Sulfur Induces As Tolerance in Barley Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Analysis
2.2.1. Physiological Parameters
2.2.2. Analysis of Nutrients and As Content
2.2.3. Oxidative Stress Parameters
2.3. Soil Analysis
2.3.1. Chemical Analysis
2.3.2. Biological Properties
2.4. Statistical Analyses
3. Results and Discussion
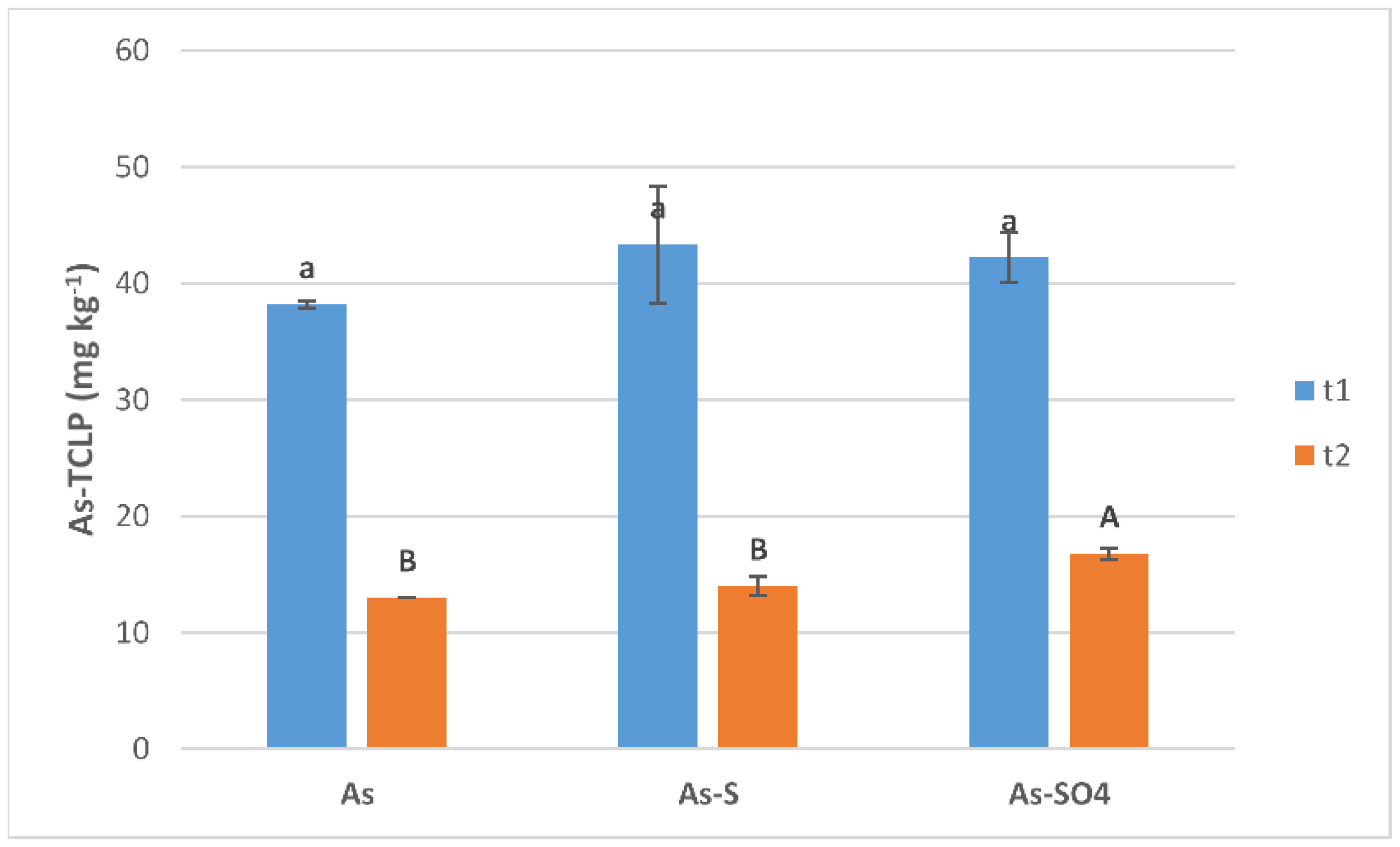
3.1. Impact on Soil Chemical Properties
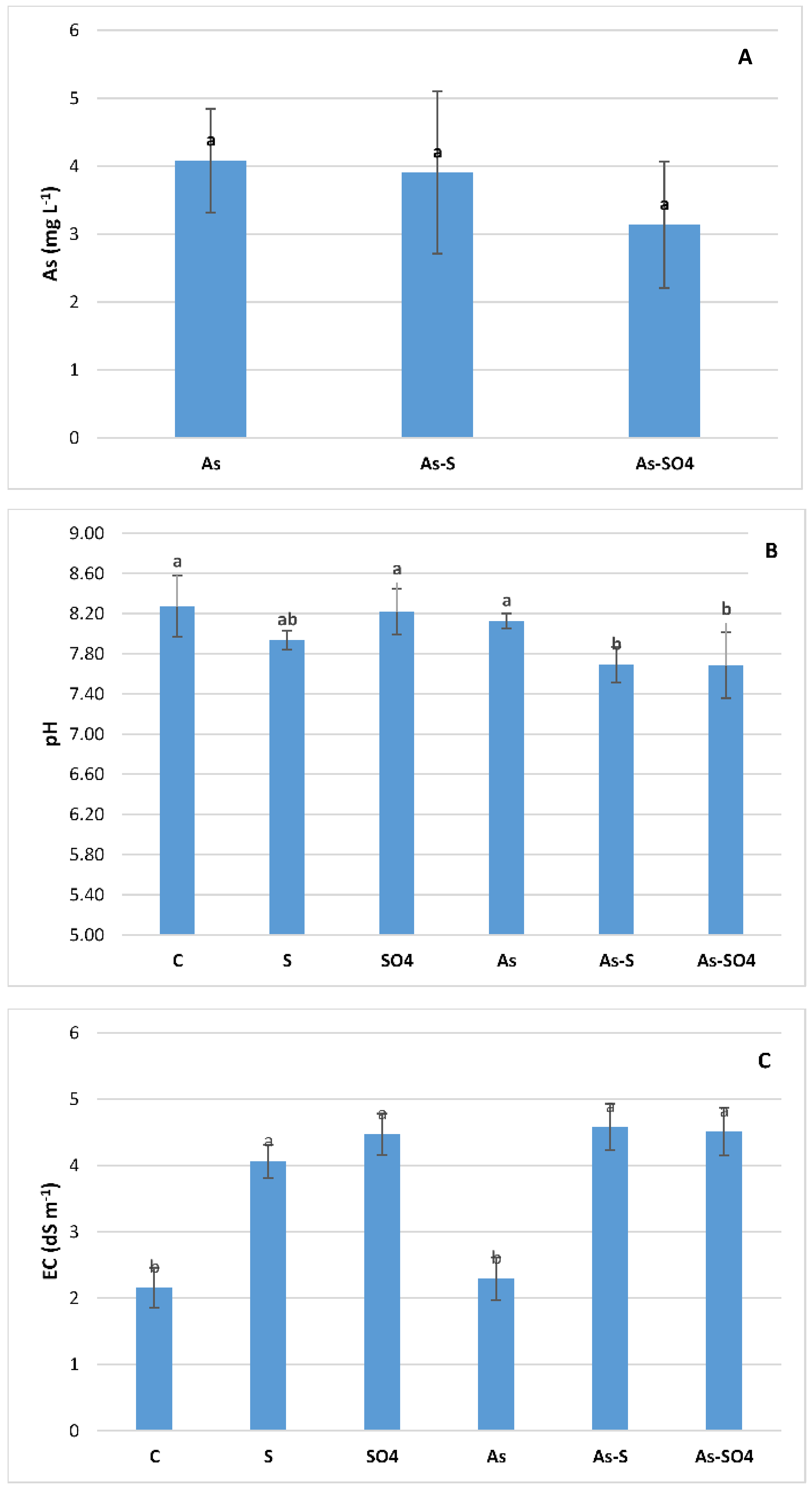
3.2. Impact on Rhizosphere
3.2.1. Pore Water
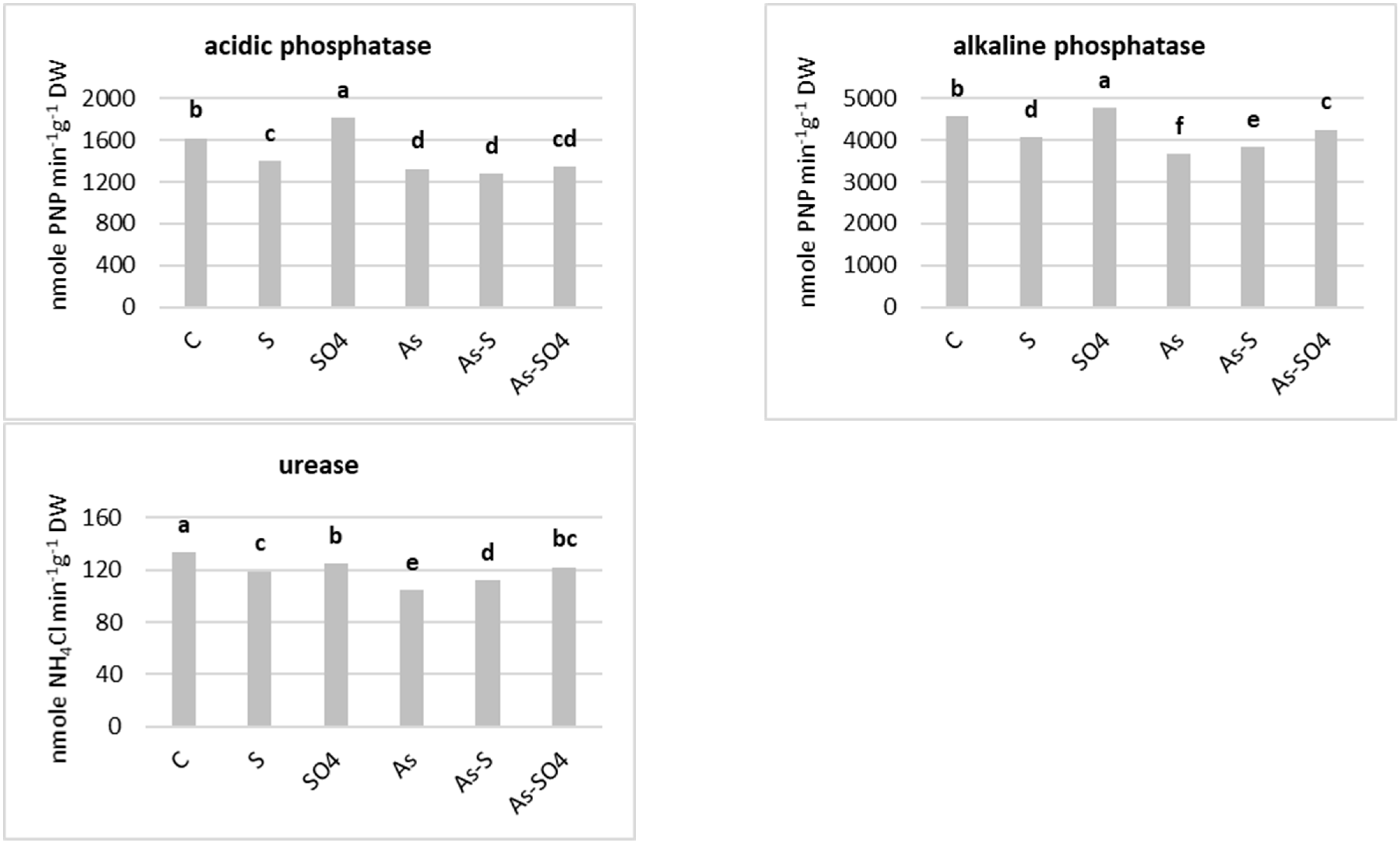
3.2.2. Soil Biological Properties
3.3. Impact on Barley Plants
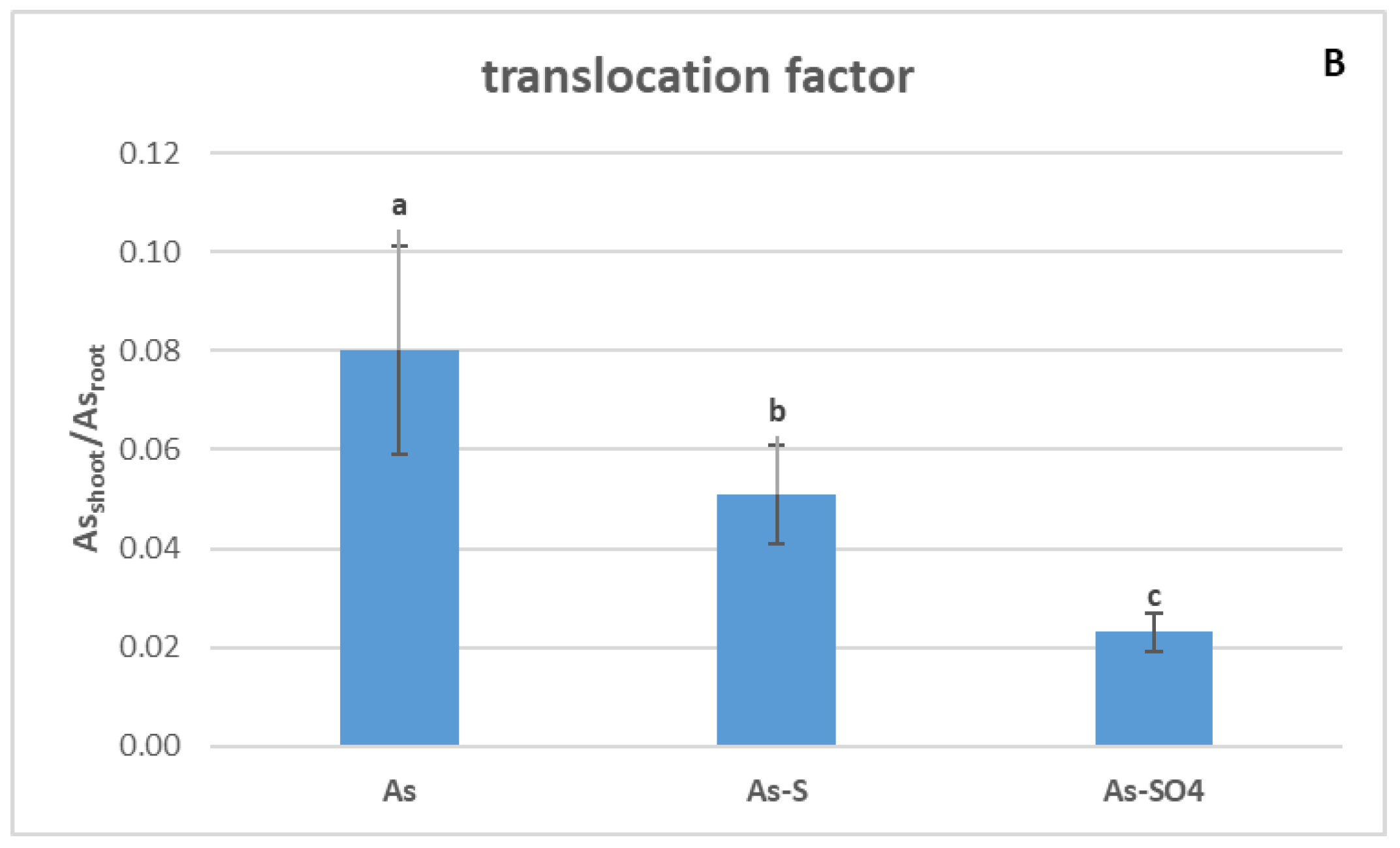
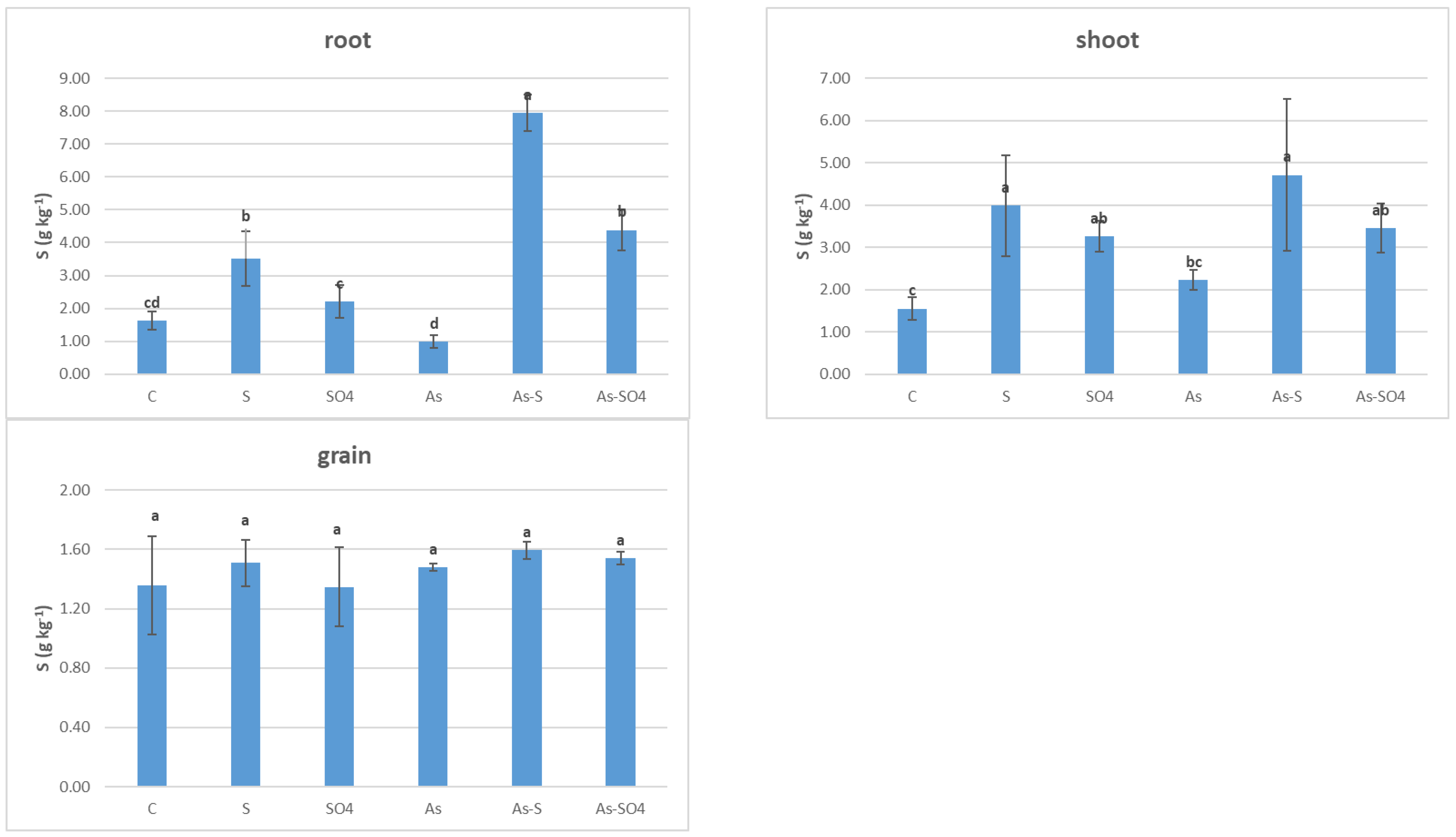
3.3.1. As Accumulation in Barley Plants
3.3.2. Nutrient Accumulation in Barley Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Van Liedekerke, M.; Prokop, G.; Rabl-Berger, S.; Kibblewhite, M.; Louwagie, G. Progress in Management of Contaminated Sites in Europe; Joint Research Centre: Changchun, China, 2014; ISBN 9789279348464. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC85913 (accessed on 20 October 2024).
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal-contaminated land. A review. Environ. Chem. Lett. 2010, 8, 1–17. [Google Scholar] [CrossRef]
- Toth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2010; ISBN 9780429192036. [Google Scholar]
- Rahman, M.M.; Naidu, R.; Bhattacharya, P. Arsenic contamination in groundwater in the Southeast Asia region. Environ. Geochem. Health 2009, 31, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Owens, G.; Naidu, R. Arsenic levels in rice grain and assessment of daily dietary intake of arsenic from rice in arsenic-contaminated regions of Bangladesh—Implications to groundwater irrigation. Environ. Geochem. Health 2009, 31, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Lee, C.-C.; Huang, W.-J.; Huang, H.-T.; Wu, Y.-C.; Hsu, Y.-C.; Kao, Y.-T. Arsenic speciation in rice and risk assessment of inorganic arsenic in Taiwan population. Environ. Sci. Pollut. Res. Int. 2016, 23, 4481–4488. [Google Scholar] [CrossRef]
- Kumar, M.; Rahman, M.M.; Ramanathan, A.; Naidu, R. Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: Health risk index. Sci. Total Environ. 2016, 539, 125–134. [Google Scholar] [CrossRef]
- Rasheed, H.; Slack, R.; Kay, P. A comparative assessment of arsenic distribution in rice produced in Pakistan and other geographical regions. In Arsenic Research and Global Sustainability. 6th International Congress on Arsenic in the Environment (As2016); Bhattacharya, P., Vahter, M., Jarsjö, J., Kumpiene, J., Ahmad, A., Sparrenbom, C., Jacks, G., Donselaar, M.E., Bundschuh, J., Eds.; CRC Press (Taylor & Francis Group): Stockholm, Sweden, 2016; ISBN 9781138029415. [Google Scholar]
- Li, H.-B.; Li, J.; Zhao, D.; Li, C.; Wang, X.-J.; Sun, H.-J.; Juhasz, A.L.; Ma, L.Q. Arsenic Relative Bioavailability in Rice using a Mouse Arsenic Urinary Excretion Bioassay and Its Application to Assess Human Health Risk. Environ. Sci. Technol. 2017, 51, 4689–4696. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Hytochelatins and m etallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Bhatti, A.A.; Dar, Z.A.; Bhat, S.A. Phytoremediation of Heavy Metals: An Eco-Friendly and Sustainable Approach. In Bioremediation and Biotechnology; Springer International Publishing: Cham, Switzerland, 2020; pp. 215–231. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef] [PubMed]
- González, Á.; Gil-Diaz, M.M.; Lobo, M.C. Metal tolerance in barley and wheat cultivars: Physiological screening methods and application in phytoremediation. J. Soils Sediments 2017, 17, 5. [Google Scholar] [CrossRef]
- González, Á.; Lobo, M.C. Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits. Am. J. Plant Sci. 2013, 4, 1799–1810. [Google Scholar] [CrossRef]
- Zhang, G.P.; Fukami, M.; Sekimoto, H. Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage. F. Crop. Res. 2002, 77, 93–98. [Google Scholar] [CrossRef]
- Xue, D.; Chen, M.; Zhang, G. Mapping of QTLs associated with cadmium tolerance and accumulation during seedling stage in rice (Oryza sativa L.). Euphytica 2009, 165, 587–596. [Google Scholar] [CrossRef]
- Qiu, B.; Zhou, W.; Xue, D.; Zeng, F.; Ali, S.; Zhang, G. Identification of Cr-tolerant lines in a rice (Oryza sativa) DH population. Euphytica 2010, 174, 199–207. [Google Scholar] [CrossRef]
- González, A.; Gil-Díaz, M.M.; Pinilla, P.; Lobo, M.C. Impact of Cr and Zn on Growth, Biochemical and Physiological Parameters, and Metal Accumulation by Wheat and Barley Plants. Water Air Soil Pollut. 2017, 228, 419. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Mubsher, A.; Rizwan, M.; Usman, M.; Jafir, M.; Umair, M.; Alharby, H.F.; Bamagoos, A.A.; Alshamrani, R.; Ali, S. Effect of farmyard manure, elemental sulphur and EDTA on growth and phytoextraction of cadmium by spider plants (Chlorophytum comosum L.) under Cd stress. Chemosphere 2023, 313, 137385. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.-J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Yu, H.; Tan, Q.; Parkash, O.; White, J.C. The role of sulfur nutrition in plant response to metal (loid) stress: Facilitating biofortification and phytoremediation. J. Hazard. Mater. 2023, 443, 130283. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.W.; Wise, B.E. Mitigating Cadmium Accumulation in Spinach and Onions by the Application of Silicon Fertilizer to Soil. Soil Sediment Contam. An Int. J. 2020, 29, 532–544. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Zhang, W.; Gao, Y.; Jie, H.; Rao, W.; Jiang, Y.; Wang, D. Remediation of arsenic-contaminated paddy soil: Effects of elemental sulfur and gypsum fertilizer application. Ecotoxicol. Environ. Saf. 2021, 223, 112606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yang, Y.; Zhang, L.; Zhang, J.; Zhou, Y.; Huang, H.; Luo, S.; Luo, L. Silicon-based additive on heavy metal remediation in soils: Toxicological effects, remediation techniques, and perspectives. Environ. Res. 2022, 205, 112244. [Google Scholar] [CrossRef]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron alleviates cadmium toxicity in Brassica napus by promoting the chelation of cadmium onto the root cell wall components. Sci. Total Environ. 2020, 728, 138833. [Google Scholar] [CrossRef]
- Sun, L.; Gao, G.; Sun, Y.; Yang, S.; Qin, Q.; Ye, J.; Xue, Y. Appropriate sulfur fertilization in contaminated soil enhanced the cadmium uptake by hyperaccumulator Sedum alfredii Hance. Ecotoxicol. Environ. Saf. 2024, 283, 116870. [Google Scholar] [CrossRef]
- Xu, C.; Qin, L.; Li, Y.; Zu, Y.; Wang, J. Effects of Different Sulfur Compounds on the Distribution Characteristics of Subcellular Lead Content in Arabis alpina L. var. parviflora Franch under Lead Stress. Plants 2023, 12, 874. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Ma, L.Q.; Santos, J.A.G.; Guilherme, L.R.G.; Lessl, J.T. Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata L. Environ. Pollut. 2014, 184, 187–192. [Google Scholar] [CrossRef]
- Srivastava, S.; D’Souza, S.F. Increasing sulfur supply enhances tolerance to arsenic and its accumulation in Hydrilla verticillata (L.f.) Royle. Environ. Sci. Technol. 2009, 43, 6308–6313. [Google Scholar] [CrossRef]
- Srivastava, S.; Akkarakaran, J.J.; Sounderajan, S.; Shrivastava, M.; Suprasanna, P. Arsenic toxicity in rice (Oryza sativa L.) is influenced by sulfur supply: Impact on the expression of transporters and thiol metabolism. Geoderma 2015, 270, 33–42. [Google Scholar] [CrossRef]
- Dixit, G.; Singh, A.P.; Kumar, A.; Singh, P.K.; Kumar, S.; Dwivedi, S.; Trivedi, P.K.; Pandey, V.; Norton, G.J.; Dhankher, O.P.; et al. Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J. Hazard. Mater. 2015, 298, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Zakari, S.; Jiang, X.; Zhu, X.; Liu, W.; Allakonon, M.G.B.; Singh, A.K.; Chen, C.; Zou, X.; Akponikpè, P.B.I.; Dossa, G.G.O.; et al. Influence of sulfur amendments on heavy metals phytoextraction from agricultural contaminated soils: A meta-analysis. Environ. Pollut. 2021, 288, 117820. [Google Scholar] [CrossRef] [PubMed]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Saifullah; Khan, M.N.; Iqbal, M.; Naeem, A.; Bibi, S.; Waraich, E.A.; Dahlawi, S. Elemental sulfur improves growth and phytoremediative ability of wheat grown in lead-contaminated calcareous soil. Int. J. Phytoremediat. 2016, 18, 1022–1028. [Google Scholar] [CrossRef]
- Thouin, H.; Le Guédard, M.; Hellal, J.; Joulian, C.; Charron, M.; Devau, N.; Battaglia-Brunet, F. Effects of ammonium sulphate fertilization on arsenic mobility, speciation, and toxicity in soils planted with barley. J. Soils Sediments 2022, 22, 2422–2434. [Google Scholar] [CrossRef]
- Shi, G.; Lu, H.; Liu, H.; Lou, L.; Zhang, P.; Song, G.; Zhou, H.; Ma, H. Sulfate application decreases translocation of arsenic and cadmium within wheat (Triticum aestivum L.) plant. Sci. Total Environ. 2020, 713, 136665. [Google Scholar] [CrossRef]
- Kovar, J.L.; Grant, C.A. Nutrient Cycling in Soils: Sulfur. In Soil Management: Building a Stable Base for Agriculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 103–115. ISBN 9780891181958. [Google Scholar]
- Fässler, E.; Stauffer, W.; Gupta, S.K.; Schulin, R. Effects and limitations of elemental sulphur applications for enhanced phytoextraction. Int. J. Phytoremediat. 2012, 14, 681–690. [Google Scholar] [CrossRef]
- MAPA. Métodos Oficiales de Análisis; Secretaría General Técnica, Ministerio de Agricultura, Pesca y Alimentación Madrid: Madrid, Spain, 1994; Volume III, pp. 205–324.
- Aggrawal, M.; Rohrer, J. Determination of Chloride and Sulfate in Water and Soil. Application Note 1113 Thermo Fisher Scientific. 2016. Available online: https://tools.thermofisher.com/content/sfs/brochures/AN-1113-IC-Chloride-Sulfate-Water-Soil-AN71389-EN.pdf (accessed on 20 October 2024).
- Hoefer, C.; Santner, J.; Puschenreiter, M.; Wenzel, W.W. Localized Metal Solubilization in the Rhizosphere of Salix smithiana upon Sulfur Application. Environ. Sci. Technol. 2015, 49, 4522–4529. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Gonzalez, A. Aplicación del medidor portátil de clorofila en programas de mejora de trigo y cebada. Agroecología 2009, 4, 111–116. [Google Scholar]
- Fang, S.; Sun, W.; Hu, H.; Zhang, H.; Zheng, K. Chlorophyll Content of Barley (Hordeum vulgare L.) Estimation from Leaf SPAD, Chlorophyll Fluorescence and Reflectance Properties. Adv. Sci. Lett. 2012, 11, 702–705. [Google Scholar] [CrossRef]
- Zhao, F.; McGrath, S.P.; Crosland, A.R. Comparison of 3 wet digestion methods for the determination of plant sulfur by inductively-coupled plasma-atomic emission-spectroscopy (ICPAES). Commun. Soil Sci. Plant Anal. 1994, 25, 407–418. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol. J. 2004, 2, 477–486. [Google Scholar] [CrossRef] [PubMed]
- US-EPA. Environmental Protection Agency Method 1311. Toxicity Characteristic Leaching Procedure; US-EPA: Washington, DC, USA, 1992.
- ISO 20130:2018; Soil Quality—Measurement of Enzyme Activity Patterns in Soil Samples using colorimetric Substrates in Micro-Well Plates. ISO: Geneva, Switzerland, 2018.
- ISO-17155-2012; Soil Quality—Determination of Abundance and Activity of Soil Microflora using Respiration Curves. ISO: Geneva, Switzerland, 2012.
- Gil-Díaz, M.; Pérez-Sanz, A.; Ángeles Vicente, M.; Lobo, M.C. Immobilisation of Pb and Zn in soils using stabilised zero-valent iron nanoparticles: Effects on soil properties. Clean-Soil Air Water 2014, 42, 1776–1784. [Google Scholar] [CrossRef]
- Tichý, R.; Fajtl, J.; Kužel, S.; Kolář, L. Use of elemental sulphur to enhance a cadmium solubilization and its vegetative removal from contaminated soil. Nutr. Cycl. Agroecosystems 1996, 46, 249–255. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Liu, J.; Yang, D.; Hong, H.; Yan, C.; Lu, H. Effect of sulfate on arsenic migration and transformation in micro-cosmic experiments simulating mangrove sediment environment. Catena 2024, 236, 107719. [Google Scholar] [CrossRef]
- Martínez-Iñigo, M.J.; Pérez-Sanz, A.; Ortiz, I.; Alonso, J.; Alarcón, R.; García, P.; Lobo, M.C. Bulk soil and rhizosphere bacterial community PCR–DGGE profiles and β-galactosidase activity as indicators of biological quality in soils contaminated by heavy metals and cultivated with Silene vulgaris (Moench) Garcke. Chemosphere 2009, 75, 1376–1381. [Google Scholar] [CrossRef]
- Liu, H.; Luo, L.; Jiang, G.; Li, G.; Zhu, C.; Meng, W.; Zhang, J.; Jiao, Q.; Du, P.; Li, X.; et al. Sulfur enhances cadmium bioaccumulation in Cichorium intybus by altering soil properties, heavy metal availability and microbial community in contaminated alkaline soil. Sci. Total Environ. 2022, 837, 155879. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, K.; Zhou, J.; Zhang, W.; Liu, L.; Han, X. Spatial distribution and toxicity of cadmium in the joint presence of sulfur in rice seedling. Environ. Toxicol. Pharmacol. 2013, 36, 1235–1241. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Garcia-Gonzalo, P.; Gil-Diaz, M.M.; Alonso, J.; Lobo, M.C. Compost-assisted phytoremediation of As-polluted soil. J. Soils Sediments 2019, 19, 2971–2983. [Google Scholar] [CrossRef]
- Song, W.Y.; Mendoza-Cózatl, D.G.; Lee, Y.; Schroeder, J.I.; Ahn, S.N.; Lee, H.S.; Wicker, T.; Martinoia, E. Phytochelatin-metal(loid) transport into vacuoles shows different substrate preferences in barley and Arabidopsis. Plant Cell Environ. 2014, 37, 1192–1201. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Mean Value | |
|---|---|---|
| pH | 8.22 | |
| electrical conductivity (dS m−1) | 0.33 | |
| CO32− (%) | 5.75 | |
| N (%) | 0.20 | |
| Organic matter (%) | 1.40 | |
| Available nutrients (mg kg−1) | P | 19 |
| Ca2+ | 3076 | |
| Mg2+ | 469 | |
| Na+ | 56 | |
| K+ | 347 | |
| Total metal(loid)s (mg kg−1) | As | 8.5 |
| Cd | <LD | |
| Cr | 31 | |
| Cu | 14 | |
| Ni | 10 | |
| Pb | 8.5 | |
| Zn | 49 | |
| Anions (mg kg−1) | Cl− | 82 |
| NO2− | 0.80 | |
| NO3− | 132 | |
| PO43− | 5.20 | |
| SO42− | 33 | |
| C | S | SO4 | As | As-S | As-SO4 | |
|---|---|---|---|---|---|---|
| pH | 8.43 a | 8.06 c | 8.23 b | 8.34 ab | 8.00 c | 8.06 c |
| EC (dS m−1) | 0.27 c | 1.10 a | 0.65 b | 0.36 c | 1.06 a | 1.03 a |
| N (%) | 0.09 a | 0.10 a | 0.09 a | 0.09 a | 0.10 a | 0.10 a |
| OM (%) | 1.54 a | 1.49 a | 1.42 a | 1.56 a | 1.59 a | 1.54 a |
| Ca (mg kg−1) | 3427 ab | 3790 a | 3306 b | 3152 b | 3772 a | 3718 a |
| Mg (mg kg−1) | 390 a | 378 a | 363 a | 372 a | 364 a | 376 a |
| Na (mg kg−1) | 76 a | 70 a | 65 a | 68 a | 69 a | 101 a |
| K (mg kg−1) | 195 b | 172 b | 178 b | 282 a | 279 a | 318 a |
| Cl− (mg kg−1) | 22.2 b | 29.6 b | 41.4 b | 23.9 b | 24.6 b | 71.6 ab |
| NO2− (mg kg−1) | <LQ | <LQ | <LQ | <LQ | <LQ | <LQ |
| NO3− (mg kg−1) | 279 bc | 381 b | 415 b | 313 b | 139 c | 741 a |
| PO43− (mg kg−1) | 0.82 | 5.44 | <LQ | 2.43 | 5.53 | <LQ |
| SO42− (mg kg−1) | 36.1 c | 718 b | 1453 a | 38.8 c | 246 b | 1192 a |
| Part of the Plant | Nutrient | C | S | SO4 | As | As-S | As-SO4 |
|---|---|---|---|---|---|---|---|
| root | K (g kg−1) | 4.84 b | 4.86 b | 4.39 b | 8.16 a | 6.10 ab | 7.93 a |
| Na (g kg−1) | 0.85 a | 1.09 a | 0.58 a | 1.05 a | 0.66 a | 1.30 a | |
| Ca (g kg−1) | 20.3 a | 12.7 b | 15.8 ab | 5.29 c | 3.88 c | 3.25 c | |
| Mg (g kg−1) | 1.65 ab | 1.52 b | 2.42 a | 1.33 b | 0.81 b | 1.39 b | |
| stem | K (g kg−1) | 24.9 c | 23.3 c | 24.6 c | 36.1 b | 32.8 b | 43.3 a |
| Na (g kg−1) | 2.64 ab | 2.63 ab | 1.44 b | 3.35 a | 3.59 a | 3.36 a | |
| Ca (g kg−1) | 5.77 b | 7.18 b | 5.94 b | 9.09 ab | 8.47 ab | 11.1 a | |
| Mg (g kg−1) | 2.51 a | 2.63 a | 2.73 a | 2.47 a | 3.10 a | 2.65 a | |
| N (%) | 0.77 a | 0.84 a | 0.84 a | 1.41 a | 1.16 a | 1.49 a | |
| grain | K (g kg−1) | 4.73 ab | 3.60 b | 4.14 ab | 4.52 b | 3.83 b | 5.07 a |
| Na (g kg−1) | 0.044 c | 0.046 c | 0.045 c | 0.230 a | 0.069 bc | 0.108 b | |
| Ca (g kg−1) | 0.057 ab | 0.039 c | 0.049 bc | 0.039 c | 0.039 c | 0.047 bc | |
| Mg (g kg−1) | 0.84 a | 0.82 a | 0.69 a | 0.84 a | 0.92 a | 0.85 a | |
| N (%) | 2.25 a | 2.47 a | 2.18 a | 2.67 a | 2.63 a | 2.58 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Díaz, M.; Alonso, J.; Mancho, C.; García-Gonzalo, P.; Lobo, M.C. Sulfur Induces As Tolerance in Barley Plants. Agriculture 2024, 14, 2110. https://doi.org/10.3390/agriculture14122110
Gil-Díaz M, Alonso J, Mancho C, García-Gonzalo P, Lobo MC. Sulfur Induces As Tolerance in Barley Plants. Agriculture. 2024; 14(12):2110. https://doi.org/10.3390/agriculture14122110
Chicago/Turabian StyleGil-Díaz, Mar, Juan Alonso, Carolina Mancho, Pilar García-Gonzalo, and M. Carmen Lobo. 2024. "Sulfur Induces As Tolerance in Barley Plants" Agriculture 14, no. 12: 2110. https://doi.org/10.3390/agriculture14122110
APA StyleGil-Díaz, M., Alonso, J., Mancho, C., García-Gonzalo, P., & Lobo, M. C. (2024). Sulfur Induces As Tolerance in Barley Plants. Agriculture, 14(12), 2110. https://doi.org/10.3390/agriculture14122110








