Crop Performance and Photochemical Processes Under a UV-to-Red Spectral Shifting Greenhouse: A Study on Aubergine and Strawberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Crop Management
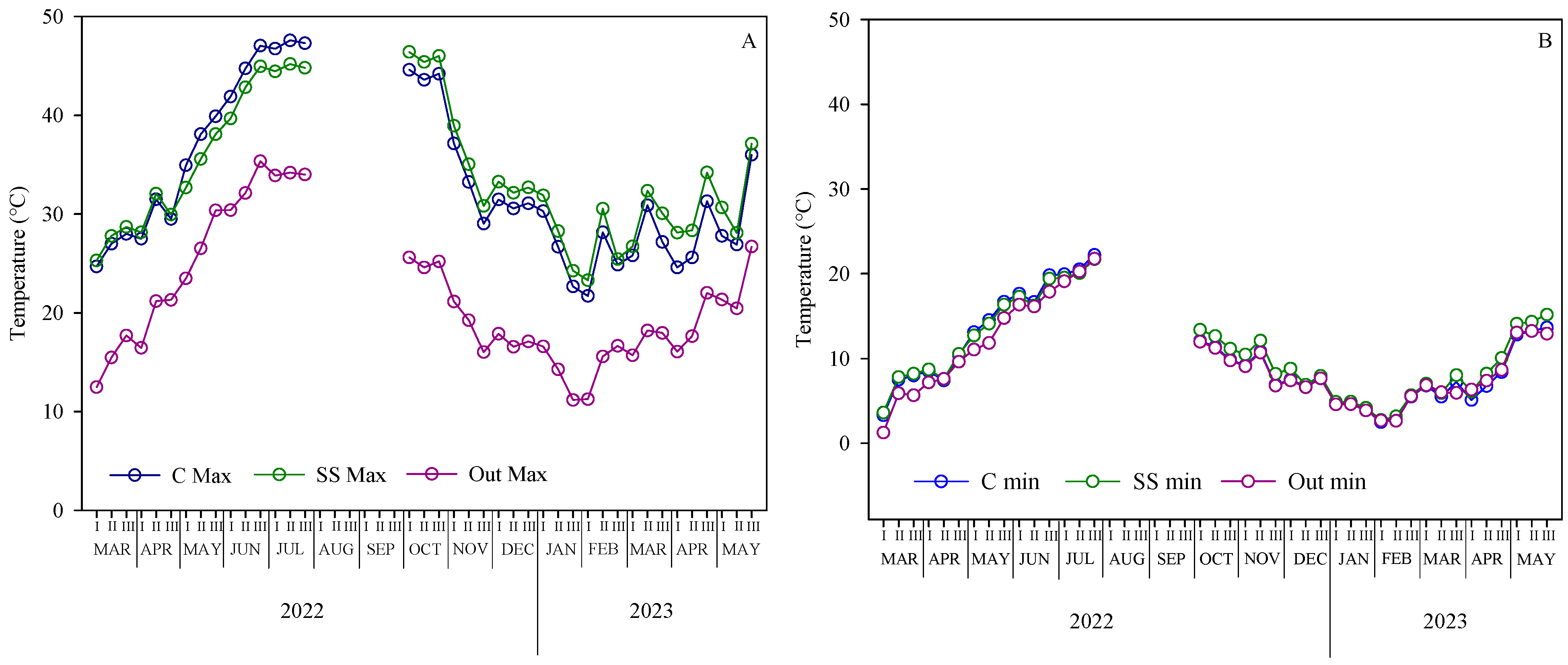
2.2. Temperature Pattern Under the Greenhouse
2.3. Analytical Methods
2.3.1. Yield
2.3.2. Fruit Quality Parameters
2.3.3. Chemical Analyses
2.4. Chlorophyll Fluorescence Measurements
| Parameters | Definitions |
|---|---|
| F0 ≅ F40µs | minimal fluorescence, when all PSII RCs are open (≅to the minimal reliable recorded fluorescence) |
| FJ ≡ F2ms | fluorescence at the J-step (2 ms) of OJIP |
| FI ≡ F30ms | fluorescence at the I-step (30 ms) of OJIP |
| FM (=FP) | maximal fluorescence, when all PSII RCs are closed (=FP when the actinic light intensity is above 500 µmol photons m−2 s−1) |
| Fv = FM − F0 | maximal variable fluorescence |
| VJ = FJ/Fv = (FJ − F0)/(FM − F0) | relative variable fluorescence at the J-step (2 ms) |
| VI = FI/Fv = (FI − F0)/(FM − F0) | relative variable fluorescence at the I-step (30 ms) |
| FM/F0 | Maximum to background chlorophyll fluorescence ratio |
| Fv/F0 | Variable to background chlorophyll fluorescence ratio |
| Fv/FM (=φP0) | maximum quantum yield of PSII photochemistry |
| M0 | approx. initial slope (in ms−1) of the fluorescence transient normalised on the maximal variable fluorescence Fv = FM − F0 |
| Area | total complementary area between the fluorescence induction curve and F = FP |
| Sm = Area/Fv = EC0/RC | Normalized area, a measure of the energy needed to close all Reaction Centres. Refers to the multiple turn-over in the closure of the reaction centres from time F0 up to the time FM. |
| Ss | The smallest Sm, corresponding to the case when every QA is reduced only once. Single turn-over. |
| N = Sm/Ss | Turn-over number, expresses how many times QA is reduced in the time interval from F0 to FM |
| Quantum yields and efficiencies or probabilities | |
| φP0 (=Fv/FM) | Maximum quantum yield of PSII photochemistry |
| ψ0 | Efficiency with which a trapped exciton can move an electron further than QA into the electron transport chain |
| φE0 | Quantum yield of the electron transport flux from QA to QB |
| φD0 | Quantum yield for energy dissipation |
| φPav | Quantum yield of the electron transport flux until the PSI electron acceptors |
| Specific energy fluxes (per active PSII RC) | |
| ABS/RC | Absorbed photon flux per PSII Reaction Centre (apparent antenna size of an active PSII) |
| TR0/RC | Trapped energy flux (leading to QA reduction) per PSII Reaction Centre |
| ET0/RC | Electron transport flux beyond QA per PSII Reaction Centre |
| DI0/RC | Dissipated energy flux per PSII Reaction Centre |
| Performance index | |
| PIAbs | Performance index for energy conservation from photons absorbed by PSII antenna until the reduction in intersystem electron acceptors |
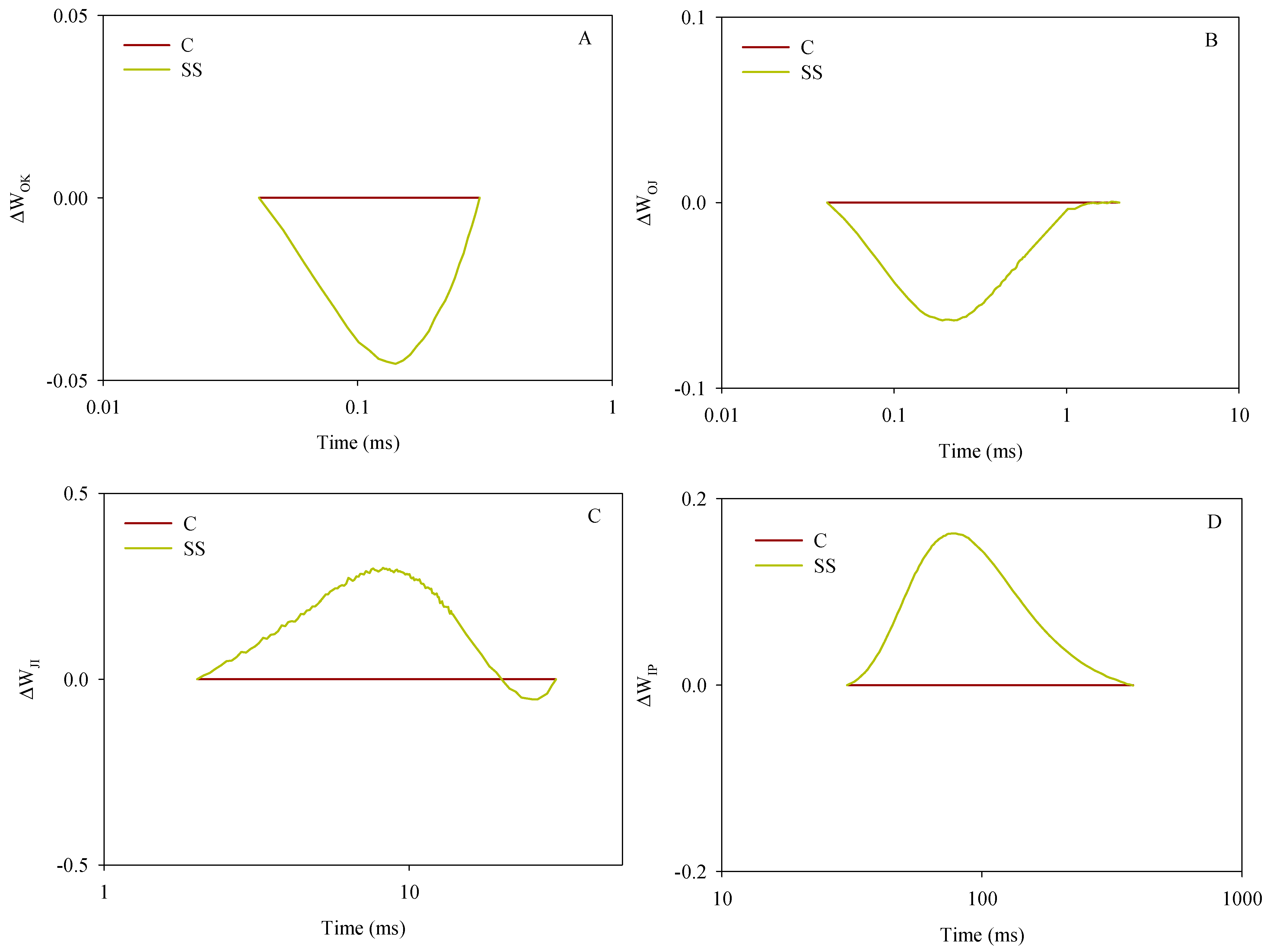
- Between O (at 40 µs on the fluorescence induction curve) and K (at 300 μs), calculated as WOK = (Ft − F0)/(FK − F0); after normalization, the difference kinetics were calculated as ΔWOK = WOK(SS) − WOK(C), evidencing the L-band;
- Between O and J (2 ms), calculated as WOJ = (Ft − F0)/(FJ − F0), with the ΔWOJ evidencing the K-band;
- Between J and I (30 ms), calculated as WJI = (Ft − FJ)/(FI − FJ), with the ΔWJI evidencing the H-band;
- Between I and P (peak of the induction curve), calculated as WIP = (Ft − FI)/(FP − FI), with the ΔWIP evidencing the G-band.
2.5. Statistical Analysis
3. Results
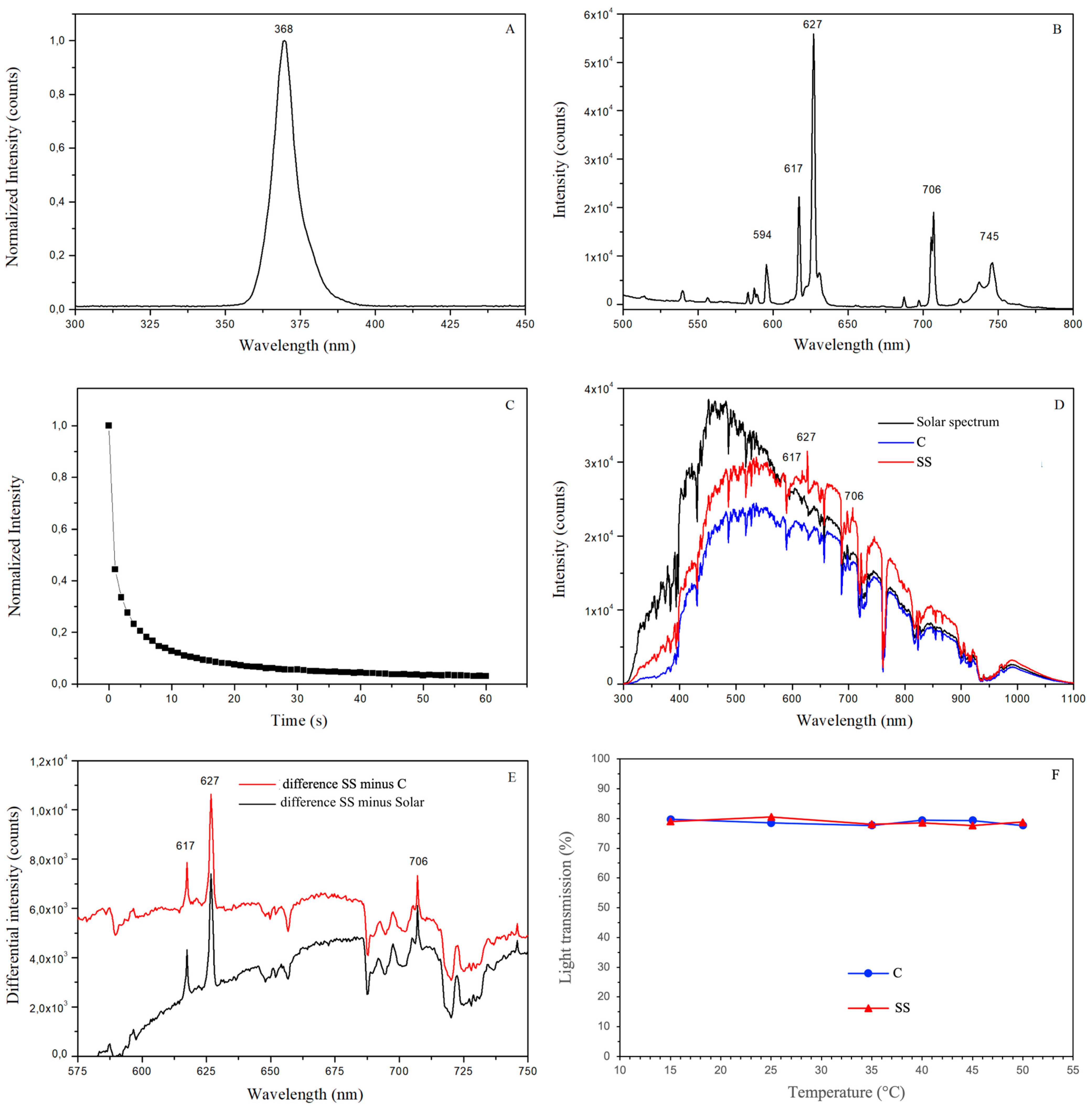
3.1. Optical Properties of the Spectral Shifting Greenhouse Cover
3.1.1. Spectrophotometric Analysis
3.1.2. Light Transmission at Variable Incident Radiation Intensity
3.1.3. Light Transmission at Variable Temperatures of the Greenhouse Cover Film
3.2. Aubergine (Spring–Summer Crop)
3.2.1. Production and Quality
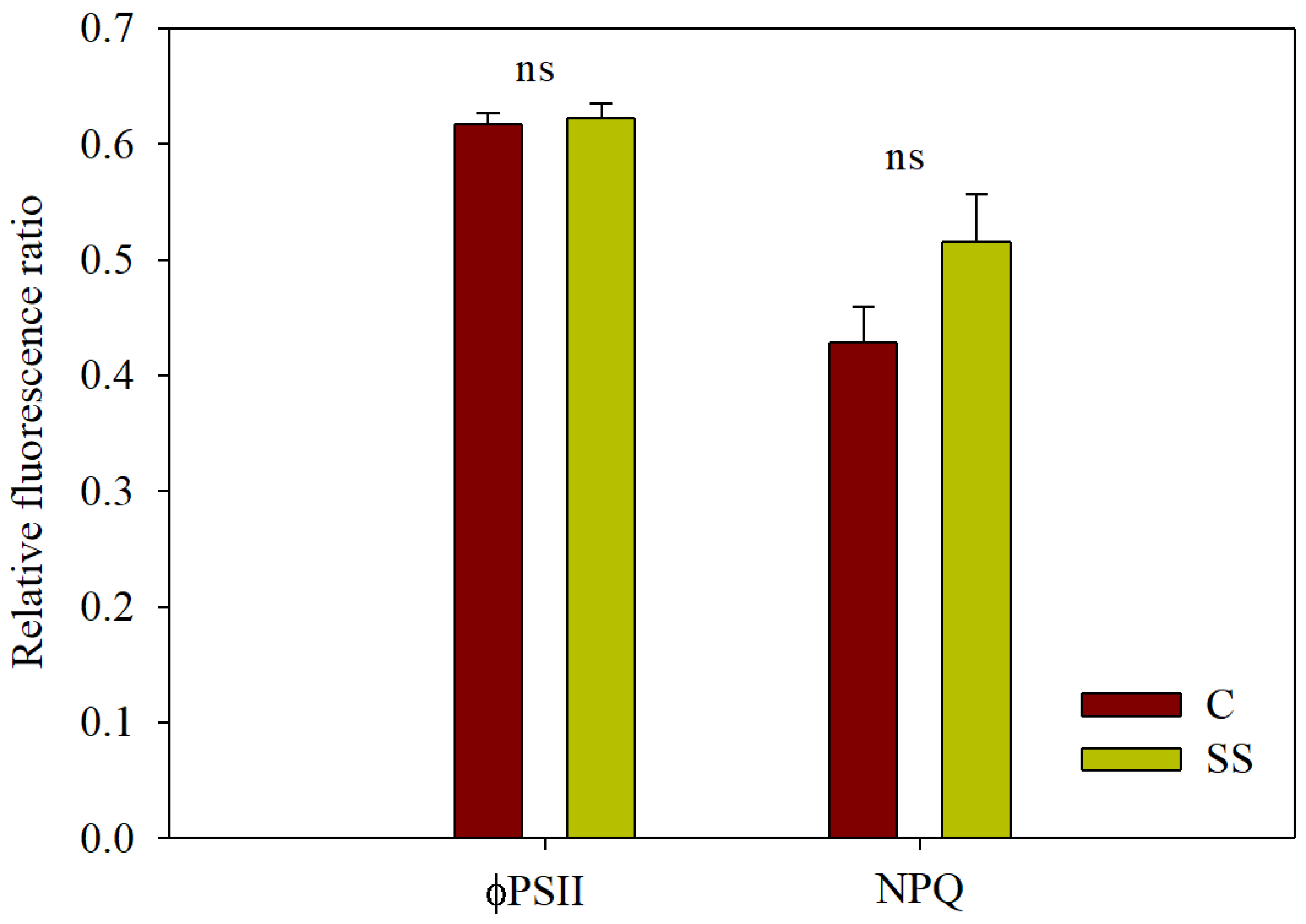
3.2.2. Chl a Fluorescence and JIP-Test Parameters
3.3. Strawberry (Autumn–Spring Crop)
3.3.1. Production and Quality
3.3.2. Chl a Fluorescence and JIP-Test Parameters
4. Discussion
4.1. Cover Film Properties
4.2. Aubergine (Spring–Summer Crop)
4.3. Strawberry (Autumn–Spring Crop)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Govindjee, G.; Govindjee, R. Introduction to Photosynthesis. In Bioenergetics of Photosynthesis; Govindjee, Ed.; Academic Press: New York, NY, USA, 1975; pp. 1–50. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. (Eds.) World Agriculture Towards 2030/2050: The 2012 Revision; ESA Working Papers 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- WMO (World Meteorological Organization). The Global Climate 2011-2020 A Decade of Accelerating Climate Change; WMO: Geneva, Switzerland, 2023; Volume 1338, ISBN 978-92-63-11338-2. [Google Scholar]
- WMO (World Meteorological Organization). State of the Global Climate 2023; WMO: Geneva, Switzerland, 2024; Volume 1347, ISBN 978-92-63-11347-4. [Google Scholar]
- Devlin, P.F.; Christie, J.M.; Terry, M.J. Many Hands Make Light Work. J. Exp. Bot. 2007, 58, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Hisamatsu, T. Light Acts as a Signal for Regulation of Growth and Development. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Shimizu, H. Effect of Light Quality on Secondary Metabolite Production in Leafy Greens and Seedlings. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of Photosynthetic Processes and the Accumulation of Secondary Metabolites in Plants in Response to Monochromatic Light Environments: A Review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Kudo, R.; Yamamoto, K. Induction of Plant Disease Resistance and Other Physiological Responses by Green Light Illumination. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Lauria, G.; Lo Piccolo, E.; Ceccanti, C.; Guidi, L.; Bernardi, R.; Araniti, F.; Cotrozzi, L.; Pellegrini, E.; Moriconi, M.; Giordani, T.; et al. Supplemental Red LED Light Promotes Plant Productivity, “Photomodulates” Fruit Quality and Increases Botrytis Cinerea Tolerance in Strawberry. Postharvest Biol. Technol. 2023, 198, 112253. [Google Scholar] [CrossRef]
- Mitchell, K.J. Influence of Light and Temperature on the Growth of Ryegrass (Lolium Spp.): I. Pattern of Vegetative Development. Physiol. Plant. 1953, 6, 21–46. [Google Scholar] [CrossRef]
- Wilson, D.; Cooper, J.P. Effect of Light Intensity during Growth on Leaf Anatomy and Subsequent Light-Saturated Photosynthesis among Contrasting Lolium Genotypes. New Phytol 1969, 68, 1125–1135. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.; Park, J.; Lee, J. Influence of Green, Red and Blue Light Emitting Diodes on Multiprotein Complex Proteins and Photosynthetic Activity under Different Light Intensities in Lettuce Leaves (Lactuca Sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological Responses of Cucumber Seedlings under Different Blue and Red Photon Flux Ratios Using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee; Zhu, X.-G. Changes in the Photosynthesis Properties and Photoprotection Capacity in Rice (Oryza Sativa) Grown under Red, Blue, or White Light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Lauria, G.; Ceccanti, C. Effects of Light Modulation on Plant Photosynthesis Process and Secondary Metabolism. Agrochimica 2021, 65, 45–51. [Google Scholar] [CrossRef]
- Lu, N.; Mitchell, C.A. Supplemental lighting for greenhouse-grown fruiting vegetables. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Ilić, Z.S.; Fallik, E. Light Quality Manipulation Improves Vegetable Quality at Harvest and Postharvest: A Review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Wondraczek, L.; Tyystjärvi, E.; Méndez-Ramos, J.; Müller, F.A.; Zhang, Q. Shifting the Sun: Solar Spectral Conversion and Extrinsic Sensitization in Natural and Artificial Photosynthesis. Adv. Sci. 2015, 2, 1500218. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Lou, R.; Park, Y.; Guo, Y.; Stallknecht, E.J.; Xiao, Y.; Rieder, D.; Yang, R.; Runkle, E.S.; Yin, X. Increasing Greenhouse Production by Spectral-Shifting and Unidirectional Light-Extracting Photonics. Nat. Food 2021, 2, 434–441. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Costa, J.M.; Heuvelink, E. Achieving Sustainable Greenhouse Production: Present Status, Recent Advances and Future Developments. In Achieving Sustainable Greenhouse Production; Heuvelink, E., Marcelis, L.F.M., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 1–14. ISBN 978-0-429-26674-4. [Google Scholar]
- Katzin, D.; Marcelis, L.F.M.; Mourik, S. Energy Savings in Greenhouses by Transition from High-Pressure Sodium to LED Lighting. Appl. Energy 2021, 281, 116019. [Google Scholar] [CrossRef]
- Liu, Y.; Gui, Z.; Liu, J. Research Progress of Light Wavelength Conversion Materials and Their Applications in Functional Agricultural Films. Polymers 2022, 14, 851. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Bartak, M.; Cozzolino, E.; Ottaiano, L.; Giordano, D.; Melchionna, G.; Mormile, P.; Rippa, M.; Beltrame, L.; et al. Greenhouse Photoluminescent PMMA Panels Improve the Agronomical and Physiological Performances of Lettuce (Lactuca Sativa L.). Horticulturae 2022, 8, 913. [Google Scholar] [CrossRef]
- Paradiso, R.; Mola, I.; Conti, S.; Ottaiano, L.; Cozzolino, E.; Rippa, M.; Mormile, P.; Melchionna, G.; Testa, A.; Beltrame, L.; et al. Photosynthesis, Yield and Quality in Wild Rocket (Diplotaxis Tenuifolia L.) under Photoluminescent Greenhouse Covers. Agronomy 2023, 13, 2372. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Roháček, K.; Soukupová, J.; Barták, M. Chlorophyll Fluorescence: A Wonderful Tool to Study Plant Physiology and Plant Stress. In Plant Cell Compartments—Selected Topics; Schoefs, B., Ed.; Research Signpost: Kerala, India, 2008; pp. 41–104. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence—A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19. [Google Scholar]
- Tsimilli-Michael, M. Revisiting JIP-Test: An Educative Review on Concepts, Assumptions, Approximations, Definitions and Terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Hargreaves, G.H.; Samani, Z.A. Reference Crop Evapotranspiration from Temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and Determination of Ascorbate and Dehydroascorbate from Plant Tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for Measuring Antioxidant Activity and Its Application to Monitoring the Antioxidant Capacity of Wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Paradiso, R.; Di Mola, I.; Cozzolino, E.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Nutrient and Nutraceutical Quality of Rocket as a Function of Greenhouse Cover Film, Nitrogen Dose and Biostimulant Application. Agronomy 2023, 13, 638. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the Xanthophyll Cycle in Photoprotection Elucidated by Measurements of Light-Induced Absorbance Changes, Fluorescence and Photosynthesis in Leaves of Hedera Canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Dangre, D.; Rai, M. Biophysical Phenomics Reveals Functional Building Blocks of Plants Systems Biology: A Case Study for the Evaluation of the Impact of Mycorrhization with Piriformospora Indica. In Advanced Techniques in Soil Microbiology. Soil Biology; Varma, A., Oelmüller, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 11. [Google Scholar]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll Fluorescence as a Tool for Nutrient Status Identification in Rapeseed Plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Stirbet, A. Govindjee On the Relation between the Kautsky Effect (Chlorophyll a Fluorescence Induction) and Photosystem II: Basics and Applications of the OJIP Fluorescence Transient. J. Photochem. Photobiol. B 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Strasser, R.J.; Stirbet, A.D. Heterogeneity of Photosystem II Probed by the Numerically Simulated Chlorophyll a Fluorescence Rise (O-J-I-P). Math. Comput. Simul. 1998, 48, 3–9. [Google Scholar] [CrossRef]
- Ruban, A. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Correlation between Strawberry (Fragaria Ananassa Duch.) Productivity and Photosynthesis-Related Parameters under Various Growth Conditions. Front. Plant Sci. 2016, 7, 1607. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Nomura, K.; Saito, M.; Ito, M.; Yamane, S.; Iwao, T.; Tada, I.; Yamazaki, T.; Ono, S.; Yasutake, D.; Kitano, M. Diurnal Decline in the Photosynthetic Capacity of Uppermost Leaves in an Eggplant Canopy Grown in a Horticultural Greenhouse. Photosynthetica 2022, 60, 457–464. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Goltsev, V.N.; Zuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Holleboom, C.-P.; Gacek, D.A.; Liao, P.-N.; Negretti, M.; Croce, R.; Walla, P.J. Carotenoid–Chlorophyll Coupling and Fluorescence Quenching in Aggregated Minor PSII Proteins CP24 and CP29. Photosynth. Res. 2015, 124, 171–180. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Sieczko, L.; Borucki, W.; Sujkowska-Rybkowska, M.; Mirgos, M.; Niedzińska, M.; Bederska-Błaszczyk, M.; Kowalczyk, W.; Geszprych, A.; Gajc-Wolska, J. The Effect of LED and HPS Assimilation Lighting on Leaf Anatomy, Chlorophyll and Carotenoid Autofluorescence Signals, and Some Physiological and Chemical Leaf Traits Related to the Productivity of Cucumber (Cucumis Sativus L.) in High-Wire Cultivation. Agronomy 2022, 12, 2004. [Google Scholar] [CrossRef]
- Sardar, S.; Caferri, R.; Camargo, F.V.A.; Capaldi, S.; Ghezzi, A.; Dall’Osto, L.; D’Andrea, C.; Cerullo, G.; Bassi, R. Site-Directed Mutagenesis of the Chlorophyll-Binding Sites Modulates Excited-State Lifetime and Chlorophyll–Xanthophyll Energy Transfer in the Monomeric Light-Harvesting Complex CP29. J. Phys. Chem. Lett. 2024, 15, 3149–3158. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in Vivo Recording of Prompt and Delayed Fluorescence and 820-Nm Reflection Changes during Drying and after Rehydration of the Resurrection Plant Haberlea Rhodopensis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Esmaeilizadeh, M.; Shamsabad, M.R.M.; Roosta, H.R.; Dąbrowski, P.; Rapacz, M.; Zieliński, A.; Wróbel, J.; Kalaji, H.M. Manipulation of Light Spectrum Can Improve the Performance of Photosynthetic Apparatus of Strawberry Plants Growing under Salt and Alkalinity Stress. PLoS ONE 2021, 16, e0261585. [Google Scholar] [CrossRef]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S.; Seif, M. Photosynthetic and Growth Responses of Green and Purple Basil Plants under Different Spectral Compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef]
- Cammarisano, L. Illuminating Future Food Production: The Impacts of Tuneable Artificial Lighting on Food Crop Biology and Productivity. Ph.D. Thesis, Institute of Biological, Environmental and Rural Sciences, Aberystwyth University, Aberystwyth, UK, 2020. [Google Scholar]
- Küpper, H.; Benedikty, Z.; Morina, F.; Andresen, E.; Mishra, A.; Trtílek, M. Analysis of OJIP Chlorophyll Fluorescence Kinetics and QA Reoxidation Kinetics by Direct Fast Imaging. Plant Physiol. 2019, 179, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Vredenberg, W.J. Algorithm for Analysis of OJDIP Fluorescence Induction Curves in Terms of Photo- and Electrochemical Events in Photosystems of Plant Cells: Derivation and Application. J. Photochem. Photobiol. B Biol. 2008, 91, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tsimilli-Michael, M.; Strasser, R.J. The Energy Flux Theory 35 Years Later: Formulations and Applications. Photosynth. Res. 2013, 117, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of (γ-Tocopherol Methyl Transferase Gene in Transgenic Brassica Juncea Plants Alleviates Abiotic Stress: Physiological and Chlorophyll a Fluorescence Measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.C.; Yang, L.Y.; Zhou, L. Effect of Different LED-Lighting Quality Conditions on Growth and Photosynthetic Characteristics of Saffron Plants (Crocus Sativus L.). Photosynthetica 2022, 60, 497–507. [Google Scholar] [CrossRef]
- Dimitrova, S.; Paunov, M.; Pavlova, B.; Dankov, K.; Kouzmanova, M.; Velikova, V.; Tsonev, T.; Kalaji, H.M.; Goltsev, V. Photosynthetic Efficiency of Two Platanus Orientalis L. Ecotypes Exposed to Moderately High Temperature—JIP-Test Analysis. Photosynthetica 2020, 58, 657–670. [Google Scholar] [CrossRef]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP Amplitude of the Fluorescence Rise OJIP Is Sensitive to Changes in the Photosystem I Content of Leaves: A Study on Plants Exposed to Magnesium and Sulfate Deficiencies, Drought Stress and Salt Stress. Physiol. Plant. 2012, 144, 277–288. [Google Scholar] [CrossRef]
- Zagorchev, L.; Atanasova, A.; Albanova, I.; Traianova, A.; Mladenov, P.; Kouzmanova, M.; Goltsev, V.; Kalaji, H.M.; Teofanova, D. Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta Campestris by JIP-Test. Cells 2021, 10, 1399. [Google Scholar] [CrossRef]
- Novoplansky, A.; Sachs, T.; Cohen, D.; Bar, R.; Bodenheimer, J.; Reisfeld, R. Increasing Plant Productivity by Changing the Solar Spectrum. Sol. Energy Mater. 1990, 21, 17–23. [Google Scholar] [CrossRef]
- Hemming, S.; van Os, E.A.; Hemming, J.; Dieleman, J.A. The Effect of New Developed Fluorescent Greenhouse Films on the Growth of Fragaria x Ananassa ‘Elsanta’. Eur. J. Hort. Sci. 2006, 71, 145–154. [Google Scholar]
- Hidaka, K.; Yoshida, K.; Shimasaki, K.; Murakami, K.; Yasutake, D.; Kitano, M. Spectrum Conversion Film for Regulation of Plant Growth. J. Fac. Agric. Kyushu Univ. 2008, 53, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, D.; Yoon, H.I.; Son, J.E. Growth, Morphology, and Photosynthetic Activity of Chinese Cabbage and Lettuce Grown under Polyethylene and Spectrum Conversion Films. Hortic. Environ. Biotechnol. 2023, 64, 593–603. [Google Scholar] [CrossRef]
- Li, Y.; Tu, W.; Liu, C.; Liu, W.; Hu, G.; Liu, X.; Chen, Z.; Yang, C. Light Conversion Film Promotes CO 2 Assimilation by Increasing Cyclic Electron Flow around Photosystem I in Arabidopsis Thaliana. Int. J. Hydrog. Energy 2017, 42, 8545–8553. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kang, J.H.; Kang, W.H.; Son, J.E. Subtle Changes in Solar Radiation under a Green-to-Red Conversion Film Affect the Photosynthetic Performance and Chlorophyll Fluorescence of Sweet Pepper. Photosynthetica 2020, 58, 1107–1115. [Google Scholar] [CrossRef]
- Hernández-Martínez, N.R.; Blanchard, C.; Wells, D.; Salazar-Gutiérrez, M.R. Current State and Future Perspectives of Commercial Strawberry Production: A Review. Sci. Hortic. 2023, 312, 111893. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED Light on the Production of Strawberry during Cultivation in a Plastic Greenhouse and in a Growth Chamber. Sci. Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, X.; Gu, X.; Deng, M.; Li, X.; Zhou, A.; Suo, M.; Gao, W.; Lin, Y.; Wang, Y. Light Quality and Sucrose-Regulated Detached Ripening of Strawberry with Possible Involvement of Abscisic Acid and Auxin Signaling. Int. J. Mol. Sci. 2023, 24, 5681. [Google Scholar] [CrossRef]
- Roosta, H.R.; Bikdeloo, M.; Ghorbanpour, M. The Growth, Nutrient Uptake and Fruit Quality in Four Strawberry Cultivars under Different Spectra of LED Supplemental Light. BMC Plant Biol. 2024, 24, 179. [Google Scholar] [CrossRef]
- Lauria, G.; Lo Piccolo, E.; Pellegrini, E.; Bellini, E.; Giordani, T.; Guidi, L.; Lorenzini, G.; Malorgio, F.; Massai, R.; Nali, C.; et al. Photosynthetic Traits and Biochemical Responses in Strawberry (Fragaria × Ananassa Duch.) Leaves Supplemented with LED Lights. Photosynthetica 2021, 59, 557–569. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; Kooten, O.; Harbinson, J.; Ieperen, W. Plasticity of Photosynthesis after the ‘Red Light Syndrome’ in Cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Kang, J.H.; Yoon, H.I.; Lee, J.M.; Kim, J.P.; Son, J.E. Electron Transport and Photosynthetic Performance in Fragaria × Ananassa Duch. Acclimated to the Solar Spectrum Modified by a Spectrum Conversion Film. Photosynth. Res. 2022, 151, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Lo Piccolo, E.; Ceccanti, C.; Paoli, L.; Giordani, T.; Guidi, L.; Malorgio, F.; Massai, R.; Nali, C.; Pellegrini, E.; et al. Supplemental Red Light More than Other Wavebands Activates Antioxidant Defenses in Greenhouse-Cultivated Fragaria x Ananassa Var. Elsanta Plants. Sci. Hortic. 2023, 321, 112319. [Google Scholar] [CrossRef]
- Guiamba, H.D.S.S.; Zhang, X.; Sierka, E.; Lin, K.; Ali, M.M.; Ali, W.M.; Lamlom, S.F.; Kalaji, H.M.; Telesiński, A.; Yousef, A.F.; et al. Enhancement of Photosynthesis Efficiency and Yield of Strawberry (Fragaria Ananassa Duch.) Plants via LED Systems. Front. Plant Sci. 2022, 13, 918038. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of Red and Blue Light on Anthocyanin Accumulation and Differential Gene Expression in Strawberry (Fragaria × Ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef]
- Canora, D.D.; Guasch, L.L.; Zuazo, R.S. Species-Specific Responses of Antarctic Terrestrial Microalgae to Salinity Stress. Comparative Study in Klebsormidium sp. and Stigeoclonium sp. Czech Polar Rep. 2022, 12, 89–102. [Google Scholar] [CrossRef]
- Hussain, N.; Sohail, Y.; Shakeel, N.; Javed, M.; Bano, H.; Gul, H.S.; Zafar, Z.U.; Frahat Zaky Hassan, I.; Ghaffar, A.; Athar, H.U.; et al. Role of Mineral Nutrients, Antioxidants, Osmotic Adjustment and PSII Stability in Salt Tolerance of Contrasting Wheat Genotypes. Sci. Rep. 2022, 12, 12677. [Google Scholar] [CrossRef]
- Arnold, A.L.M.; McGrath, C.; Reinhardt, A. Effects of Oak Processionary Moth (Thaumetopoea Processionea L.) Outbreaks on the Leaf Performance and Health of Urban and Forest Oak Trees (Quercus Robur L.) in Brandenburg, Germany. Forests 2023, 14, 124. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of Chlorophyll Fluorescence Can Improve Crop Production Strategies: An Examination of Future Possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]


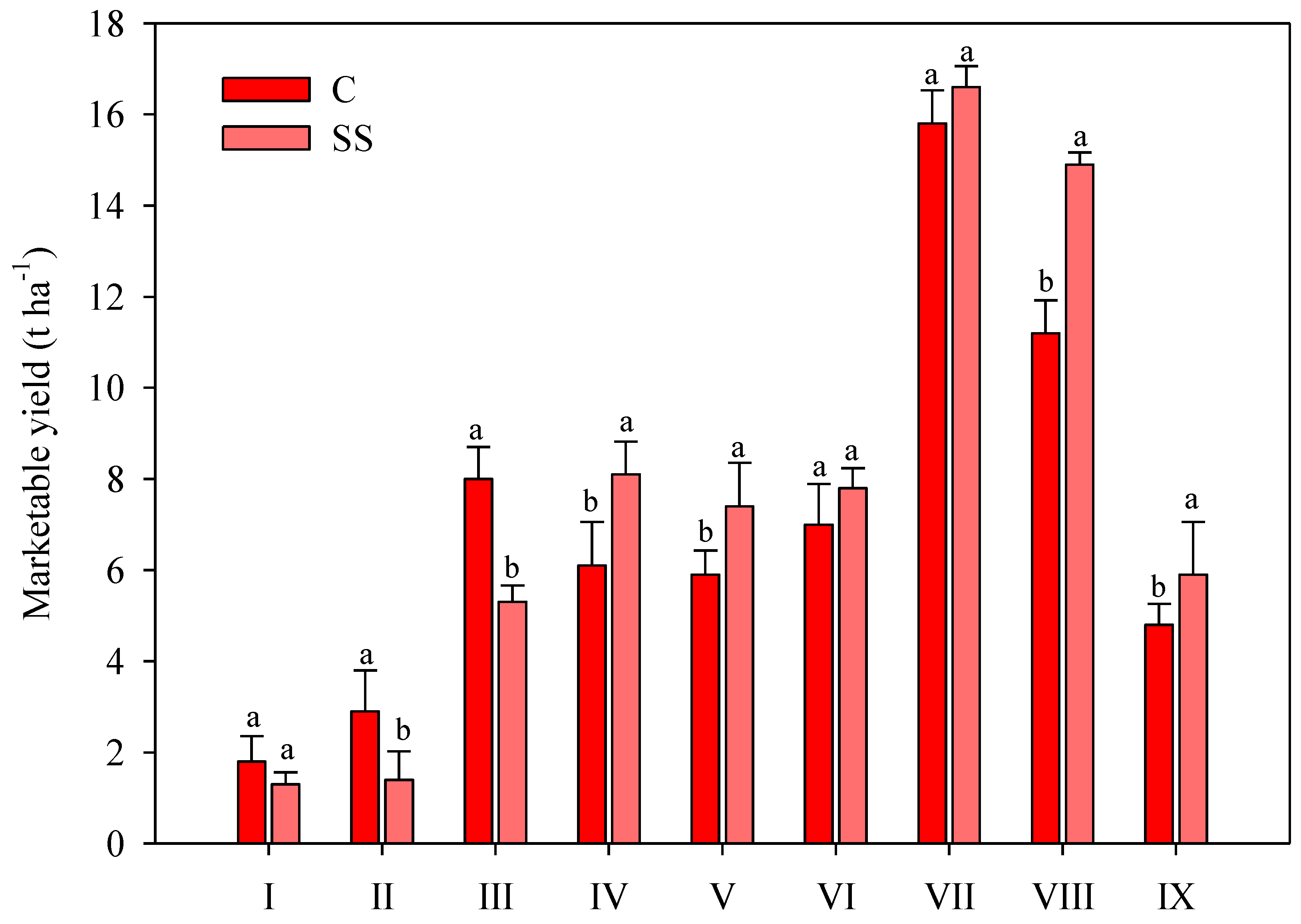
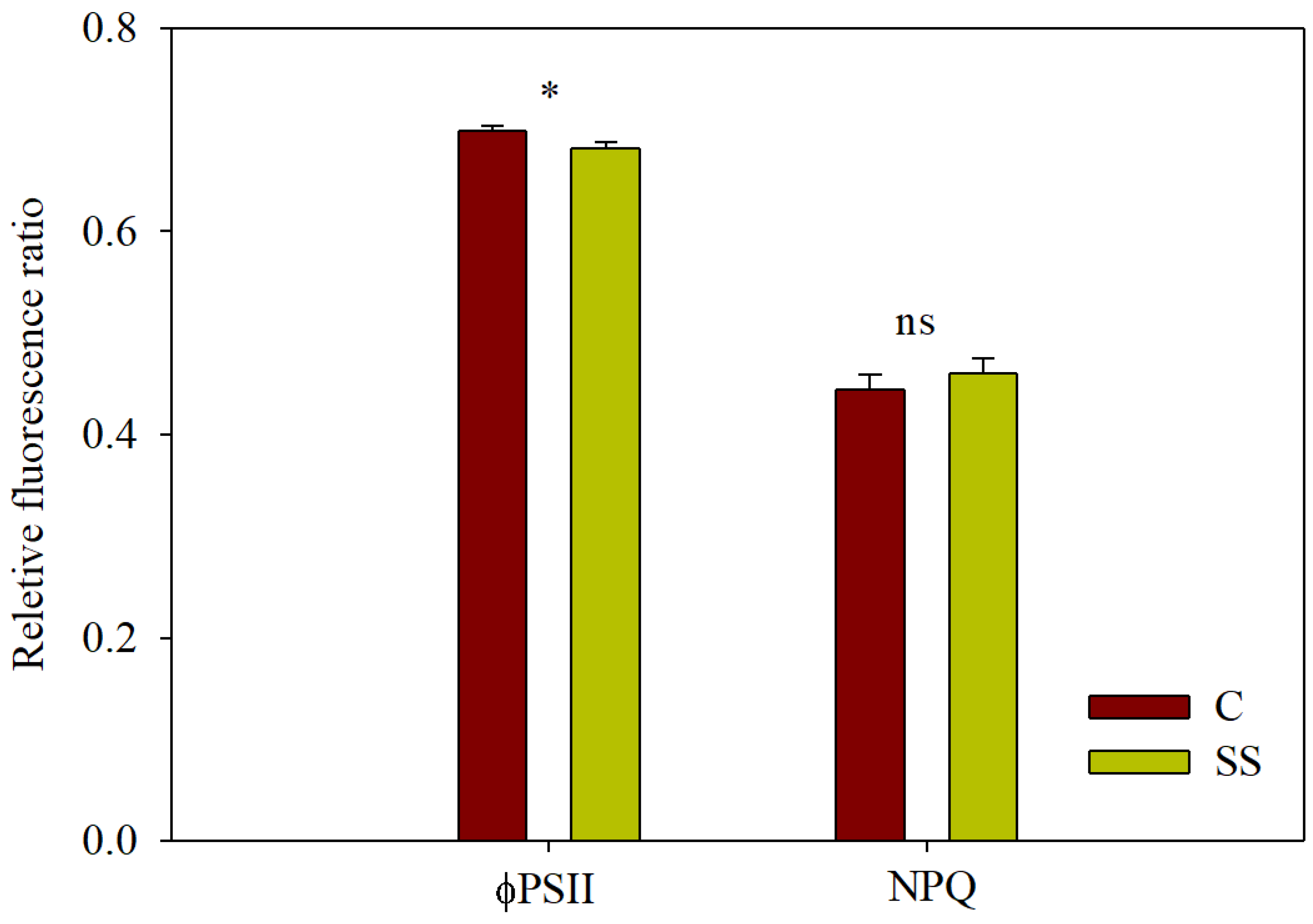
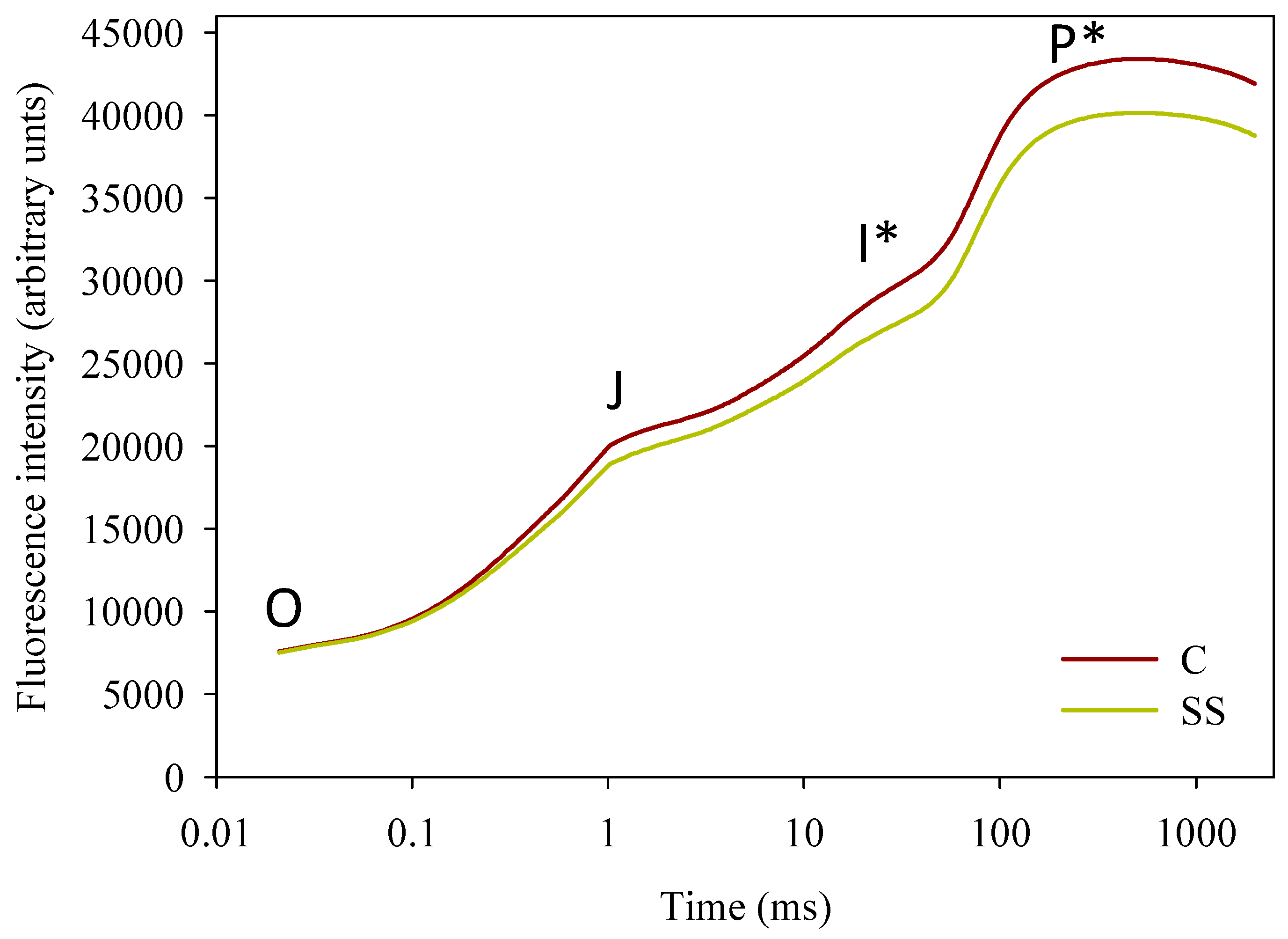

| Treatments | Cumulative Yield t ha−1 | Total Fruits n° m−2 | Mean Weight g fruit−1 | Dry Matter % | Firmness kg cm−2 |
|---|---|---|---|---|---|
| Control | 63.9 ± 3.0 | 30.2 ± 1.08 | 213.0 ± 1.58 | 7.6 ± 0.14 | 1.69 ± 0.009 |
| SS | 70.8 ± 2.4 | 33.8 ± 0.94 | 210.7 ± 0.72 | 8.0 ± 0.16 | 1.93 ± 0.013 |
| * | * | ns | ns | ** |
| Treatments | HAA mmol AsA 100 g−1 dw | LAA mmol Trolox 100 g−1 dw | Phenols mg GAE g−1 dw | AsA mg 100 g−1 fw | Carotenoids mg g−1 fw |
|---|---|---|---|---|---|
| Control | 3.86 ± 0.29 | 24.9 ± 1.09 | 3.12 ± 0.08 | 59.4 ± 1.46 | 0.037 ± 0.001 |
| SS | 3.06 ± 0.04 | 26.4 ± 0.53 | 3.18 ± 0.08 | 61.2 ± 4.48 | 0.041 ± 0.002 |
| ns | ns | ns | ns | * |
| Treatments | L* | a* | b* |
|---|---|---|---|
| Control | 24.0 ± 0.1 | 3.6 ± 0.1 | −0.17 ± 0.02 |
| SS | 24.3 ± 0.2 | 4.3 ± 0.2 | −0.18 ± 0.03 |
| ns | * | ns |
| JIP-Test Parameter | Control | Spectral Shifting | t-Test |
|---|---|---|---|
| F0 | 13,677 ± 479 | 12,198 ± 392 | * |
| FJ | 25,415 ± 907 | 26,852 ± 1402 | ns |
| FI | 21,966 ± 1278 | 25,667 ± 2062 | ns |
| FM | 39,398 ± 1744 | 42,505 ± 2442 | ns |
| Fv | 25,721 ± 1783 | 30,307 ± 2443 | ns |
| VJ | 0.470 ± 0.027 | 0.476 ± 0.023 | ns |
| VI | 0.294 ± 0.035 | 0.389 ± 0.044 | ns |
| FM/F0 | 2.934 ± 0.150 | 3.535 ± 0.201 | * |
| Fv/F0 | 1.934 ± 0.150 | 2.535 ± 0.201 | * |
| Fv/FM | 0.641 ± 0.019 | 0.695 ± 0.022 | ns |
| M0 | 1.288 ± 0.053 | 1.180 ± 0.041 | ns |
| Area | 12,903,958 ± 602,835 | 12,066,264 ± 450,842 | ns |
| Sm | 523 ± 23 | 436 ± 27 | * |
| Ss | 0.366 ± 0.015 | 0.409 ± 0.020 | ns |
| N | 1497 ± 104 | 1175 ± 139 | ns |
| ΦP0 | 0.641 ± 0.019 | 0.695 ± 0.022 | ns |
| Ψ0 | 0.530 ± 0.027 | 0.524 ± 0.023 | ns |
| ΦE0 | 0.344 ± 0.022 | 0.361 ± 0.017 | ns |
| ΦD0 | 0.359 ± 0.019 | 0.305 ± 0.022 | ns |
| ΦPav | 870 ± 7 | 885 ± 8 | ns |
| PiAbs | 0.613 ± 0.091 | 0.854 ± 0.101 | ns |
| ABS/RC | 4.528 ± 0.279 | 3.988 ± 0.450 | ns |
| TR0/RC | 2.834 ± 0.133 | 2.607 ± 0.178 | ns |
| ET0/RC | 1.546 ± 0.141 | 1.427 ± 0.167 | ns |
| DI0/RC | 1.694 ± 0.182 | 1.382 ± 0.288 | ns |
| Treatments | Cumulative Yield t ha−1 | Total Fruits n° m−2 | Mean Weight g fruit−1 | DM % | TSS °Brix | Firmness kg cm−2 |
|---|---|---|---|---|---|---|
| Control | 63.5 ± 7.02 | 226.8 ± 9.7 | 27.8 ± 1.21 | 9.2 ± 0.15 | 9.4 ± 0.11 | 0.43 ± 0.002 |
| SS | 68.7 ± 5.24 | 247.1 ± 3.7 | 28.0 ± 1.11 | 9.2 ± 0.20 | 9.8 ± 0.11 | 0.46 ± 0.003 |
| ns | ns | ns | ns | ns | ** |
| Treatments | HAA mmol AsA 100 g−1 dw | LAA mmol Trolox 100 g−1 dw | Phenols mg GAE g−1 dw | AsA mg 100 g−1 fw | Carotenoids mg g−1 fw |
|---|---|---|---|---|---|
| Control | 11.1 ± 0.02 | 27.9 ± 1.0 | 4.6 ± 0.10 | 63.6 ± 2.0 | 0.017 ± 0.0003 |
| SS | 11.5 ± 0.11 | 26.5 ± 0.8 | 4.8 ± 0.01 | 50.6 ± 0.9 | 0.010 ± 0.0001 |
| ns | ns | ns | * | ** |
| Treatments | L* | a* | b* |
|---|---|---|---|
| Control | 33.9 ± 0.5 | 24.9 ± 1.09 | 3.12 ± 0.08 |
| SS | 35.7 ± 0.2 | 26.4 ± 0.53 | 3.18 ± 0.08 |
| * | ns | ns |
| JIP-Test Parameter | Control | Spectral Shifting | t-Test |
|---|---|---|---|
| F0 | 8190 ± 162 | 8128 ± 196 | ns |
| FJ | 21,361 ± 486 | 20,223 ± 543 | ns |
| FI | 29,769 ± 746 | 27,469 ± 705 | * |
| FM | 43,422 ± 956 | 40,201 ± 813 | * |
| Fv | 35,232 ± 838 | 32,074 ± 720 | * |
| VJ | 0.374 ± 0.005 | 0.378 ± 0.009 | ns |
| VI | 0.611 ± 0.005 | 0.602 ± 0.007 | ns |
| FM/F0 | 5.306 ± 0.076 | 4.973 ± 0.102 | * |
| Fv/F0 | 4.306 ± 0.076 | 3.973 ± 0.102 | * |
| Fv/FM | 0.811 ± 0.003 | 0.797 ± 0.004 | * |
| M0 | 0.611 ± 0.014 | 0.620 ± 0.025 | ns |
| Area | 19,347,982 ± 582,933 | 17,040,625 ± 577,748 | * |
| Sm | 550 ± 12 | 534 ± 18 | ns |
| Ss | 0.616 ± 0.008 | 0.617 ± 0.011 | ns |
| N | 894 ± 18 | 867 ± 28 | ns |
| ΦP0 | 0.811 ± 0.003 | 0.797 ± 0.004 | * |
| Ψ0 | 0.626 ± 0.005 | 0.622 ± 0.009 | ns |
| ΦE0 | 0.508 ± 0.005 | 0.497 ± 0.009 | ns |
| ΦD0 | 0.189 ± 0.003 | 0.203 ± 0.004 | * |
| ΦPav | 928 ± 1 | 925 ± 2 | ns |
| PiAbs | 3.651 ± 0.164 | 3.403 ± 0.274 | ns |
| ABS/RC | 2.011 ± 0.028 | 2.049 ± 0.045 | ns |
| TR0/RC | 1.630 ± 0.021 | 1.630 ± 0.029 | ns |
| ET0/RC | 1.019 ± 0.011 | 1.010 ± 0.010 | ns |
| DI0/RC | 0.381 ± 0.009 | 0.418 ± 0.017 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, S.; Di Mola, I.; Barták, M.; Cozzolino, E.; Melchionna, G.; Mormile, P.; Ottaiano, L.; Paradiso, R.; Rippa, M.; Testa, A.; et al. Crop Performance and Photochemical Processes Under a UV-to-Red Spectral Shifting Greenhouse: A Study on Aubergine and Strawberry. Agriculture 2025, 15, 569. https://doi.org/10.3390/agriculture15060569
Conti S, Di Mola I, Barták M, Cozzolino E, Melchionna G, Mormile P, Ottaiano L, Paradiso R, Rippa M, Testa A, et al. Crop Performance and Photochemical Processes Under a UV-to-Red Spectral Shifting Greenhouse: A Study on Aubergine and Strawberry. Agriculture. 2025; 15(6):569. https://doi.org/10.3390/agriculture15060569
Chicago/Turabian StyleConti, Stefano, Ida Di Mola, Miloš Barták, Eugenio Cozzolino, Giuseppe Melchionna, Pasquale Mormile, Lucia Ottaiano, Roberta Paradiso, Massimo Rippa, Antonino Testa, and et al. 2025. "Crop Performance and Photochemical Processes Under a UV-to-Red Spectral Shifting Greenhouse: A Study on Aubergine and Strawberry" Agriculture 15, no. 6: 569. https://doi.org/10.3390/agriculture15060569
APA StyleConti, S., Di Mola, I., Barták, M., Cozzolino, E., Melchionna, G., Mormile, P., Ottaiano, L., Paradiso, R., Rippa, M., Testa, A., & Mori, M. (2025). Crop Performance and Photochemical Processes Under a UV-to-Red Spectral Shifting Greenhouse: A Study on Aubergine and Strawberry. Agriculture, 15(6), 569. https://doi.org/10.3390/agriculture15060569









