1. Introduction
Meeting the food demands of a growing global population requires effective strategies to minimize the environmental impact and cost of agriculture, especially due to the intensive use of mineral fertilizers and agrochemicals. Biofertilizers, composed of eco-friendly microorganisms, offer a promising alternative for enabling more sustainable, low-cost, and high-yield agricultural practices [
1,
2]. One example among many others includes inoculants composed of nitrogen-fixing bacteria (BNF), which can reduce or even eliminate the need for soluble nitrogen fertilizers, resulting in a positive economic impact [
3]. In addition to pre-selected efficient diazotrophic strains, inoculants must be formulated to ensure cell viability for adequate periods until use. This is achieved through the use of carriers and additives with specific characteristics.
The development of effective solid or liquid bioinoculant formulations involves several steps: inoculum preparation, addition of cell protectants, use of suitable carrier materials, appropriate packaging, and selection of optimal delivery methods [
2]. Furthermore, producing a biofertilizer must consider not only the interests of the agricultural sector, such as ease of use and ensuring bacterial survival during the infection process but also the demands of manufacturers, reducing the risk of the presence of hazardous materials and maintaining the product’s shelf life according to crop-specific needs [
4].
Peat, a non-renewable natural resource formed from the microbial decomposition of organic matter, is a common carrier used in formulating rhizobial inoculants that include various species of
Rhizobium,
Bradyrhizobium, and
Mesorhizobium [
5,
6]. Given its characteristics, it maintains the viability of rhizobial strains for up to six months of storage, facilitating their manipulation and adhesion to seeds [
7]. However, the use of peat has several drawbacks, such as the extraction of this finite resource, which is regulated by local environmental laws, and the high microbial load that necessitates rigorous sterilization conditions, thereby increasing production costs [
5,
6,
7,
8,
9].
As a peat alternative, biochar, a carbon-rich solid material produced from biomass pyrolysis, has been evaluated in various studies and has shown the potential to yield successful outcomes [
10,
11].
Liquid formulations are widely used for large-scale inoculations in mechanized planting systems. These formulations employ additives that enhance stability and maintain rhizobial viability during storage [
12,
13]. Compared to peat, these carriers are sensitive to excessive temperature increases, which reduce the microbial population [
14,
15,
16]. Generally, the shelf life of liquid inoculants is shorter than that of peat inoculants, especially when stored at room temperature [
17].
Recent reviews have explored innovative biofertilizer formulations derived from a diverse range of materials. Among these emerging solutions for sustainable agriculture, biopolymeric matrices designed as carriers for PGPB have shown significant potential [
12,
18,
19,
20,
21].
Some techniques, such as bacterial encapsulation, produce high-quality polymers that maintain cell viability for extended periods. However, these techniques are expensive and involve complex production steps. The effectiveness of microorganism immobilization depends on the carrier material, such as alginate-based biopolymers, which can be optimized by the inclusion of additives [
22]. In contrast, using biofilm-based biofertilizers has the potential to create inoculant carriers that benefit from low-cost raw materials and ease of acquisition [
4,
23]. Polymeric blends serve as inoculation carriers that promote biofilm formation.
Forty blends of carboxymethylcellulose (CMC) and starch were evaluated, and those that best maintained cell viability for 168 days were selected for further testing as
Bradyrhizobium carriers for inoculating cowpea seeds [
24]. The CMC/starch blends demonstrated a performance similar to that of the peat-based inoculant, indicating their potential as viable alternatives. In another study, CMC/starch blends selected as carriers for liquid and gel-based formulations were also capable of maintaining the viability of a mixture of five strains of diazotrophic bacteria (
Gluconacetobacter diazotrophicus,
Herbaspirillum seropedicae,
H. rubrisubalbicans, Azospirillum amazonense, and
Burkholderia tropica) [
7]. A short-term greenhouse experiment showed that inoculated sugarcane positively responded to colonization by the diazotrophic strains.
The current study aimed to optimize the effectiveness of CMC/starch blends for their application as inoculation carriers for a strain of Bradyrhizobium pachyrhizi. Various brands of component materials were evaluated, along with their concentrations and autoclaving times, to standardize the conditions necessary for producing effective carriers.
2. Materials and Methods
Biological material: The BR 3262 strain of
Bradyrhizobium pachyrhizi, identified as BRM 006350 in the database of the Brazilian Agricultural Research Corporation, Embrapa, under the “Linhagem” (Lineage) section;
https://am.cenargen.embrapa.br/amconsulta, accessed on 25 April 2025. A cell suspension was prepared in Yeast Extract Mannitol (YEM) culture medium [
25] and then added to an aqueous glycerol solution (15%) before being stored in a super freezer at approximately −80 °C.
Preparation of inoculation carriers: Blends of CMC and starch were used to formulate the BR 3262 strain [
24]. They were prepared in a 60/40 dry basis proportion (64 g L
−1) in distilled water, containing 38.40 g L
−1 of CMC and 25.60 g L
−1 of starch compatibilized with magnesium oxide (MgO). The product specifications for CMC, starch, and MgO are detailed in
File S5. Initially, starch, MgO, and distilled water were homogenized in a blender for 30 s. Then, CMC was added, followed by additional stirring 30 s Twenty grams of each CMC/starch blend was transferred to polypropylene bags, labelled, sealed, and sterilized in an autoclave at 121 °C and 1 atm for 30 or 60 min, depending on the trial. According to the experimental procedure, the following raw materials were tested: two commercial brands of CMC designated as CMC-I and CMC-II, four commercial brands of starch: starch A, starch B, starch C, and starch D, and six concentrations of MgO (
w/
v): 0.1% (64 mg L
−1), 0.2% (128 mg L
−1), 0.3% (192 mg L
−1), 0.4% (256 mg L
−1), 0.5% (512 mg L
−1), and 1.0% (1024 mg L
−1).
Inoculation of blends: The BR 3262 strain was cultivated in Yeast Extract Mannitol (YEMA) culture medium from the cell stock [
25] at 28 °C for 7 days. Subsequently, the cell mass was transferred to a 250 mL Erlenmeyer flask containing 100 mL of YEM medium [
25] and placed in an orbital shaker at 150 rpm and 28 °C. After 4 days, the broth was centrifuged at approximately 350 RCF for 10 min at 4 °C; the supernatant was discarded, and the pellet was resuspended in sterile distilled water. A new culture was initiated from the suspension, with an OD of around 0.1 at 540 nm. After 10 days of stirring at 28 °C, the culture was inoculated into the blends, reaching final concentrations of 1.2 to 2 × 10
8 CFU mL
−1. The inoculation carriers were stored at room temperature, approximately 25 °C.
Blend pH and cell viability determinations: The pH levels of the inoculated and non-inoculated blends were measured after dilution in distilled water (1:6) using a bench pH meter (BioVera–PB1800). The cell viability of the BR 3262 strain suspensions was assessed after each collection time by placing 5 g of the blend in 50 mL autoclaved plastic tubes, to which 25 mL of sterile distilled water was added. The tubes were vortexed for 1 min. Subsequently, a 1 mL aliquot was transferred to a microtube, from which a serial dilution between 10
−1 and 10
−8 in sterile saline solution (8.5 g L
−1) was performed. Next, 100 µL aliquots of the 10
−3 to 10
−8 suspensions were spread on the YEMA medium using a Drigalski loop. The plates were placed in a BOD incubator at 28 °C for 8 days, after which the colonies were counted. From this procedure, nine colonies in the 10
−3 dilution were identified as the minimum detectable number of viable cells or 5.4 × 10
5 CFU ml
−1, considering the initial dilution factor of the blend (×6). The recommended cell viability for commercial inoculants should be in the range of 10
8 or 10
9 CFU mL
−1 [
26,
27], which means that the minimum detectable number represents approximately 1:1000 of the optimal value. Samples with viable cells equal to or below 5.4 × 10
5 CFU ml
−1 were classified as “not determined” and were excluded from the statistical analysis.
Four experiments were conducted between March 2016 and September 2018. They were set up using a completely randomized design with two replicates, each of which was sampled and assessed twice. The factorial arrangement for each experiment is outlined as follows:
Polymeric blends consisting of CMC-I and starch A were prepared as previously described, with or without the addition of MgO (0.1%, 0.2%, 0.3%, 0.4%, 0.5%, and 1.0%). After preparation, the mixtures were packaged in polypropylene bags, autoclaved for 30 min, and inoculated with a suspension of strain BR 3262 to achieve an initial concentration of 1.2 × 108 CFU mL−1. Cell viability and pH were measured 30 days post inoculation.
Polymeric mixtures composed of CMC-I and four commercial starch types, compatibilized with 0.3% or 1.0% MgO, were formulated in this study. The pH of the freshly prepared mixtures was measured, followed by distribution into polypropylene bags and autoclaving for 30 min. The pH was remeasured 24 h post autoclaving. A 4 × 2 × 2 factorial experiment was conducted to investigate the influence of 4 different starch types, 2 concentration levels of MgO (0.3% and 1.0%), and 2 evaluation times before and after autoclaving.
Polymeric blends containing CMC-I and four types of commercial starch were compatibilized with either 0.3% or 1.0% MgO and evaluated. After preparation, the mixtures were packaged in polypropylene bags and autoclaved for 30 or 60 min. The pH of the blends was measured at 1, 14, 30, 90, and 180 days after autoclaving. A 4 × 2 × 2 × 5 factorial scheme was employed, comprising 4 starches, 2 concentration levels of MgO, 2 autoclaving times, and 5 evaluation periods.
The combined effects of CMC type, starch type, MgO concentration, and autoclaving time on the cell viability were investigated. Furthermore, a new commercial brand of CMC was used in this study. A total of 32 treatment combinations were evaluated, including two commercial CMCs (CMC-I and CMC-II), four commercial starches (A, B, C, and D), two concentration levels of MgO (0.3% and 1.0%), and two autoclaving durations (30 and 60 min). Following preparation, the polymeric blends were packaged in polypropylene bags, autoclaved, and inoculated with strain BR 3262 to achieve an initial concentration of 2 × 108 CFU mL−1. Cell viability was assessed at 7, 28, 56, 84, and 168 days post autoclaving. A 2 × 4 × 2 × 2 × 5 factorial scheme was applied: 2 CMCs, 4 starches, 2 MgO concentrations, 2 autoclaving times, and 5 evaluation times.
Statistical analysis: Statistical analyses were conducted to evaluate the cell viability and pH values. The residuals from the design models underwent the Shapiro-Wilk test to assess normality and the Bartlett test to analyze the homogeneity of variances using R software [
28]. Analysis of variance (ANOVA), followed by Tukey’s test (
p < 0.05), was conducted to compare the treatment means using Sisvar software (v5.7) [
29]. Additionally, regression models were fitted to the quantitative factors. The Spearman correlation coefficient (ρ) and
p-value of significance were also determined.
3. Results
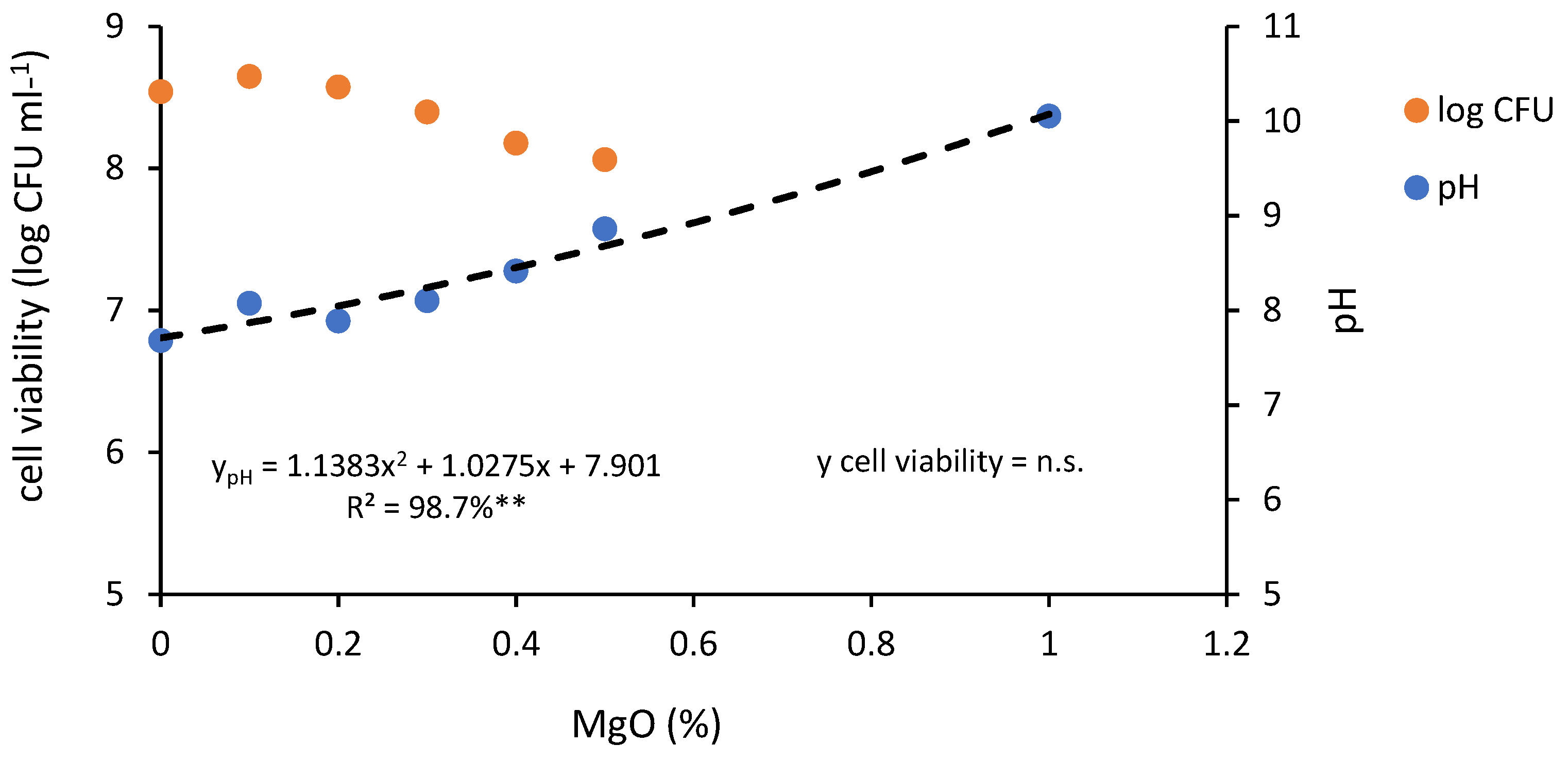
3.1. The Effect of Varying Concentrations of the Compatibilizer MgO on the pH of the Polymeric Blend and the Cell Viability of Strain BR 3262 (Experiment 1)
The addition of MgO as a compatibilizer to the CMC/starch blend helped prevent phase separation, which impacted bacterial cell viability. A range of MgO concentrations up to 1.0% was added to the blends inoculated with 1.2 × 10
8 CFU mL
−1 of
B. pachyrhizi strain BR 3262. After one month at room temperature, the pH of the blends compatibilized with 1.0% MgO was approximately 10. With up to 0.5% MgO, the observed pH varied from 8.0 to 8.8 (
Figure 1;
File S1). A decrease in cell viability was observed with increasing pH, establishing an inverse correlation (ρ = −0.89;
p = 0.0333). At a pH of approximately 8.8, cell viability remained close to 10
8 CFU mL
−1, while at pH 10, it dropped to log ≤ 5.732 (N.D.).
3.2. The Impact of Autoclaving on Polymer Blends (Experiment 2)
Table 1 (
File S4) illustrates the impact of autoclaving on the performance of polymer blends made from four different commercial starches compatibilized with either 0.3% or 1.0% MgO. Autoclaving significantly decreased the pH of the blends; however, the degree of pH reduction varied depending on the type of starch and the concentration of MgO. Before autoclaving, the overall average pH values were approximately 10.73 for blends compatibilized with 0.3% and 10.98 for those with 1.0% MgO.
The most significant reduction in pH due to autoclaving was observed in the blends containing A and B starches, which were compatibilized with 0.3% MgO. The pH of these blends decreased from approximately 10.7 before autoclaving to around 8.0 afterward. A reduction in pH ranging from roughly 0.3 to 0.7 occurred for all blends compatibilized with 1.0% MgO, as well as for blends containing C and D starches compatibilized with 0.3% MgO.
3.3. Impact of Autoclave Exposure Duration on the pH of Polymeric Blends (Experiment 3)
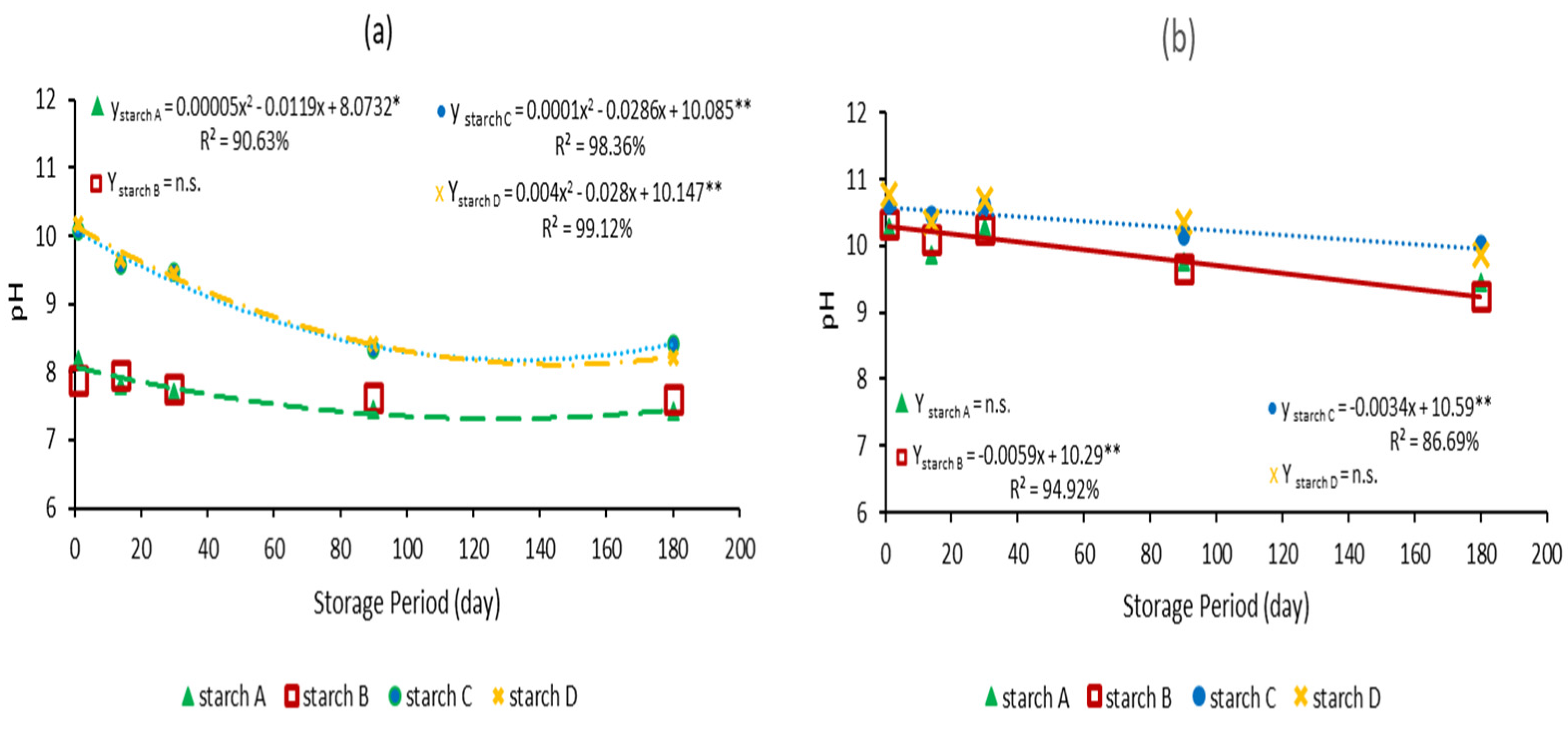
The pH behavior of the CMC/starch blends was evaluated over 180 days at room temperature, showing a decreasing trend. Blends compatibilized with 0.3% MgO exhibited a quadratic decrease in pH, reaching a minimum value after approximately 119 days for blend A and 141 days for blends C and D (
Figure 2a;
File S2). Conversely, blends with 1.0% MgO displayed a linear reduction in pH throughout the storage period (
Figure 2b and
File S2). As indicated in
Table 1, blends containing starches A and B with 0.3% MgO initially showed lower pH values after autoclaving than those containing starches C and D. The CMC/starch A blend exhibited an initial estimated pH of 8.1, which gradually decreased to 7.4 after 120 days. However, blends containing starches C and D initially had an estimated average pH of 10.1, eventually achieving a minimum average pH of 8.1 after 140 days, which is still considered suitable for maintaining cell viability. Blends containing starches B and C and 1.0% MgO had initial pH values of 10.3 and 10.6, respectively, and maintained pH levels above 9 throughout the experiment. This higher pH range may have limited their suitability as carriers for the target strain.
3.4. Effect of Polymeric Blends on the Cell Viability of Strain BR 3262 (Experiment 4)
Experiment 4 builds on previous results that investigated the pH of polymeric blends made with four types of starch, two MgO concentrations, and two autoclaving times (
Table 1;
Figure 2). This study examined the cell viability of the BR 3262 strain within these blends by introducing a new factor: two types of CMC.
The interactions between the type of starch and autoclaving time were assessed for each combination of CMC type and storage period in the blends compatibilized with 0.3% MgO (
Table 2) and 1.0% MgO (
File S6). The original data for both tables are presented in
File S3. As previously noted, most blends compatibilized with 1.0% MgO exhibited low cell viability over time. In contrast, most blends composed of starches A, B, and C, formulated with 0.3% MgO, maintained cell counts of approximately 10
8 CFU mL
−1 for up to 84 days, which is significantly higher than starch D. The CMC-I/starches A, B, or C blends displayed this pattern at 7, 28, 56, and 84 days of storage after being autoclaved for 30 min and at 7, 56, and 84 days of storage after autoclaving for 60 min (
Table 2). The CMC-II/starch D blends at 7 and 168 days of storage showed cell viability below the detectable limit of the analytical method (log ≤ 5.73) for both autoclaving times. The CMC-II/starches A, B, or C blends exhibited significantly higher cell viability than starch D at 56 d of storage when autoclaved for 30 min and at 56 and 84 d of storage when autoclaved for 60 min.
These results indicate that most blends containing starches A, B, and C, compatibilized with 0.3% MgO, achieved log values for cell viability of around 8, corresponding to 108 CFU mL−1, which are typically found in commercial inoculants. A specific response was also observed in maintaining cell viability, depending on the various preparation conditions of the blends tested in this study.
Some significant differences in cell viability were also observed based on autoclaving time. In instances where differences occurred, the five blends autoclaved for 60 min demonstrated significantly higher cell viability than those autoclaved for 30 min. This was evident in the blends composed of CMC-I with starch D at 56 days of storage, with starch B at 84 days, and with starch A at 168 days, as well as the blends composed of CMC-II with starch C at 7 days and with starch D at 56 days.
Most blends compatibilized with 1.0% MgO exhibited cell viability values below the minimum detectable limit of the method (log ≤ 5.73) during the storage period. Among these, the majority of the undetermined data were observed in the presence of starches C and D (S6).
Blends compatibilized with 1.0% MgO composed of starch B demonstrated significantly higher cell viability than those produced with starch A in the presence of CMC-I after 60 min of autoclaving at 84 days of storage, achieving log values of approximately 8.
Since cell viability during storage is a fundamental characteristic of an inoculant, regression models were used to estimate the duration for which each blend could maintain 10
8 CFU ml
−1. Unlike the mean comparison test, regression models are recommended for quantitative factors, such as storage time, enabling us to calculate the adjustment between the levels of this factor (7 to 168 days) for each combination of MgO concentration, starch, CMC, and autoclaving time. The models and R
2 values are listed in
Table 3 (
File S3).
The CMC-I/starch B blend and CMC-II/starch C blend, compatibilized with 0.3% MgO and autoclaved for 60 or 30 min, respectively, demonstrated the best performance, maintaining a cell viability of 108 CFU mL−1 for approximately 130 days. Both curves were quadratic and reached their maximum at 61 and 63 days after formulation preparation, respectively.
Blends composed of starch C with 0.3% MgO, along with CMC-I and CMC-II, both autoclaved for 60 min, can maintain cell viability of around 108 CFU mL−1 for 106 and 86 days, respectively.
In addition, seven blends compatibilized with 0.3% MgO maintained cell viability of around 108 CFU mL−1 for 61 to 70 days of storage: CMC-I/starch A or CMC-II starch A, autoclaved for 30 or 60 min; CMC-I/starch B or CMC-II starch B, autoclaved for 30 min; and CMC-II/starch B, autoclaved for 60 min. In this category, a CMC-I/starch A blend compatibilized with 1.0% MgO and autoclaved for 60 min was also capable of maintaining around 108 CFU mL−1 during 61 days. Despite the maintenance of viable cells for only a short period, approximately 2 months, in some instances, such as on-farm inoculant production, a shorter storage time might be sufficient, especially when considering a significantly better cost-benefit ratio.
Blends composed of both CMCs and starch D, compatibilized with 0.3% or 1.0% of MgO, could not maintain cell viability at around 108 CFU mL−1 and did not achieve log values above the minimum detectable limit of the method (log ≤ 5.73). Blends compatibilized with 1.0% MgO, including both CMCs and starch C or starch B, also did not achieve log values above the detectable limit of the method (log ≤ 5.73) or were unable to maintain cell viability above 108 CFU mL−1.
In addition to the above results, the other blends exhibited linear, quadratic, or cubic models after adjusting the regression of the cell viability data, which maintained cell viability above 108 CFU mL−1 for various periods. At the time of collection, the cells of strain BR 3262 were in the exponential phase of bacterial growth. Thus, it is estimated that the cells added to the blends were viable at the beginning of the storage period. These suspensions were quantified to ensure a sufficient number of cells to reach a concentration of 108 CFU mL−1. However, after 7 days of storage, a continuous range of responses was observed, varying from an insufficient number of cells to reach the minimum detectable limit (log ≤ 5.73) to a maximum log value of 8.7, which corresponds to approximately 5 × 108 CFU mL−1. These changes, influenced by the characteristics of each blend, may explain the different regression models obtained, suggesting that a differentiated adaptation of the cells to this new environment occurred. This behavior warrants further investigation in future studies.
When comparing the cell viability results (
Table 2 and
Table 3;
File S6) with the pH evaluation (
Figure 2), it is clear that pH alone does not determine the viability of bacterial cells. The regression models demonstrated that the blends produced with starches B and C yielded carriers capable of sustaining cells for an extended period, while
Figure 2 indicates that these blends exhibited distinct pH behaviors.
While the blend with starch B maintained a pH range of 8–7.5 during the 180-day storage period, the blend with starch C varied from 10 to 8. Therefore, starch B maintained a lower pH range than starch C. The data in
Table 1 indicate that the blends with starch B experienced a pH drop immediately after autoclaving to approximately 8.0. In contrast, those prepared with starch C maintained a pH of around 10.0 after autoclaving. These findings suggest that specific mechanisms are likely activated to ensure the survival of rhizobial cells under different pH conditions.
This study emphasizes that each blend possesses a unique characteristic. Optimizing an inoculant carrier requires a synergistic combination of properties among the various constituents that function effectively together. The final properties of each blend depend on the specific interactions established between each component and its quantity in the mixture.
4. Discussion
Biofertilizer formulations based on natural biopolymers are attracting increasing interest due to their biodegradability and potential to promote the use of renewable and cost-effective resources [
30]. Regarding CMC/starch blends, the roles and characteristics of the individual components—starch, CMC, and MgO—should be considered. Among the natural biopolymers, starch, renowned for its film-forming ability and biocompatibility, is highly versatile because of its physicochemical properties, which enable its use in a wide range of products. Starch consists of two glucan polymers: amylopectin, a highly branched molecule, and primarily linear amylose. These polymers arrange themselves within the starch granule in semi-crystalline and amorphous concentric layers [
31]. Amylopectin’s hydrogen bonding facilitates water absorption, leading to increased viscosity and enhanced gelatinization properties. In contrast, amylose contributes to the hydrophobicity of starch. The relative proportions of these components define the unique properties of different starches, tailoring them for specific applications [
32]. Starch is a widely available biopolymer extracted from various plant sources, including potato, maize, wheat, and cassava [
31,
33]. The molecular structure, which varies according to botanical origin, influences the shape, size, and composition of starch granules [
32].
In this study, polymer blends were prepared using starches from four different commercial products (5S), resulting in varied responses from the tested compositions. Further studies on the characterization of these starches based on their rheological properties and amylopectin/amylose ratio may enable the prediction of the optimal starch type for maintaining prolonged cell viability. For example, cassava starch served as a carrier for
Bacillus subtilis and
B. pumilus strains and was able to maintain cell viability at approximately 10
8 CFU mL
−1 after six months at room temperature or under refrigeration [
34].
Modified starch is also used as a coating material to encapsulate bioactive compounds, a process suitable for producing efficient inoculant formulations [
32]. Encapsulation effectively protects PGPB from unfavorable soil conditions. This protection is further enhanced by the starch’s ability to retain substantial amounts of water within its structure, thereby supporting bacterial survival and promoting healthy plant growth [
35].
Starch-based carriers combined with other polymers have gained interest in formulation designs. Several applications have been reported, such as the ability of optimized alginate-starch beads to maintain a stable
B. pumilus cell concentration exceeding 10⁹ CFU mL
−1 and a water-retention capacity of approximately 37% [
35]. The co-immobilization of
Azospirillum argentinense and
Pseudomonas rhodesiae in chitosan/starch beads has proven to be an effective strategy for application to maize seeds [
36]. This formulation provides greater stability and ensures increased crop productivity compared to liquid inoculants. The controlled release of beneficial microorganisms is a key advantage of encapsulation using biopolymeric matrices. A modified starch-based system was designed to protect and release
Priestia megaterium; however, to achieve efficacy, careful modulation of the film microstructure and composition is necessary [
37]. In addition to forming polymeric matrices, starch can also function as an additive, such as in a lignin-alginate hydrogel capable of immobilizing and releasing rhizobial cells [
38] or as a cell protectant [
4].
However, despite their advantageous characteristics for various applications, biodegradable materials often exhibit inferior mechanical properties compared to petroleum-derived polymers [
39]. To address this limitation, one approach involves mixing starch with another biopolymer with superior mechanical strength, such as carboxymethyl cellulose (CMC). The CMC/starch blend developed has been utilized for a biofilm-based biofertilizer and has obtained patent protection filed under the PCT (Patent Cooperation Treaty) on 24 June 2008, granted as “Polymeric compositions containing rhizobial inoculant, their use, and seeds treated with the compositions” (original title in Spanish: “Composiciones polimericas conteniendo inoculante rizobiano, uso de las mismas y simientes tratadas con las composiciones”) (AR051081A1) in Argentina [
24]. CMC is a white, granular, polymeric substance that is soluble in both cold and hot water and is available in various viscosity grades [
40]. It is produced by reacting cellulose with sodium monochloroacetate, which enables it to acquire gel-forming, water-retention, and polyelectrolyte properties [
41]. Notably, its viscosity-building capacity is significant. [
42]. Despite the high molecular weights of starch and CMC, which typically hinder miscibility, they share a degree of structural similarity that contributes to their partial compatibility and enhances gel stability [
43].
CMC/starch blends demonstrate potential as carriers for PGPB, including
Bradyrhizobium bacteria, which can promote legume nodulation and enhance biological nitrogen fixation. These blends, particularly those containing 50–60% CMC, effectively maintain cell viability for approximately one month under room temperature storage [
24].
Due to the specific characteristics of starch and CMC, including their structure, types of interactions, solubilization, and rheological behavior, the mixture of these polymers with water gradually forms a heterogeneous system, indicating immiscibility. Pure CMC/starch mixtures (without ions) exhibited phase separation after 5 months, demonstrating their low stability. To address this issue, ions that can promote interactions within the system and enhance compatibility were added to the mixtures. Da Silva et al. [
44] investigated the effect of MgO and ZnO as compatibilizing agents for CMC/starch blends. Compatibility refers to polymeric systems that may exhibit phase separation but also present desirable properties and good adhesion between the phases.
Formulations containing 1.0% ZnO exhibited phase separation and low mechanical resistance, compromising the system’s integrity. Rheological tests indicate that the presence of Zn
2+ ions weakens the gel structure, particularly in mixtures with starch. In contrast, mixtures with 1.0% MgO remain stable for up to one year without phase separation [
24]. The rheological results also show that Mg
2+ increases the elastic modulus, enhancing the cohesion between the CMC and starch phases.
The absence of phase separation is crucial for ensuring a homogeneous distribution and protection of bacteria over time, thus preserving their viability and effectiveness in biological nitrogen fixation (BNF) and other plant growth-promoting activities. Therefore, MgO is an ideal compatibilizing agent for inoculant formulations, especially considering prolonged storage periods.
This suggests a possible barrier to adhesion between the polymeric carboxyl and hydroxyl groups. In simpler terms, ZnO is a less effective compatibilizer compared to MgO.
This presents a potential for significantly improved retention of cell viability for up to 165 days at room temperature. Bacterial cell numbers decreased by only one order of magnitude (from 10
9 to 10
8 CFU mL
−1), which is comparable to the performance of traditional peat-based inoculants [
24].
Building on the promising properties of MgO as a compatibilizing agent, this study investigated blends containing two concentrations of MgO: 0.3% and 1.0%. Under our experimental conditions, the primary factor influencing the performance of the blends formulated with 0.3% MgO appeared to be the type of starch. In contrast, most blends formulated with 1.0% MgO exhibited phase separation, except for the CMC-I/starch B blend, which was autoclaved for 60 min and demonstrated behavior similar to that reported by Fernandes Júnior et al. [
24], suggesting potential benefits to this specific formulation.
In addition to its compatibilizing effect, MgO hydration is a multifaceted process that involves both dissolution and crystallization. Mg ions can either dissolve in the surrounding solution or be adsorbed onto the MgO surface [
45]. Despite its low solubility in water, the hydration of MgO results in the formation of Mg (OH)
2, which significantly increases the pH of the solution. This pH can even exceed 10.4 [
45,
46], which may be detrimental to the maintenance of rhizobial cells.
The optimal pH for different Rhizobium species can vary significantly; however, the recommended optimal culture medium pH for growing rhizobia is between 6.5 and 7.0 [
46,
47]. Nevertheless, the ability of some Rhizobium species to tolerate elevated pH levels in culture media suggests that high pH alone is unlikely to be the sole factor responsible for the reduced bacterial viability observed in some blends in the present study. We can further strengthen the argument that high pH is not the main cause of reduced viability by considering the findings of other studies. Datta et al. [
48] reported that strains from
Rhizobium leguminosarum,
R. phaseoli,
R. trifolii, and even
Bradyrhizobium japonicum could grow within a pH range of 8 to 9 or even higher. Similarly, O’Hara et al. [
49] demonstrated tolerance to pH 10 and above in four rhizobia genera:
Mesorhizobium,
Neorhizobium,
Rhizobium, and
Sinorhizobium. Notably,
Azorhizobium and
Bradyrhizobium species were found to tolerate a range of 8 to 10 [
46].
Unlike bulk Mg(OH)
2, which primarily influences the external environment of rhizobial cells through its pH, a new mechanism of action has been proposed for Mg(OH)
2 nanoparticles [
50,
51]. Mg(OH)
2 nanoparticles can cross the bacterial cell wall and enter the cells via endocytosis. The accumulation of nanoparticles in vivo promotes a greater release of OH
− within the cellular aqueous environment, which increases the pH and leads to cell death [
52]. In this study, the observed reduction in cell viability may also result from the particle size of Mg(OH)
2, suggesting the potential presence of nanoparticles.
The development of nano-biofertilizers, which integrate nanotechnology with biofertilizers, may significantly improve agricultural productivity. Nano-biofertilizers enhance nutrient delivery, improve plant growth, and increase resistance to environmental stress [
53]. While nanotechnology is recognized as a potentially transformative innovation, it is currently essential to deepen our understanding of how nano-agrochemicals affect beneficial organisms, pollinators, overall ecosystems, and the composition of soil life.
In the present study, blends were produced from four different types of commercial starches, and their pH values exhibited varying behaviors, regardless of whether the blends were subjected to autoclaving (
Table 1) or stored for 180 days at room temperature (
Figure 2). The results reveal two distinct patterns. Blends prepared with either 0.3% or 1.0% MgO exhibited very similar pH values before autoclaving, measuring 10.8 and 11.0, respectively. However, after autoclaving, a wider difference was observed depending on the type of starch and MgO concentration. At 0.3% MgO, an average pH of 8.0 was found for starches A and B, while a pH of 10.1 was noted for starches C and D. At 1.0% MgO, the differences were not as dramatic, although the blends prepared with starches A and B still had a slightly lower pH than those prepared with starches C and D.
The quality of raw materials, as specified, significantly impacts the characteristics of final products. Therefore, proper selection of materials is essential to ensure good carrier quality.
In summary, using a CMC/starch blend for the inoculant formulation presents several advantages: (1) it is a low-cost product; (2) it is easy to produce; (3) it has low potential toxicity to humans and other living beings; and (4) it has a low potential for environmental pollution.
First, the different blends were evaluated using a mean comparison. Later, because the maintenance of cell viability throughout the storage period is one of the main characteristics of an inoculant, regression models were used over 168 days. These models are recommended for quantitative factors, and they allowed us to estimate how long each blend was able to maintain 10
8 CFU ml
−1, which is a concentration reported to give efficient agronomical results [
26,
27].
Additionally, our results demonstrate that some CMC/starch blends maintained excellent cell viability for up to four months at room temperature. This characteristic makes them ideal for production in biofactories serving agricultural units located near the inoculant production site, thereby reducing the need for cold storage and transportation costs. These formulations are also suitable for family farming areas that require small amounts of a wide variety of inoculants to meet the diverse needs of calendar-driven agricultural practices. The CMC/starch blend provides a convenient solution for short-term storage. Future studies should explore the possibility of using refrigeration to extend the viability of inoculants for long-term storage. Another important point is the difference in autoclaving costs when comparing formulations based on the CMC/starch blend with peat inoculants. Due to its diverse and rich microbial flora, peat requires a longer autoclaving time and rigorous quality control to ensure that any residual contaminating microorganisms are within acceptable limits, as mandated by local legislation. It is also worth emphasizing that, in contrast to peat, which relies on the extraction of non-renewable resources from peatlands, the CMC/starch blend offers a more environmentally friendly solution.