Synergistic Approaches for Sustainable Remediation of Organic Contaminated Soils: Integrating Biochar and Phytoremediation
Abstract
1. Introduction
2. Phytoremediation of Organic Contaminated Soils
2.1. Phytoremediation Mechanisms
2.2. The Current Status of Phytoremediation Applications
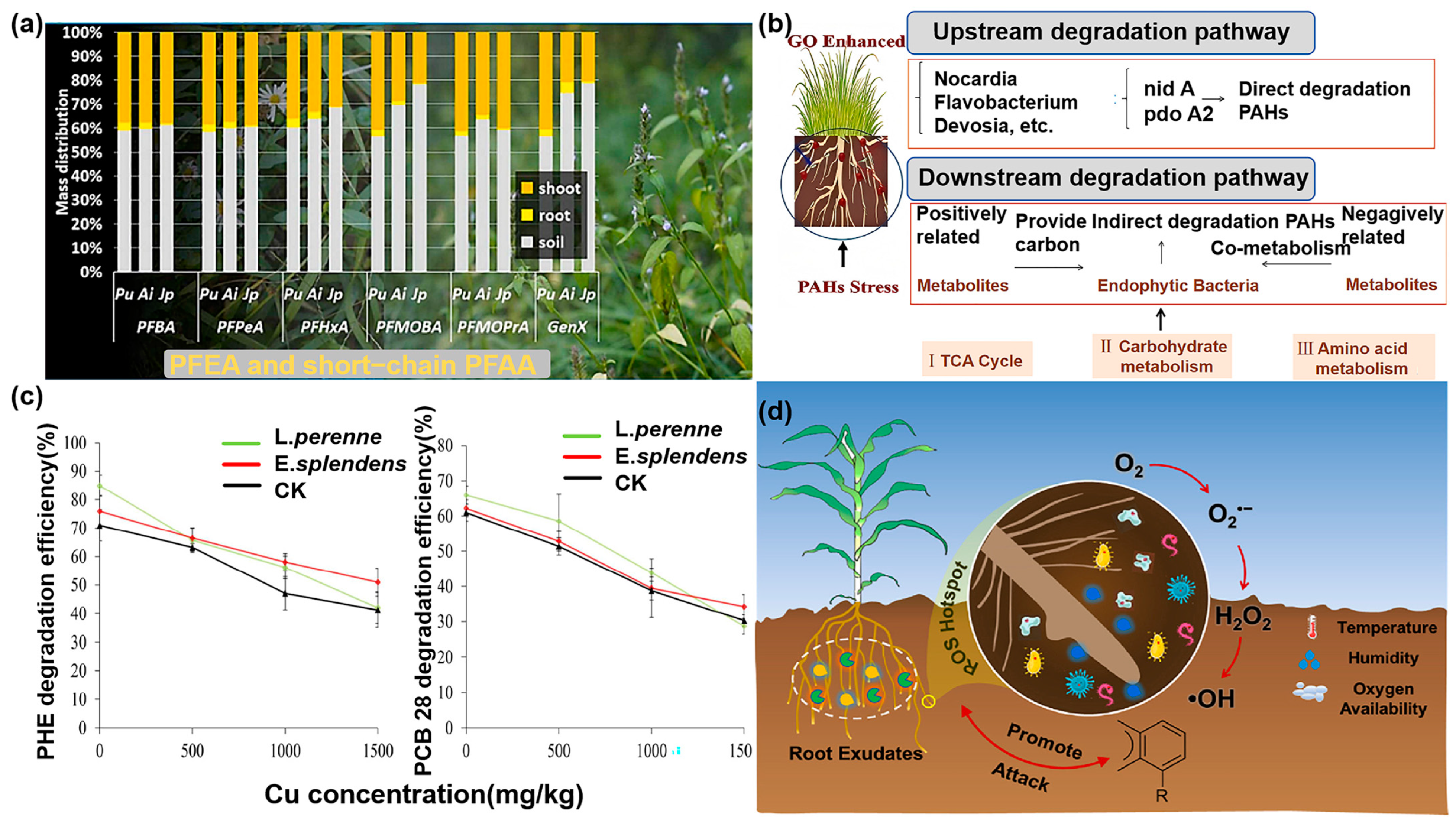
2.2.1. Applications of Direct Phytoremediation
2.2.2. Applications of Indirect Phytoremediation
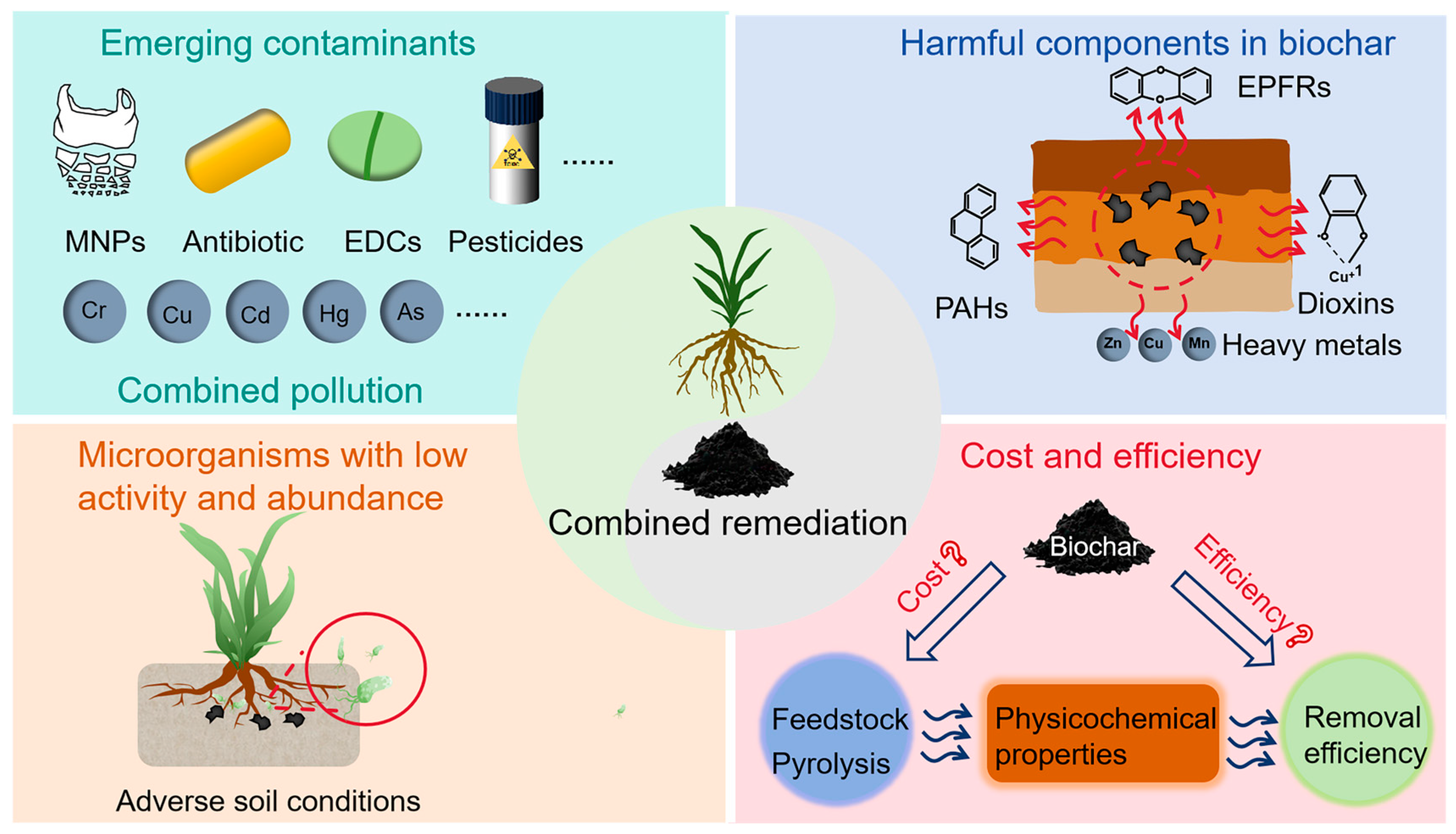
2.3. Limitations of Phytoremediation
3. Remediation of Organic Pollutants by Biochar
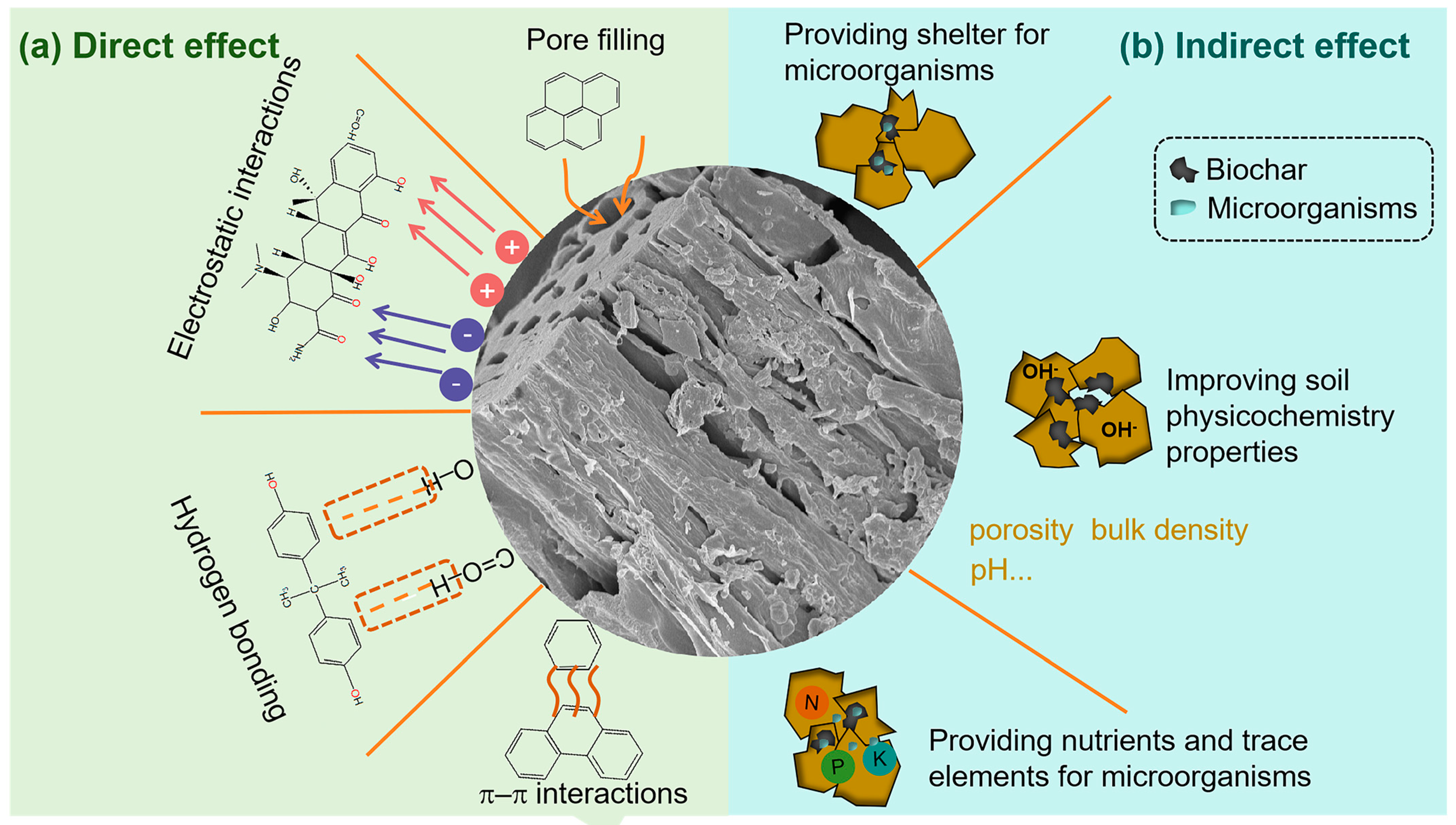
3.1. Remediation Mechanism of Biochar
3.1.1. Direct Adsorption
3.1.2. Enhancing Microbial Metabolic Activity
3.2. Current Status of Biochar Applications
| Feedstock | Pyrolysis Condition | Organic Pollutants | Removal Efficiency | Main Removal Mechanisms | Reference |
|---|---|---|---|---|---|
| Wheat straw | 500 °C | Tetracycline | 96% | π–π interactions, hydrophobic interaction, and hydrogen bonding | [85] |
| Wheat straw | 700 °C | PAHs | 40% | Adsorption, redox, aggregation | [64] |
| Corn straw | 600 °C | Sulfamethoxazole | 67.5% | •OH- and O2−-mediated oxidation | [63] |
| Reed straw | 800 °C | Herbicide | 46% | Pore filling, hydrogen bonding, π–π conjunction, electrostatic attractions, and other chemical interactions | [90] |
| Wood | 450 °C | Pesticide thiamethoxam | 22.8% | Pore filling and complexation, and oxygen-containing | [62] |
| Beech wood | 900 °C | PAH | 100% | π–π electron donor–acceptor interactions | [91] |
| Rice hull | 500 °C | Oxyfluorfen | Pore-filling mechanism, hydrophobic and π–π interactions | [84] | |
| Algal | 800 °C | Atrazine | 78.4% | Hydrogen bonding formation and π–π interactions | [86] |
| Waste timber | 800–900 °C | PFAS | 23–100% | Hydrophobic interactions | [92] |
| Bamboo | 820 °C | PCBs | 78.9% | Enhanced microbial degradation in the soil and biochar-induced redox or adsorption | [93] |
| Corn straw | 300 °C | PAHs | 31% | Promoting the growth of Sphingobacteria and Agaricomycetes to degrade pollutants | [94] |
| Wheat straws | 300 °C, 500 °C | Phenanthrene | 44.7% | The selective stimulation of specific degrading genera and PAH-degradative nidA gene | [88] |
| Rice straw | 550 °C | Di-(2-ethylhexyl) phthalate | 68.6% | π–π electron donor–acceptor interactions, chemisorption, and coordination | [95] |
| Salix viminalis | 800 °C | PAHs | 70% | Pore adsorption | [96] |
| Peanut shell | 450 °C | Atrazine | 71.4% | Hydrophobic partition, π–π electron donor–acceptor interactions, H-bonding, and pore-filling mechanism | [97] |
| Burcucumber | 700 °C | Sulfamethazine | 69% | Cation exchange process of dominant cationic forms as well as the sorption of zwitterionic forms. | [98] |
| Maize straw | 500 °C | Atrazine | 47.8% | Stimulating atrazine-degrading microbial communities in soil | [99] |
| Pine | 750 °C | PFOS | 88.7% | Electrostatic interactions and hydrophobic interactions | [100] |
| Mentha arvensis | Chlorpyrifos atrazine | 76% 77% | Promoting bacterial degradation of pollutants and upregulation of related functional genes | [101] |
3.3. Limitations of Biochar Remediation
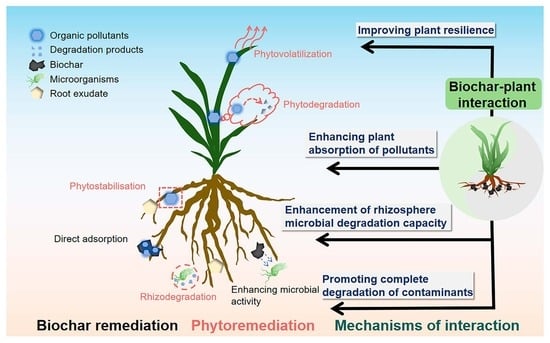
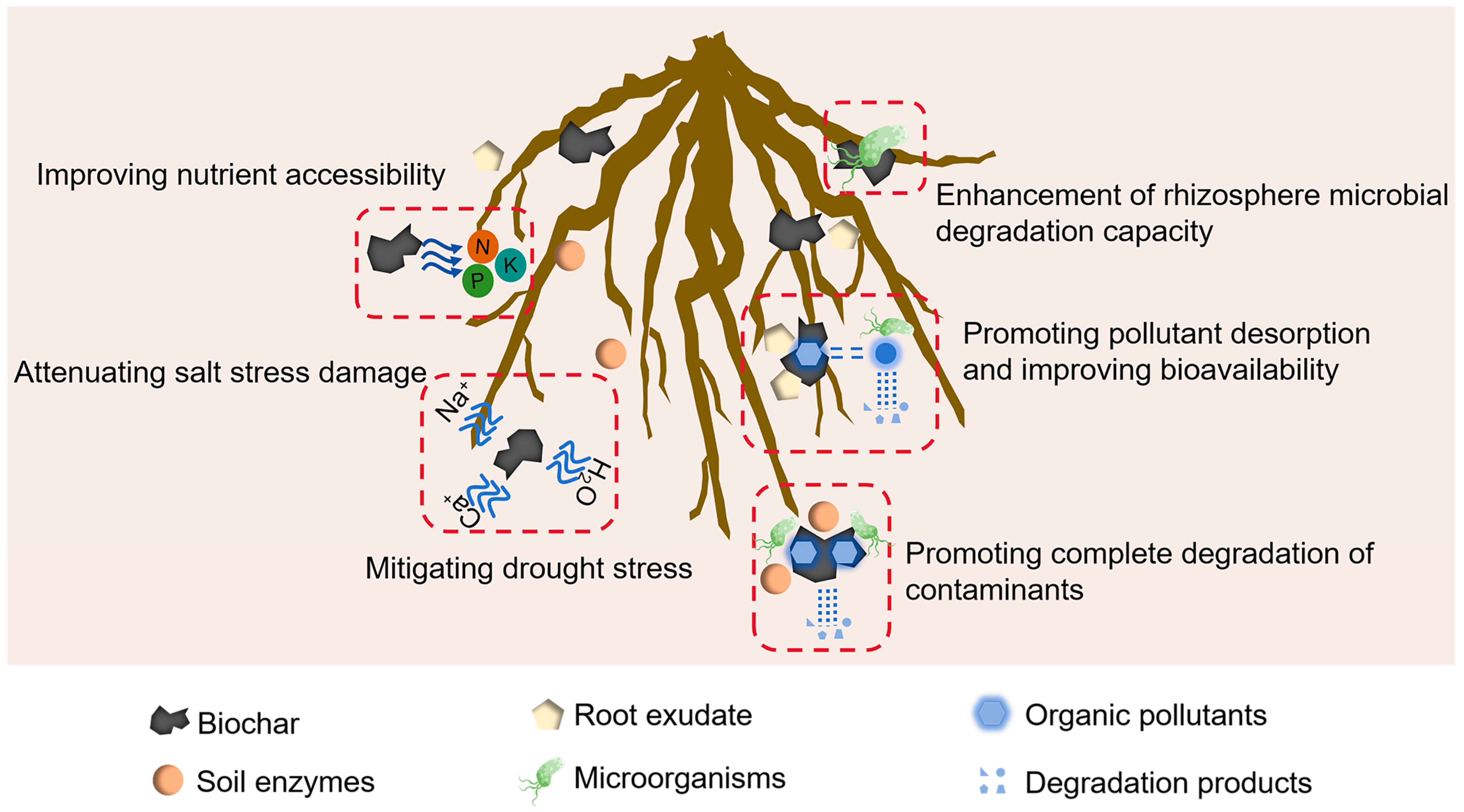
4. Biochar Combined with Phytoremediation
4.1. Improving Plant Resilience
4.2. Enhancing Plant Absorption of Pollutants
4.3. Enhancement of Rhizosphere Microbial Degradation Capacity
4.4. Promoting Complete Degradation of Contaminants
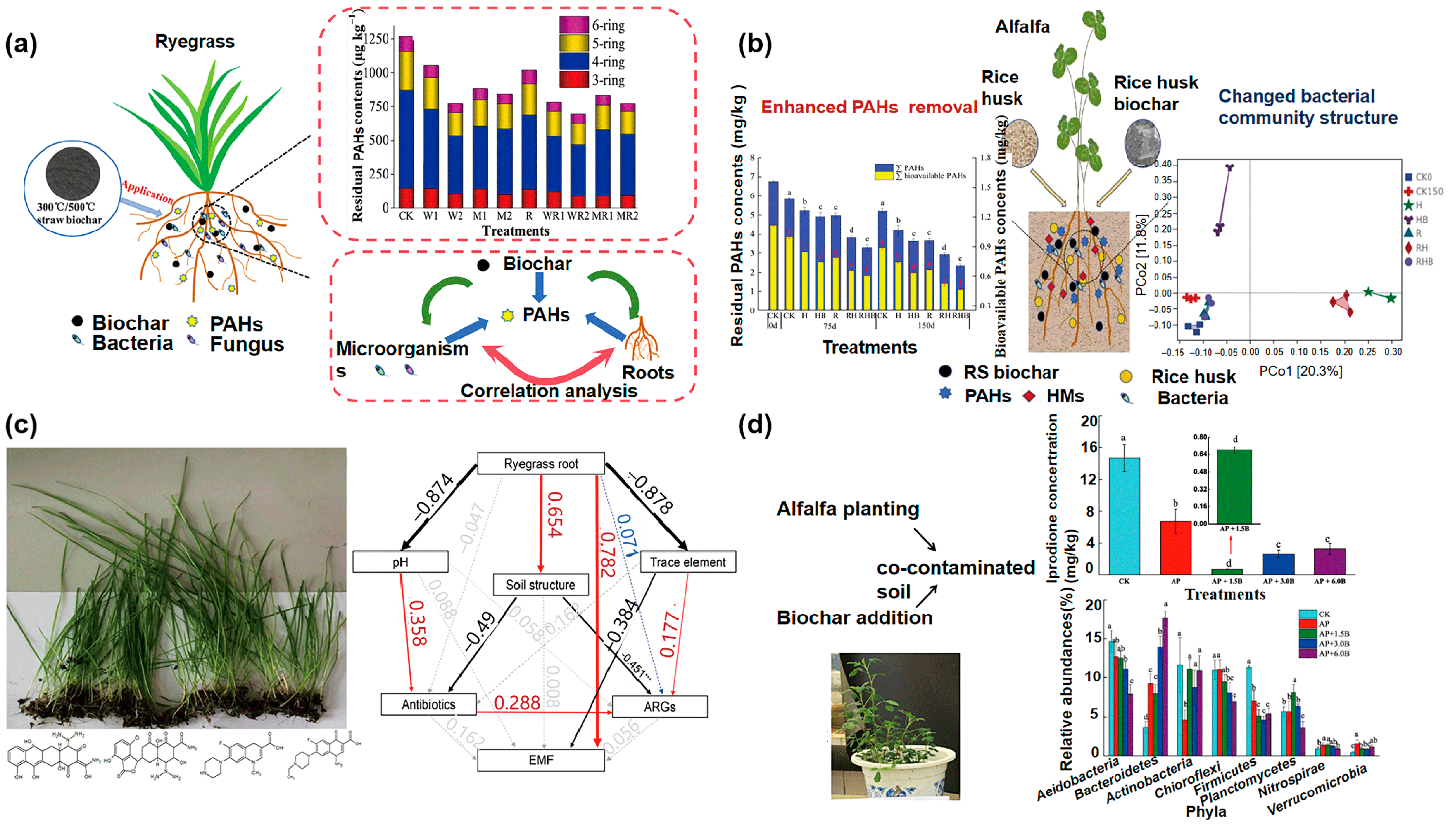
4.5. Application of Biochar Combined with Phytoremediation Technology for Organic Contaminants
| BC Type | Plant Species | Organic Pollutants | Removal Efficiency | Mechanism of Synergism | Reference |
|---|---|---|---|---|---|
| Maize straw biochar | Ryegrass | PAHs | About 55% | Biochar and root exudates enhanced microbial biomass and activity to promote PAHs dissipation. | [121] |
| Wheat straw biochar | Lolium multiflorum L. | PAHs | 62.5% | Direct adsorption of biochar and its synergistic stimulation of microbial activity involved in the degradation of PAHs. | [126] |
| Woody biomass biochar | Buchloe dactyloides | PAHs | 27.1% | The combination of plants and biochar increased soil enzyme activity, altered the structure and function of soil microorganisms, and promoted the expression of functional genes. | [127] |
| Maize straw biochar | Ryegrass | PAHs | 15.9% | The enhanced symbiosis among bacterial members is beneficial for the resistance of the soil microbiome to PAH stress. | [128] |
| Rice husk biochar | Alfalfa | PAHs | 65.3% | Rice husk biochar and alfalfa enhanced the growth of Steroidobacter, Bacillus, and Sphingomonas in rhizosphere soils to remove PAH. | [129] |
| Oak leaves biochar | Trifolium arvense | Total petroleum hydrocarbons | 56.4% | The positive interactions in the rhizosphere between the microorganisms and root were responsible for the decomposition and/or removal of the TPH. | [130] |
| Woodchip biochar | White clover | Total petroleum hydrocarbons | 68% | Direct uptake of biochar and its effect on promoting the degradation of TPHs by inter-root and endophytic bacteria. | [131] |
| Wheat stalks Biochar | Ryegrass | Antibiotics | The enzymes secreted by the roots of ryegrass and the decomposition of surrounding microorganisms and the strong direct adsorption capacity of biochar to antibiotics. | [132] | |
| Forest wood waste biochar | Timothy-grass | PFAS | 9.1% | Biochar promotes the direct uptake of PFAs by forage grasses. | [117] |
| Wood biochar | Grass | PFAS | 10% | The biochar stabilizes the soil, and the shoots directly take up nutrients. | [118] |
4.5.1. Polycyclic Aromatic Hydrocarbons (PAHs)
4.5.2. Antibiotics
4.5.3. Chlorinated Organics
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-B.; Duan, Y.-P.; Zhang, Z.-J.; Tu, Y.-J.; Luo, P.-C.; Gao, J.; Dai, C.-M.; Zhou, L. Multimedia fate model and risk assessment of typical antibiotics in the integrated demonstration zone of the Yangtze River Delta, China. Sci. Total Environ. 2022, 805, 150258. [Google Scholar] [CrossRef]
- Devendrapandi, G.; Liu, X.; Balu, R.; Ayyamperumal, R.; Valan Arasu, M.; Lavanya, M.; Minnam Reddy, V.R.; Kim, W.K.; Karthika, P.C. Innovative remediation strategies for persistent organic pollutants in soil and water: A comprehensive review. Environ. Res. 2024, 249, 118404. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Barone, G.D.; Jin, D.; Mao, Y.; Nan, F.; Xu, L.; Wang, Z.; Deng, Y.; Cernava, T. Pollution Status, Ecological Effects, and Bioremediation Strategies of Phthalic Acid Esters in Agricultural Ecosystems: A Review. J. Agric. Food Chem. 2024, 72, 27668–27678. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Jiang, L.; Chen, X.; Zhao, Y.; Shi, W.; Xing, Z. From organic fertilizer to the soils: What happens to the microplastics? A critical review. Sci. Total Environ. 2024, 919, 170217. [Google Scholar] [CrossRef]
- Lü, H.; Wei, J.-L.; Tang, G.-X.; Chen, Y.-S.; Huang, Y.-H.; Hu, R.; Mo, C.-H.; Zhao, H.-M.; Xiang, L.; Li, Y.-W.; et al. Microbial consortium degrading of organic pollutants: Source, degradation efficiency, pathway, mechanism and application. J. Clean. Prod. 2024, 451, 141913. [Google Scholar] [CrossRef]
- Hoyeck, M.P.; Matteo, G.; MacFarlane, E.M.; Perera, I.; Bruin, J.E. Persistent organic pollutants and β-cell toxicity: A comprehensive review. Am. J. Physiol.-Endocrinol. Metab. 2022, 322, E383–E413. [Google Scholar] [CrossRef]
- Kadhel, P.; Monnier, P.; Boucoiran, I.; Chaillet, N.; Fraser, W.D. Organochlorine Pollutants and Female Fertility: A Systematic Review Focusing on In Vitro Fertilization Studies. Reprod. Sci. 2012, 19, 1246–1259. [Google Scholar] [CrossRef]
- Latchney, S.E.; Majewska, A.K. Persistent organic pollutants at the synapse: Shared phenotypes and converging mechanisms of developmental neurotoxicity. Dev. Neurobiol. 2021, 81, 623–652. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Marquès, M.; Nadal, M.; Schuhmacher, M. Health risks for the population living near petrochemical industrial complexes. 1. Cancer risks: A review of the scientific literature. Environ. Res. 2020, 186, 109495. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, N.F.; Ching-López, A.; Olry de Labry Lima, A.; Salamanca-Fernández, E.; Pérez-Gómez, B.; Jiménez-Moleón, J.J.; Sánchez, M.J.; Rodríguez-Barranco, M. Relationship between exposure to mixtures of persistent, bioaccumulative, and toxic chemicals and cancer risk: A systematic review. Environ. Res. 2020, 188, 109787. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Q.-m.; Han, L.-j.; Li, J.-s. Synergistic remediation strategies for soil contaminated with compound heavy metals and organic pollutants. J. Environ. Chem. Eng. 2024, 12, 113145. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, H.; Yang, E.; Lv, J.; Wen, B.; Sun, F.; Luo, L.; Liu, Z. Distribution and bioaccessibility of polycyclic aromatic hydrocarbons in industrially contaminated site soils as affected by thermal treatment. J. Hazard. Mater. 2021, 411, 125129. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, F.; O’Connor, D.; Hou, D. Solidification/Stabilization for Soil Remediation: An Old Technology with New Vitality. Environ. Sci. Technol. 2019, 53, 11615–11617. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kumar, A.; Bindra, S.; Sharma, A. Phytoremediation: An emerging green technology for dissipation of PAHs from soil. J. Geochem. Explor. 2024, 259, 107426. [Google Scholar] [CrossRef]
- Song, B.; Xu, P.; Chen, M.; Tang, W.; Zeng, G.; Gong, J.; Zhang, P.; Ye, S. Using nanomaterials to facilitate the phytoremediation of contaminated soil. Crit. Rev. Environ. Sci. Technol. 2019, 49, 791–824. [Google Scholar] [CrossRef]
- Naveed, S.; Oladoye, P.O.; Kadhom, M.; Oladipo, M.E.; Alli, Y.A.; Anjum, N. The potential of phytoremediation technology as a panacea for per- and poly-fluoroalkyl substances-contaminated soil. Chem. Pap. 2024, 78, 2079–2099. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Shen, Z.; Zhu, J.; Jia, X.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J. Field trials of phytomining and phytoremediation: A critical review of influencing factors and effects of additives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2724–2774. [Google Scholar] [CrossRef]
- Silambarasan, S.; Vangnai, A.S. Plant-growth promoting Candida sp. AVGB4 with capability of 4-nitroaniline biodegradation under drought stress. Ecotoxicol. Environ. Saf. 2017, 139, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Hu, B.; Ai, Y.; Jin, J.; Hayat, T.; Alsaedi, A.; Zhuang, L.; Wang, X. Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar 2020, 2, 47–64. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Bolan, N.; Sarmah, A.K.; Bordoloi, S.; Bolan, S.; Padhye, L.P.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.M.; et al. Soil acidification and the liming potential of biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Ghadirnezhad Shiade, S.R.; Fathi, A.N.M.; Minkina, T.M.; Wong, M.H.; Rajput, V.D.J.E. Development; Sustainability, Biochar application in agroecosystems: A review of potential benefits and limitations. Environ. Dev. Sustain. 2024, 26, 19231–19255. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef]
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.; He, C.; Muhoza, B.; Brahushi, F.; Bolan, N.; et al. Integrating Biochar, Bacteria, and Plants for Sustainable Remediation of Soils Contaminated with Organic Pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef]
- Wang, W.; Wu, S.; Sui, X.; Cheng, S. Phytoremediation of contaminated sediment combined with biochar: Feasibility, challenges and perspectives. J. Hazard. Mater. 2024, 465, 133135. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Lekshmi, S.; Ardra Lekshmi, A.; Achuthavarier, D.; Smitha Chandran, S. Critical analysis of sustainable ways of removing insidious pollutants from the environment through phytoremediation techniques. Chem. Ecol. 2024, 40, 892–917. [Google Scholar] [CrossRef]
- Tufail, M.A.; Iltaf, J.; Zaheer, T.; Tariq, L.; Amir, M.B.; Fatima, R.; Asbat, A.; Kabeer, T.; Fahad, M.; Naeem, H.; et al. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ. 2022, 850, 157961. [Google Scholar] [CrossRef]
- Limmer, M.; Burken, J. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef] [PubMed]
- Aken, B.V.; Correa, P.A.; Schnoor, J.L. Phytoremediation of Polychlorinated Biphenyls: New Trends and Promises. Environ. Sci. Technol. 2010, 44, 2767–2776. [Google Scholar] [CrossRef]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kamalesh, T.; Senthil Kumar, P.; Rangasamy, G. An insights of organochlorine pesticides categories, properties, eco-toxicity and new developments in bioremediation process. Environ. Pollut. 2023, 333, 122114. [Google Scholar]
- Kumar, V.; Kaushal, A.; Shah, M.P.; Singh, K. Chapter 16—Phytoaugmentation technology for phytoremediation of environmental pollutants: Current scenario and future prospects. In Bioremediation for Environmental Sustainability; Kumar, V., Saxena, G., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 329–381. [Google Scholar]
- Turkovskaya, O.; Muratova, A. Plant–Bacterial Degradation of Polyaromatic Hydrocarbons in the Rhizosphere. Trends Biotechnol. 2019, 37, 926–930. [Google Scholar] [CrossRef]
- He, Q.; Yan, Z.; Qian, S.; Xiong, T.; Grieger, K.D.; Wang, X.; Liu, C.; Zhi, Y. Phytoextraction of per- and polyfluoroalkyl substances (PFAS) by weeds: Effect of PFAS physicochemical properties and plant physiological traits. J. Hazard. Mater. 2023, 454, 131492. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, Y.; Fan, Q.; Li, P.; Liang, J.; Liu, Y.; Ma, R.; Li, R.; Shi, L. Remediating petroleum hydrocarbons in highly saline–alkali soils using three native plant species. J. Environ. Manag. 2023, 339, 117928. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L.; Li, Y. Bioremediation of a polycyclic aromatic hydrocarbon–contaminated urban soil: Degradation dynamics and phytotransformation pathways. J. Soils Sediments 2022, 22, 797–808. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Lv, Y.; Jia, S.; Chen, F.; Liu, Y.; Huang, L. Effect of culturing ryegrass (Lolium perenne L.) on Cd and pyrene removal and bacteria variations in co-contaminated soil. Environ. Technol. Innov. 2021, 24, 101963. [Google Scholar] [CrossRef]
- Wang, Z.; Teng, Y.; Wang, X.; Xu, Y.; Li, R.; Hu, W.; Li, X.; Zhao, L.; Luo, Y. Removal of cadmium and polychlorinated biphenyls by clover and the associated microbial community in a long-term co-contaminated soil. Sci. Total Environ. 2023, 871, 161983. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoory, A.F.; Idris, M.; Abdullah, S.R.S.; Anuar, N. Phytoremediation of contaminated soils containing gasoline using Ludwigia octovalvis (Jacq.) in greenhouse pots. Environ. Sci. Pollut. Res. 2017, 24, 11998–12008. [Google Scholar] [CrossRef]
- Fu, W.; Chen, X.; Zheng, X.; Liu, A.; Wang, W.; Ji, J.; Wang, G.; Guan, C. Phytoremediation potential, antioxidant response, photosynthetic behavior and rhizosphere bacterial community adaptation of tobacco (Nicotiana tabacum L.) in a bisphenol A-contaminated soil. Environ. Sci. Pollut. Res. 2022, 29, 84366–84382. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, L.; Lu, W.; Luo, C.; Song, M. Elsholtzia splendens promotes phenanthrene and polychlorinated biphenyl degradation under Cu stress through enrichment of microbial degraders. J. Hazard. Mater. 2022, 438, 129492. [Google Scholar] [CrossRef]
- Ding, Y.; Ren, H.; Hao, X.; Zhang, R.; Hao, J.; Liu, J.; Pan, H.; Wang, Y. Enhanced phytoremediation of PCBs-contaminated soil by co-expressing tfdB and bphC in Arabidopsis aiding in metabolism and improving toxicity tolerance. Environ. Exp. Bot. 2024, 217, 105548. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Zhang, F.; Li, R.; Hu, Y.; Yu, H.; Tuyiringire, D.; Wang, L. Effects of bok choy on the dissipation of dibutyl phthalate (DBP) in mollisol and its possible mechanisms of biochemistry and microorganisms. Ecotoxicol. Environ. Saf. 2019, 181, 284–291. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Chen, J.; Tai, P.; Bi, X.; Zou, P.; Wang, Y.; Xiao, Y. Biotrophic interactions between plant and endophytic bacteria in removal of PAHs and Cd from contaminated soils enhanced by graphene oxide. J. Clean. Prod. 2023, 417, 137996. [Google Scholar] [CrossRef]
- Liu, J.; Shen, S.; Zhu, K.; Li, Z.; Chen, N.; Lichtfouse, E.; Jia, H. Novel insights into the factors influencing rhizosphere reactive oxygen species production and their role in polycyclic aromatic hydrocarbons transformation. Soil Biol. Biochem. 2024, 198, 109562. [Google Scholar] [CrossRef]
- Yang, S.-H.; Shan, L.; Chu, K.-H. Root exudates enhanced 6:2 FTOH defluorination, altered metabolite profiles and shifted soil microbiome dynamics. J. Hazard. Mater. 2024, 466, 133651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, C.; Gu, Y.; Shi, Y.; Gao, X. Microplastics in plant-soil ecosystems: A meta-analysis. Environ. Pollut. 2022, 308, 119718. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Liu, S.; Junaid, M.; Wang, J. Effects of micro(nano)plastics on higher plants and the rhizosphere environment. Sci. Total Environ. 2022, 807, 150841. [Google Scholar] [CrossRef]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Ikan, C.; Raja, B.-L.; Redouane, O.; Cherki, G.; Meddich, A. Co-inoculation of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria can mitigate the effects of drought in wheat plants (Triticum durum). Plant Biosyst.—Int. J. Deal. All Asp. Plant Biology 2023, 157, 907–919. [Google Scholar] [CrossRef]
- Dinneny, J.R. Traversing organizational scales in plant salt-stress responses. Curr. Opin. Plant Biol. 2015, 23, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Duan, B.; Li, C. Physiological responses to drought and enhanced UV-B radiation in two contrasting Picea asperata populations. Can. J. For. Res. 2007, 37, 1253–1262. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Haider, F.U.; Wang, X.; Zulfiqar, U.; Farooq, M.; Hussain, S.; Mehmood, T.; Naveed, M.; Li, Y.; Liqun, C.; Saeed, Q.; et al. Biochar application for remediation of organic toxic pollutants in contaminated soils; An update. Ecotoxicol. Environ. Saf. 2022, 248, 114322. [Google Scholar] [CrossRef]
- Fakhar, A.; Galgo, S.J.C.; Canatoy, R.C.; Rafique, M.; Sarfraz, R.; Farooque, A.A.; Khan, M.I. Advancing modified biochar for sustainable agriculture: A comprehensive review on characterization, analysis, and soil performance. Biochar 2025, 7, 8. [Google Scholar] [CrossRef]
- You, X.; Jiang, H.; Zhao, M.; Suo, F.; Zhang, C.; Zheng, H.; Sun, K.; Zhang, G.; Li, F.; Li, Y. Biochar reduced Chinese chive (Allium tuberosum) uptake and dissipation of thiamethoxam in an agricultural soil. J. Hazard. Mater. 2020, 390, 121749. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cheng, Z.; Li, X.; Wang, C.; Yan, L.; Shen, G.; Shen, Z. Degradation mechanism and QSAR models of antibiotic contaminants in soil by MgFe-LDH engineered biochar activating urea-hydrogen peroxide. Appl. Catal. B Environ. 2022, 302, 120866. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Kołtowski, M. Effect of co-application of nano-zero valent iron and biochar on the total and freely dissolved polycyclic aromatic hydrocarbons removal and toxicity of contaminated soils. Chemosphere 2017, 168, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Y.; Li, J.; Fang, Z.; Bolan, N.; Bhatnagar, A.; Gao, B.; Hou, D.; Wang, S.; Song, H.; et al. Engineered biochar for environmental decontamination in aquatic and soil systems: A review. Carbon Res. 2022, 1, 4. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef]
- Qu, J.; Meng, Q.; Peng, W.; Shi, J.; Dong, Z.; Li, Z.; Hu, Q.; Zhang, G.; Wang, L.; Ma, S.; et al. Application of functionalized biochar for adsorption of organic pollutants from environmental media: Synthesis strategies, removal mechanisms and outlook. J. Clean. Prod. 2023, 423, 138690. [Google Scholar] [CrossRef]
- Jin, J.; Kang, M.; Sun, K.; Pan, Z.; Wu, F.; Xing, B. Properties of biochar-amended soils and their sorption of imidacloprid, isoproturon, and atrazine. Sci. Total Environ. 2016, 550, 504–513. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.J.; Saravanan, A. Advances in production and application of biochar from lignocellulosic feedstocks for remediation of environmental pollutants. Bioresour. Technol. 2019, 292, 122030. [Google Scholar] [CrossRef]
- Dong, W.; Xing, J.; Chen, Q.; Huang, Y.; Wu, M.; Yi, P.; Pan, B.; Xing, B. Hydrogen bonds between the oxygen-containing functional groups of biochar and organic contaminants significantly enhance sorption affinity. Chem. Eng. J. 2024, 499, 156654. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sun, L.; Asadullah, M.; Belhaj, D. Sorption of hydrophobic organic contaminants on functionalized biochar: Protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. J. Hazard. Mater. 2018, 360, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Norgaard, T.; Pugliese, L.; Carvalho, P.N.; Wu, S. Global meta-analysis and machine learning reveal the critical role of soil properties in influencing biochar-pesticide interactions. Environ. Int. 2024, 193, 109131. [Google Scholar] [CrossRef]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C.W. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Adhikari, S.; Ma, H.; Kim, D.-H.; Bai, Y.; Hou, D. Progress and future prospects in biochar composites: Application and reflection in the soil environment. Crit. Rev. Environ. Sci. Technol. 2021, 51, 219–271. [Google Scholar] [CrossRef]
- Abhishek, K.; Shrivastava, A.; Vimal, V.; Gupta, A.K.; Bhujbal, S.K.; Biswas, J.K.; Singh, L.; Ghosh, P.; Pandey, A.; Sharma, P.; et al. Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: A state-of-the-art review. Sci. Total Environ. 2022, 853, 158562. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Sherpa, K.; Bui, X.-T.; Nguyen, V.-T.; Vo, T.-D.-H.; Ho, H.-T.-T.; Chen, C.-W.; Dong, C.-D. Biochar for soil remediation: A comprehensive review of current research on pollutant removal. Environ. Pollut. 2023, 337, 122571. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, W.; Dong, J.; Yang, T.; Shangguan, Z.; Qu, J.; Li, X.; Tan, X. The effects of biochar and its applications in the microbial remediation of contaminated soil: A review. J. Hazard. Mater. 2022, 438, 129557. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.; Wu, X.; Dong, F.; Xu, J.; Zheng, Y. Sorption, degradation and bioavailability of oxyfluorfen in biochar-amended soils. Sci. Total Environ. 2019, 658, 87–94. [Google Scholar] [CrossRef]
- Sun, Y.; Lyu, H.; Cheng, Z.; Wang, Y.; Tang, J. Insight into the mechanisms of ball-milled biochar addition on soil tetracycline degradation enhancement: Physicochemical properties and microbial community structure. Chemosphere 2022, 291, 132691. [Google Scholar] [CrossRef]
- Gao, Z.; Dai, Z.; Wang, R.; Li, Y. Adsorption kinetics and mechanism of atrazine on iron-modified algal residue biochar in the presence of soil. Environ. Sci. Pollut. Res. 2023, 30, 70506–70518. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhou, H.; Wang, J.; Yang, J.; Lai, J.; Chen, Y.; Sun, F.; Ye, X.; Wu, Y. Responses of soil microbial metabolism, function and soil quality to long-term addition of organic materials with different carbon sources. Biochar 2024, 6, 80. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Y.; Zhu, Y.; Zhang, H.; Wang, X.; Li, W.; Li, P.; Han, J. Insights into the mechanisms underlying the biodegradation of phenanthrene in biochar-amended soil: From bioavailability to soil microbial communities. Biochar 2023, 5, 14. [Google Scholar] [CrossRef]
- de Resende, M.F.; Brasil, T.F.; Madari, B.E.; Pereira Netto, A.D.; Novotny, E.H. Polycyclic aromatic hydrocarbons in biochar amended soils: Long-term experiments in Brazilian tropical areas. Chemosphere 2018, 200, 641–648. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhu, W.; Yao, X.; Ye, C.; Lei, B.; Rong, X.; Zheng, J.; Liu, X.; Wu, J.; Liu, X.; et al. ZnCl2 and thiourea co-modified biochar for effectively removing quinclorac in water and soil: Mechanism and alleviating its phytotoxicity on tobacco plants. Sep. Purif. Technol. 2024, 350, 127865. [Google Scholar] [CrossRef]
- Carlini, C.; Chaudhuri, S.; Mann, O.; Tomsik, D.; Hüffer, T.; Greggio, N.; Marazza, D.; Hofmann, T.; Sigmund, G. Benchmarking biochar with activated carbon for immobilizing leachable PAH and heterocyclic PAH in contaminated soils. Environ. Pollut. 2023, 325, 121417. [Google Scholar] [CrossRef]
- Sørmo, E.; Silvani, L.; Bjerkli, N.; Hagemann, N.; Zimmerman, A.R.; Hale, S.E.; Hansen, C.B.; Hartnik, T.; Cornelissen, G. Stabilization of PFAS-contaminated soil with activated biochar. Sci. Total Environ. 2021, 763, 144034. [Google Scholar] [CrossRef]
- Huang, S.; Bao, J.; Shan, M.; Qin, H.; Wang, H.; Yu, X.; Chen, J.; Xu, Q. Dynamic changes of polychlorinated biphenyls (PCBs) degradation and adsorption to biochar as affected by soil organic carbon content. Chemosphere 2018, 211, 120–127. [Google Scholar] [CrossRef]
- Ni, N.; Li, X.; Yao, S.; Shi, R.; Kong, D.; Bian, Y.; Jiang, X.; Song, Y. Biochar applications combined with paddy-upland rotation cropping systems benefit the safe use of PAH-contaminated soils: From risk assessment to microbial ecology. J. Hazard. Mater. 2021, 404, 124123. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Luo, S.; Chen, Y.; Li, G.; Li, H.; Wei, T.; Huang, F.; Ren, L.; Liang, Y.-Q.; Lin, Z.; et al. Performance and mechanisms of biochar-assisted vermicomposting in accelerating di-(2-ethylhexyl) phthalate biodegradation in farmland soil. J. Hazard. Mater. 2023, 443, 130330. [Google Scholar] [CrossRef] [PubMed]
- Kołtowski, M.; Hilber, I.; Bucheli, T.D.; Charmas, B.; Skubiszewska-Zięba, J.; Oleszczuk, P. Activated biochars reduce the exposure of polycyclic aromatic hydrocarbons in industrially contaminated soils. Chem. Eng. J. 2017, 310, 33–40. [Google Scholar] [CrossRef]
- Wang, P.; Cao, J.; Mao, L.; Zhu, L.; Zhang, Y.; Zhang, L.; Jiang, H.; Zheng, Y.; Liu, X. Effect of H3PO4-modified biochar on the fate of atrazine and remediation of bacterial community in atrazine-contaminated soil. Sci. Total Environ. 2022, 851, 158278. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Rajapaksha, A.U.; Zhang, M.; Thiele-Bruhn, S.; Lee, S.S.; Ok, Y.S. Acid-activated biochar increased sulfamethazine retention in soils. Environ. Sci. Pollut. Res. 2015, 22, 2175–2186. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; He, C.; Wen, X.; Liu, Y.; Wang, M.; Fu, Y.; Xiang, L.; Jiang, J.; Jiang, X.; Wang, F. A comparative evaluation of biochar and Paenarthrobacter sp. AT5 for reducing atrazine risks to soybeans and bacterial communities in black soil. Environ. Res. 2024, 252, 119055. [Google Scholar] [CrossRef]
- Askeland, M.; Clarke, B.O.; Cheema, S.A.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Biochar sorption of PFOS, PFOA, PFHxS and PFHxA in two soils with contrasting texture. Chemosphere 2020, 249, 126072. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, R.; Pandey, V.; Singh, A.; Singh, M.; Shanker, K.; Khare, P. Effect of biochar on soil microbial community, dissipation and uptake of chlorpyrifos and atrazine. Biochar 2024, 6, 17. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.M.; Zwieten, L.V.; Kimber, S.; Cowie, A.L.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Wang, L.; O’Connor, D.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C.W.; Shen, Z.; Hou, D. Biochar Aging: Mechanisms, Physicochemical Changes, Assessment, and Implications for Field Applications. Environ. Sci. Technol. 2020, 54, 14797–14814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liang, Y. Changing bioavailability of per- and polyfluoroalkyl substances (PFAS) to plant in biosolids amended soil through stabilization or mobilization. Environ. Pollut. 2022, 308, 119724. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Wang, F.; Bian, Y.; Jiang, X. Combined effects of maize straw biochar and oxalic acid on the dissipation of polycyclic aromatic hydrocarbons and microbial community structures in soil: A mechanistic study. J. Hazard. Mater. 2019, 364, 325–331. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Zhang, W.; Wang, F.; Bian, Y.; Boughner, L.A.; Jiang, X. Novel Biochar-Plant Tandem Approach for Remediating Hexachlorobenzene Contaminated Soils: Proof-of-Concept and New Insight into the Rhizosphere. J. Agric. Food Chem. 2016, 64, 5464–5471. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F.; Cao, F.; Guo, J.; Sun, H. Desorption of atrazine in biochar-amended soils: Effects of root exudates and the aging interactions between biochar and soil. Chemosphere 2018, 212, 687–693. [Google Scholar] [CrossRef]
- Ai-Karaki, G.N. Barley response to salt stress at varied levels of phosphorus. J. Plant Nutr. 1997, 20, 1635–1643. [Google Scholar] [CrossRef]
- Alkorta, I.; Garbisu, C. Phytoremediation of organic contaminants in soils. Bioresour. Technol. 2001, 79, 273–276. [Google Scholar] [CrossRef]
- Khan, Z.; Yang, X.-J.; Fu, Y.; Joseph, S.; Khan, M.N.; Khan, M.A.; Alam, I.; Shen, H. Engineered biochar improves nitrogen use efficiency via stabilizing soil water-stable macroaggregates and enhancing nitrogen transformation. Biochar 2023, 5, 52. [Google Scholar] [CrossRef]
- Chi, W.; Nan, Q.; Liu, Y.; Dong, D.; Qin, Y.; Li, S.; Wu, W. Stress resistance enhancing with biochar application and promotion on crop growth. Biochar 2024, 6, 43. [Google Scholar] [CrossRef]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tan, Z.; Gong, H.; Huang, Q. Migration and Transformation Mechanisms of Nutrient Elements (N, P, K) within Biochar in Straw–Biochar–Soil–Plant Systems: A Review. ACS Sustain. Chem. Eng. 2019, 7, 22–32. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Mukherjee, S.; Sarkar, B. A perspective on biochar for repairing damages in the soil–plant system caused by climate change-driven extreme weather events. Biochar 2022, 4, 22. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, J.; Guo, L.; Fang, H.; Liu, X.; Liang, K.; Niu, W.; Liu, F.; Siddique, K.H.M. Effects of Two Biochar Types on Mitigating Drought and Salt Stress in Tomato Seedlings. Agronomy 2023, 13, 1039. [Google Scholar] [CrossRef]
- Hasnain, M.; Munir, N.; Abideen, Z.; Zulfiqar, F.; Koyro, H.W.; El-Naggar, A.; Caçador, I.; Duarte, B.; Rinklebe, J.; Yong, J.W.H. Biochar-plant interaction and detoxification strategies under abiotic stresses for achieving agricultural resilience: A critical review. Ecotoxicol. Environ. Saf. 2023, 249, 114408. [Google Scholar] [CrossRef]
- Shahzadi, A.; Noreen, Z.; Alamery, S.; Zafar, F.; Haroon, A.; Rashid, M.; Aslam, M.; Younas, A.; Attia, K.A.; Mohammed, A.A.; et al. Effects of biochar on growth and yield of Wheat (Triticum aestivum L.) under salt stress. Sci. Rep. 2024, 14, 20024. [Google Scholar] [CrossRef]
- Scearce, A.E.; Goossen, C.P.; Schattman, R.E.; Mallory, E.B.; MacRae, J.D. Linking drivers of plant per- and polyfluoroalkyl substance (PFAS) uptake to agricultural land management decisions. Biointerphases 2023, 18, 040801. [Google Scholar] [CrossRef]
- Ilango, A.K.; Zhang, W.; Liang, Y. Uptake of per- and polyfluoroalkyl substances by Conservation Reserve Program’s seed mix in biosolids-amended soil. Environ. Pollut. 2024, 363, 125235. [Google Scholar] [CrossRef]
- Pathak, H.K.; Chauhan, P.K.; Seth, C.S.; Dubey, G.; Upadhyay, S.K. Mechanistic and future prospects in rhizospheric engineering for agricultural contaminants removal, soil health restoration, and management of climate change stress. Sci. Total Environ. 2024, 927, 172116. [Google Scholar] [CrossRef]
- Sarma, H.; Shyam, S.; Zhang, M.; Guerriero, G. Nano-biochar interactions with contaminants in the rhizosphere and their implications for plant-soil dynamics. Soil Environ. Health 2024, 2, 100095. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Han, F.; Cong, H.; Zhou, W. Co-application of phytoremediation with iron-loaded biochar in petroleum and zinc co-contaminated soil. Environ. Res. 2024, 263, 120037. [Google Scholar] [CrossRef] [PubMed]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Shang, X.; Ma, Y.; Zhang, K.; Zhang, L.; Zhou, Y.; Gong, Z.; Miao, R. Biochars assisted phytoremediation of polycyclic aromatic hydrocarbons contaminated agricultural soil: Dynamic responses of functional genes and microbial community. Environ. Pollut. 2024, 345, 123476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, A.; Zou, B.; Qian, Y.; Li, X.; Sun, Z. The Combination of Buchloe dactyloides Engelm and Biochar Promotes the Remediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbons. Microorganisms 2024, 12, 968. [Google Scholar] [CrossRef]
- Li, X.; Yao, S.; Bian, Y.; Jiang, X.; Song, Y. The combination of biochar and plant roots improves soil bacterial adaptation to PAH stress: Insights from soil enzymes, microbiome, and metabolome. J. Hazard. Mater. 2020, 400, 123227. [Google Scholar] [CrossRef]
- Shang, X.; Wu, S.; Liu, Y.; Zhang, K.; Guo, M.; Zhou, Y.; Zhu, J.; Li, X.; Miao, R. Rice husk and its derived biochar assist phytoremediation of heavy metals and PAHs co-contaminated soils but differently affect bacterial community. J. Hazard. Mater. 2024, 466, 133684. [Google Scholar] [CrossRef]
- Abbaspour, A.; Zohrabi, F.; Dorostkar, V.; Faz, A.; Acosta, J.A. Remediation of an oil-contaminated soil by two native plants treated with biochar and mycorrhizae. J. Environ. Manag. 2020, 254, 109755. [Google Scholar] [CrossRef]
- Yousaf, U.; Ali Khan, A.H.; Farooqi, A.; Muhammad, Y.S.; Barros, R.; Tamayo-Ramos, J.A.; Iqbal, M.; Yousaf, S. Interactive effect of biochar and compost with Poaceae and Fabaceae plants on remediation of total petroleum hydrocarbons in crude oil contaminated soil. Chemosphere 2022, 286, 131782. [Google Scholar] [CrossRef]
- Liang, Y.; Pei, M.; Wang, D.; Cao, S.; Xiao, X.; Sun, B. Improvement of Soil Ecosystem Multifunctionality by Dissipating Manure-Induced Antibiotics and Resistance Genes. Environ. Sci. Technol. 2017, 51, 4988–4998. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Lin, M.; Tang, J.; Liu, R.; Yang, Y.; Yu, Y.; Li, G.; An, T. Occurrence and fate of polycyclic aromatic hydrocarbons from electronic waste dismantling activities: A critical review from environmental pollution to human health. J. Hazard. Mater. 2022, 424, 127683. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Niu, L.; Su, A.; Li, M.; Wang, Y.; Xu, Y. Sustained and efficient remediation of biochar immobilized with Sphingobium abikonense on phenanthrene-copper co-contaminated soil and microbial preferences of the bacteria colonized in biochar. Biochar 2023, 5, 43. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, L.; Tang, J.; Fang, L.; Yu, X.; Li, M.; Chen, L. Urbanization–land-use interactions predict antibiotic contamination in soil across urban–rural gradients. Sci. Total Environ. 2023, 867, 161493. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Zhao, Y.; Zhu, D.; Gillings, M.; Penuelas, J.; Ok, Y.S.; Capon, A.; Banwart, S. Soil biota, antimicrobial resistance and planetary health. Environ. Int. 2019, 131, 105059. [Google Scholar] [CrossRef]
- Sun, B.; Lian, F.; Bao, Q.; Liu, Z.; Song, Z.; Zhu, L. Impact of low molecular weight organic acids (LMWOAs) on biochar micropores and sorption properties for sulfamethoxazole. Environ. Pollut. 2016, 214, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yuan, J.; Shi, J.; Xu, J.; He, Y. Chlorinated organic pollutants in global flooded soil and sediments: Pollution status and potential risk. Environ. Pollut. 2023, 323, 121270. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Bai, S.H.; Zhang, Y.; Teng, Y.; Xu, Z. Assisted phytoremediation of a co-contaminated soil with biochar amendment: Contaminant removals and bacterial community properties. Geoderma 2019, 348, 115–123. [Google Scholar] [CrossRef]
- Vargas, A.; López, J.E.; Jaimes, A.; Saldarriaga, J.F. Phytoremediation of Hg and chlorpyrifos contaminated soils using Phaseolus vulgaris L. with biochar, mycorrhizae, and compost amendments. Environ. Geochem. Health 2024, 46, 478. [Google Scholar] [CrossRef]
- Hayat, A.; Hussain, I.; Soja, G.; Iqbal, M.; Shahid, N.; Syed, J.H.; Yousaf, S. Organic and chemical amendments positively modulate the bacterial proliferation for effective rhizoremediation of PCBs-contaminated soil. Ecol. Eng. 2019, 138, 412–419. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The Dark Side of Black Gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Yang, X.; Ng, W.; Wong, B.S.E.; Baeg, G.H.; Wang, C.-H.; Ok, Y.S. Characterization and ecotoxicological investigation of biochar produced via slow pyrolysis: Effect of feedstock composition and pyrolysis conditions. J. Hazard. Mater. 2019, 365, 178–185. [Google Scholar] [CrossRef]
- Weidemann, E.; Buss, W.; Edo, M.; Mašek, O.; Jansson, S. Influence of pyrolysis temperature and production unit on formation of selected PAHs, oxy-PAHs, N-PACs, PCDDs, and PCDFs in biochar—A screening study. Environ. Sci. Pollut. Res. 2018, 25, 3933–3940. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, R.; Zimmerman, A.R.; Wang, H.; Gao, B. Applications, impacts, and management of biochar persistent free radicals: A review. Environ. Pollut. 2023, 327, 121543. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mehejabin, F.; Chowdhury, A.A.; Almomani, F.; Khan, N.A.; Badruddin, I.A.; Kamangar, S. Biochar produced from waste-based feedstocks: Mechanisms, affecting factors, economy, utilization, challenges, and prospects. GCB Bioenergy 2024, 16, e13175. [Google Scholar] [CrossRef]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The costs and benefits of biochar production and use: A systematic review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Galinato, S.P.; Yoder, J.K.; Granatstein, D. The economic value of biochar in crop production and carbon sequestration. Energy Policy 2011, 39, 6344–6350. [Google Scholar] [CrossRef]
- Khan, N.; Chowdhary, P.; Gnansounou, E.; Chaturvedi, P. Biochar and environmental sustainability: Emerging trends and techno-economic perspectives. Bioresour. Technol. 2021, 332, 125102. [Google Scholar] [CrossRef]

| Plants | Organic Pollutants | Removal Efficiency | Main Removal Mechanisms | Reference |
|---|---|---|---|---|
| Weeds | PFAS | 41.4% | Weeds transported PFAS to roots and buds by direct absorption. | [40] |
| Lolium perenne L. | PAHs | 55.7% | Plants promoted the degradation of high-cyclic PAHs by Pseudomonas and Bacillus species. | [42] |
| Lolium perenne L. | Pyrene | 59.4% | Ryegrass increased the abundance of PAHs-degrading genera Gemmatimonas, Ohtaekwangia, Luteimonas, Lacibacterium, and Steroidobacter to remove pyrene. | [43] |
| Clover | PCBs | 60.0% | Root exudates improved the bioavailability of pollutants and promoted the degradation of PCBs by Rhizobiales, Burkholderiales, and Xanthomonadales. | [44] |
| Alfalfa | TPHs | 74.1% | Low molecular weight organic acids enhanced soil desorption of oil and improved the potential for microbial degradation by root-associated microbes. | [41] |
| Ludwigia octovalvis | Gasoline | 79.8% | Plants provided suitable conditions for rhizosphere bacteria to degrade hydrocarbons. | [45] |
| Nicotiana tabacum L. | Bisphenol A | 80% | Nicotiana tabacum L. promoted the degradation of Bisphenol A by promoting the activities of Proteobacteria, Acidobacteria, and soil enzymes in the rhizosphere bacterial community. | [46] |
| Elsholtzia splendens | Phenanthrene biphenyl 28 | 84.9%, 65.9% | Elsholtzia splendens shifted the soil microbial community, harbored unique degraders’ community, and enriched degradation genes. | [47] |
| Transgenic Arabidopsis thaliana | PCBs | 85.9% | Plants took up PCBs from the soil and their aerobic conversion to chlorobenzoic and chlorinated fatty acids. | [48] |
| Bok choy | Dibutyl phthalate (DBP) | 91% | Bok choy promoted the dissipation of DBP by regulating the DOM in rhizosphere soil and the enrichment of rhizosphere secretions. | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, H.; Zhou, C.; Guan, D.-X.; Azeem, M.; Li, G. Synergistic Approaches for Sustainable Remediation of Organic Contaminated Soils: Integrating Biochar and Phytoremediation. Agriculture 2025, 15, 905. https://doi.org/10.3390/agriculture15080905
Fang H, Zhou C, Guan D-X, Azeem M, Li G. Synergistic Approaches for Sustainable Remediation of Organic Contaminated Soils: Integrating Biochar and Phytoremediation. Agriculture. 2025; 15(8):905. https://doi.org/10.3390/agriculture15080905
Chicago/Turabian StyleFang, Hao, Cailing Zhou, Dong-Xing Guan, Muhammad Azeem, and Gang Li. 2025. "Synergistic Approaches for Sustainable Remediation of Organic Contaminated Soils: Integrating Biochar and Phytoremediation" Agriculture 15, no. 8: 905. https://doi.org/10.3390/agriculture15080905
APA StyleFang, H., Zhou, C., Guan, D.-X., Azeem, M., & Li, G. (2025). Synergistic Approaches for Sustainable Remediation of Organic Contaminated Soils: Integrating Biochar and Phytoremediation. Agriculture, 15(8), 905. https://doi.org/10.3390/agriculture15080905