Organic Fertilization Enhances Microbial-Mediated Dissolved Organic Matter Composition and Transformation in Paddy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Field Experiment Design
2.3. Soil Sampling and Determination of Physicochemical Properties
2.4. DOM Extraction and FT-ICR-MS Determination
2.5. Assessment of Thermodynamic Stability and Transformation Potential of DOM
2.6. DNA Extraction and Metagenomic Sequencing
2.7. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
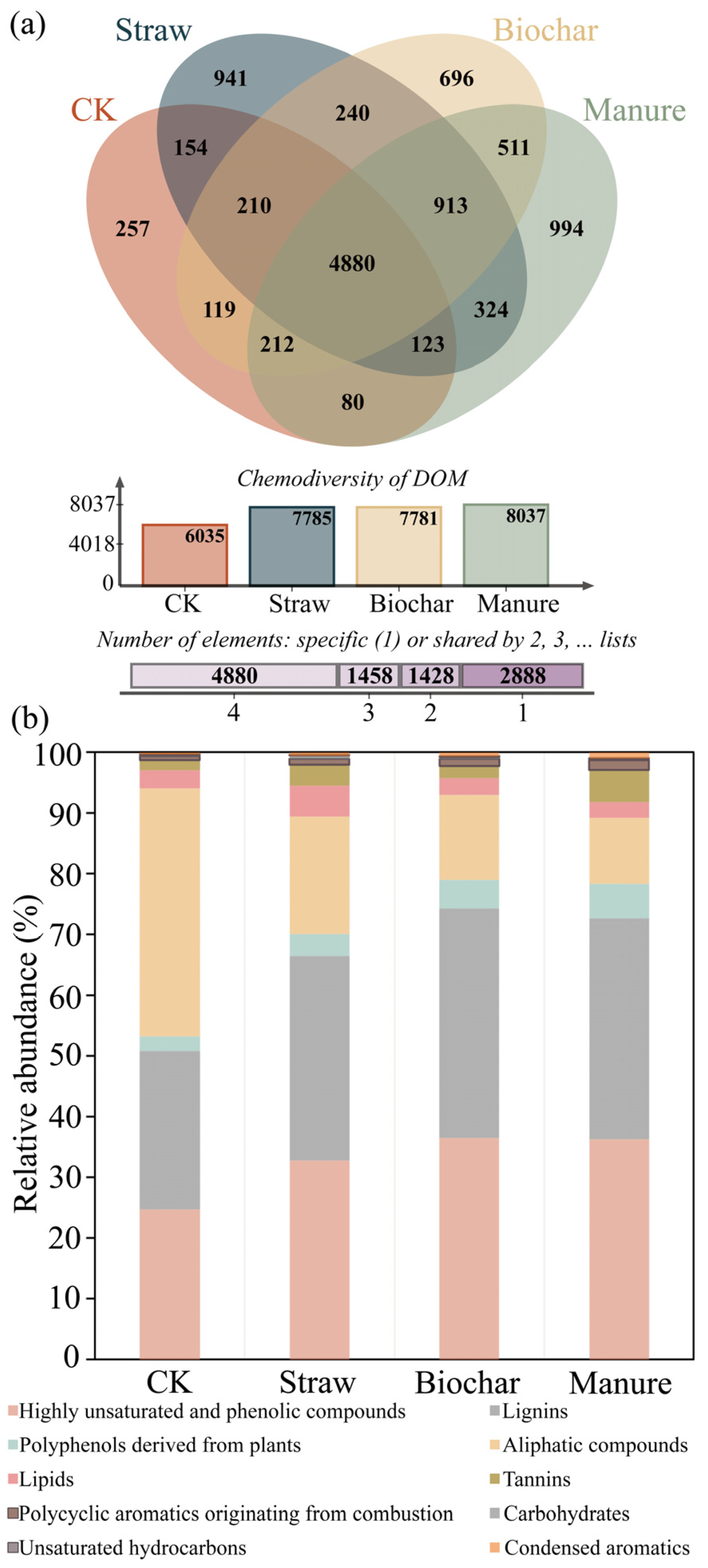
3.2. Chemodiversity and Composition of DOM Molecules
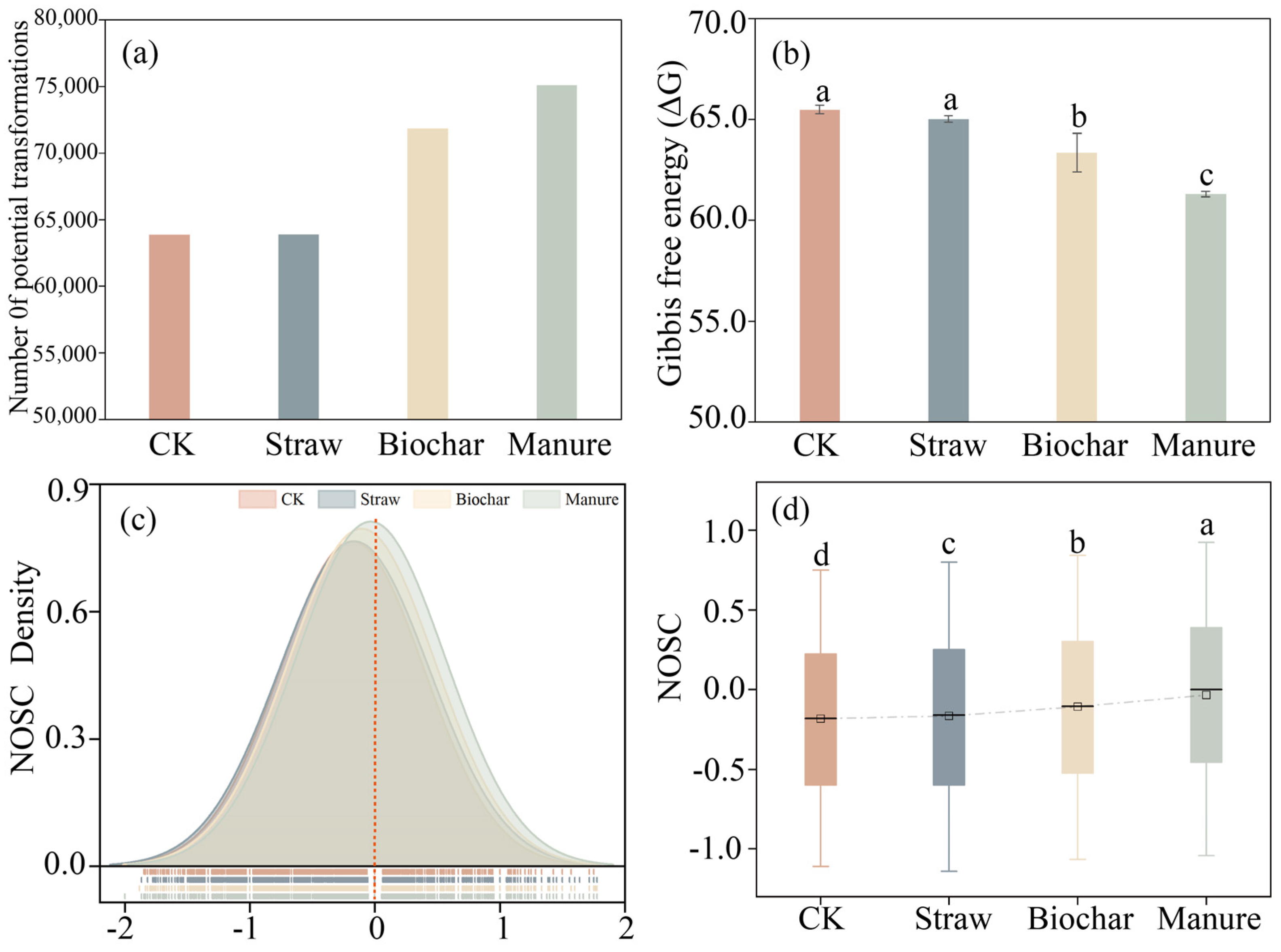
3.3. DOM Molecular Potential Transformations
3.4. Soil Microbial Diversity and Functional Gene Abundance
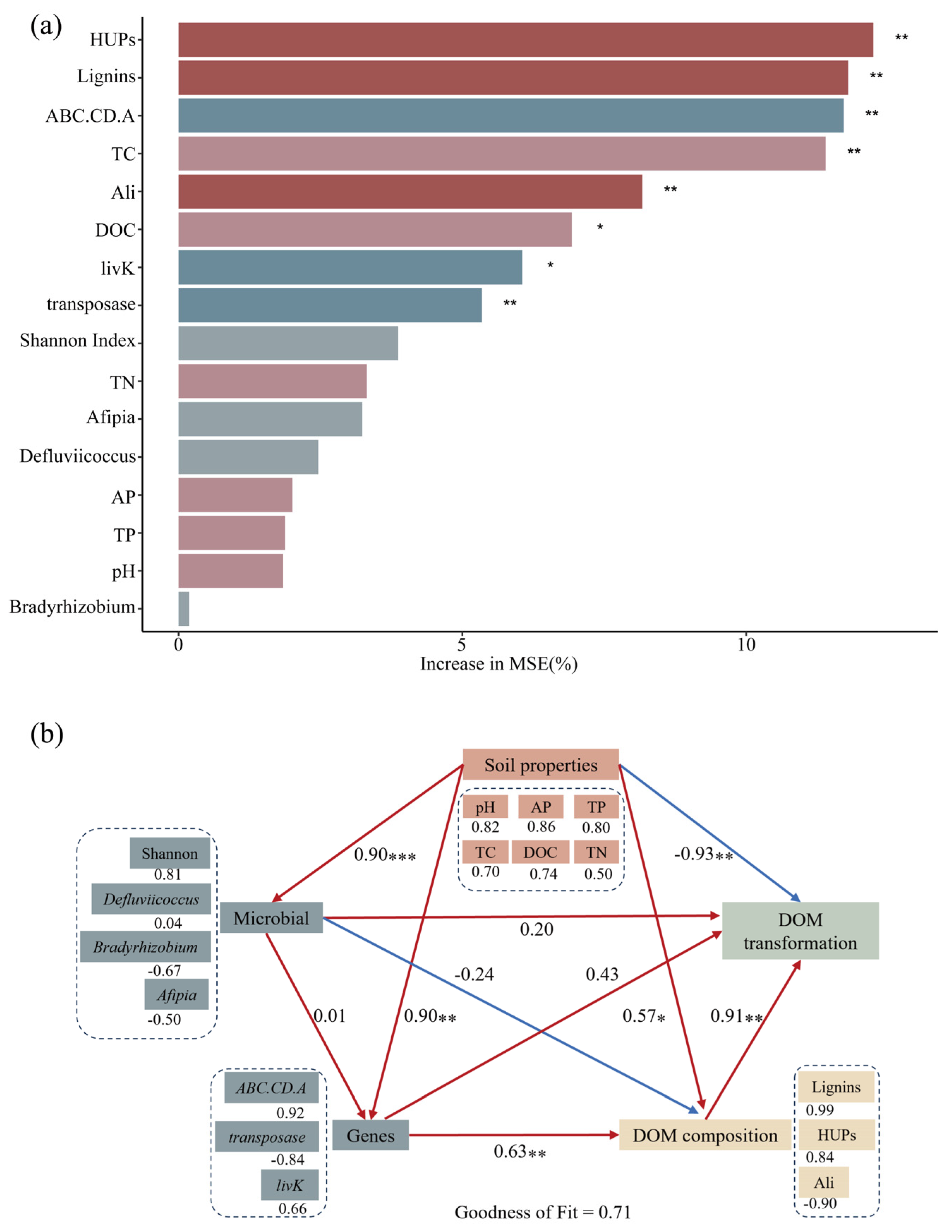
3.5. Relationships Among DOM Molecules, Soil Properties and Microbial Communities
3.6. Drivers of DOM Molecular Transformation
4. Discussion
4.1. Soil Physicochemical Modulation and Its Impact on DOM Composition
4.2. Thermodynamic Shifts and Enhanced Transformation Potential of DOM
4.3. Organic Fertilization Increase the DOM Transformation Potential via Microbial Functional Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Ge, T.; Van Groenigen, K.J.; Yang, Y.; Wang, P.; Cheng, K.; Zhu, Z.; Wang, J.; Li, Y.; Guggenberger, G.; et al. Rice Paddy Soils Are a Quantitatively Important Carbon Store According to a Global Synthesis. Commun. Earth Environ. 2021, 2, 154. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the Dynamics of Dissolved Organic Matter in Soils: A Review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Marschner, B.; Kalbitz, K. Controls of Bioavailability and Biodegradability of Dissolved Organic Matter in Soils. Geoderma 2003, 113, 211–235. [Google Scholar] [CrossRef]
- Basile-Doelsch, I.; Balesdent, J.; Pellerin, S. Reviews and Syntheses: The Mechanisms Underlying Carbon Storage in Soil. Biogeosciences 2020, 17, 5223–5242. [Google Scholar] [CrossRef]
- McDowell, W.H.; Magill, A.H.; Aitkenhead-Peterson, J.A.; Aber, J.D.; Merriam, J.L.; Kaushal, S.S. Effects of Chronic Nitrogen Amendment on Dissolved Organic Matter and Inorganic Nitrogen in Soil Solution. For. Ecol. Manag. 2004, 196, 29–41. [Google Scholar] [CrossRef]
- Jaffe, R.; McKnight, D.; Maie, N.; Cory, R.; McDowell, W.H.; Campbell, J.L. Spatial and Temporal Variations in DOM Composition in Ecosystems: The Importance of Long-Term Monitoring of Optical Properties. J. Geophys. Res. Biogeosci. 2008, 113, G04032. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D. Dissolved Organic Carbon Concentration and Biodegradability across the Global Rivers: A Meta-Analysis. Sci. Total. Environ. 2022, 818, 151828. [Google Scholar] [CrossRef] [PubMed]
- Dalzell, B.J.; Minor, E.C.; Mopper, K.M. Photodegradation of Estuarine Dissolved Organic Matter: A Multi-Method Assessment of DOM Transformation. Org. Geochem. 2009, 40, 243–257. [Google Scholar] [CrossRef]
- Roth, V.-N.; Lange, M.; Simon, C.; Hertkorn, N.; Bucher, S.; Goodall, T.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Mommer, L.; Oram, N.J.; et al. Persistence of Dissolved Organic Matter Explained by Molecular Changes during Its Passage through Soil. Nat. Geosci. 2019, 12, 755–761. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, A.; Wang, R.; Hu, Q.; Zhou, J.; Li, M.; Wang, T.; He, D.; Zhu, L. Long-Term Straw Return Promotes Accumulation of Stable Soil Dissolved Organic Matter by Driving Molecular-Level Activity and Diversity. Agric. Ecosyst. Environ. 2024, 374, 109155. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, Y.; Hu, A.; Wang, X. Exploring the Molecular Composition of Dissolved Organic Matter and Its Connection to Microbial Communities in Industrial-Scale Anaerobic Digestion of Chicken Manure. Toxics 2025, 13, 49. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Shi, Z.; Hao, M.; Wei, X.; Sun, L.; He, Y.; Wang, X. Manure Application Affects Microbial Metabolic Quotient through DOM Recalcitrance and Microbial Strategy Shifts in a Mollisol. Soil Tillage Res. 2025, 252, 106616. [Google Scholar] [CrossRef]
- Azeem, M.; Sun, T.-R.; Jeyasundar, P.G.S.A.; Han, R.-X.; Li, H.; Abdelrahman, H.; Shaheen, S.M.; Zhu, Y.-G.; Li, G. Biochar-Derived Dissolved Organic Matter (BDOM) and Its Influence on Soil Microbial Community Composition, Function, and Activity: A Review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1912–1934. [Google Scholar] [CrossRef]
- Li, T.; Li, P.; Qin, W.; Wu, M.; Saleem, M.; Kuang, L.; Zhao, S.; Tian, C.; Li, Z.; Jiang, J.; et al. Fertilization Weakens the Ecological Succession of Dissolved Organic Matter in Paddy Rice Rhizosphere Soil at the Molecular Level. Environ. Sci. Technol. 2023, 57, 19782–19792. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Yu, S.; Lv, J.; Jiang, L.; Geng, P.; Cao, D.; Wang, Y. Changes of Soil Dissolved Organic Matter and Its Relationship with Microbial Community along the Hailuogou Glacier Forefield Chronosequence. Environ. Sci. Technol. 2023, 57, 4027–4038. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone Taxa as Drivers of Microbiome Structure and Functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Delgado-Baquerizo, M.; Jeffries, T.C.; Trivedi, C.; Anderson, I.C.; Lai, K.; McNee, M.; Flower, K.; Singh, B.P.; Minkey, D.; et al. Soil Aggregation and Associated Microbial Communities Modify the Impact of Agricultural Management on Carbon Content. Environ. Microbiol. 2017, 19, 3070–3086. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; Van Agtmaal, M.; et al. Land Use Driven Change in Soil pH Affects Microbial Carbon Cycling Processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Xia, M.; Li, P.; Liu, J.; Qin, W.; Dai, Q.; Wu, M.; Li, Z.; Li, D.; Liu, M. Long-Term Fertilization Promotes the Microbial-Mediated Transformation of Soil Dissolved Organic Matter. Commun. Earth Environ. 2025, 6, 114. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hu, H.; Anderson, I.C.; Jeffries, T.C.; Zhou, J.; Singh, B.K. Microbial Regulation of the Soil Carbon Cycle: Evidence from Gene–Enzyme Relationships. ISME J. 2016, 10, 2593–2604. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; Li, G.; Petropoulos, E.; Feng, Y.; Li, Z. The Chemodiversity of Paddy Soil Dissolved Organic Matter Is Shaped and Homogenized by Bacterial Communities That Are Orchestrated by Geographic Distance and Fertilizations. Soil Biol. Biochem. 2021, 161, 108374. [Google Scholar] [CrossRef]
- Arnosti, C. Microbial Extracellular Enzymes and the Marine Carbon Cycle. In Annual Review of Marine Science; Carlson, C.A., Giovannoni, S.J., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 3, pp. 401–425. ISBN 978-0-8243-4503-7. [Google Scholar]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial Extracellular Enzymes in Biogeochemical Cycling of Ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Schneider, D.; Dippold, M.A.; Poehlein, A.; Wu, W.; Gui, H.; Ge, T.; Wu, J.; Thiel, V.; Kuzyakov, Y.; et al. Active Metabolic Pathways of Anaerobic Methane Oxidation in Paddy Soils. Soil Biol. Biochem. 2021, 156, 108215. [Google Scholar] [CrossRef]
- McCarren, J.; Becker, J.W.; Repeta, D.J.; Shi, Y.; Young, C.R.; Malmstrom, R.R.; Chisholm, S.W.; DeLong, E.F. Microbial Community Transcriptomes Reveal Microbes and Metabolic Pathways Associated with Dissolved Organic Matter Turnover in the Sea. Proc. Natl. Acad. Sci. USA 2010, 107, 16420–16427. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Zhang, W.; Lin, L.; Wang, L.; Niu, L.; Zhang, H.; Wang, P.; Wang, C. Response of Bacterial Community in Composition and Function to the Various DOM at River Confluences in the Urban Area. Water Res. 2020, 169, 115293. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Y.; Ye, L.; Qian, Y.; Shi, Y.; Xu, K.; Ren, H.; Geng, J. Microbial Roles in Dissolved Organic Matter Transformation in Full-Scale Wastewater Treatment Processes Revealed by Reactomics and Comparative Genomics. Environ. Sci. Technol. 2021, 55, 11294–11307. [Google Scholar] [CrossRef] [PubMed]
- Kujawinski, E.B.; Behn, M.D. Automated Analysis of Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectra of Natural Organic Matter. Anal. Chem. 2006, 78, 4363–4373. [Google Scholar] [CrossRef]
- D’Andrilli, J.; Cooper, W.T.; Foreman, C.M.; Marshall, A.G. An Ultrahigh-Resolution Mass Spectrometry Index to Estimate Natural Organic Matter Lability. Rapid Commun. Mass Spectrom. 2015, 29, 2385–2401. [Google Scholar] [CrossRef]
- Kellerman, A.M.; Dittmar, T.; Kothawala, D.N.; Tranvik, L.J. Chemodiversity of Dissolved Organic Matter in Lakes Driven by Climate and Hydrology. Nat. Commun. 2014, 5, 3804. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, H.; Wang, H.-T.; Xin, P.-Y.; Xu, X.-H.; Ma, Y.; Liu, W.-P.; Teng, C.-Y.; Jiang, C.-L.; Lou, L.-P.; et al. The Chemodiversity of Paddy Soil Dissolved Organic Matter Correlates with Microbial Community at Continental Scales. Microbiome 2018, 6, 187. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, S.; Fang, H.; Geng, J.; Li, Y.; Shi, F.; Wang, H.; Chen, L.; Zhou, Y. Copper and Cadmium Co-Contamination Increases the Risk of Nitrogen Loss in Red Paddy Soils. J. Hazard. Mater. 2024, 479, 135626. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental Evaluation of Methods to Quantify Dissolved Organic Nitrogen (DON) and Dissolved Organic Carbon (DOC) in Soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- NY/T 4606-2025; Determination of Total Carbon and Organic Matter in Soil by Elemental Analyzer Method. Ministry of Agriculture and Rural Affairs of China: Beijing, China, 2025.
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; ISBN 7-109-06644-4. [Google Scholar]
- Kome, G.K.; Enang, R.K.; Yerima, B.P.K.; Lontsi, M.G.R. Models Relating Soil pH Measurements in H2O, KCl and CaCl2 for Volcanic Ash Soils of Cameroon. Geoderma Reg. 2018, 14, e00185. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, T.; Bao, Y.; He, P.; Yang, K.; Mei, X.; Wei, Z.; Xu, Y.; Shen, Q.; Banerjee, S. Network Analysis and Subsequent Culturing Reveal Keystone Taxa Involved in Microbial Litter Decomposition Dynamics. Soil Biol. Biochem. 2021, 157, 108230. [Google Scholar] [CrossRef]
- Yang, M.; Chen, T.; Liu, Y.; Huang, L. Visualizing Set Relationships: EVenn’s Comprehensive Approach to Venn Diagrams. iMeta 2024, 3, e184. [Google Scholar] [CrossRef]
- Li, X.-M.; Chen, Q.-L.; He, C.; Shi, Q.; Chen, S.-C.; Reid, B.J.; Zhu, Y.-G.; Sun, G.-X. Organic Carbon Amendments Affect the Chemodiversity of Soil Dissolved Organic Matter and Its Associations with Soil Microbial Communities. Environ. Sci. Technol. 2018, 53, 50–59. [Google Scholar] [CrossRef]
- Wang, L.; Yan, H.; Wang, X.W.; Wang, Z.; Yu, S.X.; Wang, T.W.; Shi, Z.H. The Potential for Soil Erosion Control Associated with Socio-Economic Development in the Hilly Red Soil Region, Southern China. CATENA 2020, 194, 104678. [Google Scholar] [CrossRef]
- Li, N.; Ma, X.; Xu, H.; Feng, Y.; Ren, G.; Yang, G.; Han, X.; Wang, X.; Ren, C. Biochar Addition Mitigates Nitrogen Loss Induced by Straw Incorporation and Nitrogen Fertilizer Application. Soil Use Manag. 2020, 36, 751–765. [Google Scholar] [CrossRef]
- Huang, R.; Li, Z.; Xiao, Y.; Liu, J.; Jiang, T.; Deng, O.; Tang, X.; Wu, Y.; Tao, Q.; Li, Q.; et al. Composition of DOM along the Depth Gradients in the Paddy Field Treated with Crop Straw for 10 Years. J. Environ. Manag. 2024, 353, 120084. [Google Scholar] [CrossRef]
- Jia, J.; De Goede, R.; Li, Y.; Zhang, J.; Wang, G.; Zhang, J.; Creamer, R. Unlocking Soil Health: Are Microbial Functional Genes Effective Indicators? Soil Biol. Biochem. 2025, 204, 109768. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Zhou, Y.; Zhang, B.; Peng, Y.; Zhuo, Y.; Ai, W.; Gao, C.; Wu, B.; Liu, D.; et al. Straw and Straw Biochar Differently Affect Fractions of Soil Organic Carbon and Microorganisms in Farmland Soil under Different Water Regimes. Environmental Technol. Innov. 2023, 32, 103412. [Google Scholar] [CrossRef]
- Novair, S.B.; Cheraghi, M.; Faramarzi, F.; Lajayer, B.A.; Senapathi, V.; Astatkie, T.; Price, G.W. Reviewing the Role of Biochar in Paddy Soils: An Agricultural and Environmental Perspective. Ecotoxicol. Environ. Saf. 2023, 263, 115228. [Google Scholar] [CrossRef]
- Liu, S.; Liang, C.; Zhang, J.; Chen, S.; Cheng, J.; Chang, M.; Xu, J. Effects of Manure Application on Paddy Soil Phosphorus in China Based on a Meta-Analysis. Sci. Rep. 2025, 15, 36272. [Google Scholar] [CrossRef]
- Said-Pullicino, D.; Cucu, M.A.; Sodano, M.; Birk, J.J.; Glaser, B.; Celi, L. Nitrogen Immobilization in Paddy Soils as Affected by Redox Conditions and Rice Straw Incorporation. Geoderma 2014, 228, 44–53. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Li, L.; Liu, X.; Zhang, B.; Zheng, J.; Pan, G. Change in Active Microbial Community Structure, Abundance and Carbon Cycling in an Acid Rice Paddy Soil with the Addition of Biochar. Eur. J. Soil Sci. 2016, 67, 857–867. [Google Scholar] [CrossRef]
- Holatko, J.; Bielska, L.; Hammerschmiedt, T.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Baltazar, T.; Latal, O.; Brtnicky, M. Cattle Manure Fermented with Biochar and Humic Substances Improve the Crop Biomass, Microbiological Properties and Nutrient Status of Soil. Agronomy 2022, 12, 368. [Google Scholar] [CrossRef]
- Hu, A.; Jang, K.-S.; Tanentzap, A.J.; Zhao, W.; Lennon, J.T.; Liu, J.; Li, M.; Stegen, J.; Choi, M.; Lu, Y.; et al. Thermal Responses of Dissolved Organic Matter under Global Change. Nat. Commun. 2024, 15, 576. [Google Scholar] [CrossRef]
- Wu, D.; Ren, C.; Ren, D.; Tian, Y.; Li, Y.; Wu, C.; Li, Q. New Insights into Carbon Mineralization in Tropical Paddy Soil under Land Use Conversion: Coupled Roles of Soil Microbial Community, Metabolism, and Dissolved Organic Matter Chemodiversity. Geoderma 2023, 432, 116393. [Google Scholar] [CrossRef]
- Yang, X.; Peng, X.; Feng, K.; Wang, S.; Zou, X.; Deng, Y. Organic Molecular Network Analysis Reveals Transformation Signatures of Dissolved Organic Matter during Anaerobic Digestion Process. Water Res. 2025, 282, 123777. [Google Scholar] [CrossRef]
- Stegen, J.C.; Garayburu-Caruso, V.A.; Danczak, R.E.; Goldman, A.E.; Renteria, L.; Torgeson, J.M.; Hager, J. Maximum Respiration Rates in Hyporheic Zone Sediments Are Primarily Constrained by Organic Carbon Concentration and Secondarily by Organic Matter Chemistry. Biogeosciences 2023, 20, 2857–2867. [Google Scholar] [CrossRef]
- Duan, X.; Rui, Y.; Xia, Y.; Hu, Y.; Ma, C.; Qiao, H.; Zeng, G.; Su, Y.; Wu, J.; Chen, X. Higher Microbial C Use Efficiency in Paddy than in Adjacent Upland Soils: Evidence from Continental Scale. Soil Tillage Res. 2024, 235, 105891. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Badri, D.V.; Quintana, N.; El Kassis, E.G.; Kim, H.K.; Choi, Y.H.; Sugiyama, A.; Verpoorte, R.; Martinoia, E.; Manter, D.K.; Vivanco, J.M. An ABC Transporter Mutation Alters Root Exudation of Phytochemicals That Provoke an Overhaul of Natural Soil Microbiota. Plant Physiol. 2009, 151, 2006–2017. [Google Scholar] [CrossRef]
- Thomas, C.; Tampe, R. Structural and Mechanistic Principles of ABC Transporters. In Annual Review of Biochemistry; Kornberg, R.D., Ed.; Annual Reviews: Palo Alto, CA, USA, 2020; Volume 89, pp. 605–636. ISBN 978-0-8243-0889-6. [Google Scholar]
- Kalbitz, K.; Kaiser, K. Contribution of Dissolved Organic Matter to Carbon Storage in Forest Mineral Soils. J. Plant Nutr. Soil Sci. 2008, 171, 52–60. [Google Scholar] [CrossRef]
- Shi, S.; Chang, D.; Liang, T.; Gao, S.; Zhou, G.; Cao, W. Long-Term Organic Fertilization Decreases Soil Carbon Biodegradability by Mediating Molecular Transformation of Dissolved Organic Matter. Resour. Environ. Sustain. 2025, 22, 100261. [Google Scholar] [CrossRef]
- Si, Q.; Chen, K.; Wei, B.; Zhang, Y.; Sun, X.; Liang, J. Dissolved Carbon Flow to Particulate Organic Carbon Enhances Soil Carbon Sequestration. SOIL 2024, 10, 441–450. [Google Scholar] [CrossRef]
- Camenzind, T.; Mason-Jones, K.; Mansour, I.; Rillig, M.C.; Lehmann, J. Formation of Necromass-Derived Soil Organic Carbon Determined by Microbial Death Pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Floc’h, J.-B.; Hamel, C.; Laterriere, M.; Tidemann, B.; St-Arnaud, M.; Hijri, M. Inter-Kingdom Networks of Canola Microbiome Reveal Bradyrhizobium as Keystone Species and Underline the Importance of Bulk Soil in Microbial Studies to Enhance Canola Production. Microb. Ecol. 2022, 84, 1166–1181. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, S.; Wang, Y.; Liu, S.; Sun, H. Greenhouse Gas Emissions of Rice Straw Return Varies with Return Depth and Soil Type in Paddy Systems of Northeast China. Arch. Agron. Soil Sci. 2021, 67, 1591–1602. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Wu, Y.; Wang, H.; Chen, Y.; Wu, W. Reducing CH4 and CO2 Emissions from Waterlogged Paddy Soil with Biochar. J. Soils Sediments 2011, 11, 930–939. [Google Scholar] [CrossRef]
- Kong, D.; Li, S.; Jin, Y.; Wu, S.; Chen, J.; Hu, T.; Wang, H.; Liu, S.; Zou, J. Linking Methane Emissions to Methanogenic and Methanotrophic Communities under Different Fertilization Strategies in Rice Paddies. Geoderma 2019, 347, 233–243. [Google Scholar] [CrossRef]
- Zhao, Z.; Amano, C.; Reinthaler, T.; Orellana, M.V.; Herndl, G.J. Substrate Uptake Patterns Shape Niche Separation in Marine Prokaryotic Microbiome. Sci. Adv. 2024, 10, eadn5143. [Google Scholar] [CrossRef]
- Zhang, Y.; Naafs, B.D.A.; Huang, X.; Zhao, M.; Zeng, L.; Blewett, J.; Pancost, R.D.; Xie, S. The Stable Carbon and Hydrogen Isotopic Composition of Microbial Fatty Acids Traces Microbial Metabolism in Soils and Peats. Geochim. Cosmochim. Acta 2024, 365, 85–100. [Google Scholar] [CrossRef]
- Duan, J. Agricultural Management Practices in China Enhance Nitrogen Sustainability and Benefit Human Health. Nat. Food 2024, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, Z.; Gong, H.; He, K.; Miao, Q.; Tian, X.; Wang, Z.; Wang, Y.; Zheng, H.; Cui, Z. Soil Organic Carbon Formation Efficiency from Straw/Stover and Manure Input and Its Drivers: Estimates from Long-term Data in Global Croplands. Glob. Change Biol. 2024, 30, e17460. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, W.; Fu, J.; Ning, Y.; Cui, H.; Cheng, D.; Zhang, N.; Zhou, W.; Liao, W. Effect of Long-Term Straw Return on Organic Matter Transformation by Hydroxyl Radical during Paddy Soil Oxygenation. Chem. Eng. J. 2024, 482, 148974. [Google Scholar] [CrossRef]
- Luo, L.; Wang, J.; Lv, J.; Liu, Z.; Sun, T.; Yang, Y.; Zhu, Y.-G. Carbon Sequestration Strategies in Soil Using Biochar: Advances, Challenges, and Opportunities. Environ. Sci. Technol. 2023, 57, 11357–11372. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Choi, M.; Tanentzap, A.J.; Liu, J.; Jang, K.-S.; Lennon, J.T.; Liu, Y.; Soininen, J.; Lu, X.; Zhang, Y.; et al. Ecological Networks of Dissolved Organic Matter and Microorganisms under Global Change. Nat. Commun. 2022, 13, 3600. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Fang, H.; Cheng, S.; Wang, H.; Guo, Y.; Shi, F.; Liu, B.; Pu, H. Organic Fertilization Enhances Microbial-Mediated Dissolved Organic Matter Composition and Transformation in Paddy Soil. Agriculture 2025, 15, 2412. https://doi.org/10.3390/agriculture15232412
Chen L, Fang H, Cheng S, Wang H, Guo Y, Shi F, Liu B, Pu H. Organic Fertilization Enhances Microbial-Mediated Dissolved Organic Matter Composition and Transformation in Paddy Soil. Agriculture. 2025; 15(23):2412. https://doi.org/10.3390/agriculture15232412
Chicago/Turabian StyleChen, Long, Huajun Fang, Shulan Cheng, Hui Wang, Yifan Guo, Fangying Shi, Bingqian Liu, and Haiguang Pu. 2025. "Organic Fertilization Enhances Microbial-Mediated Dissolved Organic Matter Composition and Transformation in Paddy Soil" Agriculture 15, no. 23: 2412. https://doi.org/10.3390/agriculture15232412
APA StyleChen, L., Fang, H., Cheng, S., Wang, H., Guo, Y., Shi, F., Liu, B., & Pu, H. (2025). Organic Fertilization Enhances Microbial-Mediated Dissolved Organic Matter Composition and Transformation in Paddy Soil. Agriculture, 15(23), 2412. https://doi.org/10.3390/agriculture15232412







