Effect of Plant Hormones and Preservative Solutions on Post-Harvest Quality and Physiological Senescence Parameters of Cut Leaves of Hosta Tratt. ‘Krossa Regal’ and Polygonatum multiflorum (L.) All. ‘Variegatum’
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Procedure
2.3. Chemical Measurements and Analyses
- -
- Leaf blade discoloration—yellowing (the gradual loss of chlorophyll and emerging leaf chlorosis);
- -
- The browning of leaf margins and the appearance of necrotic spots;
- -
- The drying of the leaf blade and the curling of the margins;
- -
- Leaf drops from the stems.
2.4. Statistical Analysis
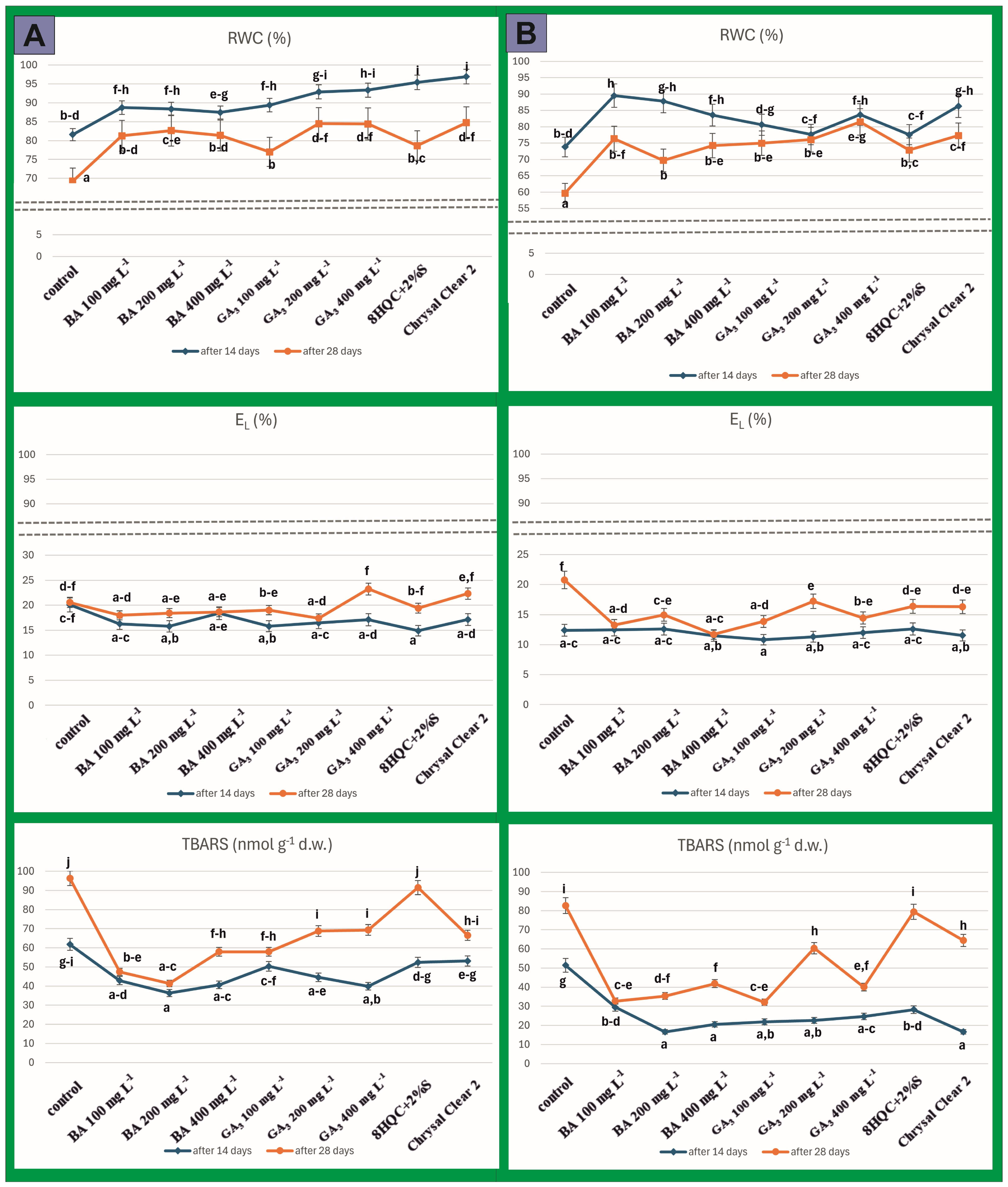
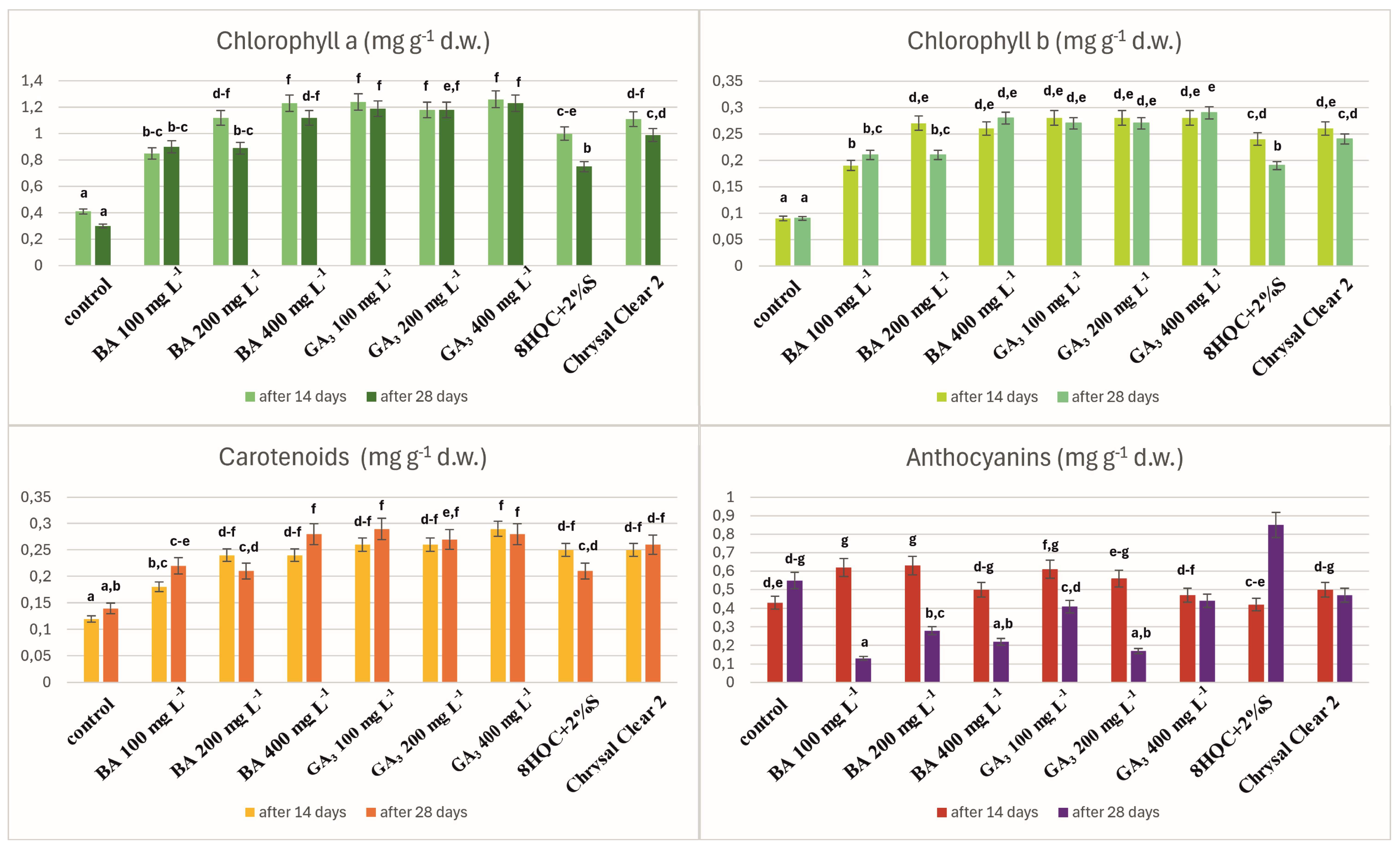
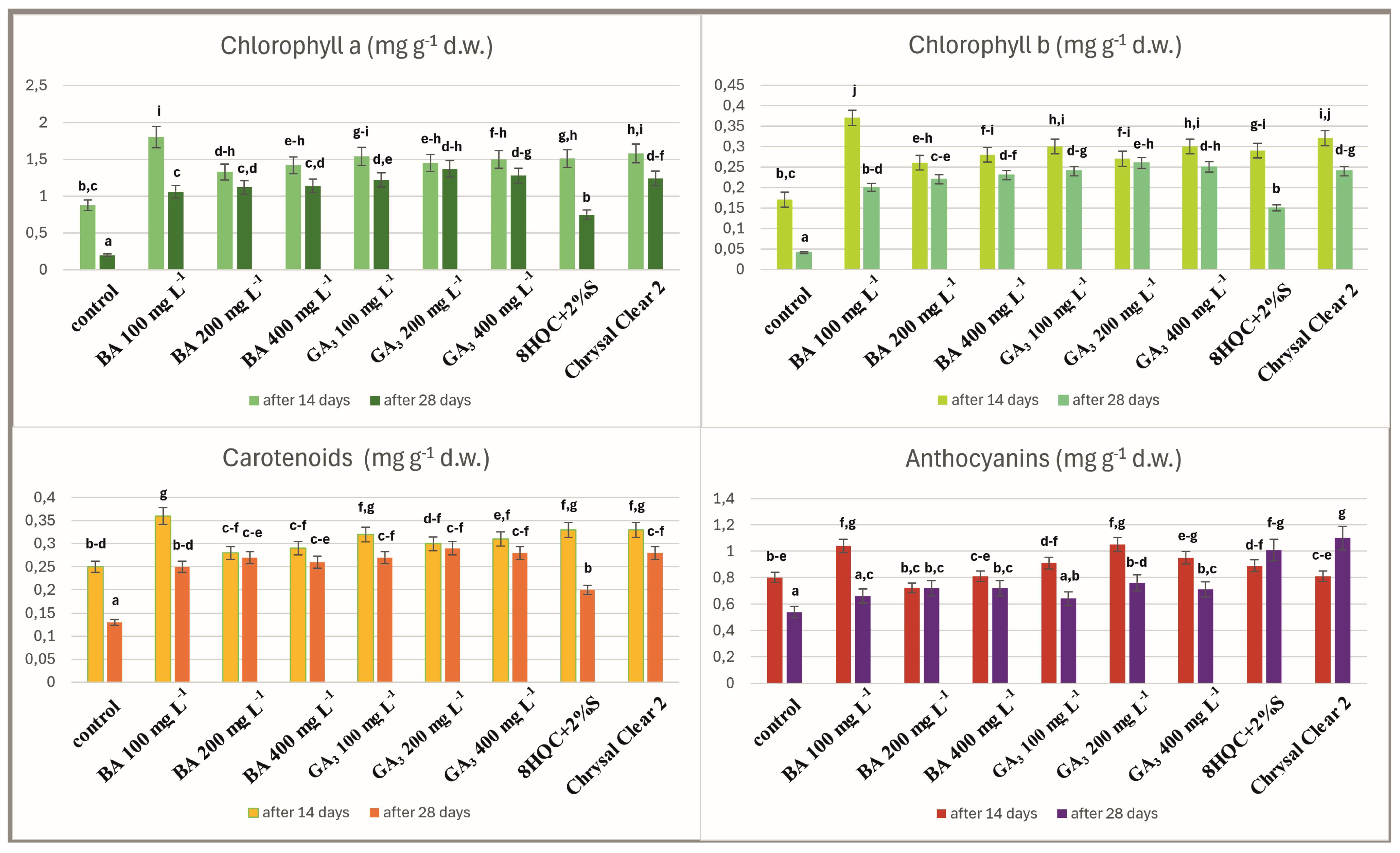
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulczycka-Walorska, M.P.; Krzymińska, A. Influence of various chemical compounds on postharvest longevity of cut leaves of Waldsteinia geoides Willd. Acta Agrobot. 2014, 67, 83–90. [Google Scholar] [CrossRef][Green Version]
- Garg, A.; Thakur, T. Cut Foliage and Cut Greens. Agric. Food E-Newsl. 2024, 6, 412–414. [Google Scholar]
- Stefanowicz, J.; Ochocka, J.R. Solomon’s seal (Polygonatum multiflorum (L.) All.)—Common medicinal plant of ours forests. Postępy Fitoter. 2004, 4, 163–168. [Google Scholar]
- Teka, T.; Wang, L.; Gao, J.; Mou, J.; Pan, G.; Yu, H.; Gao, X.; Han, L. Polygonatum multiflorum: Recent updates on newly isolated compounds, potential hepatotoxic compounds and their mechanisms. J. Ethnopharmacol. 2021, 271, 113864. [Google Scholar] [CrossRef]
- Krzymińska-Bródka, A.; Ulczycka-Walorska, M.; Łysiak, G.P.; Czuchaj, P. The longevity of cut Polygonatum multiflorum (L.) All. shoots depending on postharvest handling. Acta Agrobot. 2023, 76, 176280. [Google Scholar] [CrossRef]
- Aelenei, R.I.; Petra, S.; Toma, F. Studies and reaearch on the species and varieties of Hosta in cultivation. Sci. Pap. Ser. B Hortic. 2020, 64, 511. [Google Scholar]
- Isgandarova, T.Y.; Rustamova, S.M.; Aliyeva, D.R.; Rzayev, F.H.; Gasimov, E.K.; Huseynova, I.M. Antioxidant and Ultrastructural Alterations in Wheat During Drought-Induced Leaf Senescence. Agronomy 2024, 14, 2924. [Google Scholar] [CrossRef]
- Liu, R.; Zuo, X.; Chen, Y.; Qian, Z.; Xu, C.; Wang, L.; Chen, S. Transcriptional regulation in leaves of cut chrysanthemum (Chrysanthemum morifolium) ‘FenDante’ in Response to Post-Harvest Ethylene Treatment. Horticulturae 2022, 8, 573. [Google Scholar] [CrossRef]
- Liu, K.; Jing, T.; Wang, Y.; Ai, X.; Bi, H. Melatonin delays leaf senescence and improves cucumber yield by modulating chlorophyll degradation and photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915. [Google Scholar] [CrossRef]
- Qu, Y.; Jiang, L.; Wuyum, T.; Mu, S.; Xie, F.; Chen, Y.; Zhang, L. Effects of exogenous putrescine on delaying senescence of cut foliage of Nephrolepis cordifolia. Front. Plant Sci. 2020, 11, 566824. [Google Scholar] [CrossRef]
- Kwon, H.S.; Leporini, C.; Kim, S.; Heo, S. Prolonged vase life by salicylic acid treatment and prediction of vase life using petal color senescence of cut lisianthus. Postharvest Biol. Technol. 2024, 209, 112726. [Google Scholar] [CrossRef]
- Haq, A.U.; Farooq, S.; Lone, M.L.; Altaf, F.; Parveen, S.; Tahir, I. “Blossoming beyond time”: Proline orchestrates flower senescence in Ranunculus asiaticus L. by modulating biochemical and antioxidant machinery. J. Plant Growth Regul. 2024, 1–11. [Google Scholar] [CrossRef]
- Haq, A.U.; Farooq, S.; Lone, M.L.; Parveen, S.; Altaf, F.; Tahir, I. Flower Senescence Coordinated by Ethylene: An Update and Future Scope on Postharvest Biology in the “Buttercup” Family. J. Plant Growth Regul. 2024, 43, 402–422. [Google Scholar] [CrossRef]
- Trivellini, A.; Franzoni, G.; Ferrante, A.; Mensuali, A. Melatonin improves the postharvest life of cut ruscus foliage after a long storage condition. Postharvest Biol. Technol. 2025, 222, 113419. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. A composite vase solution using Silicon (Si) and other preservatives improved the vase quality of cut lily (Lilium ‘Siberia’) Flowers. Horticulturae 2025, 11, 112. [Google Scholar] [CrossRef]
- da Cunha Neto, A.R.; de Oliveira Paiva, P.D.; Nogueira, M.R.; Pereira do Nascimento, A.M.; dos Santos, H.O.; dos Reis, M.V. Exploring the impact of dry conditioning on the postharvest quality and longevity of torch ginger flowers stems. Acta Bot. Bras. 2023, 37, e20230100. [Google Scholar] [CrossRef]
- Janowska, B.; Stanecka, A.; Czarnecka, B. Post harvest longevity of the leaves of the calla lily (Zantedeschia Spreng.). Acta Sci. Pol. Hortorum Cultus 2012, 11, 121–131. [Google Scholar] [CrossRef][Green Version]
- Janowska, B.; Nowińska, M.; Andrzejak, R. The vase life of the leaves of selected perennial species after the application of growth regulators. Agronomy 2022, 12, 805. [Google Scholar] [CrossRef]
- Da Gama, A.B.N.; de Oliveira Paiva, P.D.; Brito, O.G.; da Costa, A.L.; da Silva, D.P.C.; Paiva, R. Role of preservative solutions on physiological mechanisms for delaying leaf yellowing in alstroemeria flowers. Ciênc. Agrotecnol. 2025, 49, e023024. [Google Scholar] [CrossRef]
- Wachowicz, M.; Rabiza-Świder, J.; Skutnik, E.; Łukaszewska, A. The short-term cold storage effect on vase life of cut Hosta leaves. Acta Sci. Pol. Hortorum Cultus 2007, 6, 3–13, ISSN 2545-1405. [Google Scholar]
- Pogroszewska, E.; Szot, P.; Rubinowska, K.; Konopińska-Mamej, A.; Świstowska, A.; Zdybel, A.; Parzymies, M.; Durlak, W. The effect of silicon on morphological traits and mechanical properties of Polygonatum multiflorum (L.) All. ‘Variegatum’ cut shoots. Acta Sci. Pol. Hortorum Cultus 2018, 17, 157–166. [Google Scholar] [CrossRef]
- Barrs, H.D. Determination of water deficits in plant tissues. In Water Deficits and Plant Growth, Vol. I: Development, Control and Measurement; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1968; pp. 235–368. [Google Scholar]
- Kościelniak, J. Wpływ następczy temperatur chłodowych w termoperiodyzmie dobowym na produktywność fotosyntetyczną kukurydzy (Zea mays L.). Zesz. Nauk. Akad. Rol. Krakowie 1993, 174. [Google Scholar]
- Heath, R.L.; Packer, L. Effect of light on lipid peroxidation in chloroplasts. Biochem. Biophys. Res. Commun. 1968, 19, 716–720. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Leng, P.; Qi, J.X. Effect of anthocyanin on David peach (Prunus davidiana Franch) under low temperature stress. Sci. Hortic. 2003, 97, 27–39. [Google Scholar] [CrossRef]
- Skutnik, E.; Rabiza-Świder, J.; Wachowicz, M.; Łukaszewska, A. Starzenie się ciętych liści Zantedeschia aethiopica i Z. elliottiana. Część, I. Degradacja chlorofilu. Acta Sci. Pol. Hortorum Cultus 2004, 3, 57–65. [Google Scholar]
- Janowska, B.; Andrzejak, R. The role of cytokinins and gibberellins on post-harvest longevity of florist’s greens. Agriculture 2022, 12, 1375. [Google Scholar] [CrossRef]
- Dai, J.W.; Paull, R.E. Postharvest handling of Alstroemeria. HortScience 1991, 26, 314. [Google Scholar] [CrossRef]
- Hicklenton, P.R. GA3 and benzylaminopurine delay leaf chlorosis in cut Alstroemeria stems. HortScience 1991, 26, 1198–1199. [Google Scholar] [CrossRef]
- Yeat, C.S.; Szydlik, M.; Łukaszewska, A.J. The effect of postharvest treatments on flower quality and vase life of cut Alstroemeria ‘Dancing Queen’. J. Fruit Ornam. Plant Res. 2012, 20, 147–160. [Google Scholar] [CrossRef]
- Janowska, B. Effect of conditioning on the longevity of leaves of the Italian arum (Arum italicum Mill.) kept at a low temperature. Nauka Przyr. Technol. 2010, 4, 12. [Google Scholar]
- Szymaniak, D.; Pernak, J.; Rzemieniecki, T.; Kaczmarek, D.K.; Andrzejak, R.; Kosiada, T.; Janowska, B. Synthesis and characterization of bio-based quaternary ammonium salts with gibberellate or α-tryptophanate anion. Monatshefte Chem. Chem. Mon. 2020, 151, 1365–1373. [Google Scholar] [CrossRef]
- Łukaszewska, A.; Skutnik, E. Przewodnik Florysty; Wydawnictwo SGGW: Warszawa, Poland, 2003. [Google Scholar]
- Han, S.S. Growth regulators delay foliar chlorosis of Easter lily leaves. J. Am. Soc. Hortic. Sci. 1995, 120, 254–258. [Google Scholar] [CrossRef]
- Skutnik, E.; Łukaszewska, A.J.; Serek, M.; Rabiza, J. Effect of growth regulators on postharvest characteristics of Zantedeschia aethiopica. Postharvest Biol. Technol. 2001, 21, 241–246. [Google Scholar] [CrossRef]
- Ulczycka-Walorska, M.P.; Krzymińska, A. The effect of 8-hydroxyquinoline sulphate and gibberellic acid on postharvest Viola odorata L. leaf longevity. Agriculture 2022, 12, 247. [Google Scholar] [CrossRef]
- Ulczycka-Walorska, M.P.; Krzymińska, A. Longevity of Sedum spectabile Boreau cut flowering shoots depending on postharvest treatment. Bulg. J. Agric. Sci. 2015, 21, 851–854. [Google Scholar]
- Soad, M.M.I.; Lobna, S.T.; Rawia, A.E. Extending postharvest life and keeping quality of Gerbera cut-flowers using some chemical preservatives. J. Appl. Sci. Res. 2011, 7, 1233–1239. [Google Scholar]
- Elhindi, K.M. Evaluation of several holding solutions for prolonging vase-life and keeping quality of cut sweet pea flowers (Lathyrus odoratus L.). Saudi J. Biol. Sci. 2012, 19, 195–202. [Google Scholar] [CrossRef]
- Krzymińska, A. Flower longevity of ornamental al liums depending on cutting stage and post-harvest treatment. Ann. Wars. Univ. Life Sci.—SGGW 2009, 30, 11–16. [Google Scholar]
- Asrar, A.W.A. Effects of some preservative solutions on vase life and keeping quality of snapdragon (Antirrhinum majus L.) cut flowers. J. Saudi Soc. Agric. Sci. 2012, 11, 29–35. [Google Scholar] [CrossRef]
- Rubinowska, K.; Michałek, W.; Pogroszewska, E. The effects of chemical substances on senescence of Weigela florida (Bunge) A.DC. ‘Variegata nana’ cut steams. Acta Sci. Pol. Hortorum Cultus 2012, 11, 17–28. [Google Scholar]
- Han, S. Preventing postproduction leaf yellowing in Easter lily. J. Am. Soc. Hortic. Sci. 1997, 122, 869–872. [Google Scholar] [CrossRef]
- Kappers, I.F.; Jordi, W.; Maas, F.M.; Stoopen, G.M.; van der Plas, L.H.W. Gibberellin and phytochrome control senescence in alstroemeria leaves independently. Physiol. Plant. 1998, 103, 91–98. [Google Scholar] [CrossRef]
- Janowska, B.; Schroeter-Zakrzewska, A. Wpływ regulatorów wzrostu na trwałość liści lawendy morskiej (Limonium latifolium/Sm./Kuntze). Nauka Przyr. Technol. 2010, 4, 3. [Google Scholar]
- Janowska, B.; Stanecka, A. Wpływ regulatorów wzrostu na trwałość kwiatów ciętych i liści lilii Calla (Zantedeschia Spreng) po zbiorach. Acta Agrobot. 2011, 64, 91–98. [Google Scholar] [CrossRef][Green Version]
- Nakajima, S.; Ito, H.; Tanaka, R.; Tanaka, A. Chlorophyll b reductase plays an essential role in maturation and storability of Arabidopsis seeds. Plant Physiol. 2012, 160, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Gamian, A.; Cisowski, W. Niskocząsteczkowe związki przeciwutleniające po chodzenia roślinnego. / Low-molecular antioxidant compounds of plant origin. Postępy Hig. Med. Doświadczalnej 2005, 59, 34–41. [Google Scholar]
- Krstulović, A.; Prebeg, T.; Generalić Mekinić, I.; Han Dovedan, I.; Gunjača, J. GA4+7 plus benzyladenine in combination with sucrose improves postharvest leaf and inflorescence quality in Lilium ‘Alma Ata’. Acta Sci. Pol. Hortorum Cultus 2018, 17, 29–40. [Google Scholar] [CrossRef]
- Rabiza-Świder, J.; Skutnik, E.; Chodorska, M. The effect of growth regulators and preservative on senescence of cut oriental lily ‘Helvetia’. Acta Sci. Pol. Hortorum Cultus 2012, 11, 183–194. [Google Scholar]
- Han, S.S. Role of sugar in the vase solution on postharvest flower and leaf quality of Oriental lily ‘Stargazer’. HortScience 2003, 38, 412–416. [Google Scholar] [CrossRef]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of an thocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Murakami, P.F.; Schaberg, P.G.; Shane, J.B. Stem girdling manipulates leaf sugar concentrations and anthocyanin expression in sugar maple trees during autumn. Tree Physiol. 2008, 28, 1467–1473. [Google Scholar] [CrossRef]
- van Doorn, W.G. Water relations of cut flowers. Hortic. Rev. 1997, 18, 1–85. [Google Scholar] [CrossRef]
- Sankat, C.K.; Maharaj, V. Shelf life of green herb ‘shado beni’ (Eryngium foetidum L. ) stored under refrigerated conditions. Postharvest Biol. Technol. 1996, 7, 109–118. [Google Scholar] [CrossRef]
- Bunya-Atichart, K.; Ketsa, S.; van Doorn, W.G. Postharvest physiology of Curcuma alismatifolia flowers. Postharvest Biol. Technol. 2004, 34, 219–226. [Google Scholar] [CrossRef]
- Janowska, B.; Schroeter-Zakrzewska, A. Effect of gibberellic acid benzyladenine and 8-hydroxyquinoline sulphate on postharvest leaf longevity of Arum italicum Mill. Zesz. Probl. Postępów Nauk Rol. 2008, 525, 181–187. [Google Scholar]
- Danaee, E.; Mostofi, Y.; Moradi, P. Effect of GA3 and BA on postharvest quality and vase life of Gerbera (Gerbera jamesonii cv. Good Timing) cut flowers. Hortic. Environ. Biotech. 2011, 52, 140–144. [Google Scholar] [CrossRef]
- Koziara, Z.; Suda, B. Przedłużanie trwałości wybranych gatunków kordylin stosowanych na zieleń ciętą. /Extension of the shelf life of some cordylines species used for cut florist greens. Zesz. Probl. Postępów Nauk Rol. 2008, 525, 203–210. [Google Scholar]
- Łukaszewska, A.J. Niech Żyją Kwiaty w Wazonie/Long Live Flowers in a Vase; Drukrol: Kraków, Poland, 2009. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1–26. [Google Scholar] [CrossRef]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.-S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed]
- Michałek, W.; Pogroszewska, E.; Rubinowska, K.; Sadkowska, P. Starzenie się liści piwonii chińskiej (Paeonia lactiflora) w zależności od sposobu pozbiorczego traktowania pędów. Acta Agrobot. 2006, 59, 421–430. [Google Scholar] [CrossRef][Green Version]
- Gaur, K.; Jhandji, S.; Dhatt, K.K. Effects of benzyladenine (BA) and gibberellic acid (GA3) on physiological and biochemical attributes of Gladiolus (Gladiolus grandifloras L.) spikes. Bangladesh J. Bot. 2022, 51, 297–304. [Google Scholar] [CrossRef]
- Hossain, Z.; Mandal, A.K.A.; Datta, S.K.; Biswas, A.K. Decline in ascorbate peroxidase activity–A prerequisite factor for tepal senescence in gladiolus. J. Plant Physiol. 2006, 163, 186–194. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, G.C.; Dixit, K. Role of superoxide dismutases during petal senescence in rose (Rosa hybrida L.). J. Hortic. Sci. Biotechnol. 2007, 82, 673–678. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, J.; Kumar, P. Effects of plant growth regulators and sucrose on post-harvest physiology, membrane stability and vase life of cut spikes of gladiolus. Plant Growth Regul. 2008, 55, 221–229. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubinowska, K.; Szot, P.; Pogroszewska, E.; Podolak, I.; Wróbel-Biedrawa, D. Effect of Plant Hormones and Preservative Solutions on Post-Harvest Quality and Physiological Senescence Parameters of Cut Leaves of Hosta Tratt. ‘Krossa Regal’ and Polygonatum multiflorum (L.) All. ‘Variegatum’. Agriculture 2025, 15, 842. https://doi.org/10.3390/agriculture15080842
Rubinowska K, Szot P, Pogroszewska E, Podolak I, Wróbel-Biedrawa D. Effect of Plant Hormones and Preservative Solutions on Post-Harvest Quality and Physiological Senescence Parameters of Cut Leaves of Hosta Tratt. ‘Krossa Regal’ and Polygonatum multiflorum (L.) All. ‘Variegatum’. Agriculture. 2025; 15(8):842. https://doi.org/10.3390/agriculture15080842
Chicago/Turabian StyleRubinowska, Katarzyna, Paweł Szot, Elżbieta Pogroszewska, Irma Podolak, and Dagmara Wróbel-Biedrawa. 2025. "Effect of Plant Hormones and Preservative Solutions on Post-Harvest Quality and Physiological Senescence Parameters of Cut Leaves of Hosta Tratt. ‘Krossa Regal’ and Polygonatum multiflorum (L.) All. ‘Variegatum’" Agriculture 15, no. 8: 842. https://doi.org/10.3390/agriculture15080842
APA StyleRubinowska, K., Szot, P., Pogroszewska, E., Podolak, I., & Wróbel-Biedrawa, D. (2025). Effect of Plant Hormones and Preservative Solutions on Post-Harvest Quality and Physiological Senescence Parameters of Cut Leaves of Hosta Tratt. ‘Krossa Regal’ and Polygonatum multiflorum (L.) All. ‘Variegatum’. Agriculture, 15(8), 842. https://doi.org/10.3390/agriculture15080842






