Ripening Dynamics and Optimal Harvest Timing of ‘Fantastico’ and ‘Femminello’ Bergamot Fruit
Abstract
1. Introduction
2. Materials and Methods
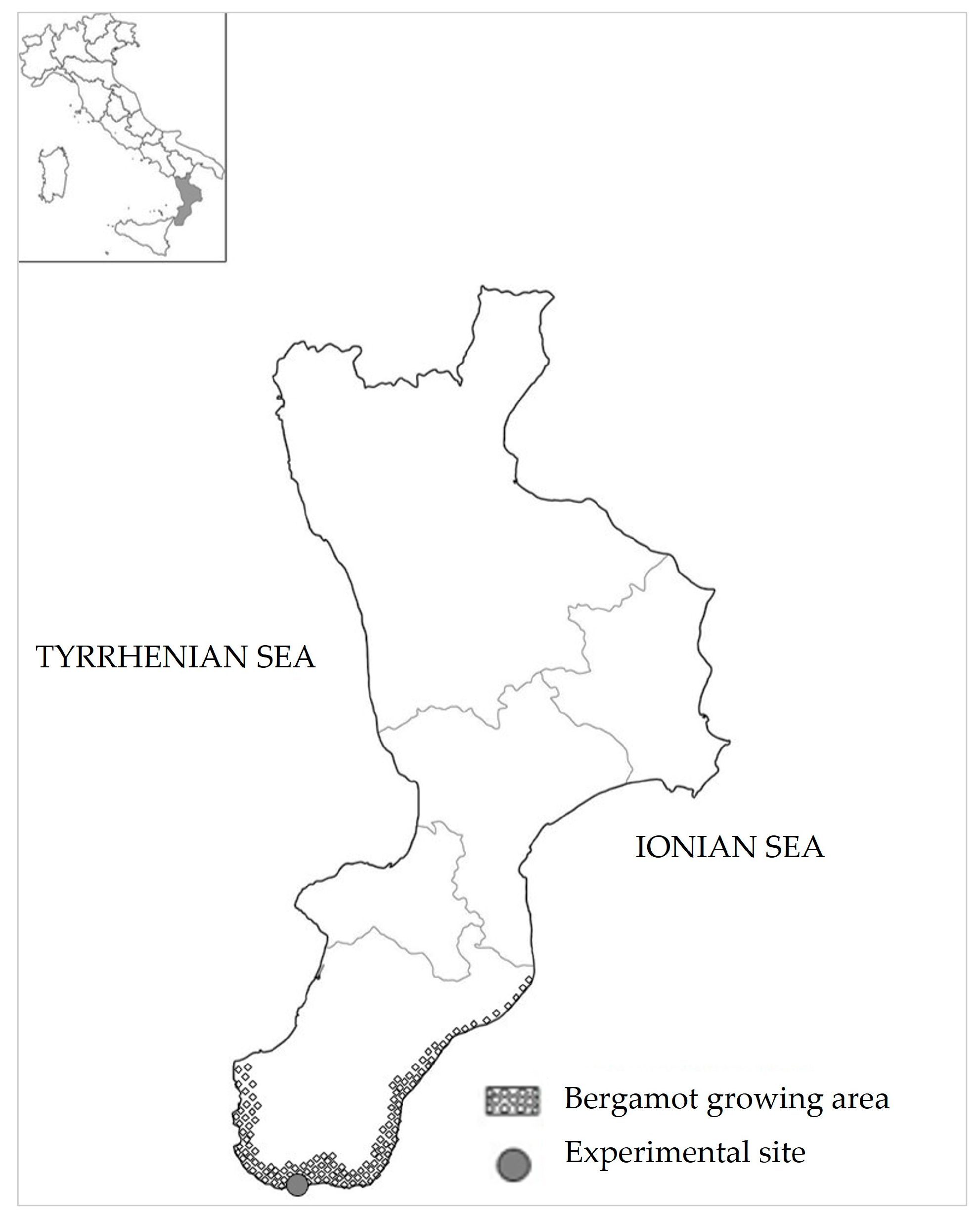
2.1. Plant Material and Site Location
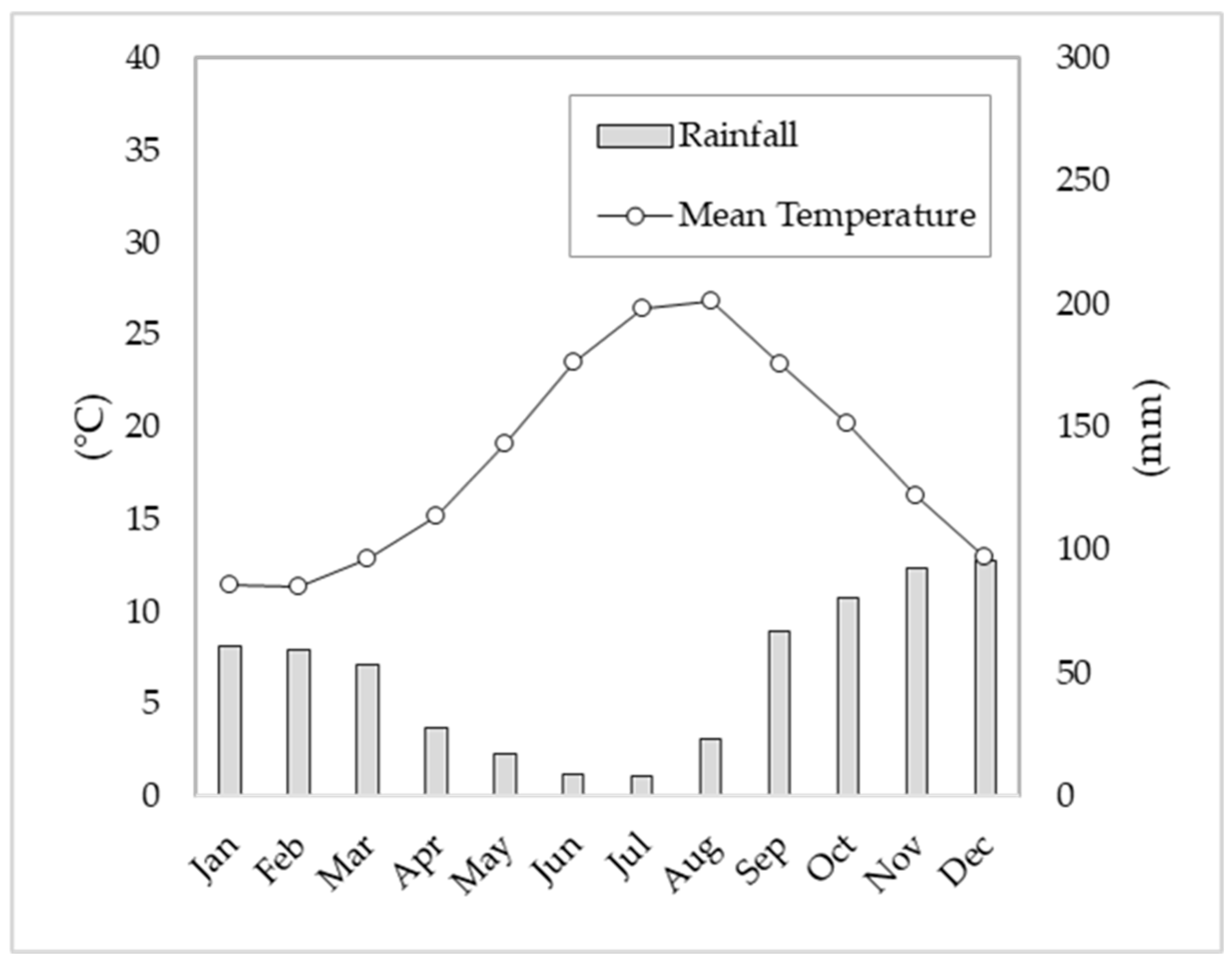
2.2. Climate Parameters
2.3. Phenological Observations
2.4. Fruit Drop Monitoring
2.5. Yield Determination
2.6. Fruit Sampling for Physical and Qualitative Analyses
2.7. Carpometric and Physical Parameters Measurements
- V is the volume of the individual fruit (cm3);
- V1 is the initial volume of water (cm3);
- V2 is the volume registered after the immersion of the bergamot fruit in the graduated beaker (cm3).
2.8. Juice Quality Parameters Dermination
2.9. Statistical Analysis
3. Results and Discussion
3.1. Climatic Conditions and Main Phenological Phases
3.2. Fruit Drop
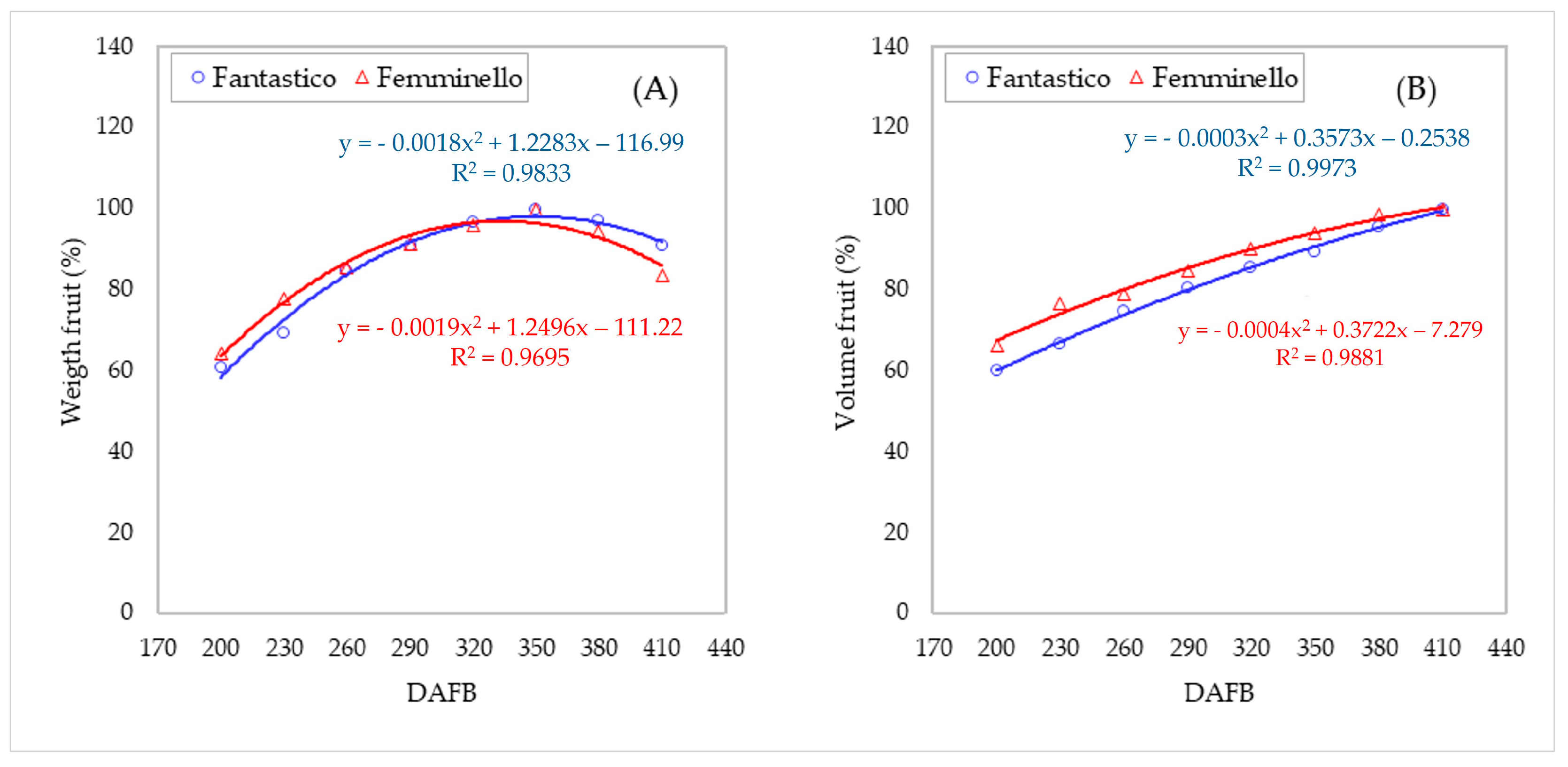
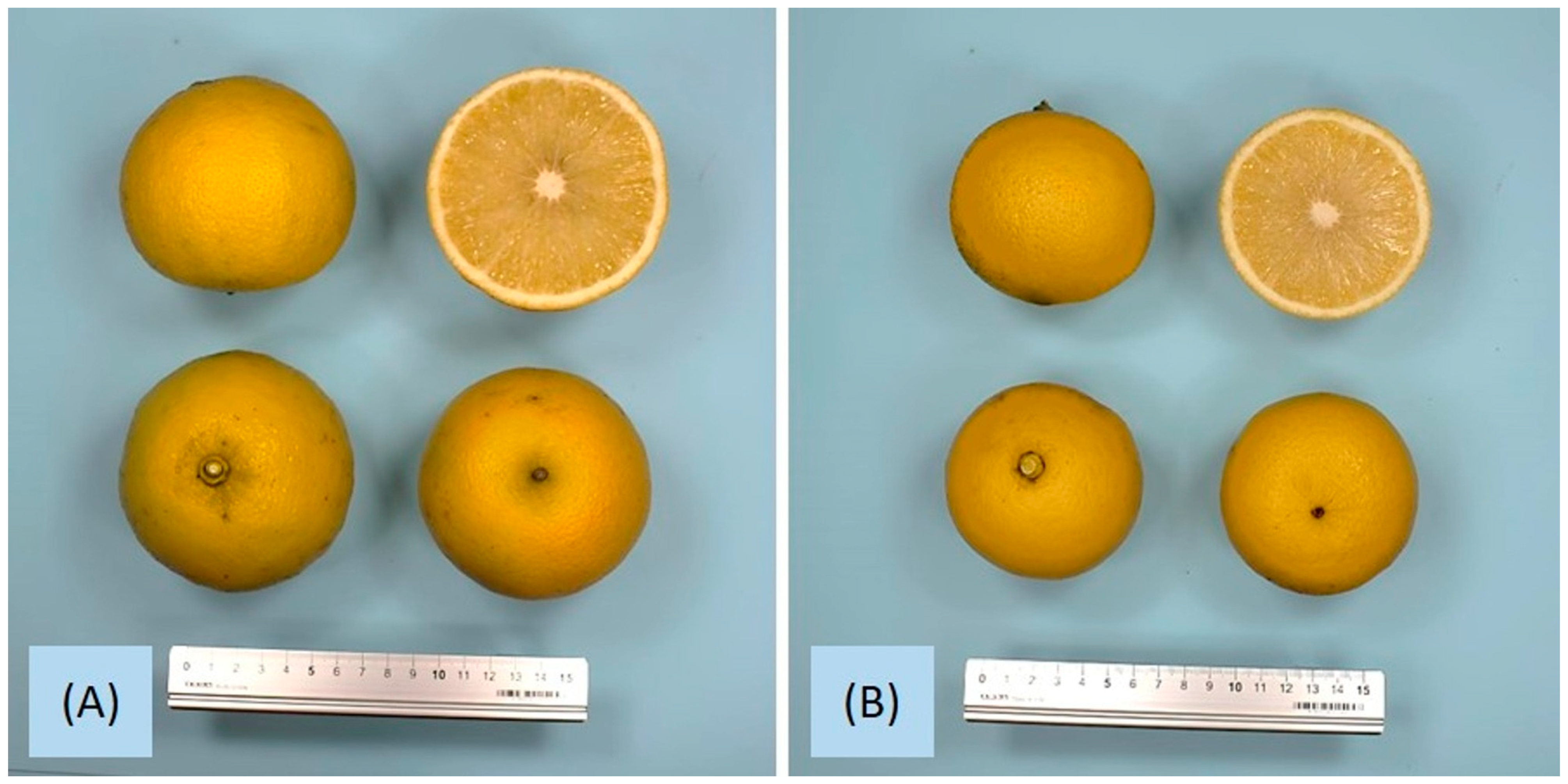
3.3. Carpometric and Physical Parameters of the Fruits
3.4. Color and Thickness of the Peel
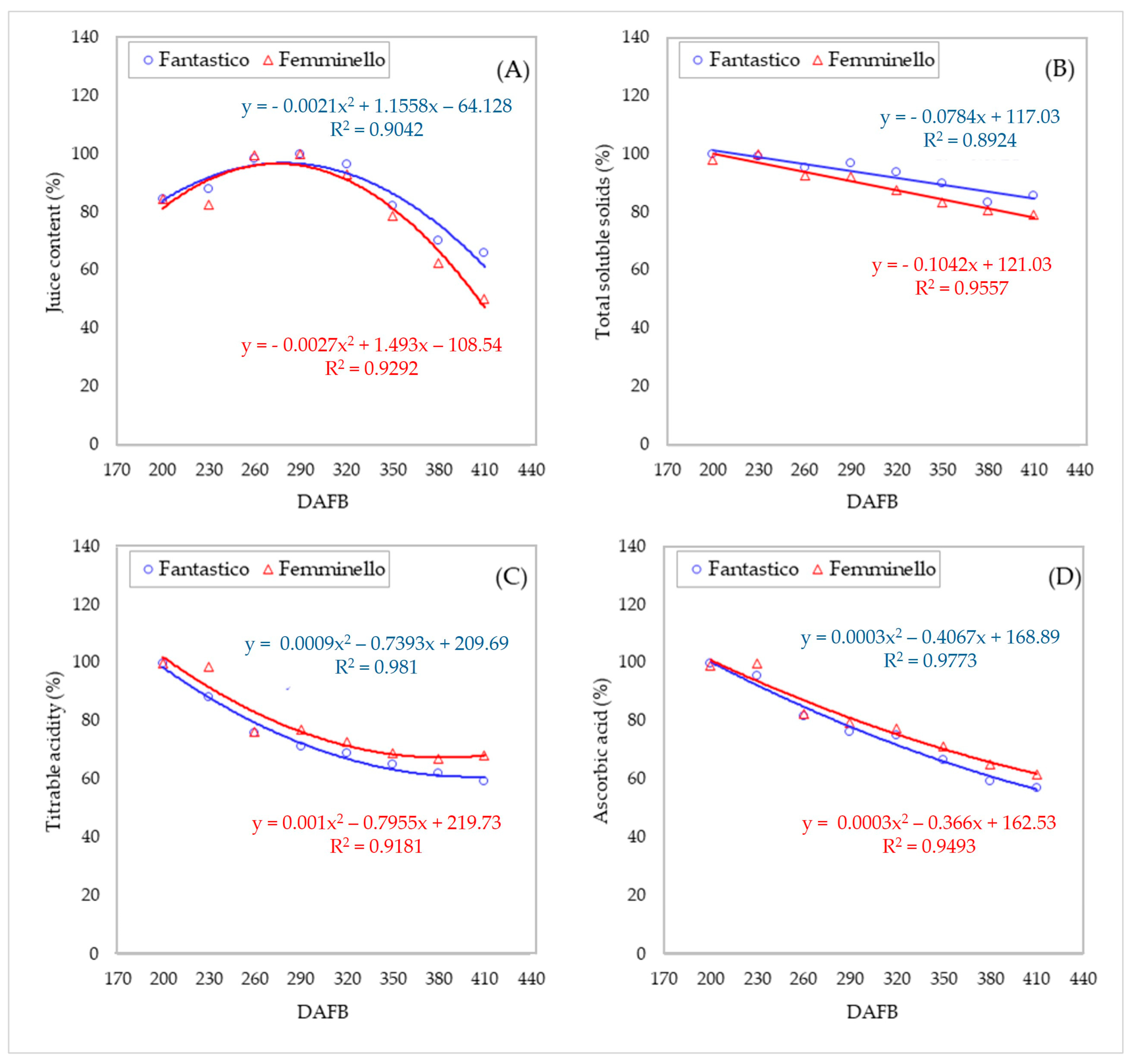
3.5. Qualitative Characteristics of the Fruits
3.6. Definition of Optimal Harvest Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 2000, 100, 1155–1166. [Google Scholar]
- Li, X.; Xie, R.; Lu, Z.; Zhou, Z. The origin of cultivated citrus as inferred from internal transcribed spacer and chloroplast DNA sequence and amplified fragment length polymorphism fingerprints. J. Am. Soc. Hort. Sci. 2010, 135, 341–350. [Google Scholar]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of edible coating enriched with natural antioxidant extract and bergamot essential oil on the shelf life of strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef]
- Franceschi, E.; Grings, M.B.; Frizzo, C.D.; Vladimir Oliveira, J.; Dariva, C. Phase behavior of lemon and bergamot peel oils in supercritical CO2. Fluid Phase Equilibria 2004, 226, 1–8. [Google Scholar] [CrossRef]
- Figoli, A.; Donato, L.; Carnevale, R.; Tundis, R.; Statti, G.A.; Menichini, F.; Drioli, E. Bergamot essential oil extraction by pervaporation. Desalination 2006, 193, 160–165. [Google Scholar]
- Rombolà, L.; Tridico, L.; Scuteri, D.; Sakurada, T.; Sakurada, S.; Mizoguchi, H.; Avato, P.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Bergamot essential oil attenuates anxiety-like behaviour in rats. Molecules 2017, 22, 614. [Google Scholar] [CrossRef]
- Valussi, M.; Donelli, D.; Firenzuoli, F.; Antonelli, M. Bergamot oil: Botany, production, pharmacology. Encyclopedia 2021, 1, 152–176. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Visalli, G.; Smeriglio, A.; Cirmi, S.; Lombardo, G.E.; Campiglia, P.; Di Pietro, A.; Navarra, M. Flavonoid fraction of orange and bergamot juices protect human lung epithelial cells from hydrogen peroxide-induced oxidative stress. Evid.-Based Complement. Altern. Med. 2015, 2015, 957031. [Google Scholar]
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia Risso et Poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016, 30, 1404–1411. [Google Scholar]
- Da Pozzo, E.; De Leo, M.; Faraone, I.; Milella, L.; Cavallini, C.; Piragine, E.; Testai, L.; Calderone, V.; Pistelli, L.; Braca, A.; et al. Antioxidant and antisenescence effects of bergamot juice. Oxidative Med. Cell. Longev. 2018, 2018, 9395804. [Google Scholar]
- Nauman, M.C.; Johnson, J.J. Clinical application of bergamot (Citrus bergamia) for reducing high cholesterol and cardiovascular disease markers. Integr. Food Nutr. Metab. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Cirmi, S.; Maugeri, A.; Russo, C.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Neuroprotective effect of bergamot juice in 6-OHDA-induced SH-SY5Y cell death, an in vitro model of Parkinson’s disease. Pharmaceutics 2020, 12, 326. [Google Scholar] [CrossRef]
- Adorisio, S.; Muscari, I.; Fierabracci, A.; Thi Thuy, T.; Marchetti, M.C.; Ayroldi, E.; Delfino, D.V. Biological effects of bergamot and its potential therapeutic use as an anti-inflammatory, antioxidant, and anticancer agent. Pharm. Biol. 2023, 61, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Mafrica, R.; De Bruno, A.; Lanza, D.; Poiana, M. Effects of altering carbohydrate supply to fruit during development on the carpometric and qualitative characteristics of “Feminello Zagara Bianca” lemon. Horticulturae 2023, 9, 71. [Google Scholar] [CrossRef]
- Gattuso, A.; Mafrica, R.; Cannavò, S.; Mafrica, D.; De Bruno, A.; Poiana, M. Quality evaluation of bergamot juice produced in different areas of Calabria region. Foods 2024, 13, 2080. [Google Scholar] [CrossRef]
- Manera, F.J.; Brotons, J.M.; Conesa, A.; Porras, I. Influence of temperature on the beginning of de-greening in lemon peel. Sci. Hortic. 2012, 145, 34–38. [Google Scholar] [CrossRef]
- Porras, I.; Brotons, J.M.; Conesa, A.; Manera, F.J. Influence of temperature and net radiation on the natural degreening process of grapefruit (Citrus paradisi Macf.) cultivars Rio Red and Star Ruby. Sci. Hortic. 2014, 173, 45–53. [Google Scholar] [CrossRef]
- Liu, X.; Hu, C.; Liu, X.; Riaz, M.; Liu, Y.; Dong, Z.; Tan, Q.; Sun, X.; Wu, S.; Tan, Z. Effect of magnesium application on the fruit coloration and sugar accumulation of navel orange (Citrus sinensis Osb.). Sci. Hortic. 2022, 304, 111282. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- Tadeo, F.R.; Cercós, M.; Colmenero–Flores, J.M.; Iglesias, D.J.; Naranjo, M.A.; Rios, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; et al. Molecular physiology of development and quality of Citrus. Adv. Bot. Res. 2008, 47, 147–223. [Google Scholar]
- Monselise, S.P. Citrus fruit development, endogenous systems, and external regulation. Proc. Int. Soc. Citric. 1977, 2, 664–668. [Google Scholar]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar]
- Agustí, M.; Zaragoza, S.; Bleiholder, H.; Buhr, L.; Hack, H.; Klose, R.; Staub, R. Escala BBCH para la descripción de los estadios fenológicos del desarrollo de los agrios (Gén. Citrus). Levante Agric. 1995, 332, 189–199. [Google Scholar]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants: BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry, Blackwell: Oxford, UK, 2001; p. 158. [Google Scholar]
- Pauli, H. Proposed extension of the CIE recommendation on “Uniform color spaces, color difference equations, and metric color terms”. J. Opt. Soc. Am. 1976, 66, 866. [Google Scholar]
- Robertson, A.R. The CIE 1976 Color-Difference Formulae. Color Res. Appl. 1977, 2, 7–11. [Google Scholar] [CrossRef]
- Jiménez-Cuesta, M.; Cuquerella, J.; Martinez-Javaga, J.M. Determination of a Color Index for Citrus Fruit Degreening. In Proceedings of the International Citrus Congress, Tokyo, Japan, 9–12 November 1981. [Google Scholar]
- Boninsegna, M.A.; De Bruno, A.; Piscopo, A. Quality evaluation of ready-to-eat coated clementine (Citrus × Clementina) fruits. Coatings 2023, 13, 1562. [Google Scholar] [CrossRef]
- Huberman, M.; Goren, R.; Zamski, E. Anatomical aspects of hormonal regulation of abscission in citrus—The shoot peduncle abscission zone in the non-abscising stage. Physiol. Plant. 1983, 59, 445–454. [Google Scholar]
- Iwahori, S.; Van Steveninck, R.F.M. Ultrastructural observation of lemon fruit abscission. Sci. Hortic. 1976, 4, 235–246. [Google Scholar] [CrossRef]
- Sexton, R.; Roberts, J.A. Cell biology of abscission. Annu. Rev. Plant Physiol. 1982, 33, 133–162. [Google Scholar] [CrossRef]
- Zanchin, A.; Marcato, C.; Trainotti, L.; Casadoro, G.; Rascio, N. Characterization of abscission zones in the flowers and fruits of peach (Prunus persica (L.) Batsch). New Phytol. 1995, 129, 345–354. [Google Scholar]
- Brown, K.M. Ethylene and abscission. Physiol. Plant. 1997, 100, 567–576. [Google Scholar]
- Abeles, F.B. Ethylene in Plant Biology; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Biale, J.B. Respiration of fruits. In Handbuch der Pflanzenphysiologie; Ruhland, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1960; Volume 12, Part II; pp. 536–592. [Google Scholar]
- Sawamura, M. Levels of Endogenous Ethylene in Attached Citrus Fruits. Agric. Biol. Chem. 1981, 45, 2935–2937. [Google Scholar]
- Kazokas, W. Activities of Cell Wall Hydrolases and Cellulase Gene Expression in Valencia Orange and Tahiti Lime Calyx Abscission Zones During Intermittent Ethylene Treatments. Master’s Thesis, University of Florida, Gainesville, FL, USA, 1997. [Google Scholar]
- Burns, J.K.; Nairn, C.J.; Lewandowski, D.J. Cell wall hydrolase activity and cellulase gene expression during abscission of Valencia citrus fruit and leaves. Proc. Fla. State Hortic. Soc. 1995, 108, 254–258. [Google Scholar]
- Huberman, M.; Goren, R. Exo- and endo-cellular cellulase and polygalacturonase in abscission zones of developing orange fruits. Physiol. Plant. 1979, 45, 189–196. [Google Scholar]
- Greenberg, J.; Goren, R.; Riov, J. The role of cellulase and polygalacturonase in abscission of young and mature Shamouti orange fruits. Physiol. Plant. 1975, 34, 1–7. [Google Scholar]
- Cheng, C.; Zhang, L.; Yang, X.; Zhong, G. Profiling gene expression in citrus fruit calyx abscission zone (AZ-C) treated with ethylene. Mol. Genet. Genom. 2015, 290, 1991–2006. [Google Scholar] [CrossRef]
- Hartmond, U.; Yuan, R.; Burns, J.K.; Grant, A.; Kender, W.J. Citrus fruit abscission induced by methyl-jasmonate. J. Am. Soc. Hortic. Sci. 2000, 125, 547–552. [Google Scholar] [CrossRef]
- Dutta, S.K.; Gurung, G.; Yadav, A.; Laha, R.; Mishra, V.K. Factors associated with citrus fruit abscission and management strategies developed so far: A review. N. Zealand J. Crop Hortic. Sci. 2022, 51, 467–488. [Google Scholar]
- Rasmussen, G.K. Cellulase activity, endogenous abscisic acid, and ethylene in four citrus cultivars during maturation. Plant Physiol. 1975, 56, 765–767. [Google Scholar]
- Rasmussen, G.K. Cellulase activity in separation zones of citrus fruit treated with abscisic acid under normal and hypobaric atmospheres. J. Am. Soc. Hortic. Sci. 1974, 99, 229–231. [Google Scholar]
- Riov, J.A. Polygalacturonase from citrus leaf explants. Plant Physiol. 1974, 53, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, G.K. Changes in cellulase and pectinase activities in fruit tissues and separation zones of citrus treated with cycloheximide. Plant Physiol. 1973, 51, 626–628. [Google Scholar] [CrossRef][Green Version]
- Osborne, D.J.; Jackson, M.B.; Milborrow, B.V. Physiological properties of abscission accelerator from senescent leaves. Nat. New Biol. 1972, 240, 98–101. [Google Scholar] [CrossRef]
- Kang, C.; Cao, J.; Wang, Y.; Sun, C. Advances of section drying in citrus fruit: The metabolic changes, mechanisms and prevention methods. Food Chem. 2022, 395, 133499. [Google Scholar] [CrossRef]
- Giuffré, A.M. Bergamot (Citrus bergamia, Risso): The Effects of Cultivar and Harvest Date on Functional Properties of Juice and Cloudy Juice. Antioxidants 2019, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.M. Morphological, anatomical, and physiological changes in the developing fruit of the Valencia orange, Citrus sinensis (L.) Osbeck. Aust. J. Bot. 1958, 6, 1–23. [Google Scholar] [CrossRef]
- Farooq Rab, A.; Khan, N.; Rahman, H. Fruit growth and development in three cultivars of citrus: Orange, Kinnow and Feutrall’s early. FUUAST J. Biol. 2011, 1, 145–147. [Google Scholar]
- Tadeo, F.R.; Moya, J.L.; Iglesias, D.J.; Talon, M.; Primo-Millo, E. Histología y Citología de Cítricos; Generalitat Valenciana, Conselleria d’Agricultura, Pesca y Alimentación: Valencia, Spain, 2003. [Google Scholar]
- Bustan, A.; Goldschmidt, E.E.; Erner, Y. Carbohydrate supply and demand during fruit development in relation to productivity of grapefruit and Murcott mandarin. Acta Hortic. 1996, 416, 81–88. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant growth regulators II: Cytokinins, their analogues and antagonists. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 205–226. [Google Scholar]
- Mauk, C.S.; Bausher, M.G.; Yelenosky, G. Influence of growth regulator treatments on dry matter production, fruit abscission, and 14C-assimilate partitioning in citrus. J. Plant Growth Regul. 1986, 5, 111–120. [Google Scholar] [CrossRef]
- Shishido, Y.; Hori, Y.; Shikano, S. Effects of benzyladenine on translocation and distribution of photoassimilates during fruit setting and development in cucumber plants. J. Jpn. Soc. Hortic. Sci. 1990, 59, 129–136. [Google Scholar] [CrossRef][Green Version]
- Talon, M.; Tadeo, F.R.; Ben-Cheikh, W.; Gomez-Cadenas, A.; Mehouachi, J.; Pérez-Botella, J.; Primo-Millo, E. Hormonal regulation of fruit set and abscission in citrus: Classical concepts and new evidence. Acta Hortic. 1998, 463, 209–217. [Google Scholar]
- Guardiola, J.L.; García-Luis, A. Increasing fruit size in citrus. Thinning and stimulation of fruit growth. Plant Growth Regul. 2000, 31, 121–132. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiología Vegetal; 3a. Edición; Universitat Jaume: Castellón de la Plana, Spain, 2006. [Google Scholar]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, E.E. Carbohydrate supply as a critical factor for citrus fruit development and productivity. Hort. Sci. 1999, 34, 1020–1024. [Google Scholar]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar]
- Du Plessis, S.F.; Koen, T.J. Effect of nutrition on fruit size of citrus. Proc. Int. Soc. Citric. 1984, 1, 148–150. [Google Scholar]
- Gilfillan, I.M. Fruit size improvement. In Production Guidelines for Export Citrus; Netterville, R.M., Ed.; South African Co-op Citrus Exchange: Hennopsmeer, South Africa, 1990; Volume II, pp. 1–11. [Google Scholar]
- Zhang, L.; Shen, G.; Zhang, J.; Chen, C. Study of the law of orange fruit growth and development and the influence of meteorological factors. Proc. Intl. Soc. Citricult. 1994, 1, 432–434. [Google Scholar]
- Krajewski, A. Pruning of citrus in Southern Africa: A hacker’s guide. Citrus J. 1996, 6, 19–23. [Google Scholar]
- Guardiola, J.L.; García-Luis, A. Thinning effects on citrus yield and fruit size. Acta Hortic. 1997, 463, 463–473. [Google Scholar]
- Albrigo, L.G. Climatic effects on flowering, fruit set and quality of citrus—A review. In Proceedings of the International Society of Citriculture X Congress, Agadir, Morocco, 15–20 February 2004. [Google Scholar]
- Romero, P.; Navarro, J.M.; Pérez-Pérez, J.; García-Sánchez, F.; Gómez-Gómez, A.; Porras, I.; Martinez, V.; Botía, P. Deficit irrigation and rootstock: Their effects on water relations, vegetative development, yield, fruit quality and mineral nutrition of Clemenules mandarin. Tree Physiol. 2006, 26, 1537–1548. [Google Scholar]
- Treeby, M.T.; Henroid, R.E.; Bevington, K.B.; Milne, D.J.; Storey, R. Irrigation management and rootstock effects on navel orange [Citrus sinensis (L.) Osbeck] fruit quality. Agric. Water Manag. 2007, 91, 24–32. [Google Scholar]
- Khokhar, U.U.; Sharma, R. Maturity Indices for Sweet Orange CV. Blood Red. Haryana J. Hortic. Sci. 1984, 13, 22–25. [Google Scholar]
- Bhullar, J.S. Determination of Maturity Standards of Sweet Oranges in Himanchal Pradesh. Haryana J. Hortic. Sci. 1983, 12, 183–188. [Google Scholar]
- Singh, S.; Aulakh, P.S.; Gill, P.P. Physicochemical changes during fruit development and maturation in grapefruit (Citrus paradisi Macf.) cv. Star Ruby. Ecoscan 2015, 9, 17–20. [Google Scholar]
- Rokaya, P.R.; Baral, D.R.; Gautam, D.M.; Shrestha, A.K.; Paudyal, K.P. Effect of altitude and maturity stages on quality attributes of mandarin (Citrus reticulata Blanco). Am. J. Plant Sci. 2016, 7, 958. [Google Scholar]
- Shahida, C.; Manisha, K.; Mridul, D. Physico-Chemical Changes in Assam Lemon (Citrus limon Burm.) During Fruit Development and Maturation. Int. J. Environ. Clim. Change 2022, 12, 90–94. [Google Scholar]
- Porat, R. Degreening of citrus fruit. Tree For. Sci. Biotechnol. 2008, 2, 71–76. [Google Scholar]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesium-dechelatase. Plant Cell 2006, 28, 2147–2160. [Google Scholar]
- Yin, X.R.; Xie, X.L.; Xia, X.J.; Yu, J.Q.; Ferguson, I.B.; Giovannoni, J.J.; Chen, K.S. Involvement of an ethylene response factor in chlorophyll degradation during citrus fruit degreening. Plant J. 2016, 86, 403–412. [Google Scholar]
- Cunningham, F.X., Jr.; Pogson, B.; Sun, Z.; McDonald, K.A.; DellaPenna, D.; Gantt, E. Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 1996, 8, 1613–1626. [Google Scholar] [PubMed]
- Ohmiya, A.; Kato, M.; Shimada, T.; Nashima, K.; Kishimoto, S.; Nagata, M. Molecular basis of carotenoid accumulation in horticultural crops. Hortic. J. 2019, 88, 135–149. [Google Scholar]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Spiegel-Roy, P.; Goldschmidt, E.E. Biology of Citrus; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Manera, F.J.; Brotons, J.M.; Conesa, A.; Porras, I. Relation between temperature and the beginning of peel color change in grapefruit (Citrus paradisi Macf.). Sci. Hortic. 2013, 160, 292–299. [Google Scholar] [CrossRef]
- Conesa, A.; Manera, F.C.; Brotons, J.M.; Fernandez-Zapata, J.C.; Simón, I.; Simón-Grao, S.; Alfosea-Simón, M.; Nicolása, J.J.M.; Valverde, J.M.; García-Sanchez, F. Changes in the content of chlorophylls and carotenoids in the rind of ‘Fino 49’ lemons during maturation and their relationship with parameters from the CIELAB color space. Sci. Hortic. 2019, 243, 252–260. [Google Scholar]
- Stearns, C.J.; Young, G.T. The relation of climatic conditions to color development in citrus fruit. Proc. Fla. State Hortic. Soc. 1942, 56, 39–61. [Google Scholar]
- Agustí, M.; Almela, V. Aplicación de fitorreguladores en citricultura; AEDOS: Barcelona, Spain, 1991. [Google Scholar]
- Davies, F.S.; Albrigo, L.G. Citrus; Cab International: Wallingford, UK, 1994. [Google Scholar]
- Alós, E.; Cercós, M.; Rodrigo, M.J.; Zacarías, L.; Talón, M. Regulation of color break in citrus fruits. Changes in pigment profiling and gene expression induced by gibberellins and nitrate, two ripening retardants. J. Agric. Food Chem. 2006, 54, 4888–4895. [Google Scholar] [CrossRef]
- Chen, Q.X.; Xu, C.J.; Wang, W.J.; Zheng, J.; Chen, K.S. Effect of artificial pollination on fruit development and quality in storage of Yongjiazaoxiangyou pomelo. J. Fruit Sci. 2005, 22, 412–415. [Google Scholar]
- Nie, Z.P.; Huang, Q.; Chen, C.Y.; Wan, C.P.; Chen, J.Y. Chitosan coating alleviates postharvest juice sac granulation by mitigating ROS accumulation in harvested pummelo (Citrus grandis L. Osbeck) during room temperature storage. Postharvest Biol. Technol. 2020, 169, 111309. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, P.; Qi, Y.P.; Zhou, C.P.; Yang, L.T.; Liao, X.Y.; Chen, L.S. Effects of granulation on organic acid metabolism and its relation to mineral elements in Citrus grandis juice sacs. Food Chem. 2014, 145, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Goto, A. Relationship between pectic substances and calcium in healthy, gelated and granulated juice sacs of Sanbokan (Citrus sulcata hort. ex Takahashi) fruit. Plant Cell Physiol. 1989, 30, 801–806. [Google Scholar] [CrossRef]
- Shomer, I.; Chalutz, E.; Vasiliver, R.; Lomaniec, E.; Berman, M. Sclerification of juice sacs in pummelo (Citrus gramdis) fruit. Can. J. Bot. 1989, 67, 625–632. [Google Scholar] [CrossRef]
- Wu, L.M.; Wang, C.; He, L.G.; Wang, Z.J.; Tong, Z.; Song, F.; Peng, S.A. Transcriptome analysis unravels metabolic and molecular pathways related to fruit sac granulation in a late-ripening navel orange (Citrus sinensis Osbeck). Plants 2020, 9, 95. [Google Scholar] [CrossRef]
- Dai, Y.L.; Zhao, Q.Y.; Liu, R.N.; Liu, L.T.; Zhang, M.L.; Li, Y.; Wang, P. Analysis of cellulose content and synthase gene expression in juice sacs secondary wall during granulation of Guanxi pomelo. J. Fruit Sci. 2021, 38, 1435–1443. [Google Scholar]
- El-Zeftawi, B.M. Granulation of Valencia oranges. Food Technol. Aust. 1972, 25, 103–105. [Google Scholar]
- She, W.Q.; Pan, D.M.; Lin, H.T. Relationship between granulation and active oxygen metabolism of juice sac in pummelo fruit during maturation. Sci. Agric. Sin. 2009, 42, 1737–1743. [Google Scholar]
- Shi, M.Y.; Liu, X.; Zhang, H.P.; He, Z.Y.; Yang, H.B.; Chen, J.J.; Xu, J. The IAA- and ABA-responsive transcription factor CgMYB58 upregulates lignin biosynthesis and triggers juice sac granulation in pummelo. Hortic. Res. 2020, 7, 139. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Cheng, F.S.; Dai, C.; Sun, Y.F.; Lu, J.; Huang, Y.; Li, M.; He, Y.; Wang, F.; et al. Identification of microRNAs correlated with citrus granulation based on bioinformatics and molecular biology analysis. Postharvest Biol. Technol. 2016, 118, 59–67. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; El-Otmani, M.; Agustí, M. Examining the impact of dry climates temperature on citrus fruit internal ripening. Sci. Hortic. 2024, 337, 113501. [Google Scholar] [CrossRef]
- Albertini, M.V.; Carcouet, E.; Pailly, O.; Gambotti, C.; Luro, F.; Berti, L. Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. J. Agric. Food Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef]
- Cercόs, M.; Soler, G.; Iglesias, D.J.; Gadea, J.; Forment, J.; Talόn, M. Global analysis of gene expression during development and ripening of citrus fruit flesh. A proposed mechanism for citric acid utilization. Plant Mol. Biol. 2006, 62, 513–527. [Google Scholar]
- Shimada, T.; Nakano, R.; Shulaev, V.; Sadka, A.; Blumwald, E. Vacuolar citrate/HC symporter of citrus juice cells. Planta 2006, 224, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Terol, J.; Soler, G.; Talon, M.; Cercos, M. The aconitate hydratase family from Citrus. BMC Plant Biol. 2010, 10, 222. [Google Scholar]
- Huang, D.; Zhao, Y.; Cao, M.; Qiao, L.; Zheng, Z.L. Integrated systems biology analysis of transcriptomes reveals candidate genes for acidity control in developing fruits of sweet orange (Citrus sinensis L. osbeck). Front. Plant Sci. 2016, 7, 486. [Google Scholar] [CrossRef]
- Moon, D.; Mizutani, F.; Moon, D.G. Changes in soluble solids, acidity, and abscisic acid contents in different portions of fruit during maturation of satsuma mandarin. J. Korean Soc. Hortic. Sci. 2002, 43, 107–112. [Google Scholar]
- Lin, Q.; Wang, C.; Dong, W.; Jiang, Q.; Wang, D.; Li, S.; Chen, M.; Liu, C.; Sun, C.; Chen, K. Transcriptome and metabolome analyses of sugar and organic acid metabolism in Ponkan (Citrus reticulata) fruit during fruit maturation. Gene 2015, 554, 64–74. [Google Scholar] [CrossRef]
- Ladaniya, M.S. Nutritive and medicinal value of citrus fruit. In Citrus Fruit; Ladaniya, M.S., Ed.; Biology Technology and Evaluation; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 501–514. [Google Scholar]
- Yang, X.Y.; Xie, J.X.; Wang, F.F.; Zhong, J.; Liu, Y.Z.; Li, G.H.; Peng, S.A. Comparison of ascorbate metabolism in fruits of two citrus species withobvious difference in ascorbate content in pulp. J. Plant Physiol. 2011, 168, 2196–2205. [Google Scholar]



| Parameter | Cv ¥ | Harvest Time (DAFB) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 230 | 260 | 290 | 320 | 350 | 380 | 410 | Sign | ||
| Fruit weight (g) | FA | 224.6 ± 1.44 e | 255.8 ± 5.80 d | 312.5 ± 1.95 c | 336.5 ± 1.94 b | 357.1 ± 0.96 a | 369.3 ± 0.78 a | 358.5 ± 4.67 a | 335.4 ± 1.05 b | ** |
| FE | 174.9 ± 3.03 f | 211.3 ± 4.11 e | 232.4 ± 1.23 d | 247.8 ± 1.34 c | 260.8 ± 0.67 b | 271.5 ± 2.57 a | 257.0 ± 0.80 bc | 227.5 ± 0.67 d | ** | |
| Sign. | ** | ** | ** | ** | ** | ** | ** | ** | ||
| Fruit height (mm) | FA | 74.4 ± 0.34 d | 79.3 ± 1.78 c | 86.0 ± 0.90 b | 87.6 ± 0.42 b | 88.7 ± 0.28 b | 90.5 ± 1.30 a | 92.2 ± 0.36 a | 93.6 ± 0.48 a | ** |
| FE | 69.5 ± 0.15 e | 75.2 ± 0.40 d | 77.8 ± 0.58 cd | 79.4 ± 0.32 bc | 81.4 ± 1.07 abc | 83.0 ± 0.38 ab | 84.6 ± 1.55 a | 85.1 ± 0.76 a | ** | |
| Sign. | ** | n.s. | ** | ** | ** | ** | ** | ** | ||
| Fruit width (mm) | FA | 80.2 ± 0.57 f | 81.1 ± 0.73 f | 87.3 ± 0.12 e | 89.8 ± 0.09 de | 91.8 ± 0.17 cd | 92.9 ± 0.40 bc | 95.1 ± 0.89 ab | 96.7 ± 0.55 a | ** |
| FE | 70.8 ± 0.60 f | 75.3 ± 0.55 e | 77.6 ± 0.14 d | 79.7 ± 0.16 c | 81.4 ± 0.36 bc | 82.3 ± 0.30 ab | 83.7 ± 0.30 a | 84.1 ± 0.44 a | ** | |
| Sign. | ** | ** | ** | ** | ** | ** | ** | ** | ||
| Ratio height/width | FA | 0.93 ± 0.010 | 0.98 ± 0.025 | 0.98 ± 0.011 | 0.98 ± 0.004 | 0.97 ± 0.004 | 0.97 ± 0.017 | 0.97 ± 0.006 | 0.97 ± 0.010 | n.s. |
| FE | 0.98 ± 0.009 | 1.00 ± 0.012 | 1.00 ± 0.007 | 1.00 ± 0.005 | 1.00 ± 0.017 | 1.01 ± 0.008 | 1.01 ± 0.015 | 1.01 ± 0.014 | n.s. | |
| Sign. | ** | n.s. | n.s. | * | n.s. | n.s. | n.s. | n.s. | ||
| Fruit volume (cm3) | FA | 262.3 ± 3.12 f | 290.8 ± 5.58 e | 327.2 ± 2.35 d | 352.4 ± 2.40 c | 373.8 ± 1.85 bc | 391.3 ± 3.04 b | 417.5 ± 9.27 a | 437.0 ± 3.43 a | ** |
| FE | 213.1 ± 3.11 f | 246.5 ± 2.32 e | 253.6 ± 2.37 e | 271.8 ± 1.37 d | 289.4 ± 1.49 c | 301.6 ± 1.05 bc | 316.3 ± 8.01 ab | 320.8 ± 0.72 a | ** | |
| Sign. | ** | ** | ** | ** | ** | ** | ** | ** | ||
| Fruit specific gravity (g/cm3) | FA | 0.86 ± 0.005 b | 0.88 ± 0.014 b | 0.96 ± 0.006 a | 0.96 ± 0.009 a | 0.96 ± 0.004 a | 0.95 ± 0.008 a | 0.86 ± 0.007 b | 0.77 ± 0.004 c | ** |
| FE | 0.83 ± 0.023 b | 0.86 ± 0.010 ab | 0.92 ± 0.004 a | 0.91 ± 0.006 a | 0.90 ± 0.006 a | 0.90 ± 0.006 a | 0.82 ± 0.018 b | 0.71 ± 0.003 c | ** | |
| Sign. | n.s. | n.s. | ** | ** | ** | ** | n.s. | ** | ||
| Parameter | Cv ¥ | Harvest Time (DAFB) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 230 | 260 | 290 | 320 | 350 | 380 | 410 | Sign | ||
| L* | FA | 56.7 ± 0.29 d | 62.9 ± 0.15 c | 65.9 ± 0.37 b | 70.1 ± 0.03 a | 70.6 ± 0.14 a | 69.7 ± 0.11 a | 70.5 ± 0.21 a | 70.2 ± 0.22 a | ** |
| FE | 59.4 ± 0.27 c | 65.5 ± 0.42 b | 70.5 ± 0.24 a | 70.6 ± 0.51 a | 70.5 ± 0.33 a | 70.3 ± 0.35 a | 70.5 ± 0.21 a | 70.3 ± 0.38 a | ** | |
| Sign | ** | ** | ** | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| a* | FA | −5.1 ± 0.14 e | 0.2 ± 0.39 d | 1.8 ± 0.15 c | 6.2 ± 0.11 b | 7.7 ± 0.32 a | 7.9 ± 0.29 a | 7.4 ± 0.05 a | 6.1 ± 0.15 b | ** |
| FE | −5.1 ± 0.20 e | 1.0 ± 0.40 d | 6.0 ± 0.12 c | 7.5 ± 0.04 b | 9.3 ± 0.25 a | 9.2 ± 0.08 a | 8.6 ± 0.03 a | 7.4 ± 0.06 b | ** | |
| Sign | n.s. | n.s. | ** | ** | * | ** | ** | ** | ||
| b* | FA | 29.9 ± 0.05 d | 36.9 ± 0.41 b | 38.4 ± 0.22 a | 38.8 ± 0.15 a | 37.7 ± 0.18 ab | 36.8 ± 0.41 b | 34.5 ± 0.11 c | 33.8 ± 0.12 c | ** |
| FE | 30.4 ± 0.44 d | 37.6 ± 0.58 b | 39.2 ± 0.40 a | 39.4 ± 0.23 a | 39.6 ± 0.04 a | 38.9 ± 0.13 ab | 37.1 ± 0.18 b | 35.0 ± 0.10 c | ** | |
| Sign | n.s. | n.s. | n.s. | n.s. | ** | ** | ** | ** | ||
| H° | FA | 99.6 ± 0.25 a | 89.8 ± 0.57 b | 87.4 ± 0.24 c | 80.9 ± 0.17 d | 78.5 ± 0.42 ef | 78.0 ± 0.31 f | 77.9 ± 0.09 f | 79.7 ± 0.27 de | ** |
| FE | 99.5 ± 0.30 a | 88.6 ± 0.66 b | 81.3 ± 0.09 c | 79.2 ± 0.11 d | 76.8 ± 0.34 ef | 76.6 ± 0.09 f | 76.9 ± 0.10 ef | 78.1 ± 0.12 de | ** | |
| Sign | n.s. | n.s. | ** | ** | * | * | ** | ** | ||
| C* | FA | 30.3 ± 0.07 e | 37.0 ± 0.42 c | 38.5 ± 0.22 ab | 39.3 ± 0.15 a | 38.5 ± 0.24 ab | 37.7 ± 0.46 bc | 35.3 ± 0.11 d | 34.4 ± 0.10 d | ** |
| FE | 30.8 ± 0.45 d | 37.7 ± 0.57 b | 39.7 ± 0.41 a | 40.1 ± 0.22 a | 40.7 ± 0.03 a | 40.0 ± 0.13 a | 38.1 ± 0.17 b | 35.8 ± 0.09 c | ** | |
| Sign | n.s. | n.s. | n.s. | * | ** | ** | ** | ** | ||
| CCI | FA | −3.0 ± 0.10 f | 0.1 ± 0.16 e | 0.7 ± 0.06 d | 2.3 ± 0.05 c | 2.9 ± 0.11 ab | 3.1 ± 0.08 a | 3.0 ± 0.01 a | 2.6 ± 0.07 bc | ** |
| FE | −2.8 ± 0.09 e | 0.3 ± 0.18 d | 2.2 ± 0.02 c | 2.7 ± 0.02 b | 3.3 ± 0.08 a | 3.4 ± 0.04 a | 3.3 ± 0.04 a | 3.0 ± 0.02 ab | ** | |
| Sign | n.s. | n.s. | ** | ** | * | * | ** | ** | ||
| Peel thickness | FA | 4.5 ± 0.10 b | 4.9 ± 0.12 ab | 5.0 ± 0.03 a | 5.2 ± 0.06 a | 5.2 ± 0.06 a | 5.3 ± 0.07 a | 5.3 ± 0.14 a | 5.3 ± 0.11 a | ** |
| (mm) | FE | 3.3 ± 0.11 b | 3.7 ± 0.12 ab | 3.9 ± 0.03 a | 4.0 ± 0.08 a | 4.0 ± 0.02 a | 4.0 ± 0.07 a | 4.0 ± 0.03 a | 4.1 ± 0.08 a | ** |
| Sign | ** | ** | ** | ** | * | * | ** | ** | ||
| Parameter | Cv ¥ | Harvest Time (DAFB) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 230 | 260 | 290 | 320 | 350 | 380 | 410 | Sign | ||
| Juice content (%) | FA | 45.4 ± 1.27 c | 47.4 ± 1.73 bc | 52.9 ± 0.61 ab | 53.7 ± 1.00 a | 51.7 ± 0.58 ab | 44.2 ± 0.58 c | 37.7 ± 1.41 d | 35.4 ± 1.61 d | ** |
| FE | 46.4 ± 0.77 b | 45.2 ± 0.44 b | 54.6 ± 0.77 a | 54.7 ± 0.84 a | 51.0 ± 0.80 a | 43.0 ± 0.97 b | 34.3 ± 2.24 c | 27.5 ± 0.25 d | ** | |
| Sign | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ** | ||
| Total soluble solids (°Brix) | FA | 9.3 ± 0.03 a | 9.3 ± 0.03 ab | 8.9 ± 0.12 bc | 9.0 ± 0.03 abc | 8.8 ± 0.07 cd | 8.4 ± 0.15 de | 7.8 ± 0.12 f | 8.0 ± 0.06 ef | ** |
| FE | 8.9 ± 0.03 ab | 9.1 ± 0.21 a | 8.4 ± 0.09 bc | 8.4 ± 0.15 bc | 8.0 ± 0.03 cd | 7.6 ± 0.15 de | 7.3 ± 0.07 e | 7.2 ± 0.12 e | ** | |
| Sign | ** | n.s. | * | * | ** | * | * | ** | ||
| Titratable acidity (g·L−1) | FA | 51.3 ± 0.92 a | 45.4 ± 1.00 b | 39.1 ± 0.61 c | 36.7 ± 0.56 cd | 35.5 ± 0.30 cde | 33.5 ± 0.35 def | 31.8 ± 1.24 ef | 30.5 ± 0.88 f | ** |
| FE | 55.5 ± 0.81 a | 54.8 ± 1.17 a | 42.4 ± 0.94 bc | 42.9 ± 1.64 b | 40.4 ± 0.71 bcd | 38.2 ± 0.51 cd | 37.3 ± 0.56 d | 37.9 ± 0.76 cd | ** | |
| Sign | * | ** | * | * | ** | ** | ** | * | ||
| TSS/TA ratio | FA | 1.8 ± 0.03 d | 2.0 ± 0.04 cd | 2.3 ± 0.06 bc | 2.5 ± 0.03 ab | 2.5 ± 0.03 ab | 2.5 ± 0.06 ab | 2.5 ± 0.12 ab | 2.6 ± 0.06 a | ** |
| FE | 1.6 ± 0.02 c | 1.7 ± 0.07 bc | 2.0 ± 0.06 a | 2.0 ± 0.09 a | 2.0 ± 0.04 a | 2.0 ± 0.05 a | 2.0 ± 0.01 a | 1.9 ± 0.02 ab | ** | |
| Sign | ** | ** | * | ** | ** | ** | * | ** | ||
| Ascorbic acid (g·L−1) | FA | 0.78 ± 0.009 a | 0.74 ± 0.013 a | 0.64 ± 0.022 b | 0.60 ± 0.024 b | 0.59 ± 0.007 bc | 0.52 ± 0.006 cd | 0.46 ± 0.015 de | 0.44 ± 0.007 e | ** |
| FE | 0.67 ± 0.007 a | 0.67 ± 0.004 a | 0.56 ± 0.010 b | 0.53 ± 0.019 b | 0.52 ± 0.003 bc | 0.48 ± 0.006 cd | 0.44 ± 0.010 de | 0.42 ± 0.009 e | ** | |
| Sign | ** | ** | * | n.s. | ** | ** | n.s. | n.s. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafrica, R.; De Bruno, A.; Mafrica, D.L.; Merlo, C.; Gattuso, A.; Poiana, M. Ripening Dynamics and Optimal Harvest Timing of ‘Fantastico’ and ‘Femminello’ Bergamot Fruit. Agriculture 2025, 15, 737. https://doi.org/10.3390/agriculture15070737
Mafrica R, De Bruno A, Mafrica DL, Merlo C, Gattuso A, Poiana M. Ripening Dynamics and Optimal Harvest Timing of ‘Fantastico’ and ‘Femminello’ Bergamot Fruit. Agriculture. 2025; 15(7):737. https://doi.org/10.3390/agriculture15070737
Chicago/Turabian StyleMafrica, Rocco, Alessandra De Bruno, Davide Leo Mafrica, Cristina Merlo, Antonio Gattuso, and Marco Poiana. 2025. "Ripening Dynamics and Optimal Harvest Timing of ‘Fantastico’ and ‘Femminello’ Bergamot Fruit" Agriculture 15, no. 7: 737. https://doi.org/10.3390/agriculture15070737
APA StyleMafrica, R., De Bruno, A., Mafrica, D. L., Merlo, C., Gattuso, A., & Poiana, M. (2025). Ripening Dynamics and Optimal Harvest Timing of ‘Fantastico’ and ‘Femminello’ Bergamot Fruit. Agriculture, 15(7), 737. https://doi.org/10.3390/agriculture15070737









