The Effect of Fertilization with Antibiotic-Contaminated Manure on Microbial Processes in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Parameters
2.2. Experimental Design
2.3. Determination of Antibiotic Concentrations in Soil and Plants
2.3.1. Quantitative Determination of Doxycycline and Enrofloxacin in Above-Ground Parts and Roots of Zea mays
Chemicals and Reagents
Sample Preparation
UHPLC-MS/MS
Validation of the Method
2.3.2. Quantitative Determination of Monensin in the Above-Ground Parts and Roots of Zea mays
Chemicals and Reagents
Sample Preparation
UHPLC-MS/MS
Validation of the Method
2.4. Chemical and Physicochemical Analyses of Soil
2.5. Biochemical Analyses of Soil
2.6. Microbiological Analyses of Soil
2.7. Statistical Analysis
3. Results
3.1. Response of Zea mays to Fertilization with Manure Containing Antibiotics
3.2. Antibiotic Residues in Soil and in the Above-Ground Parts and Roots of Zea mays Grown on Soil Fertilized with Manure
3.3. Physicochemical Properties of Soil Fertilized with Manure Containing Antibiotics
3.4. Response of Soil Enzymes to Fertilization with Manure Containing Antibiotics
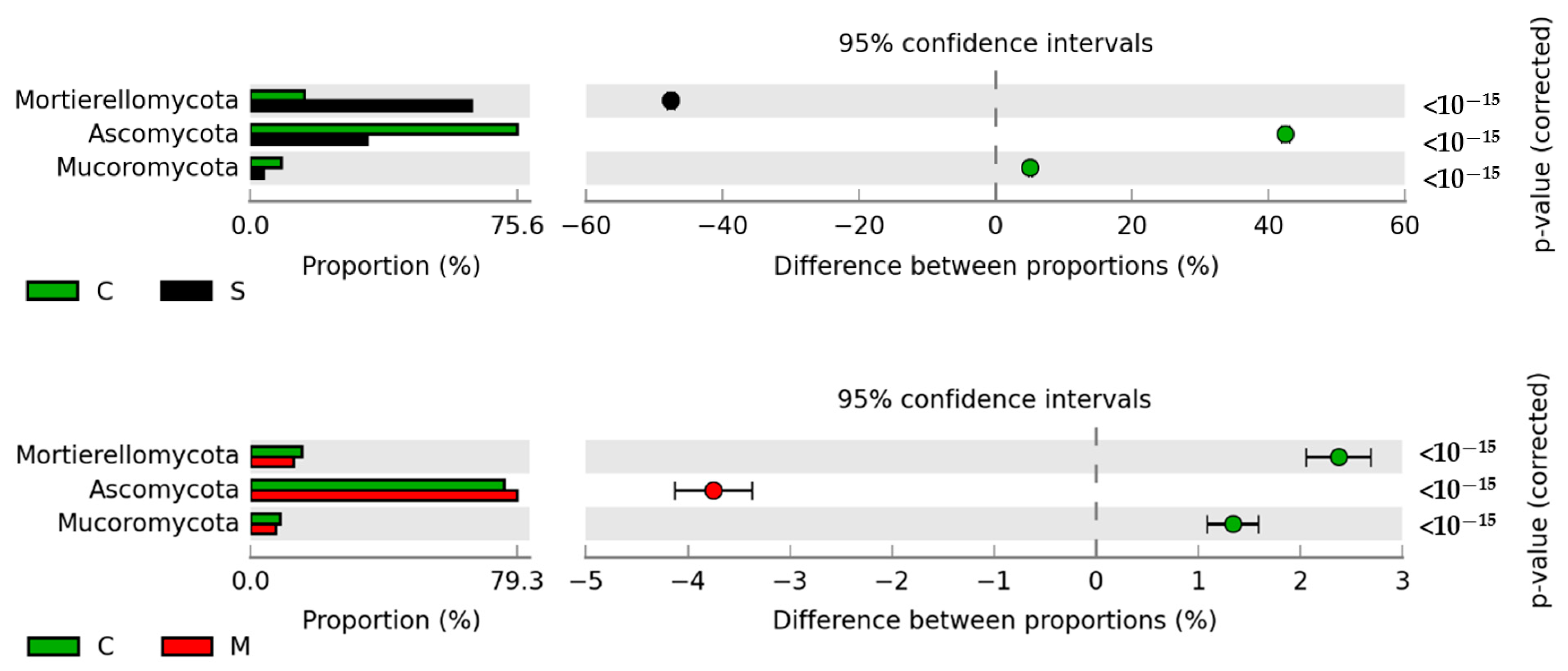
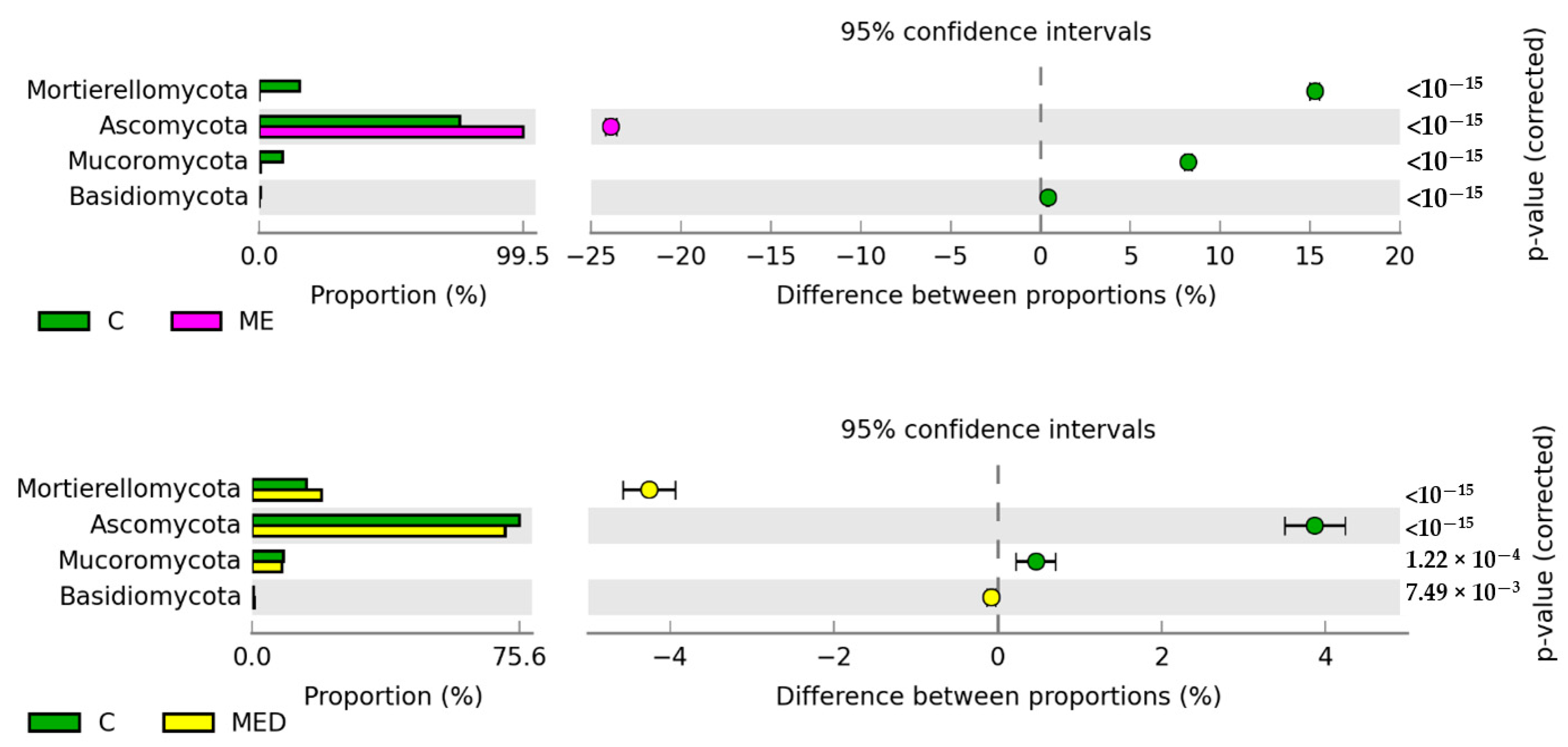
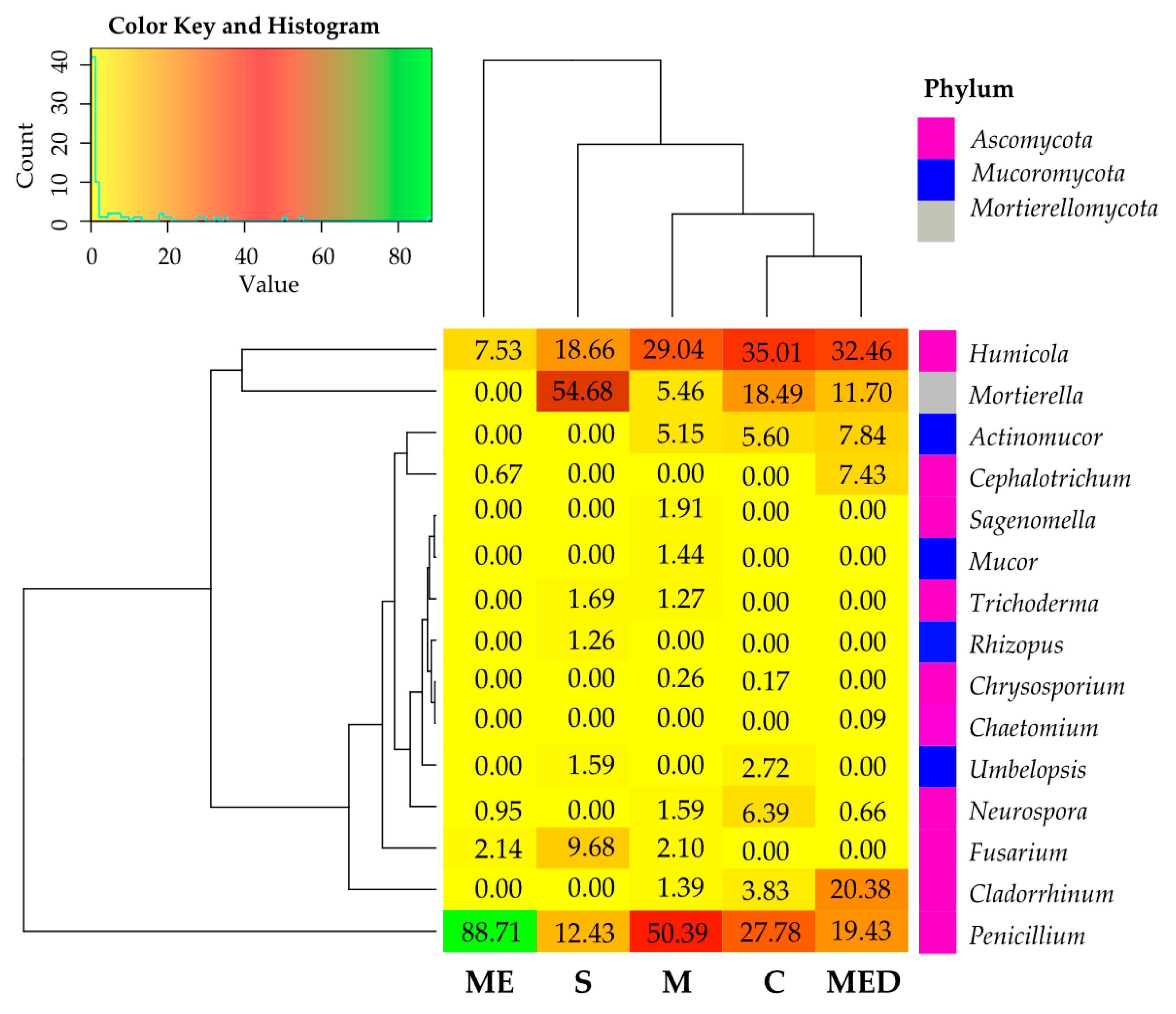
3.5. Response of the Soil Microbiome to Fertilization with Manure Containing Antibiotics
3.5.1. Culturable Microorganisms
3.5.2. Non-Culturable Microorganisms
4. Discussion
4.1. Response of Zea mays to Fertilization with Manure Containing Antibiotics
4.2. Physicochemical Properties of Soil Fertilized with Manure Containing Antibiotics
4.3. Antibiotic Concentrations in Zea mays and in Soil Fertilized with Manure Containing Antibiotics
4.4. Response of Soil Enzymes to Fertilization with Manure Containing Antibiotics
4.5. Response of the Soil Microbiome to Fertilization with Manure Containing Antibiotics
4.5.1. Culturable Microorganisms
4.5.2. Non-Culturable Microorganisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- McBride, S.G.; Wepking, C.; Hedin, M.L.; Thompson, R.C.; Barrett, J.E.; Strickland, M.S. Livestock manure and antibiotics alter extracellular enzyme activity. Appl. Soil Ecol. 2020, 155, 103667. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Zhang, X.; Wang, J.; Gao, M. Effects of chlortetracycline on soil microbial communities: Comparisons of enzyme activities to the functional diversity via Biolog EcoPlates™. Eur. J. Soil Biol. 2015, 68, 69–76. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The potential for restoring the activity of oxidoreductases and hydrolases in soil contaminated with petroleum products using perlite and dolomite. Appl. Sci. 2024, 14, 3591. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Evaluation of the effectiveness of innovative sorbents in restoring enzymatic activity of soil contaminated with bisphenol A (BPA). Molecules 2024, 29, 3113. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Li, D.; Xie, T.; Zhao, K.; Zhou, L.; Li, F. Antibiotics-heavy metals combined pollution in agricultural soils: Sources, fate, risks, and countermeasures. Green Energy Environ. 2024; in press. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Shen, Q.; Zhao, F.J. Antibiotics and antibiotic resistance from animal manures to soil: A review. Eur. J. Soil Sci. 2017, 69, 181–195. [Google Scholar] [CrossRef]
- Seo, K.W.; Lee, Y.J. Molecular characterization of fluoroquinolone-resistant Escherichia coli from broiler breeder farms. Poult. Sci. 2021, 100, 101250. [Google Scholar] [CrossRef]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Bingjie Xu, B.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef]
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 2017, 15, 422–434. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, G.F.; Zhu, B. The antibiotic resistome: Gene flow in environments, animals and human beings. Front. Med. 2017, 11, 161–168. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2014, 9, 1269–1279. [Google Scholar] [CrossRef]
- Cabezón, E.; Ripoll-Rozada, J.; Peña, A.; de la Cruz, F.; Arechaga, I. Towards an integrated model of bacterial conjugation. FEMS Microbiol. Rev. 2014, 39, 81–89. [Google Scholar] [CrossRef]
- Ling, B.; Feng, W.; Yang, N.; Fan, L.; Guo, G.; Li, X.; Zheng, J. High incidence of multiple intI1 genomic gene cassettes in Aeromonas strains. Aquaculture 2024, 579, 740171. [Google Scholar] [CrossRef]
- Pornsukarom, S.; Thakur, S. Horizontal dissemination of antimicrobial resistance determinants in multiple salmonella serotypes following isolation from the commercial swine operation environment after manure. Appl. Environ. Microbiol. 2017, 83, e01503-17. [Google Scholar] [CrossRef] [PubMed]
- Kyselkova, M.; Chrudimsky, T.; Husnik, F.; Chronakova, A.; Heuer, H.; Smalla, K.; Elhottova, D. Characterization of tet(Y)-carrying LowGC plasmids exogenously captured from cow manure at a conventional dairy farm. FEMS Microbiol. Ecol. 2016, 92, fiw075. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Fang, J.; Yang, Y.; Liu, L.; Liu, H.; Du, S. Transmission pathways and intrinsic mechanisms of antibiotic resistance genes in soil-plant systems: A review. Environ. Technol. Inno. 2025, 37, 103985. [Google Scholar] [CrossRef]
- Domingues, S.; Nielsen, K.M. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr. Opin. Microbiol. 2017, 38, 16–21. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, W.; Zhang, L.; Qin, C.H.; Wang, H.; Ling, W. Micro-interfacial behavior of antibiotic-resistant bacteria and antibiotic resistance genes in the soil environment: A review. Environ. Int. 2024, 191, 108972. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Wang, X.L.; Zhang, W.Z.; Wang, L.; Lyu, H.H.; Tang, J.C. Regulation of ARGs abundance by biofilm colonization on microplastics under selective pressure of antibiotics in river water environment. J. Environ. Manag. 2024, 355, 120402. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.; Tringe, S.; Sa, T.; Singh, B. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.; Huang, R.; Cui, Y.; Li, Q.; Zhao, Y.; Wang, X.; Mao, D.; Luo, Y.; Ren, H. Antibiotic Resistance Gene-Carrying Plasmid Spreads into the Plant Endophytic Bacteria using Soil Bacteria as Carriers. Environ. Sci. Technol. 2021, 55, 10462–10470. [Google Scholar] [CrossRef]
- Wei, Z.; Feng, K.; Wang, Z.; Zhang, Y.; Yang, M.; Zhu, Y.G.; Virta, M.P.J. High-Throughput single-cell technology reveals the contribution of horizontal gene transfer to typical antibiotic resistance gene dissemination in wastewater treatment plants. Environ. Sci. Technol. 2021, 55, 11824–11834. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Hong, Y.; Chen, M.; Zhang, H.; Peng, Y.; Liako, K.; Wang, H.; Zhu, F. Exploring the third-generation tetracycline resistance of multidrug-resistant livestock-associated methicillin-resistant Staphylococcus aureus ST9 across healthcare settings in China. J. Antimicrob. Chemoth. 2023, 78, 1871–1881. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Zhu, L.; Wang, J. Field-based evidence for enrichment of antibiotic resistance genes and mobile genetic elements in manure-amended vegetable soils. Sci. Total Environ. 2019, 654, 906–913. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Lv, Y.; Guo, X.; Lu, S. Response of Cyperus involucratus to sulfamethoxazole and ofloxacin-contaminated environments: Growth physiology, transportation, and microbial community. Ecotoxicol. Environ. Saf. 2020, 206, 111332. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Mitigating salt stress in Zea mays: Harnessing Serratia nematodiphila-biochar-based seed coating for plant growth promotion and rhizosphere microecology regulation. Ind. Crop Prod. 2025, 223, 120164. [Google Scholar] [CrossRef]
- Bonkowski, M.; Tarkka, M.; Razavi, B.S.; Schmidt, H.; Blagodatskaya, E.; Koller, R.; Yu, P.; Knief, C.; Hochholdinger, F.; Vetterlein, D. Spatiotemporal dynamics of maize (Zea mays L.) root growth and its potential consequences for the assembly of the rhizosphere microbiota. Front. Microbiol. 2021, 12, 619499. [Google Scholar] [CrossRef]
- Available online: https://pl.climate-data.org/europa/polska/warmian-masurian-voivodeship/olsztyn-758/ (accessed on 5 December 2024).
- Available online: https://olsztyn.stat.gov.pl/files/gfx/olsztyn/pl/defaultstronaopisowa/1414/1/1/2024_dzial_01_warunki_naturalne_i_ochrona_srodowiska.pdf (accessed on 5 December 2024).
- Available online: https://polska-energia.com/naslonecznienie-i-liczba-slonecznych-dni-w-polsce-co-warto-wiedziec/ (accessed on 5 December 2024).
- Available online: https://pl.weatherspark.com/y/85075/%C5%9Arednie-warunki-pogodowe-w:-Olsztyn-Polska-w-ci%C4%85gu-roku (accessed on 5 December 2024).
- Gbylik-Sikorska, M.; Gajda, A.; Felipe-Sotelo, M.; Caniça, M.; Cabal-Rosel, A.; Tenson, T.; Korínková, M.; Arbo, K.; Kisand, V.; Rab, G.; et al. Investigation of 29 Antimicrobial Compounds in Soil Using Newly Developed UHPLC-MS/MS Method. Molecules 2023, 28, 649. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to. Off. J. Eur. Union. 2021, 180, 84–109.
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; EUR 28099 EN; Publications office of the EU: Luxembourg, 2016; ISBN 978-92-79-61768-3. [Google Scholar]
- Carter, M.R. Soil Sampling and Methods of Analysis; Canadian Society of Soil Science; Lewis Publishers: London, UK, 1993. [Google Scholar]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef]
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. The International Organization for Standardization: Geneva, Switzerland, 2021.
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The usability of sorbents in restoring enzymatic activity in soils polluted with petroleum-derived products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. The role of grass compost and Zea Mays in alleviating toxic effects of tetracycline on the soil bacteria community. Int. J. Environ. Res. Public Health 2022, 19, 7357. [Google Scholar] [CrossRef]
- Sarathchandra, S.U.; Burch, G.; Cox, N.R. Growth patterns of bacterial communites in the rhizoplane and rhizosphere of with clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.) in long-term pasture. Appl. Soil Ecol. 1997, 6, 293–299. [Google Scholar]
- De Leij, F.A.A.M.; Whipps, J.M.; Lynch, J.M. The use of colony development for the characterization of bacterial communities in soil and on roots. Microb. Ecol. 1993, 27, 81–97. [Google Scholar] [CrossRef]
- Ferris, M.J.; Muyzer, G.; Ward, D.M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl. Environ. Microbiol. 1996, 62, 340–346. [Google Scholar]
- Data Analysis Software System, version 13; The R Foundation for Statistical Computing: Vienna, Austria. 2017. Available online: https://www.statistica.com (accessed on 5 December 2024).
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bio-informatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 5 December 2024).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, M.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data, version 3.20.0.; R Package; R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://rdrr.io/cran/gplots/ (accessed on 5 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 5 December 2024).
- Teressa, D.; Kibret, K.; Dechasa, N.; Wogi, L. Soil properties and nutrient uptake of maize (Zea mays) as influenced by mixed manure and blended inorganic fertilizer in Haramaya district, eastern Ethiopia. Heliyon 2024, 10, e35784. [Google Scholar] [CrossRef]
- Stehlíková, I.; Kodešová, R.; Kunzová, E.; Czakó, A.; Mayerová, M.; Madaras, M. Sixty-year impact of manure and NPK on soil aggregate stability. Geoderma Reg. 2024, 39, e00858. [Google Scholar] [CrossRef]
- Han, X.M.; Hu, H.W.; Chen, Q.L.; Yang, L.Y.; Li, H.L.; Zhu, Y.G.; Li, X.Z.; Ma, Y.B. Antibiotic resistance genes and associated bacterial communities in agricultural soils amended with different sources of animal manures. Soil Biol. Biochem. 2018, 126, 91–102. [Google Scholar] [CrossRef]
- Han, T.; Liang, Y.; Wu, Z.; Zhang, L.; Liu, Z.; Li, Q.; Chen, X.; Guo, W.; Jiang, L.; Pan, F.; et al. Effects of tetracycline on growth, oxidative stress response, and metabolite pattern of ryegrass. J. Hazard. Mater. 2019, 380, 12885. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Peng, K.; Jiang, L.; Zhang, D.; Song, D.; Chen, G.; Xu, H.; Li, Y.; Luo, C. Alleviated antibiotic-resistant genes in the rhizosphere of agricultural soils with low antibiotic concentration. Agric. Food Chem. 2020, 68, 2457–2466. [Google Scholar] [CrossRef]
- Wen, X.; Xu, J.; Xiang, G.; Cao, Z.; Yan, Q.; Mi, J.; Ma, B.; Zou, Y.; Zhang, N.; Liao, X.; et al. Multiple driving factors contribute to the variations of typical antibiotic resistance genes in different parts of soil-lettuce system. Ecotoxicol. Environ. Saf. 2021, 225, 112815. [Google Scholar] [CrossRef]
- Conte, S.; Lloyd, A. Exploring multiple drug and herbicide resistance in plants-Spotlight on transporter proteins. Plant Sci. 2011, 180, 196–203. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.; Hao, Z.; Mansoori, N.; da Rocha, U.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Zhang, D.; Hu, X.; Ding, W.; Wang, Y.; Li, G.; Dong, N.; Hu, H.; Hu, T.; et al. Integrative multi-omics analysis reveals the underlying toxicological mechanisms of enrofloxacin on the growth of wheat seedling roots. J. Hazard. Mater. 2024, 477, 135303. [Google Scholar] [CrossRef]
- Doornbos, R.; van Loon, L.; Bakker, P. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Zheng, F.; Zhou, G.; Zhu, D.; Neilson, R.; Zhu, Y.; Chen, B.; Yang, X. Does plant identity affect the dispersal of resistomes above and below ground? Environ. Sci. Technol. 2022, 56, 14904–14912. [Google Scholar] [CrossRef]
- Lebeis, S.; Paredes, S.; Lundberg, D.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; del Rio, T.; Jones, C.; Tringe, S.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef]
- Carvalhais, L.; Dennis, P.; Badri, D.; Kidd, B.; Vivanco, J.; Schenk, P. Linking Jasmonic Acid Signaling, Root Exudates, and Rhizosphere Microbiomes. Mol. Plant Microbe Interact. 2015, 28, 1049–1058. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef]
- Rath, S.; Fostier, A.; Pereira, L.; Dioniso, A.; Ferreira, F.; Doretto, K.; Peruchi, L.; Viera, A.; Neto, O.; Bosco, S.; et al. Sorption behaviors of antimicrobial and antiparasitic veterinary drugs on subtropical soils. Chemosphere 2019, 214, 111–122. [Google Scholar] [CrossRef]
- Du, H.; Du, J.; Liu, F.; Zhang, Y.; Guo, H.; Wan, D. Binding of tetracycline on soil phyllosilicates with Cd(II) as affected by pH and mineral type. J. Soil Sediment. 2021, 21, 775–783. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Chapman, H.D.; Jeffers, T.K.; Williams, R.B. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 2010, 89, 1788–1801. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Lahr, J.; Nibbeling, C.; Jansen, L.J.M.; Bongers, I.E.A.; Wipfler, E.L.; Van de Shans, M.G.M. The persistence of a broad range of antibiotics during calve, pig and broiler manure storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef]
- Hussain, S.; Naeem, M.; Chaudhry, M.N.; Iqbal, M.A. Accumulation of residual antibiotics in the vegetables irrigated by pharmaceutical wastewater. Expo. Health 2016, 8, 107–115. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Transfer of antibiotics from wastewater or animal manure to soil and edible crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef]
- Broekaert, N.; Daeseleire, E.; Delezie, E.; Vandecasteele, B.; De Beer, T.; Van Poucke, C. Can the Use of Coccidiostats in Poultry Breeding Lead to Residues in Vegetables? An Experimental Study. J. Agric. Food Chem. 2012, 60, 12411–12418. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Microbial diversity and enzyme activity as indicators of permethrin-exposed soil health. Molecules 2023, 28, 4756. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; Burns, R.G. Ecology of extracellular enzyme activities and organic matter degradation in soil: A complex community-driven process. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America; Wiley: Madison, WI, USA, 2011; pp. 35–55. [Google Scholar]
- Miśkowiec, P.; Olech, Z. Searching for the correlation between the activity of urease and the content of nickel in the soil Samples: The role of metal speciation. J. Soil Sci. Plant Nutr. 2020, 20, 1904–1911. [Google Scholar] [CrossRef]
- Unger, I.M.; Goyne, K.W.; Kennedy, A.C.; Kremer, R.J.; Mclain, J.E.T.; Williams, C.F. Antibiotic Effects on Microbial Community Characteristics in Soils under Conservation Management Practices. Soil Sci. Soc. Am. J. 2013, 77, 100–112. [Google Scholar] [CrossRef]
- Chen, W.; Liu, W.; Pan, N.; Jiao, W.; Wang, M. Oxytetracycline on functions and structure of soil microbial community. J. Soil Sci. Plant Nutr. 2013, 13, 967–975. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Díaz-Raviña, M.; Martín, A.; García-Campos, E.; Barreiro, A.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M.; Fernández-Calviño, D. Soil enzymatic activities and microbial community structure in soils polluted with tetracycline antibiotics. Agronomy 2021, 11, 906. [Google Scholar] [CrossRef]
- Molaei, A.; Lakzian, A.; Datta, R.; Haghnia, G.; Astaraei, A.; Rassouli-Sadaghiani, M.; Ceccherini, M.T. Impact of chlortetracycline and sulfapyridine antibiotics on soil enzyme activities. Int. Agrophys. 2017, 31, 499–505. [Google Scholar] [CrossRef]
- Guangming, L.; Xuechen, Z.; Xiuping, W.; Hongbo, S.; Jingsong, Y.; Xiangping, W. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Janse, I. Tetracycline amide antibiotics. In Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals, 1st ed.; Part III: Infectious Diseases, Chapter 9; Lamberth, C., Dinges, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 115–132. [Google Scholar]
- Bloor, M.C.; Kiryishina, A.; Kydralieva, K. Divergent effects of antibiotics on plants and microbiota in soils with contrasting humus content. Water Air Soil Pollut. 2021, 232, 518. [Google Scholar] [CrossRef]
- Męcik, M.; Buta-Hubeny, M.; Paukszto, Ł.; Maździarz, M.; Wolak, I.; Harnisz, M.; Korzeniewska, E. Poultry manure-derived microorganisms as a reservoir and source of antibiotic resistance genes transferred to soil autochthonous microorganisms. J. Environ. Manag. 2023, 348, 119303. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Liu, W.; Zhuang, J. The effect of manure application rates on the vertical distribution of antibiotic resistance genes in farmland soil. Soil Syst. 2024, 8, 89. [Google Scholar] [CrossRef]
- Guo, X.; Weining Qi, W.; Yao Feng, Y.; Zhaojun Li, Z. Degradation of oxytetracycline in soil by a Pseudomonas strain. J. Integr. Agric. 2024, 24, 2002–2014. [Google Scholar] [CrossRef]
- Chen, J.; Xu, H.; Sun, Y.; Huang, L.; Zhang, P.; Zou, C.; Zhao, C. Interspecific differences in growth response and tolerance to the antibiotic sulfadiazine in ten clonal wetland plants in South China. Sci. Total Environ. 2016, 543, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hou, Q.; Wang, Z.; Tian, D.; Zhang, X.; Zhang, Y.; Wu, Q.; Sun, F. Fatty acid addition strategy redirected the metabolic flux towards an ultra-high monensin productivity of Streptomyces cinnamonensis. Synth. Syst. Biotechnol. 2025, 10, 532–542. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, C.Y.; Li, X.M.; Tang, Z.K.; Qiao, J.; Zhao, G.R. DasR positively controls monensin production at two-level regulation in Streptomyces cinnamonensis. J. Ind. Microbiol. Biotechnol. 2016, 43, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Barabote, R.D.; Thekkiniath, J.; Strauss, R.E.; Vediyappan, G.; Fralick, J.A.; San Francisco, M.J. Xenobiotic efflux in bacteria and fungi: A genomics update. Adv. Enzymol. Relat. Areas Mol. Biol. 2011, 77, 237–306. [Google Scholar] [CrossRef]
- Kondratowicz-Maciejewska, K.; Lemanowicz, J.; Jaskulska, I. Effects of Long-Term Multi-Treatment Experiments on Organic Matter and Enzymatic Activity in Sandy Soil. Sustainability 2025, 17, 3252. [Google Scholar] [CrossRef]
- Gong, P.; Liu, H.; Xin, Y.; Wang, G.; Dai, X.J.; Yao, J. Composting of oxytetracycline fermentation residue in combination with hydrothermal pretreatment for reducing antibiotic resistance genes enrichment. Bioresour. Technol. 2020, 318, 124271. [Google Scholar] [CrossRef]
- Iltchenco, J.; Smiderle, M.D.; Gaio, J.; Magrini, F.E.; Paesi, S. Metataxonomic characterization of the microbial present in the anaerobic digestion of turkey litter waste with the addition of two inocula: Allochthonous and commercial. Int. Microbiol. 2025, 28, 539–551. [Google Scholar] [CrossRef]
- Dyksma, S.; Jansen, L.; Gallert, C. Syntrophic acetate oxidation replaces acetoclastic methanogenesis during thermophilic digestion of biowaste. Microbiome 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Yang, J.; Xiang, J.; Gin, K.; Xie, Y.; Yu, K.; Li, P.; He, Y. Antibiotic resistome associated with influencing factors in industrial-scale organic waste aerobic composting plants. Bioresour. Technol. 2023, 385, 129354. [Google Scholar] [CrossRef]
- Fang, D.; Zhao, G.; Xu, X.; Zhang, Q.; Shen, Q.; Fang, Z.; Huang, L.; Ji, F. Microbial community structures and functions of wastewater treatment systems in plateau and cold regions. Bioresour. Technol. 2018, 249, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Liu, H.; Wang, G.; Yao, J.; Dai, X. Enhanced depletion of antibiotics and accelerated estabilization of dissolved organic matter by hydrothermal pretreatment during composting of oxytetracycline fermentation residue. Bioresour. Technol. 2021, 339, 125618. [Google Scholar] [CrossRef]
- Feng, M.; Liu, Y.; Yang, L.; Li, Z. Antibiotics and antibiotic resistance gene dynamics in the composting of antibiotic fermentation waste—A review. Biores. Technol. 2023, 390, 129861. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yao, H. Effects of composting different types of organic fertilizer on the microbial community structure and antibiotic resistance genes. Microorganisms 2020, 8, 268. [Google Scholar] [CrossRef]
- Beyi, A.F.; Brito-Goulart, D.; Hawbecker, T.; Ruddell, B.; Hassall, A.; Dewell, R.; Dewell, G.; Sahin, O.; Zhang, Q.; Plummer, P.J. Enrofloxacin alters fecal microbiota and resistome irrespective of its dose in calves. Microorganisms 2021, 9, 2162. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, J.M.; Cano, M.E.; Velasco, C.; Martínez-Martínez, L.; Pascual, A. Plasmid-mediated quinolone resistance: An update. J. Infect. Chemother. 2011, 17, 149–182. [Google Scholar] [CrossRef]
- Frąc, M.; Pertile, G.; Panek, J.; Gryta, A.; Oszust, K.; Lipiec, J.; Usowicz, B. Mycobiome composition and diversity under the long-term application of spent mushroom substrate and chicken manure. Agronomy 2021, 11, 410. [Google Scholar] [CrossRef]
- Wen, X.; Jia, Y.; Li, J. Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from Phanerochaete chrysosporium. J. Hazard Mater. 2010, 177, 924–928. [Google Scholar] [CrossRef]
- Llorca, M.; Rodríguez-Mozaz, S.; Couillerot, O.; Panigoni, K.; de Gunzburg, J.; Bayer, S.; Czaja, R.; Barceló, D. Identification of new transformation products during enzymatic treatment of tetracycline and erythromycin antibiotics at laboratory scale by an on-line turbulent flow liquid-chromatography coupled to a high resolution mass spectrometer LTQ-Orbitrap. Chemosphere 2015, 119, 90–98. [Google Scholar] [CrossRef]
- Rajalakshmi, J.; Harish, S.; Rajendran, L.; Parthasarathy, S.; Saravanakumari, K.; Raguchander, T. Crude antibiotics and antifungal metabolites from Chaetomium globosum Cg6 suppress pythium aphanidermatum, causal agent of rhizome rot of turmeric. Curr. Microbiol. 2025, 82, 105. [Google Scholar] [CrossRef] [PubMed]
- Goda, M.S.; El-Kattan, N.; Abdel-Azeem, M.A.; Allam, K.A.M.; Badr, J.M.; Nassar, N.A.; Almalki, A.J.; Alharbi, M.; Elhady, S.S.; Eltamany, E.E. Antimicrobial potential of different isolates of Chaetomium globosum combined with liquid chromatography tandem mass spectrometry chemical profiling. Biomolecules 2023, 13, 1683. [Google Scholar] [CrossRef] [PubMed]









| Treatment | Aerial Parts | Roots | Height |
|---|---|---|---|
| S | 70.56 ± 5.55 d | 8.43 ± 0.50 c | 143.90 ± 5.91 b |
| C | 139.43 ± 4.32 bc | 15.52 ± 1.29 a | 187.65 ± 6.76 a |
| M | 146.95 ± 3.36 ab | 14.99 ± 1.76 a | 195.00 ± 9.25 a |
| ME | 151.61 ± 2.29 a | 13.13 ± 0.87 ab | 201.05 ± 3.21 a |
| MED | 138.01 ± 0.32 c | 11.99 ± 0.71 b | 191.35 ± 4.06 a |
| Treatment | 5th Leaf (Day 20) | 8th Leaf (Day 35) | 11th Leaf (Day 50) | Average |
|---|---|---|---|---|
| S | 34.55 ± 1.41 e | 24.13 ± 2.09 g | 17.48 ± 2.24 h | 25.38 D |
| C | 37.01 ± 1.44 d | 43.62 ± 3.08 a | 28.56 ± 2.57 f | 36.36 B |
| M | 39.68 ± 1.53 bc | 45.05 ± 2.07 a | 30.56 ± 2.68 f | 38.32 A |
| ME | 39.76 ± 2.38 bc | 45.05 ± 3.02 a | 30.79 ± 2.45 f | 38.53 A |
| MED | 37.50 ± 1.36 cd | 40.88 ± 2.24 b | 26.01 ± 2.66 g | 34.80 C |
| Treatment | Antibiotic Concentrations (µg kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil | Roots | Above-Ground Parts | |||||||
| M* | E* | D* | M* | E* | D* | M* | E* | D* | |
| S | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| C | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| M | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| ME | nd | 125 | nd | nd | 14.8 | nd | nd | nd | nd |
| MED | nd | 193 | 117 | nd | 33.8 | 36.2 | nd | nd | nd |
| Treatment | Corg | SOM | NTotal | C:N |
|---|---|---|---|---|
| g | ||||
| S | 6.97 ± 0.04 c | 12.02 ± 0.07 c | 1.11 ± 0.03 b | 6.31 ± 0.13 b |
| C | 7.85 ± 0.07 a | 13.53 ± 0.11 a | 1.23 ± 0.08 a | 6.40 ± 0.37 ab |
| M | 7.48 ± 0.08 b | 12.90 ± 0.14 b | 1.19 ± 0.04 ab | 6.29 ± 0.28 b |
| ME | 7.38 ± 0.14 b | 12.72 ± 0.24 b | 1.18 ± 0.02 ab | 6.25 ± 0.01 b |
| MED | 8.06 ± 0.20 a | 13.90 ± 0.34 a | 1.17 ± 0.02 ab | 6.89 ± 0.26 a |
| Treatment | pHKCl | Eh | HAC | EBC | CEC |
|---|---|---|---|---|---|
| mV | mmol | ||||
| S | 4.73 ± 0.01 e | 418.30 ± 0.08 a | 31.13 ± 0.31 c | 39.00 ± 4.08 b | 70.13 ± 4.39 b |
| C | 4.82 ± 0.01 b | 412.45 ± 0.53 b | 32.63 ± 0.31 b | 45.00 ± 0.82 a | 77.63 ± 1.12 a |
| M | 4.78 ± 0.01 c | 413.15 ± 0.78 b | 33.38 ± 0.31 a | 47.00 ± 0.82 a | 80.38 ± 0.51 a |
| ME | 4.76 ± 0.01 d | 412.95 ± 0.61 b | 33.75 ± 0.01 a | 46.00 ± 1.63 a | 79.75 ± 1.63 a |
| MED | 4.90 ± 0.01 a | 408.45 ± 0.12 c | 31.13 ± 0.31 c | 47.00 ± 0.82 a | 78.13 ± 1.12 a |
| Treatment | Deh µmol TFF | Cat mol O2 | Ure mmol N-NH4 | AcP | AlP | Aryl | Glu | BA |
|---|---|---|---|---|---|---|---|---|
| mmol PN | ||||||||
| S | 6.27 ± 0.02 d | 0.25 ± 0.01 c | 0.51 ± 0.03 c | 3.35 ± 0.01 d | 0.79 ± 0.01 b | 0.16 ± 0.01 c | 0.77 ± 0.01 c | 12.09 ± 0.01 d |
| C | 14.78 ± 0.54 a | 0.31 ± 0.01 b | 0.69 ± 0.03 b | 4.48 ± 0.06 b | 1.16 ± 0.09 a | 0.18 ± 0.01 b | 1.02 ± 0.03 b | 22.62 ± 0.38 a |
| M | 13.00 ± 0.17 b | 0.33 ± 0.01 a | 0.71 ± 0.01 ab | 4.40 ± 0.01 bc | 1.14 ± 0.01 a | 0.18 ± 0.01 b | 1.09 ± 0.01 b | 20.86 ± 0.16 b |
| ME | 12.83 ± 0.35 b | 0.34 ± 0.01 a | 0.77 ± 0.03 a | 4.68 ± 0.01 a | 1.18 ± 0.05 a | 0.21 ± 0.01 a | 1.09 ± 0.07 b | 21.09 ± 0.31 b |
| MED | 8.75 ± 0.33 c | 0.34 ± 0.01 a | 0.69 ± 0.03 b | 4.26 ± 0.17 c | 1.20 ± 0.02 a | 0.21 ± 0.01 a | 1.18 ± 0.02 a | 16.63 ± 0.45 c |
| Treatment | CFU | CD | EP |
|---|---|---|---|
| Org | |||
| S | 2.74 ± 0.18 b | 40.38 ± 1.83 a | 0.65 ± 0.10 b |
| C | 10.85 ± 0.42 a | 34.43 ± 0.14 b | 0.79 ± 0.01 a |
| M | 10.28 ± 0.78 a | 32.51 ± 1.59 b | 0.79 ± 0.04 a |
| ME | 10.54 ± 0.27 a | 27.13 ± 0.48 c | 0.88 ± 0.02 a |
| MED | 9.92 ± 0.76 a | 26.69 ± 0.91 c | 0.87 ± 0.02 a |
| Act | |||
| S | 1.60 ± 0.22 b | 22.25 ± 2.90 a | 0.76 ± 0.09 b |
| C | 4.96 ± 0.17 a | 22.02 ± 0.74 a | 0.90 ± 0.03 a |
| M | 4.85 ± 0.24 a | 21.87 ± 0.26 a | 0.86 ± 0.01 ab |
| ME | 4.70 ± 0.32 a | 20.54 ± 2.19 ab | 0.85 ± 0.05 ab |
| MED | 4.54 ± 0.12 a | 17.96 ± 0.92 b | 0.78 ± 0.06 b |
| Fun | |||
| S | 4.08 ± 0.30 a | 32.43 ± 1.28 b | 0.62 ± 0.04 b |
| C | 3.36 ± 0.25 ab | 36.17 ± 3.45 ab | 0.82 ± 0.05 a |
| M | 3.46 ± 0.13 ab | 38.59 ± 1.57 a | 0.63 ± 0.03 b |
| ME | 3.98 ± 0.89 ab | 40.17 ± 0.80 a | 0.72 ± 0.12 ab |
| MED | 3.05 ± 0.18 b | 40.02 ± 1.67 a | 0.73 ± 0.06 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowska, J.; Mikulski, D.; Borowik, A.; Zaborowska, M.; Kucharski, J.; Kozłowski, K.; Bilecka, M.; Gajda, A.; Pietruk, K.; Jedziniak, P.; et al. The Effect of Fertilization with Antibiotic-Contaminated Manure on Microbial Processes in Soil. Agriculture 2025, 15, 979. https://doi.org/10.3390/agriculture15090979
Wyszkowska J, Mikulski D, Borowik A, Zaborowska M, Kucharski J, Kozłowski K, Bilecka M, Gajda A, Pietruk K, Jedziniak P, et al. The Effect of Fertilization with Antibiotic-Contaminated Manure on Microbial Processes in Soil. Agriculture. 2025; 15(9):979. https://doi.org/10.3390/agriculture15090979
Chicago/Turabian StyleWyszkowska, Jadwiga, Dariusz Mikulski, Agata Borowik, Magdalena Zaborowska, Jan Kucharski, Krzysztof Kozłowski, Magdalena Bilecka, Anna Gajda, Konrad Pietruk, Piotr Jedziniak, and et al. 2025. "The Effect of Fertilization with Antibiotic-Contaminated Manure on Microbial Processes in Soil" Agriculture 15, no. 9: 979. https://doi.org/10.3390/agriculture15090979
APA StyleWyszkowska, J., Mikulski, D., Borowik, A., Zaborowska, M., Kucharski, J., Kozłowski, K., Bilecka, M., Gajda, A., Pietruk, K., Jedziniak, P., Ognik, K., & Jankowski, J. (2025). The Effect of Fertilization with Antibiotic-Contaminated Manure on Microbial Processes in Soil. Agriculture, 15(9), 979. https://doi.org/10.3390/agriculture15090979










