Alleviation of Chilling Injury in Postharvest Sweet Basil (Ocimum basilicum L.) with Silicon and Abscisic Acid Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Treatments
2.2.1. Trial 1: Silicon and Abscisic Acid
- CK (control) = rootzone irrigation with DI water as needed;
- CK+DI = rootzone irrigation with DI water as needed + foliar spray application with DI water a day before harvest;
- Si-irrigation+DI = rootzone irrigation with Si solution as needed + foliar spray application with DI water a day before harvest;
- DI-spray+DI = foliar spray application with DI water once a week, rootzone irrigation with DI water as needed + foliar spray application with DI water a day before harvest;
- Si-spray+DI = foliar spray application with Si solution once a week, rootzone irrigation with DI water as needed + foliar spray application with DI water a day before harvest;
- CK+ABA = rootzone irrigation with DI water as needed + foliar spray application with ABA solution a day before harvest;
- Si-irrigation+ABA = rootzone irrigation with Si solution as needed + foliar spray application with ABA solution a day before harvest;
- DI-spray+ABA = foliar spray application with DI water once a week, rootzone irrigation with DI water as needed + foliar spray application with ABA solution a day before harvest;
- Si-spray+ABA = foliar spray application with Si solution once a week, rootzone irrigation with DI water as needed + foliar spray application with ABA solution a day before harvest.
2.2.2. Trial 2: Wollastonite
2.3. Harvest and Cold Storage Test
2.4. Treatment Effects
2.4.1. Plant Growth
2.4.2. Leaf Chlorophyll Content Index (CCI)
2.4.3. Chilling Injury Index (CII) Scores
- 0 = no visible damage;
- 1 = spots covering <10% of leaf surface;
- 2 = spots covering 10–30% of leaf surface;
- 3 = spots covering 30–50% of leaf surface;
- 4 = spots covering >50% of leaf surface.
2.4.4. Fresh Weight Loss (FWL)
2.4.5. Leaf Electrolyte Leakage (LEL)
2.4.6. Statistical Analysis
3. Results
3.1. Trial 1: Silicon and Abscisic Acid
3.1.1. Plant Growth and Leaf Chlorophyll Content Index (CCI)
3.1.2. Cold Injury Index (CII) Scores
3.1.3. Fresh Weight Loss (FWL)
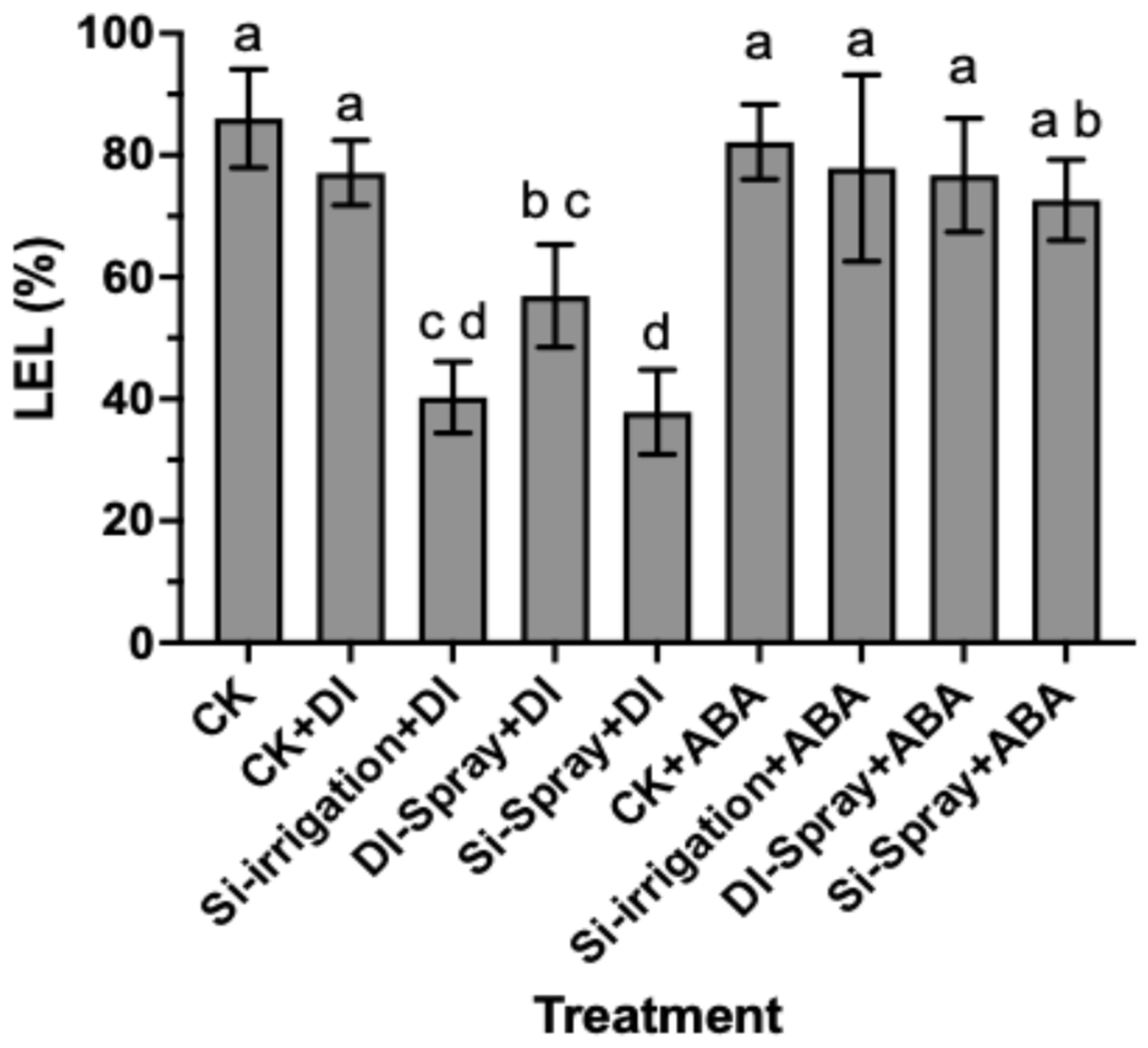
3.1.4. Leaf Electrolyte Leakage (LEL)
3.2. Trial 2: Wollastonite
3.2.1. Plant Growth and Leaf Chlorophyll Content Index (CCI)
3.2.2. Cold Injury Index (CII) Scores
3.2.3. Fresh Weight Loss (FWL)
3.2.4. Leaf Electrolyte Leakage (LEL)
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| CCI | Chlorophyll content index |
| CI | Chilling injury |
| CII | Chilling injury index |
| DI | Deionised water |
| FWL | Fresh weight loss |
| LEL | Leaf electrolyte leakage |
| RH | Relative humidity |
| ROS | Reactive oxygen species |
| Si | Silicon |
References
- Cantwell, M.; Reid, M. Postharvest physiology and handling of fresh culinary herbs. J. Herbs Spices Med. Plants 1993, 1, 93–127. [Google Scholar] [CrossRef]
- Parkin, K.L.; Marangoni, A.; Jackman, R.L.; Yada, R.Y.; Stanley, D.W. Chilling injury. A review of possible mechanisms. J. Food Biochem. 1989, 13, 127–153. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. Available online: https://www.ars.usda.gov/is/np/CommercialStorage/CommercialStorage.pdf (accessed on 24 February 2025).
- Lange, D.D.; Cameron, A.C. Postharvest shelf life of sweet basil (Ocimum basilicum). HortScience 1994, 29, 102–103. [Google Scholar] [CrossRef]
- Aharoni, N.; Kenigsbuch, D.; Chalupowicz, D.; Faura-Mlinski, M.; Aharon, Z.; Maurer, D.; Ovadia, A.; Lers, A. Reducing chilling injury and decay in stored sweet basil. Isr. J. Plant Sci. 2010, 58, 167–181. [Google Scholar] [CrossRef]
- Delbeke, S.; Ceuppens, S.; Jacxsens, L.; Uyttendaele, M. Survival of Salmonella and Escherichia coli O157:H7 on strawberries, basil, and other leafy greens during storage. J. Food Prot. 2015, 78, 652–660. [Google Scholar] [CrossRef]
- Rodeo, A.J.D.; Mitcham, E.J. Chilling temperatures and controlled atmospheres alter key volatile compounds implicated in basil aroma and flavor. Front. Plant Sci. 2023, 14, 1218734. [Google Scholar] [CrossRef]
- Lange, D.L.; Camero, A.C. Pre- and postharvest temperature conditioning of greenhouse-grown sweet basil. HortScience 1997, 32, 114–116. [Google Scholar] [CrossRef]
- Larsen, D.H.; Marcelis, L.F.M.; van Kempen, D.; Kohlen, W.; Nicole, C.C.S.; Woltering, E.J. Far-red light during cultivation improves postharvest chilling tolerance in basil. Postharvest Biol. Technol. 2023, 198, 112232. [Google Scholar] [CrossRef]
- Hammam, K.A.; Shoala, T. Influence of spraying nano-curcumin and nano-rosemarinic acid on growth, fresh herb yield, chemicals composition and postharvest criteria of French basil (Ocimum basilicum L. var. Grand Vert) plants. J. Agric. Rural. Res. 2020, 5, 1–22. Available online: http://aiipub.com/journals/jarr-200609-010106/ (accessed on 23 February 2025).
- Satpute, A.; Meyering, B.; Albrecht, U. Preharvest abscisic acid application to alleviate chilling injury of sweet basil (Ocimum basilicum L.) during cold storage. HortScience 2019, 54, 155–161. [Google Scholar] [CrossRef]
- Valiolahi, M.; Najafi, M.A.; Eskandani, M.A.; Rahnama, M. Effects of organic acid alone and in combination with H2O2 and NaCl on Escherichia coli O157:H7: An evaluation of antioxidant retention and overall acceptability in basil leaves (Ocimum basilicum). Int. J. Food Microbiol. 2019, 292, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ku, V.V.V.; Wills, R.B.H.; Leshem, Y. Use of nitric oxide to reduce postharvest water loss from horticultural produce. J. Hortic. Sci. Biotechnol. 2015, 75, 268–270. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium enrichment enhances the quality and shelf life of basil leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Heckman, J.; Wyenandt, A.; Mattson, N.; Durner, E.; Both, A.J. Potential benefits of silicon nutrition to hydroponically grown sweet basil. HortScience 2020, 55, 1799–1803. [Google Scholar] [CrossRef]
- Joudmand, A.; Hajiboland, R. Silicon mitigates cold stress in barley plants via modifying the activity of apoplasmic enzymes and concentration of metabolites. Acta Physiol. Plant. 2019, 41, 29. [Google Scholar] [CrossRef]
- Mvondo-She, M.A.; Mashilo, J.; Gatabazi, A.; Ndhlala, A.R.; Laing, M.D. Exogenous silicon application improves chilling injury tolerance and photosynthetic performance of citrus. Agronomy 2024, 14, 139. [Google Scholar] [CrossRef]
- Vu, N.-T.; Kim, S.-H.; Kim, S.-Y.; Choi, K.-Y.; Kim, I.-S. Effect of silicate fertilizer on growth, physiology and abiotic stress tolerance of Chinese cabbage seedlings. J. Bio Environ. Control. 2015, 24, 51–56. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Xu, P.; Wang, X.; Bai, J. Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed cucumber leaves. Agric. Sci. China 2009, 8, 1075–1086. [Google Scholar] [CrossRef]
- Saad, M.M. Effect of some postharvest treatments on reducing chilling injury of cucumber fruits during cold storage. Ann. Agric. Sci. Moshtohor 2019, 57, 455–468. [Google Scholar] [CrossRef][Green Version]
- Habibi, G. Effect of soil- and foliar-applied silicon on the resistance of grapevine plants to freezing stress. Acta Biol. Szeged. 2015, 59, 109–117. Available online: https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2874 (accessed on 18 November 2024).
- Habibi, G. Effect of foliar-applied silicon on photochemistry, antioxidant capacity and growth in maize plants subjected to chilling stress. Acta Agric. Slov. 2016, 107, 33–43. [Google Scholar] [CrossRef]
- Bashir, S.; John, R. Alleviation of chilling stress by supplementation of brassinosteroid and silicon in Solanum lycopersicum L. Plant Soil 2023, 486, 165–181. [Google Scholar] [CrossRef]
- Vu, N.-T.; Tran, A.-T.; Le, T.-T.-C.; Na, J.-K.; Kim, S.-H.; Park, J.-M.; Jang, D.-C.; Kim, I.-S. Improvement of tomato seedling quality under low temperature by application of silicate fertilizer. J. Bio-Environ. Control. 2017, 26, 158–166. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- He, Y.; Xiao, H.; Wang, H.; Chen, Y.; Yu, M. Effect of silicon on chilling-induced changes of solutes, antioxidants, and membrane stability in seashore paspalum turfgrass. Acta Physiol. Plant. 2009, 32, 487–494. [Google Scholar] [CrossRef]
- Wongsheree, T.; Ketsa, S.; van Doorn, W.G. The relationship between chilling injury and membrane damage in lemon basil (Ocimum×citriodourum) leaves. Postharvest Biol. Technol. 2009, 51, 91–96. [Google Scholar] [CrossRef]
- Campos, P.S.; Quartin, V.; Ramalho, J. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef]
- Nesi, C.N.; Shimakura, S.E.; Junior, P.J.R.; Mio, L.L.M.D. Survival analysis: A tool in the study of post-harvest diseases in peaches. Rev. Ceres 2015, 62, 52–61. [Google Scholar] [CrossRef]
- Bélanger, R. (Département de phytologie, Université Laval, Quebec City, ON, Canada). Silicic Acid Water Extraction and Plant-available Silicon from the Mineral Wollastonite. Unpublished Work. Available online: https://canadianwollastonite.com/wp-content/uploads/2020/05/Silicic-acid-water-extraction-and-plant-available-silicon-from-the-mineral-Wollastonite.pdf (accessed on 24 February 2025).
- Castro-Cegrí, A.; Sierra, S.; Hidalgo-Santiago, L.; Esteban-Muñoz, A.; Jamilena, M.; Garrido, D.; Palma, F. Postharvest treatment with abscisic acid alleviates chilling injury in zucchini fruit by regulating phenolic metabolism and non-enzymatic antioxidant system. Antioxidants 2023, 12, 211. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, R.G.; Gong, Z.H.; Yin, Y.X.; Ahmed, S.S.; He, Y.M. Exogenous abscisic acid increases antioxidant enzymes and related gene expression in pepper (Capsicum annuum) leaves subjected to chilling stress. Genet. Mol. Res. GMR 2012, 11, 4063–4080. [Google Scholar] [CrossRef]
- Hongtao, X.; Tongtong, W.; Dianfeng, Z.; Lizhi, W.; Yanjiang, F.; Yu, L.; Rui, L.; Zhongjie, L.; Ying, M.; Wan, L.; et al. ABA pretreatment enhances the chilling tolerance of a chilling-sensitive rice cultivar. Braz. J. Bot. 2017, 40, 853–860. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, G.; Nayyar, H. Exogenous application of abscisic acid improves cold tolerance in chickpea (Cicer arietinum L.). J. Agron. Crop Sci. 2008, 194, 449–456. [Google Scholar] [CrossRef]
- Manzi, M.; Hernández-Mazzini, F.; Pintos, P.; Lado, J. Abscisic acid preharvest application alleviates chilling injury in oranges during cold storage. J. Hortic. Sci. Biotechnol. 2022, 97, 747–756. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Marketable Plants (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| D2 | D4 | D6 | D8 | D10 | D12 | D14 | Sig. Differences | |
| CK | 71 | 14 | 14 | 14 | 0 | 0 | 0 | a |
| CK+DI | 86 | 29 | 29 | 29 | 14 | 14 | 14 | ab |
| Si-irrigation+DI | 100 | 100 | 100 | 86 | 86 | 86 | 86 | d |
| DI-spray+DI | 100 | 57 | 43 | 29 | 29 | 29 | 14 | abc |
| Si-spray+DI | 100 | 100 | 100 | 100 | 100 | 100 | 71 | d |
| CK+ABA | 100 | 86 | 71 | 71 | 43 | 0 | 0 | bc |
| Si-irrigation+ABA | 100 | 100 | 100 | 86 | 29 | 14 | 0 | c |
| DI-spray+ABA | 100 | 86 | 57 | 43 | 29 | 0 | 0 | abc |
| Si-spray+ABA | 100 | 100 | 100 | 86 | 14 | 14 | 0 | c |
| Treatment | FWL (%) | ||||||
|---|---|---|---|---|---|---|---|
| D2 | D4 | D6 | D8 | D10 | D12 | D14 | |
| CK | 1.5 ± 0.1 a | 5.6 ± 0.4 ab | 9.5 ± 0.4 a | 14.1 ± 0.6 a | 18.6 ± 0.7 a | 23.8 ± 1.0 a | 28.6 ± 1.2 a |
| CK+DI | 1.5 ± 0.1 a | 4.3 ± 0.4 abc | 7.9 ± 0.9 ab | 11.1 ± 1.0 ab | 14.3 ± 1.0 abc | 18.6 ± 1.2 ab | 21.3 ± 1.4 bc |
| Si-irrigation+DI | 1.6 ± 0.1 a | 3.9 ± 0.3 bc | 6.8 ± 0.2 b | 10.7 ± 0.4 b | 13.8 ± 0.4 c | 17.3 ± 0.4 b | 20.1 ± 0.4 c |
| DI-spray+DI | 1.5 ± 0.1 a | 3.8 ± 0.3 bc | 6.4 ± 0.4 b | 9.6 ± 0.4 b | 13.1 ± 0.4 c | 17.1 ± 0.9 b | 20.1 ± 1.1 c |
| Si-spray+DI | 1.6 ± 0.2 a | 3.5 ± 0.2 c | 6.6 ± 0.3 b | 10.9 ± 0.7 ab | 14.1 ± 0.8 bc | 17.8 ± 0.8 b | 20.6 ± 0.8 c |
| CK+ABA | 1.7 ± 0.1 a | 5.6 ± 0.2 a | 9.7 ± 0.3 a | 13.5 ± 0.3 a | 16.8 ± 0.4 ab | 21.1 ± 0.9 ab | 25.1 ± 1.1 abc |
| Si-irrigation+ABA | 2.0 ± 0.2 a | 6.0 ± 0.3 a | 8.9 ± 0.6 ab | 13.3 ± 0.9 ab | 16.9 ± 1.1 abc | 21.6 ± 1.1 ab | 26.4 ± 1.2 ab |
| DI-spray+ABA | 1.7 ± 0.1 a | 5.5 ± 0.5 abc | 10.0 ± 0.6 a | 13.4 ± 0.5 a | 16.6 ± 0.5 ab | 20.5 ± 0.8 ab | 24.4 ± 0.9 abc |
| Si-spray+ABA | 1.5 ± 0.1 a | 4.4 ± 0.4 abc | 8.3 ± 0.9 ab | 11.5 ± 0.8 ab | 14.2 ± 0.8 bc | 18.0 ± 0.9 b | 21.8 ± 1.0 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, V.; Zheng, Y. Alleviation of Chilling Injury in Postharvest Sweet Basil (Ocimum basilicum L.) with Silicon and Abscisic Acid Applications. Agriculture 2025, 15, 643. https://doi.org/10.3390/agriculture15060643
Ly V, Zheng Y. Alleviation of Chilling Injury in Postharvest Sweet Basil (Ocimum basilicum L.) with Silicon and Abscisic Acid Applications. Agriculture. 2025; 15(6):643. https://doi.org/10.3390/agriculture15060643
Chicago/Turabian StyleLy, Vivian, and Youbin Zheng. 2025. "Alleviation of Chilling Injury in Postharvest Sweet Basil (Ocimum basilicum L.) with Silicon and Abscisic Acid Applications" Agriculture 15, no. 6: 643. https://doi.org/10.3390/agriculture15060643
APA StyleLy, V., & Zheng, Y. (2025). Alleviation of Chilling Injury in Postharvest Sweet Basil (Ocimum basilicum L.) with Silicon and Abscisic Acid Applications. Agriculture, 15(6), 643. https://doi.org/10.3390/agriculture15060643








