Greenhouse Gas Emissions from Flood-Irrigated Rice as Affected by Phosphorus Fertilizer Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection, Processing, and Analyses
2.2. Treatments and Experimental Design
2.3. P Fertilizer Sources and Characterization
2.4. Soil Tub Preparation
2.5. Rice Establishment and Fertilization
2.6. Water Management
2.7. Gas Sample Collection, Analyses, and Calculations
2.8. Statistical Analyses
3. Results and Discussion
3.1. Initial Soil Properties
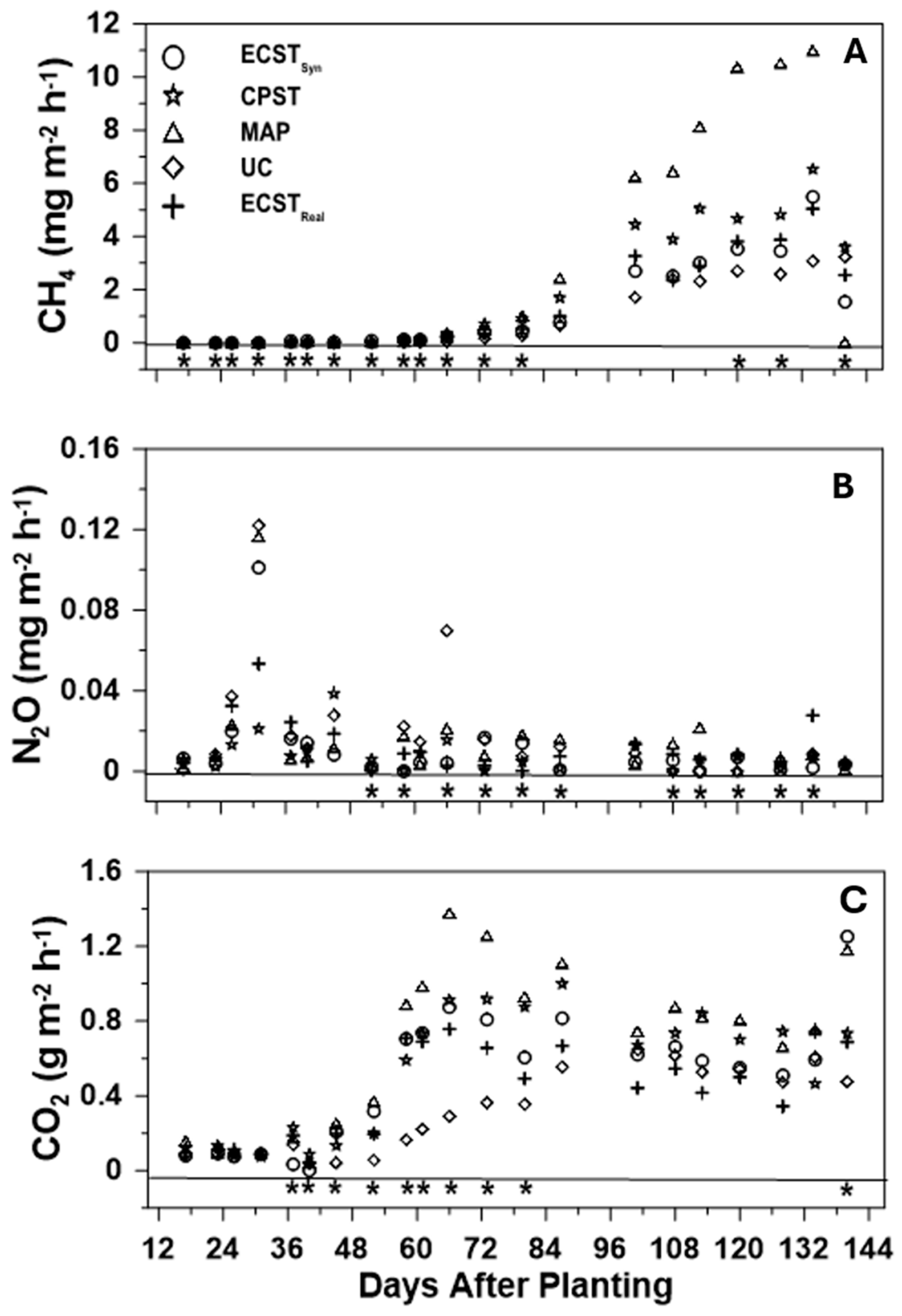
3.2. Greenhouse Gas Fluxes
3.3. Season-Long Emissions
3.4. Net Greenhouse Gas Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC) History of the IPCC. Available online: https://www.ipcc.ch/about/history/ (accessed on 27 March 2025).
- Cordell, D.; Drangert, J.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA) Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2020. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2020 (accessed on 27 March 2025).
- Brady, N.C.; Weil, R.R. The Nature and Properties Soils, 15th ed.; Pearson: London, UK, 2016. [Google Scholar]
- Motschenbacher, J.M.; Brye, K.R.; Anders, M.M.; Gbur, E.E.; Slaton, N.A.; Evans-White, M.A. Daily soil surface CO2 flux during non-flooded periods in flood-irrigated rice rotations. Agron. Sustain. Dev. 2015, 35, 771–782. [Google Scholar] [CrossRef]
- Rector, C.; Brye, K.R.; Humphreys, J.J.; Norman, R.J.; Gbur, E.E.; Hardke, J.T.; Willett, C.; Evans-White, M.A. N2O emissions and global warming potential as affected by water management and rice cultivar on an Alfisol in Arkansas, USA. Geoderma Reg. 2018, 14, e00170. [Google Scholar] [CrossRef]
- Rector, C.; Brye, K.R.; Humphreys, J.; Norman, R.J.; Slaton, N.A.; Gbur, E.E.; Willett, C.; Evans-White, M.E. Tillage and coated-urea effects on nitrous oxide emissions from direct-seeded, delayed-flood rice production in Arkansas. J. Rice Res. Dev. 2018, 1, 25–37. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Mu, Y.; Song, S.; Zhang, Y.; Tao, Y.; Lixiao, N. Alternative wetting and drying maintains rice yield and reduces global warming potential: A global meta-analysis. Field Crop Res. 2024, 318, 109603. [Google Scholar] [CrossRef]
- Liu, X.J.; Mosier, A.R.; Halvorson, A.D.; Reule, C.A.; Zhang, F.S. Dinitrogen and N2O emissions in arable soils: Effect of tillage, N source and soil moisture. Soil Biol. Biochem. 2007, 39, 2362–2370. [Google Scholar] [CrossRef]
- Dubey, S.K. Microbial ecology of methane emission in rice agroecosystem: A review. Appl. Ecol. Environ. Res. 2005, 3, 1–27. [Google Scholar] [CrossRef]
- Della Lunga, D.; Brye, K.R.; Slayden, J.M.; Henry, C.G.; Wood, L.S. Soil moisture, temperature, and oxidation-reduction potential fluctuations across a furrow-irrigated rice field on a silt-loam soil. J. Rice Res. Dev. 2020, 13, 103–114. [Google Scholar] [CrossRef]
- Della Lunga, D.; Brye, K.R.; Slayden, J.M.; Henry, C.G.; Wood, L.S. Relationships among soil factors and greenhouse gas emissions from furrow-irrigated Rice in the mid-southern, USA. Geoderma Reg. 2021, 24, e00365. [Google Scholar] [CrossRef]
- Penning, H.; Conrad, R. Quantification of carbon flow from stable isotope fractionation in rice field soils with different organic matter content. Org. Geochem. 2007, 38, 2058–2069. [Google Scholar] [CrossRef]
- Statista World Rice Acreage 2020. Available online: https://www.statista.com/statistics/271969/world-rice-acreage-since-2008/#statisticContainer (accessed on 27 March 2025).
- Hardke, J. Trends in Arkansas rice production, 2021. In BR Wells Rice Research Studies 2021; Hardke, J., Sha, X., Bateman, N., Eds.; University of Arkansas Systems Division of Agriculture Cooperative Extension Service: Little Rock, AR, USA, 2022; pp. 11–18. [Google Scholar]
- Henry, C.G.; Hirsch, S.L.; Anders, M.M.; Vories, E.D.; Reba, M.L.; Watkins, K.B.; Hardke, J.T. Annual irrigation water use for Arkansas rice production. J. Irrig. Drain. Eng. 2016, 142, 05016006. [Google Scholar] [CrossRef]
- University of Arkansas System Division of Agriculture, Cooperative Extension Service (UA-DA-CES) Rice Production Handbook. Available online: https://www.uaex.uada.edu/publications/pdf/mp192/mp192.pdf (accessed on 27 March 2025).
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Smil, V. Phosphorus in the environment: Natural flows and human interferences. Annu. Rev. Energy Environ. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- United States Geological Survey (USGS) 2022 Mineral Commodity Summaries. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-phosphate.pdf (accessed on 27 March 2025).
- Geissler, B.; Hermann, L.; Mew, M.C.; Steiner, G. Striving toward a circular economy for phosphorus: The role of phosphate rock mining. Minerals 2018, 8, 395. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A Review. Crit. Rev. Environ. Sci. Tech. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; Dijk, V.; Kimo, C. Phosphorus management in Europe in a changing world. Ambio 2015, 44, S180–S192. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Teymouri, A.; Herring, A.M.; Greenlee, L.F. Electrochemical removal and recovery of phosphorus as struvite in an acidic environment using pure magnesium vs. the AZ31 magnesium alloy as the anode. Chem. Eng. J. 2020, 380, 122480. [Google Scholar] [CrossRef]
- Muhmood, A.; Lu, J.; Dong, R.; Wu, S. Formation of struvite from agricultural wastewaters and its reuse on farmlands: Status and hindrances to closing the nutrient loop. J. Environ. Manag. 2019, 230, 1–13. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, P.; Zhang, Z.; Liu, J.; Xiao, J.; Gao, F. Simultaneous removal of ammonia nitrogen and recovery of phosphate from swine wastewater by struvite electrochemical precipitation and recycling technology. J. Clean. Prod. 2016, 127, 302–310. [Google Scholar] [CrossRef]
- Chirmuley, D.G. Struvite precipitation in WWTPs: Causes and solutions. Water (J. Aust. Water Assoc.) 1994, 21, 21–23. [Google Scholar]
- Nagy, J.; Mikola, A.; Pradhn, S.K.; Zseni, A. The utilization of struvite produced from human urine in agriculture as a natural fertilizer: A review. Period. Polytech. Chem. Eng. 2019, 63, 478–484. [Google Scholar] [CrossRef]
- Nongqwenga, N.; Muchaonyerwa, P.; Hughes, J.; Odindo, A.; Bame, I. Possible use of struvite as an alternative phosphate fertilizer. J. Soil Sci. Plant Nutr. 2017, 17, 581–593. [Google Scholar] [CrossRef]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorous fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- Ackerman, J.N.; Zvomuya, F.; Cicek, N.; Flaten, D. Evaluation of manure-derived struvite as a phosphorus source for canola. Can. J. Plant Sci. 2013, 93, 419–424. [Google Scholar] [CrossRef]
- Barak, P.; Stafford, A. Struvite: A recovered and recycled phosphorus fertilizer. In Proceedings of the 2006 Wisconsin Fertilizer, Aglime and Pest Management Conference, Madison, WI, USA, 17–19 February 2006. [Google Scholar]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A slow-release fertilizer for sustainable phosphorus management? Plant Soil. 2016, 401, 109–123. [Google Scholar] [CrossRef]
- Ylagan, S.; Brye, K.R.; Greenlee, L. Corn and soybean response to wastewater-recovered and other common phosphorus fertilizers. Agrosyst. Geosci. Environ. 2020, 3, e20086. [Google Scholar] [CrossRef]
- Brye, K.R.; Omidire, N.S.; English, L.; Parajuli, R.; Kékedy-Nagy, L.; Sultana, R.; Popp, J.; Thoma, G.; Roberts, T.L.; Greenlee, L.F. Assessment of struvite as an alternative source of fertilizer-phosphorus for flood-irrigated rice. Sustainability 2022, 14, 9621. [Google Scholar] [CrossRef]
- Omidire, N.S.; Brye, K.R.; Roberts, T.L.; Kekedy-Nagy, L.; Greenlee, L.; Gbur, E.E.; Mozzoni, L.A. Evaluation of electrochemically precipitated struvite as a fertilizer-phosphorus source in flood-irrigated rice. Agron. J. 2021, 114, 739–755. [Google Scholar] [CrossRef]
- Omidire, N.S.; Brye, K.R.; English, L.; Popp, J.; Kekedy-Nagy, L.; Greenlee, L.; Roberts, T.L.; Gbur, E.E. Wastewater-recovered struvite evaluation as a fertilizer-phosphorus source for corn in eastern Arkansas. Agron. J. 2022, 114, 2994–3012. [Google Scholar] [CrossRef]
- Omidire, N.S.; Brye, K.R. Wastewater-recycled struvite as a phosphorus source in a wheat-soybean double-crop production system in eastern Arkansas. Agrosyst. Geosci. Environ. 2022, 5, e20271. [Google Scholar] [CrossRef]
- Omidire, N.S.; Brye, K.R.; English, L.; Kekedy-Nagy, L.; Greenlee, L.; Popp, J.; Roberts, T.L. Soybean growth and production as affected by struvite as a phosphorus source in eastern Arkansas. Crop Sci. 2023, 63, 320–335. [Google Scholar] [CrossRef]
- Chien, S.H.; Prochnow, L.I.; Tu, S.; Snyder, C.S. Agronomic and environmental aspects of phosphate fertilizers varying in source and solubility: An update review. Nutr. Cycl. Agroecosyst. 2011, 89, 229–255. [Google Scholar] [CrossRef]
- United States Division of Agriculture (USDA), Natural Resources Conservation Services (NRCS) Calhoun Soil Series. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/C/CALHOUN.html (accessed on 27 March 2025).
- Gee, G.W.; Or, D. Particle size analysis. In Methods of Soil Analysis Part 4, Physical Methods Book Series No. 5; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Tucker, M.R. Determination of phosphorus by Mehlich 3 extraction. In Soil and Media Diagnostic Procedures for the Southern Region of the United States; No. 374; Donohue, S.J., Ed.; Virginia Agricultural Experiment Station: Blacksburg, VA, USA, 1992; pp. 6–8. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. In Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 3: Chemical Analysis, 3rd ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loepper, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madision, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, Revision 2. 1996. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa-3050b.pdf (accessed on 27 March 2025).
- Slayden, J.M.; Brye, K.R.; Lunga, D.D. Nitrogen fertilizer application timing effects on nitrous oxide emissions from simulated furrow-irrigated rice on a silt-loam soil in the greenhouse. J. Rice Res. Dev. 2022, 5, 366–377. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Tuong, T.P. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001, 49, 11–30. [Google Scholar] [CrossRef]
- Craswell, E.T.; Vlek, P.L.G. Greenhouse evaluation of nitrogen fertilizers for rice. Soil Sci. Soc. Am. J. 1979, 43, 1184–1188. [Google Scholar] [CrossRef]
- Smartt, A.D.; Brye, K.R.; Rogers, C.W.; Norman, R.J.; Gbur, E.E.; Hardke, J.T.; Roberts, T.L. Previous crop and cultivar effects on methane emissions from drill-seeded, delayed-flood rice grown on a clay soil. Appl. Environ. Soil Sci. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA) Soil-Plant-Atmosphere-Water Field, and Pond Hydrology. Available online: https://www.nrcs.usda.gov/resources/tech-tools/spaw-version-602 (accessed on 27 March 2025).
- Della Lunga, D.; Brye, K.R.; Roberts, T.L.; Brye, J.; Evans-White, M.; Henry, C.G.; Lessner, D. Water regime and fertilizer-phosphorus source effects on greenhouse gas emissions from rice. Agrosyst. Geosci. Environ. 2024, 7, e20482. [Google Scholar] [CrossRef]
- Sander, B.O.; Wassmann, R. Common practices for manual greenhouse gas sampling in rice production: A literature study on sampling modalities of the closed chamber method. Greenh. Gas Meas. Manag. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- Humphreys, J.J.; Brye, K.R.; Rector, C.; Gbur, E.E.; Hardke, J.T. Water management and cultivar effects on methane emissions from direct-seeded, delayed-flood rice production in Arkansas. J. Rice Res. Dev. 2018, 1, 14–24. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Global carbon and other biogeochemical cycles and feedbacks. In Climate Change 2021: The Physical Sci. Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Canadell, J.G., Ed.; Cambridge University Press: Cambridge, UK, 2021; pp. 673–816. [Google Scholar]
- Brye, K.R.; Rogers, C.W.; Smartt, A.D.; Norman, R.J. Soil texture effects on methane emissions from direct-seeded, delayed-flood rice production in Arkansas. Soil Sci. 2013, 178, 519–529. [Google Scholar] [CrossRef]
- Kongchum, M.; Harrell, D.; Barron, M.A.; Adotey, N.; Li, J.F. Methane and nitrous oxide emission from Louisiana rice fields under three management practices. J. Rice Res. Dev. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Della Lunga, D.; Brye, K.R.; Roberts, T.L.; Henry, C.G.; Evans-White, M.; Lessner, D. Struvite effects on rice growth and productivity under flood-irrigation in the greenhouse. Agric. Sci. 2023, 14, 864–877. [Google Scholar] [CrossRef]
- Della Lunga, D.; Brye, K.R.; Slayden, J.M.; Henry, C.G. Evaluation of site position and tillage effects on global warming potential from furrow-irrigated rice in the mid-southern, USA. Geoderma Reg. 2023, 32, e00625. [Google Scholar] [CrossRef]
- Karki, S.; Adviento-Borbe, M.A.A.; Runkle, B.R.K.; Moreno-García, B.; Anders, M.; Reba, M.L. Multiyear methane and nitrous oxide emissions in different irrigation management under long-term continuous rice rotation in Arkansas. J. Environ. Qual. 2022, 3, 558–572. [Google Scholar] [CrossRef]
- Della Lunga, D. Field and Greenhouse Environmental Evaluation of Struvite in Rice Production Systems. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2023. [Google Scholar]
- Arel, C. Struvite Effects on Greenhouse Gas Emissions from Flood- and Furrow-Irrigated Rice in the Greenhouse. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2024. [Google Scholar]
- Adhya, T.K.; Pattnaik, P.; Satpathy, S.N.; Kumaraswamy, S.; Sethunathan, N. Influence of phosphorous application on methane emission and production in flooded paddy soils. Soil Biol. Biochem. 1998, 30, 177–181. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Q.; Zhao, S.; Chen, D.; Gao, N.; Huana, M.; Ye, X. Citric acid secretion from rice roots contributes to reduction and immobilization of Cr(VI) by driving microbial sulfur and iron cycle in paddy soil. Sci. Total Environ. 2023, 854, 158832. [Google Scholar] [CrossRef]
- Anderson, R.; Brye, K.; Kékedy-Nagy, L.; Greenlee, L.; Gbur, E.; Roberts, T.L. Electrochemically precipitated struvite effects on extractable nutrients compared with other fertilizer phosphorus sources. Agrosyst. Geosci. Environ. 2013, 4, e20183. [Google Scholar] [CrossRef]
- Gao, D.; Sheng, R.; Whiteley, A.; Moreira-Grez, B.; Qin, H.; Zhang, W.; Zhan, Y.; Wei, W. Effect of phosphorus amendments on rice rhizospheric methanogens and methanotrophs in a phosphorus deficient soil. Geoderma 2020, 368, 114312. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Zong, L.; Zheng, X.; Sass, R.L.; Fisher, F.M. Quantitative dependence of methane emission on soil properties. Nutr. Cycl. Agroecosyst. 2002, 64, 157–167. [Google Scholar] [CrossRef]
- Humphreys, J.; Brye, K.R.; Rector, C.; Gbur, E.E. Methane emissions from rice across a soil organic matter gradient in Alfisols of Arkansas, USA. Geoderma Reg. 2019, 16, e00200. [Google Scholar] [CrossRef]
- Rogers, C.W.; Brye, K.R.; Smartt, A.D.; Norman, R.J.; Gbur, E.E.; Evans-White, M.E. Cultivar and previous crop effects on methane emissions from drill-seeded, delayed-flood rice production on a silt-loam soil. Soil Sci. 2013, 179, 28–36. [Google Scholar] [CrossRef]
- Paustian, K.; Cole, C.V.; Sauerbeck, D.; Sampson, N. CO2 mitigation by agriculture: An overview. Clim. Chang. 1998, 40, 135–162. [Google Scholar] [CrossRef]
| Soil Property | Mean (±SE) |
|---|---|
| Soil Texture (g g−1) | |
| Sand | 0.14 (<0.01) |
| Silt | 0.72 (0.01) |
| Clay | 0.14 (0.01) |
| Bulk density (g cm−3) | 1.11 (<0.01) |
| pH | 7.3 (0.05) |
| Electrical conductivity (dS m−1) | 0.173 (<0.01) |
| Extractable soil nutrients (mg kg−1) | |
| Phosphorus | 6.4 (0.8) |
| Potassium | 72.7 (1.0) |
| Calcium | 1816 (24.3) |
| Magnesium | 257 (3.2) |
| SO4-S | 4.0 (0.1) |
| Sodium | 50.1 (0.3) |
| Iron | 112 (1.4) |
| Manganese | 251 (3.0) |
| Zinc | 10.5 (0.2) |
| Copper | 2.0 (0.3) |
| Boron | 0.4 (<0.01) |
| Soil organic matter (g kg−1) | 17.4 (0.07) |
| Total carbon (g kg−1) | 5.3 (0.2) |
| Total nitrogen (g kg−1) | 0.6 (0.01) |
| Carbon–nitrogen ratio | 9.4 (0.4) |
| P Fertilizer Source | Measured Fertilizer Grade a | pH | Nutrient Concentration (±SE) | P Fertilizer Source Water Solubility c | ||
|---|---|---|---|---|---|---|
| N | P | Mg | ||||
| ______________ % ______________ | ||||||
| ECSTReal | 3–35–0 | 7.2 (<0.1) | 3.3 (0.1) | 15.5 (0.2) | 13.6 (0.3) | 2–3.8% |
| ECSTSyn | 5–37–0 | - b | 5.1 (0.2) | 16.1 (0.3) | 12.7 (0.3) | 2–3.8% |
| MAP | 11–52–0 | 4.4 (0.02) | 10.7 (0.1) | 20.9 (0.2) | 1.5 (<0.1) | 85–90% |
| CPST | 6–27–0 | 8.8 (0.13) | 5.7 (0.2) | 11.7 (0.2) | 8.3 (0.2) | 4% |
| Descriptive Statistic | Ambient Air Temperature (°C) | Chamber Air Temperature (°C) | Greenhouse Pressure (cm Hg) | Relative Humidity (%) |
|---|---|---|---|---|
| Mean | 28.9 | 28.5 | 75.4 | 62.5 |
| Maximum | 35.0 | 37.4 | 77.0 | 82.0 |
| Minimum | 20.0 | 18.9 | 59.4 | 40.0 |
| Source of Variation | CH4 | N2O | CO2 |
|---|---|---|---|
| _______________________ P_______________________ | |||
| P fertilizer source | <0.01 | 0.03 | <0.01 |
| DAP | <0.01 | <0.01 | <0.01 |
| P fertilizer source x DAP | <0.01 | <0.01 | <0.01 |
| Greenhouse Gas Property | P | P Fertilizer Source a | Overall Mean | ||||
|---|---|---|---|---|---|---|---|
| ECSTReal | ECSTSyn | CPST | MAP | UC | |||
| Season-long emissions | |||||||
| CH4 (kg ha−1) | 0.01 | 43.1 bc b | 40.3 bc | 60.7 ab | 98.7 a | 29.9 c | - |
| N2O (kg ha−1) | 0.36 | 0.34 a | 0.31 a | 0.24 a | 0.44 a | 0.53 a | 0.37 |
| CO2 (Mg ha−1) | <0.01 | 13.0 bc | 15.4 b | 17.1 ab | 21.0 a | 10.4 c | - |
| Total Net GHG Emissions (kg CO2-equivalents ha−1 season−1) | |||||||
| <0.01 | 14296 bc | 16657 b | 18865 ab | 23841 a | 11426 c | - | |
| CH4-N2O Net GHG Emissions (kg CO2-equivalents ha−1 season−1) | |||||||
| 0.01 | 1297 bc | 1211 bc | 1763 ab | 2881 a | 978 c | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arel, C.M.; Brye, K.R.; Della Lunga, D.; Roberts, T.L.; Adams, R. Greenhouse Gas Emissions from Flood-Irrigated Rice as Affected by Phosphorus Fertilizer Source. Agriculture 2025, 15, 815. https://doi.org/10.3390/agriculture15080815
Arel CM, Brye KR, Della Lunga D, Roberts TL, Adams R. Greenhouse Gas Emissions from Flood-Irrigated Rice as Affected by Phosphorus Fertilizer Source. Agriculture. 2025; 15(8):815. https://doi.org/10.3390/agriculture15080815
Chicago/Turabian StyleArel, Chandler M., Kristofor R. Brye, Diego Della Lunga, Trenton L. Roberts, and Richard Adams. 2025. "Greenhouse Gas Emissions from Flood-Irrigated Rice as Affected by Phosphorus Fertilizer Source" Agriculture 15, no. 8: 815. https://doi.org/10.3390/agriculture15080815
APA StyleArel, C. M., Brye, K. R., Della Lunga, D., Roberts, T. L., & Adams, R. (2025). Greenhouse Gas Emissions from Flood-Irrigated Rice as Affected by Phosphorus Fertilizer Source. Agriculture, 15(8), 815. https://doi.org/10.3390/agriculture15080815






