolism and improves stress responsive mechanisms to protect tomato seedlings against salt stress. Plant Physiol. Biochem. 2022, 170, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ndayiragije, A.; Lutts, S. Do exogenous polyamines have an impact on the response of a salt-sensitive rice cultivar to NaCl? J. Plant Physiol. 2006, 163, 506–516. [Google Scholar] [CrossRef]
- Ali, R.M. Role of putrescine in salt tolerance of Atropa belladonna plant. Plant Sci. 2000, 152, 173–179. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Ethylene production by leaves of rice (Oryza sativa L.) in relation to salinity tolerance and exogenous putrescine application. Plant Sci. 1996, 116, 15–25. [Google Scholar] [CrossRef]
- Wang, Q.; Yin, X. Alleviative effects of different kinds of exogenous polyamines on salt injury of soybean seedlings. J. Henan Agric. Sci. 2014, 43, 48–55. [Google Scholar]
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 2005, 162, 669–677. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Shi, Y.; Zhang, Z.; Zou, Z.; Zhang, H.; Zhao, J. Effect of exogenous spermidine on polyamine content and metabolism in tomato exposed to salinity–alkalinity mixed stress. Plant Physiol. Biochem. 2012, 57, 200–209. [Google Scholar] [CrossRef]
- Pascual, L.S.; López-Climent, M.F.; Segarra-Medina, C.; Gómez-Cadenas, A.; Zandalinas, S.I. Exogenous spermine alleviates the negative effects of combined salinity and paraquat in tomato plants by decreasing stress-induced oxidative damage. Front. Plant Sci. 2023, 14, 1193207. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Dual application of 24-epibrassinolide and spermine confers drought stress tolerance in maize (Zea mays L.) by modulating polyamine and protein metabolism. J. Plant Growth Regul. 2016, 35, 518–533. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Romero, L.; Ruiz, J.M. Accumulation of free polyamines enhanced antioxidant response in fruit of grafting tomato plants under water stress. J. Plant Physiol. 2016, 190, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.S.M.; Alharbi, K.; Rady, M.M.; Elnahal, A.S.M.; Selem, E.; Arnaout, S.M.A.I.; Selem, E.; Arnaout, S.M.A.I.; Mansour, E. Physiological, biochemical, anatomical, and agronomic responses of sesame to exogenously applied polyamines under different irrigation regimes. Agronomy 2023, 13, 875. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, H.; Lv, X.; Liu, D.; Wen, X.; Liao, Y. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol. Biochem. 2016, 100, 113–129. [Google Scholar] [CrossRef]
- Montesinos-Pereira, D.; Barrameda-Medina, Y.; Romero, L.; Ruiz, J.M.; Sánchez-Rodríguez, E. Genotype differences in the metabolism of proline and polyamines under moderate drought in tomato plants. Plant Biol. 2014, 16, 1050–1057. [Google Scholar] [CrossRef]
- Yiu, J.C.; Juang, L.D.; Fang, D.Y.T.; Liu, C.-W.; Wu, S.-J. Exogenous putrescine reduces flooding-induced oxidative damage by increasing the antioxidant properties of Welsh onion. Sci. Hortic. 2009, 120, 306–314. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, B.; Li, W.; Song, H.; Yu, Y.; Chen, J. Polyamines enhance chilling tolerance of cucumber (Cucumis sativus L.) through modulating antioxidative system. Sci. Hortic. 2009, 122, 200–208. [Google Scholar] [CrossRef]
- Shen, W.; Nada, K.; Tachibana, S. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol. 2000, 124, 431–440. [Google Scholar] [CrossRef]
- Song, Y.; Diao, Q.; Qi, H. Polyamine metabolism and biosynthetic genes expression in tomato (Lycopersicon esculentum Mill.) seedlings during cold acclimation. Plant Growth Regul. 2015, 75, 21–32. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ghorbanpour, M.; Brestic, M. Exogenous putrescine changes redox regulations and essential oil constituents in field-grown Thymus vulgaris L. under well-watered and drought stress conditions. Ind. Crops Prod. 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Hussain, S.; Farooq, M.; Wahid, M.; Wahid, A. Seed priming with putrescine improves the drought resistance of maize hybrids. Int. J. Agric. Biol. 2013, 15, 1349–1353. [Google Scholar]
- Gao, H.; Huang, H.; Lu, K.; Wang, C.; Liu, X.; Song, Z.; Zhou, H.; Yang, L.; Li, B.; Yu, C.; et al. OsCYP714D1 improves plant growth and salt tolerance through regulating gibberellin and ion homeostasis in transgenic poplar. Plant Physiol. Biochem. 2021, 168, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Napieraj, N.; Janicka, M.; Reda, M. Interactions of Polyamines and Phytohormones in Plant Response to Abiotic Stress. Plants 2023, 12, 1159. [Google Scholar] [CrossRef]
- Lightfoot, H.L.; Jonathan, H. Endogenous polyamine function—The RNA perspective. Nucleic Acids Res. 2014, 42, 11275–11290. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.; Srivastava, J.P.; Singhal, R.K.; Soufan, W.; Dadarwal, B.K.; Mishra, U.N.; Anuragi, H.; Rahman, M.A.; Sakran, M.I.; Brestic, M.; et al. EL: Alterations of Oxidative Stress Indicators, Antioxidant Enzymes, Soluble Sugars, and Amino Acids in Mustard [Brassica juncea (L.) Czern and Coss.] in Response to Varying Sowing Time, and Field Temperature. Front. Plant Sci. 2022, 13, 875009. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.Q.; Shu, S.; Shan, X.; Guo, S.R.; Sun, J. Effects of exogenous spermidine on antioxidant system of tomato seedlings exposed to high temperature stress. Russ. J. Plant Physiol. 2016, 63, 645–655. [Google Scholar] [CrossRef]
- Sang, Q.; Shan, X.; An, Y.; Shu, S.; Sun, J.; Guo, S. Proteomic analysis reveals the positive effect of exogenous spermidine in tomato seedlings’ response to high-temperature stress. Front. Plant Sci. 2017, 8, 120. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Menéndez, A.B.; Rodríguez, A.A.; Maiale, S.J.; Rodríguez-Kessler, M.; Jiménez-Bremont, J.F.; Ruiz, O.A. Polyamines contribution to the improvement of crop plants tolerance to abiotic stress. In Crop Improvement Under Adverse Conditions; Tuteja, N., Gill, S.S., Eds.; Springer: New York, NY, USA, 2013; pp. 113–136. [Google Scholar] [CrossRef]
- Alcázar, R.; Planas, J.; Saxena, T.; Zarza, X.; Bortolotti, C.; Cuevas, J.; Bitrián, M.; Tiburcio, A.F.; Altabella, T. Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous Arginine decarboxylase 2 gene. Plant Physiol. Bioch. 2010, 48, 547–552. [Google Scholar] [CrossRef]
- Chen, J. A starch- and ROS-regulating heat shock protein helps maintain male fertility in heat-stressed rice plants. Plant Physiol. 2023, 192, 2227–2229. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wen, W.; Shi, Z.; Gu, Q.; Ahammed, G.J.; Cao, K.; Shah Jahan, M.; Shu, S.; Wang, J.; et al. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 2021, 17, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Y.; Huang, Z.; Duan, H.; Tong, J.; He, X.; Gu, W.; Ma, H.; Xiao, L. Comparative proteomic analysis of seedling leaves of cold-tolerant and -sensitive spring soybean cultivars. Mol. Biol. Rep. 2015, 42, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Chen, H.C.; Jen, W.F.; Liu, L.Y.; Chang, M.C. Comparative transcriptome analysis of shoots and roots of TNG67 and TCN1 rice seedlings under cold stress and following subsequent recovery: Insights into metabolic pathways, phytohormones, and transcription factors. PLoS ONE 2015, 10, e0131391. [Google Scholar] [CrossRef] [PubMed]
- Dametto, A.; Sperotto, R.A.; Adamski, J.M.; Blasi, E.Á.R.; Cargnelutti, D.; de Oliveira, L.F.V.; Ricachenevsky, F.K.; Fregonezi, J.N.; Mariath, J.E.A.; da Cruz, R.P.; et al. Cold tolerance in rice germinating seeds revealed by deep RNA-seq analysis of contrasting indica genotypes. Plant Sci. 2015, 238, 1–12. [Google Scholar] [CrossRef]
- Espasandin, F.D.; Maiale, S.J.; Calzadilla, P.; Ruiz, O.A.; Sansberro, P.A. Transcriptional regulation of 9-cis-epoxycarotenoid dioxygenase (NCED) gene by putrescine accumulation positively modulates ABA synthesis and drought tolerance in Lotus tenuis plants. Plant Physiol. Biochem. 2014, 76, 29–35. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, R.R.; Wang, F.Y.; Peng, Z.; Kong, F.L.; Wu, J.; Lu, G. Spermidine affects the transcriptome responses to high temperature stress in ripening tomato fruit. J. Zhejiang Univ. Sci. B 2012, 13, 283–297. [Google Scholar] [CrossRef]
- Samanta, I.; Roy, P.C.; Das, E.; Mishra, S.; Chowdhary, G. Plant Peroxisomal Polyamine Oxidase: A Ubiquitous Enzyme Involved in Abiotic Stress Tolerance. Plants 2023, 12, 652. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Y.; Zhang, X.; Tian, X.; Hasan, M.M.; Yao, G.; Fang, X. Polyamines inhibit abscisic acid-induced stomatal closure by scavenging hydrogen peroxide. Physiol. Plant. 2023, 175, e13903. [Google Scholar] [CrossRef]
- Chu, G.; Chen, S.; Xu, C.; Liu, Y.; Zhang, X.; Wang, D. Ethylene and polyamines interact in rice spikelet degeneration in response to water stress during meiosis. Plant Growth Regul. 2023, 101, 617–628. [Google Scholar] [CrossRef]
- Alsharafa, K.Y. Exploring the interplay of phytohormones and polyamines in drought-stressed Cress (Lepidium sativum) leaves. J. Biol. Res. 2023, 96. [Google Scholar] [CrossRef]
- Nikhil, P.T.; Faiz, U.; Sharma, R.; Mohapatra, S. Modulation of plant polyamine and ethylene biosynthesis; and brassinosteroid signaling during Bacillus endophyticus J13-mediated salinity tolerance in Arabidopsis thaliana. J. Plant Physiol. 2024, 301, 154304. [Google Scholar] [CrossRef] [PubMed]
- Kaur-Sawhney, R.; Shih, L.M.; Cegielska, T.; Galston, A.W. Inhibition of protease activity by polyamines: Relevance for control of leaf senescence. FEBS Lett. 1982, 145, 345–349. [Google Scholar] [CrossRef]
- Murkowski, A. Heat stress and spermidine: Effect on chlorophyll fluorescence in tomato plants. Biol. Plant. 2001, 44, 53–57. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chan, Z. Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the Bermudagrass (Cynodon dactylon) response to salt and drought stresses. J. Proteome Res. 2013, 12, 4951–4964. [Google Scholar] [CrossRef]
- Su, X.Q.; Wang, M.Y.; Shu, S.; Sun, J.; Guo, S.R. Effects of exogenous spermidine on the fast Chlorophyll fluorescence induction dynamics in tomato seedlings under high temperature stress. Chin. Acta Hortic. 2013, 40, 2409–2418. [Google Scholar]
- Shan, X.; Zhou, H.; Sang, T.; Shu, S.; Sun, J.; Guo, S. Effects of exogenous spermidine on carbon and nitrogen metabolism in tomato seedlings under high temperature. J. Am. Soc. Hortic. Sci. 2016, 141, 381–388. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.F.; Alamer, K.H. Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena miller var. trigintipetala dieck. S. Afr. J. Bot. 2018, 116, 96–102. [Google Scholar] [CrossRef]
- Berahim, Z.; Dorairaj, D.; Omar, M.H.; Saud, H.M.; Ismail, M.R. Spermine mediated improvements on stomatal features, growth, grain filling and yield of rice under differing water availability. Sci. Rep. 2021, 11, 10669. [Google Scholar] [CrossRef]
- Severcan, F.; Gorgulu, G.; Gorgulu, S.T.; Guray, T. Rapid monitoring of diabetes-induced lipid peroxidation by Fourier transform infrared spectroscopy: Evidence from rat liver microsomal membranes. Anal. Biochem. 2005, 339, 36–40. [Google Scholar] [CrossRef]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Berberich, T.; Sagor, G.H.M.; Kusano, T. Polyamines in plant stress response. In Polyamines; Kusano, T., Suzuki, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 155–168. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, H.; Chander, S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J. Agron. Crop Sci. 2004, 190, 355–365. [Google Scholar] [CrossRef]
- Mehta, R.A.; Cassol, T.; Li, N.; Ali, N.; Handa, A.K.; Mattoo, A.K. Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat. Biotechnol. 2002, 20, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Nambeesan, S.; Datsenka, T.; Ferruzzi, M.G.; Malladi, A.; Mattoo, A.K.; Handa, A.K. Overexpression of yeast spermidine synthase impacts ripening, senescence, and decay symptoms in tomato. Plant J. 2010, 63, 836–847. [Google Scholar] [CrossRef]
- Gao, F.; Mei, X.; Li, Y.; Guo, J.; Shen, Y. Update on the roles of polyamines in fleshy fruit ripening, senescence, and quality. Front. Plant Sci. 2021, 12, 610313. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Kotsiras, A.; Tsafouros, A.; Roussos, P.A.; Aivalakis, G.; Katinakis, P.; Delis, C. Spatial and temporal distribution of genes involved in polyamine metabolism during tomato fruit development. Plant Physiol. Biochem. 2016, 100, 27–36. [Google Scholar] [CrossRef]
- Mattoo, A.; Cassol, T.; Mehta, R.; Handa, A.; Ali, N.; Abdul-Baki, A. Genetic engineering of tomato fruit for sustained accumulation of polyamines during ripening to study their physiological role(s). Acta Hortic. 2002, 575, 157–161. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Maodong, Q.; Dong, X.X.; Ahmad, P.; Abd_Allah, E.F.; Zhang, L. Spermine application alleviates salinity-induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najafi, R.; Kappel, N.; Mozafarian, M. The Role of Exogenously Applied Polyamines to Improve Heat Tolerance in Tomatoes: A Review. Agriculture 2025, 15, 988. https://doi.org/10.3390/agriculture15090988
Najafi R, Kappel N, Mozafarian M. The Role of Exogenously Applied Polyamines to Improve Heat Tolerance in Tomatoes: A Review. Agriculture. 2025; 15(9):988. https://doi.org/10.3390/agriculture15090988
Chicago/Turabian StyleNajafi, Raheleh, Noémi Kappel, and Maryam Mozafarian. 2025. "The Role of Exogenously Applied Polyamines to Improve Heat Tolerance in Tomatoes: A Review" Agriculture 15, no. 9: 988. https://doi.org/10.3390/agriculture15090988
APA StyleNajafi, R., Kappel, N., & Mozafarian, M. (2025). The Role of Exogenously Applied Polyamines to Improve Heat Tolerance in Tomatoes: A Review. Agriculture, 15(9), 988. https://doi.org/10.3390/agriculture15090988







