Comprehensive Quality Analysis of Common Vetch (Vicia sativa L.) Varieties Using Image Processing Techniques and Artificial Intelligence
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Nutritional Properties and Macro-Micro Mineral Content
2.2. Determination of the Physical Attributes
2.3. Binary Classification by ML
- ⮚
- MLP: batchSize: 100; debug: False; doNotCheckCapabilities: False; decay: False; hiddenLayers: ((attribs + classes)/2); normalizeNumericClass: True; momentum: 0.2; learningRate: 0.3; normalizeAttributes: True; NominalToBinaryFilter: True; validationThreshold: 20; trainingTime: 500; activation function: sigmoid; seed: 0.
- ⮚
- RF: batchSize: 100; number of trees: 100; tree depth: none; breakTiesRandomly: False; doNotCheckCapabilities: False; debug: False; numExecutionSlots: 1; numIterations: 100; seed: 1.
- ⮚
- SVM: batchSize: 100; buildCalibrationModels: False; calibrator: Logistic; doNotCheckCapabilities: False; epsilon: 1.0 × 10−12; filterType: Normalize training data; kernel: PolyKernel; numFolds: −1; randomSeed: 1; toleranceParameter: 0.001.
2.4. Evaluation of Models
2.5. Multivariate Analysis
3. Results and Discussion
3.1. Nutritional Properties and Macro–Micro Mineral Content of Different Varieties of Vetch
3.2. Seed Physical Attributes of the Varieties
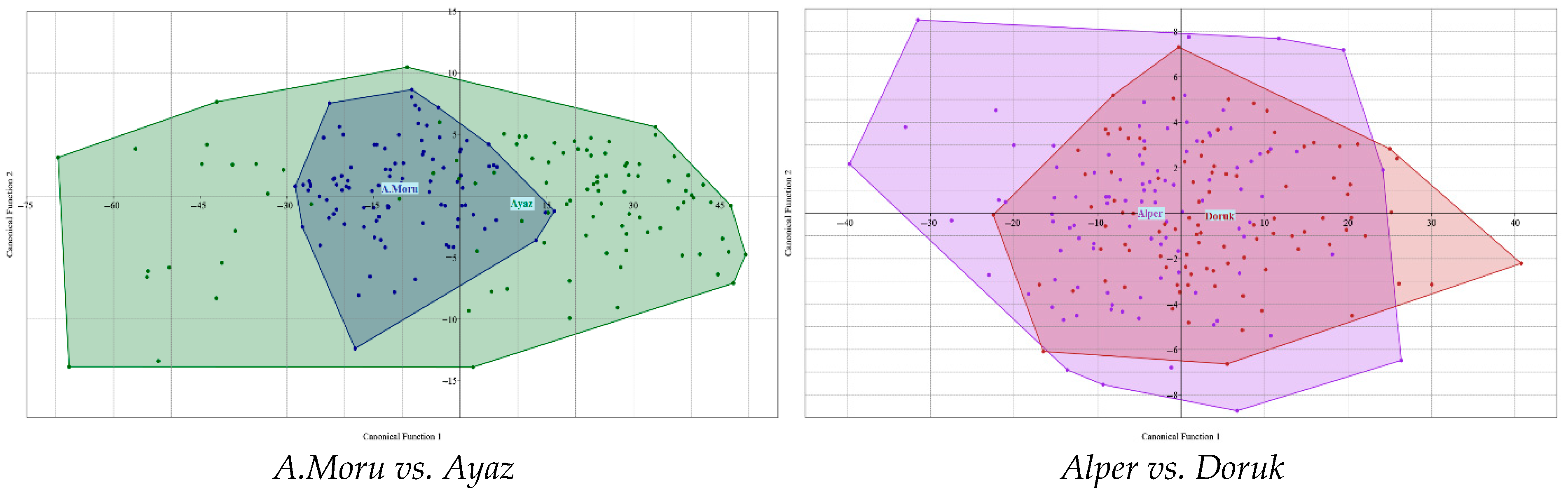
3.3. Linear Discrimination Analysis, Pairwise Comparison, and Multivariate Tests
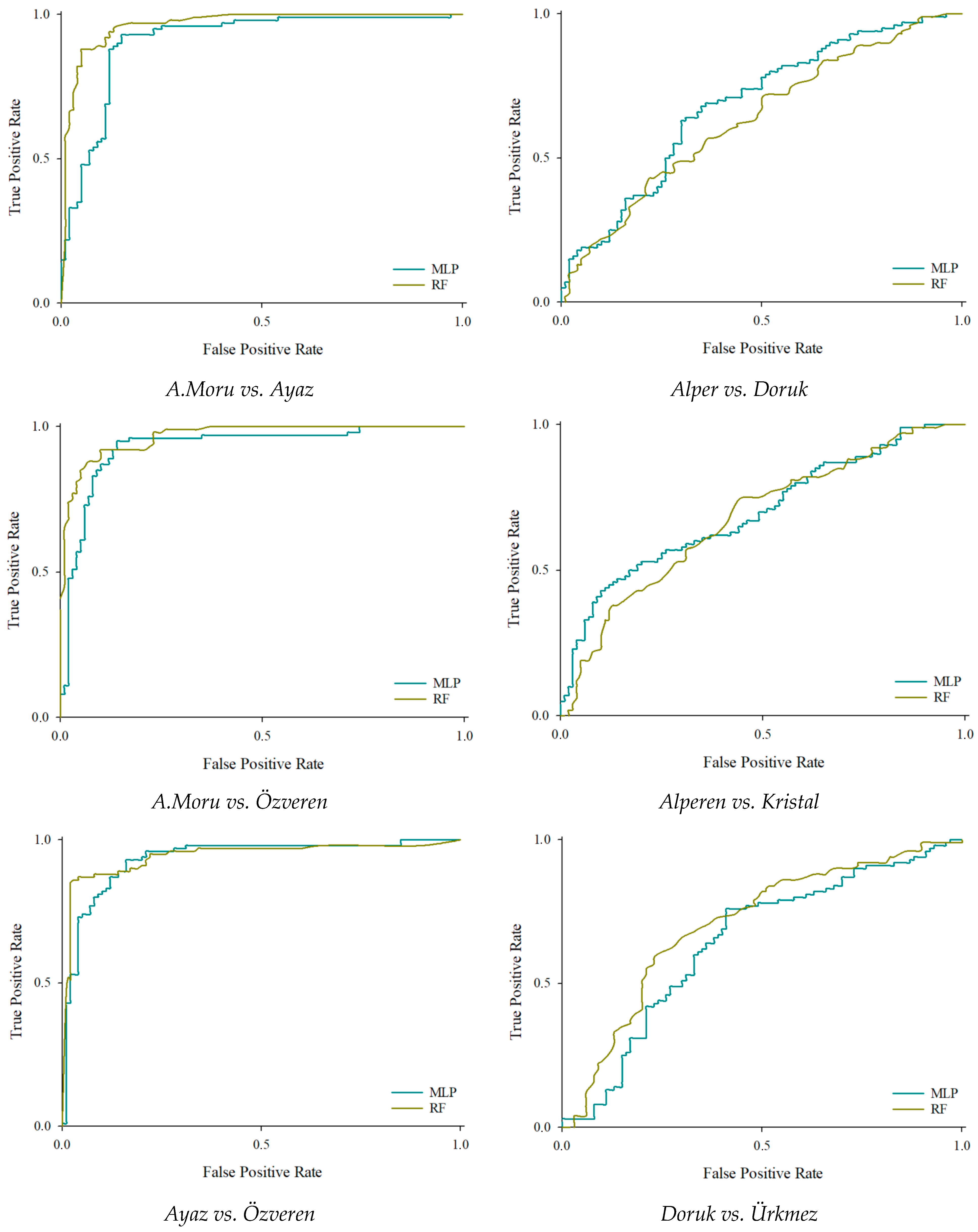
3.4. Performance Results of Binary Classification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MLP | Multi-layer perceptron |
| RF | Random forest |
| ADF | Acid detergent fiber |
| NDF | Neutral detergent fiber |
| CV | Computer vision |
| ML | Machine learning |
| ANN | Artificial neural network |
| DT | Decision trees |
| LR | Logistic regression |
| FL | Fuzzy logic |
| SVM | Support vector machine |
| KNN | K-nearest neighbor |
| MANOVA | Multivariate analysis of variance |
| BN | Bayes net |
| LB | Logit boost |
| ROC | Receiver operating characteristic |
| PRC | Precision–recall curve |
| ANOVA | Analysis of variance |
| PAST | Paleontological statistics |
| LDA | Linear discriminant analysis |
| NB | Naive bayes |
| TPR | True positive rate |
| TP | True positive |
| TN | True negative |
| FP | False positive |
| FN | False negative |
| SPSS | Statistical package for the social sciences |
| B | Boron |
| Ca | Calcium |
| Mn | Manganese |
| Na | Sodium |
| P | Phosphorus |
| S | Sulphur |
| Cu | Copper |
| Fe | Iron |
| K | Potassium |
| Mg | Magnesium |
| Zn | Zinc |
| PC | Principal component |
| CCD | Charge-coupled device |
| DL | Deep learning |
| RGB | Red, green, and blue |
References
- Sun, Y.; Zhao, N.; Sun, H.; Xu, S.; Lu, Y.; Xi, H.; Shi, H. Transcriptome profiling reveals molecular responses to salt stress in common vetch (Vicia sativa L.). Plants 2024, 13, 714. [Google Scholar] [CrossRef]
- Kartal, G.K.; Senbek, G.; Karaca, M.; Acikgoz, E. Hybridization studies in Vicia sativa complex. Euphytica 2020, 216, 29. [Google Scholar] [CrossRef]
- Martin, E.; Yıldız, H.K.; Kahraman, A.; Binzat, O.K.; Eroğlu, H.E. Detailed chromosome measurements and karyotype asymmetry of some Vicia (Fabaceae) taxa from Turkey. Caryologia 2018, 71, 224–232. [Google Scholar] [CrossRef]
- Dong, R.; Jahufer, M.Z.Z.; Dong, D.K.; Wang, Y.R.; Liu, Z.P. Characterisation of the morphological variation for seed traits among 537 germplasm accessions of common vetch (Vicia sativa L.) using digital image analysis. N. Z. J. Agric. Res. 2016, 59, 422–435. [Google Scholar] [CrossRef]
- Mao, Z.; Fu, H.; Nan, Z.; Wan, C. Fatty acid, amino acid, and mineral composition of four common vetch seeds on Qinghai-Tibetan plateau. Food Chem. 2015, 171, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Uzun, A.; Gücer, S.; Acikgoz, E. Common vetch (Vicia sativa L.) germplasm: Correlations of crude protein and mineral content to seed traits. Plant Foods Hum. Nutr. 2011, 66, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.A.; Haddad, D.A.; Ali, L.M. The phylogenetic relationships between species of Vicia L. based on morphological characteristics and proteins present in seeds. Asian J. Res. Bot. 2023, 6, 225–232. [Google Scholar]
- Chen, W.; Wang, Y.; Lv, X.; Yu, G.; Wang, Q.; Li, H.; Liu, Q. Physicochemical, structural and functional properties of protein isolates and major protein fractions from common vetch (Vicia sativa L.). Int. J. Biol. Macromol. 2022, 216, 487–497. [Google Scholar] [CrossRef]
- Grela, E.R.; Samolińska, W.; Rybiński, W.; Kiczorowska, B.; Kowalczuk-Vasilev, E.; Matras, J.; Wesołowska, S. Nutritional and anti-nutritional factors in Vicia sativa L. Seeds and the variability of phenotypic and morphological characteristics of some vetch accessions cultivated in European countries. Animals 2020, 11, 44. [Google Scholar] [CrossRef]
- Nguyen, V.; Riley, S.; Nagel, S.; Fisk, I.; Searle, I.R. Common vetch: A drought tolerant, high protein neglected leguminous crop with potential as a sustainable food source. Front. Plant Sci. 2020, 11, 818. [Google Scholar] [CrossRef]
- Butuner, R.; Cinar, I.; Taspinar, Y.S.; Kursun, R.; Calp, M.H.; Koklu, M. Classification of deep image features of lentil varieties with machine learning techniques. Eur. Food Res. Technol. 2023, 249, 1303–1316. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, Y.; Yong, K.; Zhu, M.; Wang, Y.; Wang, X.; Huang, X. Rice seed size measurement using a rotational perception deep learning model. Comput. Electron. Agric. 2023, 205, 107583. [Google Scholar] [CrossRef]
- Azizi, H.; Asli-Ardeh, E.A.; Jahanbakhshi, A.; Momeny, M. Vision-based strawberry classification using generalized and robust deep networks. J. Agric. Food Res. 2024, 15, 100931. [Google Scholar] [CrossRef]
- Goh, J.Y.; Md Yunos, Y.; Mohamed Ali, M.S. Fresh fruit bunch ripeness classification methods: A Review. Food Bioprocess Technol. 2025, 18, 183–206. [Google Scholar] [CrossRef]
- Patrício, D.I.; Rieder, R. Computer vision and artificial intelligence in precision agriculture for grain crops: A systematic review. Comput. Electron. Agric. 2018, 153, 69–81. [Google Scholar] [CrossRef]
- De-Souza, F.L.P.; Dias, M.A.; Setiyono, T.D.; Campos, S.; Shiratsuchi, L.S.; Tao, H. Identification of soybean planting gaps using machine learning. Smart Agric. Technol. 2025, 10, 100779. [Google Scholar] [CrossRef]
- Phan, T.T.H.; Nguyen, L.H.B. Enhancing rice seed purity recognition accuracy based on optimal feature selection. Ecol. Inform. 2025, 86, 103044. [Google Scholar] [CrossRef]
- Képeš, E.; Vrábel, J.; Brázdil, T.; Holub, P.; Pořízka, P.; Kaiser, J. Interpreting convolutional neural network classifiers applied to laser-induced breakdown optical emission spectra. Talanta 2024, 266, 124946. [Google Scholar] [CrossRef] [PubMed]
- Kurtulmuş, F.; Alibaş, İ.; Kavdır, I. Classification of pepper seeds using machine vision based on neural network. Int. J. Agric. Biol. Eng. 2016, 9, 51–62. [Google Scholar]
- Sabanci, K.; Aslan, M.F.; Ropelewska, E.; Unlersen, M.F. A convolutional neural network-based comparative study for pepper seed classification: Analysis of selected deep features with support vector machine. J. Food Process Eng. 2022, 45, e13955. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Yasmin, J.; Park, E.; Kim, G.; Kim, M.S.; Wakholi, C.; Cho, B.K. Classification of watermelon seeds using morphological patterns of X-ray imaging: A comparison of conventional machine learning and deep learning. Sensors 2020, 20, 6753. [Google Scholar] [CrossRef]
- Kaur, R.; Jain, A.; Kumar, S. Optimization classification of sunflower recognition through machine learning. Mater. Today Proc. 2022, 51, 207–211. [Google Scholar] [CrossRef]
- Cvejić, S.; Hrnjaković, O.; Jocković, M.; Kupusinac, A.; Doroslovački, K.; Gvozdenac, S.; Miladinović, D. Oil yield prediction for sunflower hybrid selection using different machine learning algorithms. Sci. Rep. 2023, 13, 17611. [Google Scholar] [CrossRef]
- Khatri, A.; Agrawal, S.; Chatterjee, J.M. Wheat seed classification: Utilizing ensemble machine learning approach. Sci. Program. 2022, 2022, 2626868. [Google Scholar] [CrossRef]
- Ali, A.; Qadri, S.; Mashwani, W.K.; Brahim Belhaouari, S.; Naeem, S.; Rafique, S.; Anam, S. Machine learning approach for the classification of corn seed using hybrid features. Int. J. Food Prop. 2020, 23, 1110–1124. [Google Scholar] [CrossRef]
- Ropelewska, E.; Piecko, J. Discrimination of tomato seeds belonging to different cultivars using machine learning. Eur. Food Res. Technol. 2022, 248, 685–705. [Google Scholar] [CrossRef]
- Çetin, N. Machine learning for varietal binary classification of soybean (Glycine max (L.) Merrill) seeds based on shape and size attributes. Food Anal. Methods 2022, 15, 2260–2273. [Google Scholar] [CrossRef]
- Cinar, I.; Koklu, M. Identification of rice varieties using machine learning algorithms. J. Agric. Sci. 2022, 28, 307–325. [Google Scholar]
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- Soest, P.V.; Wine, R.H. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Soest, P.V. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Say, R.; Kaplan, M.; Karaman, K. Investigation of the Effects of Different Extraction Conditions on Biochemical Properties of Barberry Fruit (Berberis crataegina DC.). ISPEC J. Agric. Sci. 2025, 9, 500–521. [Google Scholar]
- Ozkan, G.; Koyuncu, M.A. Physical and chemical composition of some walnut (Juglans regia L) genotypes grown in Turkey. Grasas y Aceites 2005, 56, 141–146. [Google Scholar] [CrossRef]
- Sayıncı, B.; Ercişli, S.; Akbulut, M.; Şavşatlı, Y.; Baykal, H. Determination of shape in fruits of cherry laurel (Prunus laurocerasus) accessions by using Elliptic Fourier analysis. Acta Sci. Pol. Hortorum Cultus 2015, 14, 63–82. [Google Scholar]
- Mohsenin, N.N. Physical Properties of Plant and Animal Materials: Structure, Physical Characteristics and Mechanical Properties; Gordon and Breach Science Publishers: New York, NY, USA, 1986. [Google Scholar]
- Fıratlıgil-Durmuş, E.; Šárka, E.; Bubník, Z.; Schejbal, M.; Kadlec, P. Size properties of legume seeds of different varieties using image analysis. J. Food Eng. 2010, 99, 445–451. [Google Scholar] [CrossRef]
- Azadnia, R.; Noei-Khodabadi, F.; Moloudzadeh, A.; Jahanbakhshi, A.; Omid, M. Medicinal and poisonous plants classification from visual characteristics of leaves using computer vision and deep neural networks. Ecol. Inform. 2024, 82, 102683. [Google Scholar] [CrossRef]
- Stegmayer, G.; Milone, D.H.; Garran, S.; Burdyn, L. Automatic recognition of quarantine citrus diseases. Expert Syst. Appl. 2013, 40, 3512–3517. [Google Scholar] [CrossRef]
- Pietersma, D.; Lacroix, R.; Lefebvre, D.; Wade, K.M. Performance analysis for machine-learning experiments using small data sets. Comput. Electron. Agric. 2003, 38, 1–17. [Google Scholar] [CrossRef]
- Bradley, A.P. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recognit. 1997, 30, 1145–1159. [Google Scholar] [CrossRef]
- Huang, Y.; Li, R.; Coulter, J.A.; Zhang, Z.; Nan, Z. Comparative grain chemical composition, ruminal degradation in vivo, and intestinal digestibility in vitro of Vicia sativa L. varieties grown on the Tibetan Plateau. Animals 2019, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Mikić, A.; Mihailović, V.; Ćupina, B.; Vasiljević, S.; Milošević, B.; Katanski, S.; Kraljević-Balalić, M. Agronomic characteristics related to grain yield and crude protein content in common vetch (Vicia sativa) accessions of diverse geographic origin. N. Z. J. Agric. Res. 2013, 56, 297–308. [Google Scholar] [CrossRef]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; Lorenzo, J.M. Tannin in ruminant nutrition. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- Kökten, K.; Koçak, A.; Bağci, E.; Akçura, M.; Çelik, S. Tannin, protein contents and fatty acid compositions of the seeds of several Vicia L. species from Turkey. Grasas y Aceites 2010, 61, 404–408. [Google Scholar] [CrossRef]
- Kelln, B.M.; Penner, G.B.; Acharya, S.N.; McAllister, T.A.; Lardner, H.A. Impact of condensed tannin-containing legumes on ruminal fermentation, nutrition, and performance in ruminants: A review. Can. J. Anim. Sci. 2020, 101, 210–223. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Grzegorczyk, S.; Alberski, J.; Olszewska, M.; Grabowski, K.; Bałuch-Małecka, A. Content of calcium and phosphorus and the Ca: P ratio is selected species of leguminous and herbaceous plants. J. Elem. 2017, 22, 663–669. [Google Scholar]
- Samarah, N.H.; Ereifej, K. Chemical composition and mineral content of common vetch seeds during maturation. J. Plant Nutr. 2009, 32, 177–186. [Google Scholar] [CrossRef]
- Yalçın, İ.; Özarslan, C. Physical properties of vetch seed. Biosyst. Eng. 2004, 88, 507–512. [Google Scholar] [CrossRef]
- Işık, E.; İzli, N. Effects of moisture content on some physical properties of the yellow lentil. J. Agric. Sci. 2016, 22, 307–316. [Google Scholar]
- Dumanoğlu, Z.; Çaçan, E.; Kökten, K. A study on the determination of some morphological and physiological characteristics of Common Vetch (Vicia sativa L.) seeds. MAS J. Appl. Sci. 2022, 7, 41–47. [Google Scholar]
- Kibar, H.; Öztürk, T.; Temizel, K.E. Effective engineering properties in the design of storage structures of postharvest dry bean grain. Acta Scientiarum. Agron. 2014, 36, 147–158. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Hu, X. Cultivar discrimination of single alfalfa (Medicago sativa L.) seed via multispectral imaging combined with multivariate analysis. Sensors 2020, 20, 6575. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Song, R.; He, X.; Mao, P.; Jia, S. Non-destructive identification of naturally aged alfalfa seeds via multispectral imaging analysis. Sensors 2021, 21, 5804. [Google Scholar] [CrossRef]
- Xu, P.; Yang, R.; Zeng, T.; Zhang, J.; Zhang, Y.; Tan, Q. Varietal classification of maize seeds using computer vision and machine learning techniques. J. Food Process Eng. 2021, 44, e13846. [Google Scholar] [CrossRef]
- Chen, B.; Shi, B.; Gong, J.; Shi, G.; Jin, H.; Qin, T.; Wang, Z. Quality detection and variety classification of pecan seeds using hyperspectral imaging technology combined with machine learning. J. Food Compos. Anal. 2024, 131, 106248. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S. Variety identification of sweet maize seeds based on hyperspectral imaging combined with deep learning. Infrared Phys. Technol. 2023, 130, 104611. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, C.; Jia, L.; Tang, Q.; Gao, L.; Zhao, G.; Qi, H. Identification of rice seed varieties based on near-infrared hyperspectral imaging technology combined with deep learning. ACS Omega 2022, 7, 4735–4749. [Google Scholar] [CrossRef]
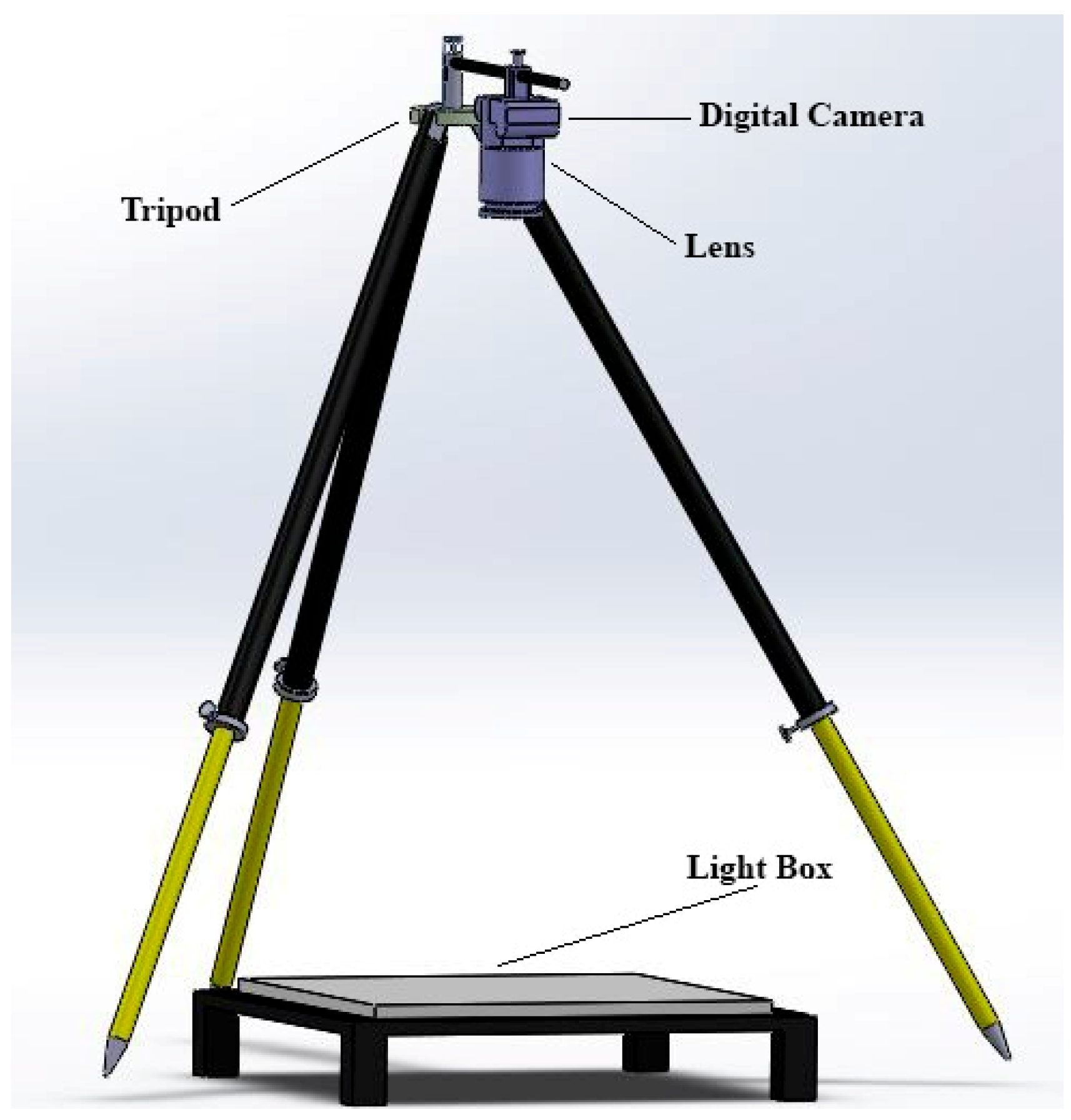



| Varieties | Institute | Coordinates |
|---|---|---|
| Alınoğlu | Field Crops Central Research Institute, Turkiye | 39°57′15.0″ N 32°48′45.8″ E |
| Ankara Moru | 39°57′14.9″ N 32°48′46.4″ E | |
| Ayaz | 39°57′15.0″ N 32°48′46.3″ E | |
| Alper | Aegean Agricultural Research Institute, Turkiye | 38°33′59.8″ N 27°03′09.7″ E |
| Doruk | 38°33′57.2″ N 27°03′09.8″ E | |
| Ürkmez | 38°33′57.9″ N 27°03′11.0″ E | |
| Alperen | Directorate of Trakya Agricultural Research Institute, Turkiye | 41°38′48.5″ N 26°35′47.7″ E |
| Kristal 2020 | 41°38′48.5″ N 26°35′48.4″ E | |
| Özveren | Eastern Mediterranean Agricultural Research Institute, Turkiye | 36°51′17.5″ N 35°20′31.3″ E |
| Varieties | Crude Protein | ADF | NDF | Tannin |
|---|---|---|---|---|
| Alper | 22.66a | 9.06bc | 13.65bc | 3.06abc |
| Alperen | 16.93e | 10.79ab | 16.47a | 2.76e |
| Alınoğlu | 20.91bc | 11.36a | 14.69ab | 3.12a |
| Ankara Moru | 19.71cd | 8.57bc | 11.92cd | 2.97bcd |
| Ayaz | 20.61bc | 9.03bc | 12.04cd | 0.48f |
| Doruk | 18.80d | 8.94bc | 15.25ab | 3.10ab |
| Kristal 2020 | 14.69f | 9.44abc | 14.68ab | 2.96cd |
| Özveren | 21.05bc | 8.19c | 11.25de | 3.07abc |
| Ürkmez | 21.96ab | 8.74bc | 9.57e | 2.90d |
| Mean | 19.70 | 9.35 | 13.28 | 2.71 |
| Varieties | B | Ca | Cu | Fe | K | Mg | Mn | Na | P | S | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alper | 5.00a | 1639.28c | 93.49a | 52.65e | 7674.08a | 1327.83c | 16.32c | 335.92a | 5393.60b | 1678.47a | 43.52c |
| Alperen | 3.22i | 1220.49i | 62.60i | 39.81h | 5061.00i | 971.09i | 22.56a | 246.39i | 4163.81i | 1258.39i | 40.64d |
| Alınoğlu | 4.08e | 1884.35b | 87.35c | 56.45c | 6627.64c | 1342.85b | 14.99d | 319.86c | 5265.08d | 1567.99e | 37.78f |
| Ankara Moru | 3.67f | 2267.34a | 79.52f | 78.17a | 5452.79h | 1232.32e | 16.65b | 270.44h | 4380.71h | 1301.24h | 31.20h |
| Ayaz | 4.14d | 1458.75h | 83.80d | 37.61i | 5778.38g | 1125.28g | 13.12f | 278.92f | 4449.36g | 1644.61b | 30.67i |
| Doruk | 4.42b | 1605.06d | 91.01b | 46.81f | 7225.46b | 1448.73a | 14.46e | 334.67b | 5697.81a | 1602.28c | 44.40b |
| Kristal 2020 | 3.27h | 1543.21g | 80.87e | 60.65b | 6230.40d | 1110.02h | 12.75h | 274.15g | 4960.85f | 1578.85d | 49.31a |
| Özveren | 4.34c | 1583.38f | 68.70h | 43.00g | 6070.57f | 1229.74f | 12.43i | 306.14d | 5268.80c | 1404.84g | 38.81e |
| Ürkmez | 3.61g | 1583.66e | 77.03g | 53.05d | 6093.19e | 1264.69d | 12.94g | 298.77e | 5034.80e | 1434.51f | 37.37g |
| Mean | 3.97 | 1642.84 | 80.49 | 52.02 | 6245.95 | 1228.06 | 15.14 | 296.14 | 4957.20 | 1496.80 | 39.30 |
| Varieties | Mass (M, g) | Volume (V, mm3) | Density (g cm−3) | Length (L, mm) | Width (W, mm) | Thickness (T, mm) | Geometric Mean Diam. (Dg, mm) | Projected Area (PA, mm2) | Surface Area (SA, mm2) |
|---|---|---|---|---|---|---|---|---|---|
| Alper | 0.07 ± 0.01c | 48.38 ± 8.45e | 1.45 ± 0.29b | 5.18 ± 0.40efg | 4.66 ± 0.37c | 3.80 ± 0.28e | 4.50 ± 0.27e | 16.00 ± 1.89e | 63.98 ± 7.56e |
| Alperen | 0.08 ± 0.02ab | 50.71 ± 9.08de | 1.58 ± 0.39a | 5.16 ± 0.40fg | 4.60 ± 0.28c | 4.05 ± 0.28bc | 4.58 ± 0.26de | 16.51 ± 1.93de | 66.03 ± 7.73de |
| Alınoğlu | 0.08 ± 0.02b | 60.75 ± 10.58b | 1.32 ± 0.32c | 5.67 ± 0.46ab | 4.86 ± 0.38b | 4.19 ± 0.33ab | 4.86 ± 0.29ab | 18.62 ± 2.18b | 74.48 ± 8.74b |
| Ankara Moru | 0.06 ± 0.01e | 52.07 ± 7.57cde | 1.15 ± 0.21d | 5.29 ± 0.32def | 4.58 ± 0.28c | 4.08 ± 0.24bc | 4.62 ± 0.22cde | 16.82 ± 1.62cde | 67.28 ± 6.50cde |
| Ayaz | 0.08 ± 0.02b | 68.80 ± 22.42a | 1.16 ± 0.42d | 5.73 ± 0.74a | 5.13 ± 0.71a | 4.27 ± 0.69a | 5.00 ± 0.68a | 19.99 ± 4.83a | 79.97 ± 19.33a |
| Doruk | 0.07 ± 0.02cd | 54.80 ± 8.61cd | 1.28 ± 0.34cd | 5.45 ± 0.37cd | 4.90 ± 0.31b | 3.90 ± 0.22de | 4.70 ± 0.24cd | 17.40 ± 1.81cd | 69.59 ± 7.26cd |
| Kristal 2020 | 0.07 ± 0.01cd | 48.96 ± 8.85e | 1.49 ± 0.33b | 5.02 ± 0.41g | 4.58 ± 0.32c | 4.03 ± 0.29cd | 4.52 ± 0.27e | 16.12 ± 1.93e | 64.49 ± 7.73e |
| Özveren | 0.08 ± 0.01a | 56.89 ± 6.59bc | 1.41 ± 0.21bc | 5.36 ± 0.25cde | 5.01 ± 0.26ab | 4.03 ± 0.23cd | 4.76 ± 0.19bc | 17.86 ± 1.39bc | 71.43 ± 5.55bc |
| Ürkmez | 0.06 ± 0.01de | 56.16 ± 8.42bc | 1.07 ± 0.23e | 5.49 ± 0.32bc | 4.88 ± 0.28b | 3.98 ± 0.30cd | 4.74 ± 0.24bc | 17.68 ± 1.79bc | 70.74 ± 7.15bc |
| Mean | 0.07 ± 0.02 | 55.28 ± 12.55 | 1.32 ± 0.34 | 5.37 ± 0.48 | 4.80 ± 0.42 | 4.04 ± 0.37 | 4.70 ± 0.36 | 17.44 ± 2.65 | 69.78 ± 10.58 |
| F-values | 36.18 ** | 34.50 ** | 32.64 ** | 30.57 ** | 28.56 ** | 16.19 ** | 24.74 ** | 29.60 ** | 29.60 ** |
| Varieties | Sphericity (S, %) | Shape Index (SI) | Roundness (R) | Aspect Ratio (AR) | Elongation (E) |
|---|---|---|---|---|---|
| Alper | 87.10 ± 3.47d | 1.23 ± 0.07ab | 0.76 ± 0.06c | 0.74 ± 0.07de | 1.37 ± 0.12ab |
| Alperen | 88.98 ± 3.92ab | 1.19 ± 0.08bc | 0.79 ± 0.07ab | 0.79 ± 0.07ab | 1.28 ± 0.11d |
| Alınoğlu | 86.06 ± 5.23d | 1.26 ± 0.12a | 0.74 ± 0.09c | 0.74 ± 0.08de | 1.36 ± 0.15ab |
| Ankara Moru | 87.45 ± 3.68bcd | 1.22 ± 0.08ab | 0.77 ± 0.06bc | 0.77 ± 0.05bc | 1.30 ± 0.10cd |
| Ayaz | 87.25 ± 4.96cd | 1.23 ± 0.11ab | 0.76 ± 0.08bc | 0.74 ± 0.08cde | 1.36 ± 0.16ab |
| Doruk | 86.44 ± 2.86d | 1.24 ± 0.06a | 0.75 ± 0.05c | 0.72 ± 0.05e | 1.40 ± 0.09a |
| Kristal 2020 | 90.28 ± 4.15a | 1.17 ± 0.08c | 0.82 ± 0.07a | 0.81 ± 0.07a | 1.25 ± 0.12d |
| Özveren | 88.92 ± 2.04abc | 1.19 ± 0.04c | 0.79 ± 0.04ab | 0.75 ± 0.05cd | 1.33 ± 0.08bc |
| Ürkmez | 86.38 ± 3.66d | 1.24 ± 0.08a | 0.75 ± 0.06c | 0.73 ± 0.06de | 1.39 ± 0.11a |
| Mean | 87.65 ± 4.10 | 1.22 ± 0.09 | 0.77 ± 0.07 | 0.75 ± 0.07 | 1.34 ± 0.13 |
| F-values | 13.57 ** | 12.62 ** | 13.80 ** | 20.79 ** | 18.96 ** |
| Eigenvalue Statistics | Function 1 | Function 2 | Function 3 | Function 4 | Function 5 | Function 6 | Function 7 | Function 8 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eigenvalues | 1.087 | 0.360 | 0.302 | 0.156 | 0.045 | 0.035 | 0.003 | 0.000 | |||||||||||||
| % of variance | 54.6 | 18.1 | 15.2 | 7.9 | 2.3 | 1.8 | 0.2 | 0.0 | |||||||||||||
| % of cumulative variance | 54.6 | 72.7 | 87.9 | 95.8 | 98.1 | 99.8 | 100.0 | 100.0 | |||||||||||||
| Canonical correlation | 0.722 | 0.514 | 0.482 | 0.368 | 0.208 | 0.185 | 0.055 | 0.012 | |||||||||||||
| MANOVA results | |||||||||||||||||||||
| Effect | Statistics | Value | Hypothesis DF | Error DF | F | p (sigma) | |||||||||||||||
| Variables | Pillai’s trace | 1.346 | 96 | 7096 | 14.95 | 0.000 ** | |||||||||||||||
| Wilks’ Lambda | 0.187 | 96 | 5938 | 17.48 | 0.000 ** | ||||||||||||||||
| Hotelling Trace | 2.182 | 96 | 7026 | 19.97 | 0.000 ** | ||||||||||||||||
| Hotelling’s pairwise comparisons. Bonferroni corrected p values in upper triangle. Mahalanobis distances in lower triangle | |||||||||||||||||||||
| Varieties | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||||||||||
| Alper | - | 1.05 × 10−9 | 9.38 × 10−17 | 4.84 × 10−11 | 2.33 × 10−47 | 2.35 × 10−2 | 2.66 × 10−8 | 9.99 × 10−15 | 1.09 × 10−6 | ||||||||||||
| Alperen | 1.99 | - | 7.10 × 10−12 | 5.90 × 10−13 | 1.21 × 10−48 | 1.02 × 10−16 | 1.33 × 10−4 | 2.19 × 10−15 | 1.42 × 10−17 | ||||||||||||
| Alınoğlu | 3.24 | 2.35 | - | 2.14 × 10−17 | 8.26 × 10−39 | 5.32 × 10−16 | 3.55 × 10−19 | 1.52 × 10−17 | 1.79 × 10−15 | ||||||||||||
| Ankara Moru | 2.21 | 2.54 | 3.37 | - | 6.84 × 10−53 | 1.00 × 10−15 | 7.23 × 10−10 | 5.82 × 10−30 | 3.70 × 10−10 | ||||||||||||
| Ayaz | 11.87 | 12.39 | 8.79 | 14.24 | - | 6.88 × 10−48 | 1.62 × 10−50 | 5.24 × 10−49 | 1.20 × 10−47 | ||||||||||||
| Doruk | 0.81 | 3.24 | 3.10 | 3.05 | 12.08 | - | 6.32 × 10−17 | 2.18 × 10−11 | 1.09 × 10+00 | ||||||||||||
| Kristal 2020 | 1.76 | 1.17 | 3.72 | 2.01 | 13.18 | 3.28 | - | 1.82 × 10−24 | 5.28 × 10−16 | ||||||||||||
| Özveren | 2.86 | 2.98 | 3.40 | 6.21 | 12.54 | 2.27 | 4.86 | - | 6.65 × 10−20 | ||||||||||||
| Ürkmez | 1.50 | 3.40 | 3.00 | 2.06 | 11.98 | 0.53 | 3.10 | 3.87 | - | ||||||||||||
| Classifiers | Predicted | Actual | Accuracy (%) | TPR | Precision | F1 | ROC | PRC | |
|---|---|---|---|---|---|---|---|---|---|
| Alper vs. Alperen | |||||||||
| MLP | Alper | Alperen | - | - | - | - | - | - | - |
| 76 | 24 | Alper | 77.00 | 0.760 | 0.776 | 0.768 | 0.788 | 0.773 | |
| 22 | 78 | Alperen | - | 0.780 | 0.765 | 0.772 | 0.788 | 0.732 | |
| RF | Alper | Alperen | - | - | - | - | - | - | - |
| 74 | 26 | Alper | 77.50 | 0.740 | 0.796 | 0.767 | 0.824 | 0.842 | |
| 19 | 81 | Alperen | - | 0.810 | 0.757 | 0.775 | 0.823 | 0.804 | |
| SVM | Alper | Alperen | - | - | - | - | - | - | - |
| 80 | 20 | Alper | 74.50 | 0.800 | 0.721 | 0.758 | 0.745 | 0.677 | |
| 31 | 69 | Alperen | - | 0.690 | 0.775 | 0.730 | 0.745 | 0.690 | |
| Alper vs. Alınoğlu | |||||||||
| MLP | Alper | Alınoğlu | - | - | - | - | - | - | - |
| 81 | 19 | Alper | 79.50 | 0.810 | 0.786 | 0.798 | 0.851 | 0.849 | |
| 22 | 78 | Alınoğlu | - | 0.780 | 0.804 | 0.792 | 0.851 | 0.831 | |
| RF | Alper | Alınoğlu | - | - | - | - | - | - | - |
| 78 | 22 | Alper | 77.00 | 0.780 | 0.765 | 0.772 | 0.858 | 0.873 | |
| 24 | 76 | Alınoğlu | - | 0.760 | 0.776 | 0.768 | 0.858 | 0.802 | |
| SVM | Alper | Alınoğlu | - | - | - | - | - | - | - |
| 83 | 17 | Alper | 78.50 | 0.830 | 0.761 | 0.794 | 0.785 | 0.717 | |
| 26 | 74 | Alınoğlu | - | 0.740 | 0.813 | 0.775 | 0.785 | 0.732 | |
| Alper vs. A. Moru | |||||||||
| MLP | Alper | A. Moru | - | - | - | - | - | - | - |
| 74 | 26 | Alper | 73.50 | 0.740 | 0.733 | 0.736 | 0.814 | 0.822 | |
| 27 | 73 | A. Moru | - | 0.730 | 0.737 | 0.734 | 0.814 | 0.756 | |
| RF | Alper | A. Moru | - | - | - | - | - | - | - |
| 77 | 23 | Alper | 78.50 | 0.770 | 0.794 | 0.782 | 0.863 | 0.886 | |
| 20 | 80 | A. Moru | - | 0.800 | 0.777 | 0.788 | 0.863 | 0.812 | |
| SVM | Alper | A. Moru | - | - | - | - | - | - | - |
| 65 | 35 | Alper | 72.50 | 0.650 | 0.765 | 0.703 | 0.725 | 0.672 | |
| 20 | 80 | A. Moru | - | 0.800 | 0.696 | 0.744 | 0.725 | 0.657 | |
| Alper vs. Ayaz | |||||||||
| MLP | Alper | Ayaz | - | - | - | - | - | - | - |
| 91 | 9 | Alper | 85.00 | 0.910 | 0.813 | 0.858 | 0.909 | 0.887 | |
| 21 | 79 | Ayaz | - | 0.790 | 0.898 | 0.840 | 0.909 | 0.886 | |
| RF | Alper | Ayaz | - | - | - | - | - | - | - |
| 89 | 11 | Alper | 87.00 | 0.890 | 0.856 | 0.879 | 0.925 | 0.891 | |
| 15 | 85 | Ayaz | - | 0.850 | 0.885 | 0.867 | 0.928 | 0.939 | |
| SVM | Alper | Ayaz | - | - | - | - | - | - | - |
| 92 | 8 | Alper | 82.00 | 0.920 | 0.767 | 0.836 | 0.820 | 0.745 | |
| 28 | 72 | Ayaz | - | 0.720 | 0.900 | 0.800 | 0.820 | 0.788 | |
| Alper vs. Doruk | |||||||||
| MLP | Alper | Doruk | - | - | - | - | - | - | - |
| 69 | 31 | Alper | 65.00 | 0.690 | 0.639 | 0.663 | 0.685 | 0.676 | |
| 39 | 61 | Doruk | - | 0.610 | 0.651 | 0.649 | 0.685 | 0.686 | |
| RF | Alper | Doruk | - | - | - | - | - | - | - |
| 57 | 43 | Alper | 60.00 | 0.570 | 0.606 | 0.588 | 0.636 | 0.621 | |
| 37 | 63 | Doruk | - | 0.630 | 0.594 | 0.612 | 0.636 | 0.643 | |
| SVM | Alper | Doruk | - | - | - | - | - | - | - |
| 60 | 40 | Alper | 60.00 | 0.600 | 0.600 | 0.597 | 0.595 | 0.557 | |
| 40 | 60 | Doruk | - | 0.600 | 0.600 | 0.597 | 0.595 | 0.557 | |
| Alper vs. Kristal | |||||||||
| MLP | Alper | Kristal | - | - | - | - | - | - | - |
| 78 | 22 | Alper | 72.00 | 0.780 | 0.696 | 0.736 | 0.738 | 0.716 | |
| 34 | 66 | Kristal | - | 0.660 | 0.750 | 0.702 | 0.738 | 0.721 | |
| RF | Alper | Kristal | - | - | - | - | - | - | - |
| 71 | 29 | Alper | 72.00 | 0.710 | 0.724 | 0.717 | 0.747 | 0.695 | |
| 27 | 73 | Kristal | - | 0.730 | 0.716 | 0.723 | 0.747 | 0.713 | |
| SVM | Alper | Kristal | - | - | - | - | - | - | - |
| 74 | 26 | Alper | 68.50 | 0.740 | 0.667 | 0.701 | 0.685 | 0.623 | |
| 37 | 63 | Kristal | - | 0.630 | 0.708 | 0.667 | 0.685 | 0.631 | |
| Alper vs. Özveren | |||||||||
| MLP | Alper | Özveren | - | - | - | - | - | - | - |
| 78 | 22 | Alper | 78.00 | 0.780 | 0.780 | 0.780 | 0.823 | 0.824 | |
| 22 | 78 | Özveren | - | 0.780 | 0.780 | 0.780 | 0.823 | 0.756 | |
| RF | Alper | Özveren | - | - | - | - | - | - | - |
| 75 | 25 | Alper | 75.50 | 0.750 | 0.758 | 0.754 | 0.831 | 0.821 | |
| 24 | 76 | Özveren | - | 0.760 | 0.752 | 0.756 | 0.831 | 0.774 | |
| SVM | Alper | Özveren | - | - | - | - | - | - | - |
| 79 | 21 | Alper | 80.50 | 0.790 | 0.814 | 0.802 | 0.805 | 0.748 | |
| 18 | 82 | Özveren | - | 0.820 | 0.796 | 0.808 | 0.805 | 0.743 | |
| Alper vs. Ürkmez | |||||||||
| MLP | Alper | Ürkmez | - | - | - | - | - | - | - |
| 65 | 35 | Alper | 69.00 | 0.650 | 0.707 | 0.677 | 0.759 | 0.745 | |
| 27 | 73 | Ürkmez | - | 0.730 | 0.676 | 0.702 | 0.759 | 0.753 | |
| RF | Alper | Ürkmez | - | - | - | - | - | - | - |
| 67 | 33 | Alper | 68.50 | 0.670 | 0.691 | 0.680 | 0.736 | 0.713 | |
| 30 | 70 | Ürkmez | - | 0.700 | 0.680 | 0.690 | 0.736 | 0.705 | |
| SVM | Alper | Ürkmez | - | - | - | - | - | - | - |
| 67 | 33 | Alper | 69.50 | 0.670 | 0.705 | 0.687 | 0.695 | 0.638 | |
| 28 | 72 | Ürkmez | - | 0.720 | 0.686 | 0.702 | 0.695 | 0.634 | |
| Classifiers | Predicted | Actual | Accuracy (%) | TPR | Precision | F1 | ROC | PRC | |
|---|---|---|---|---|---|---|---|---|---|
| Alperen vs. Alınoğlu | |||||||||
| MLP | Alperen | Alınoğlu | - | - | - | - | - | - | - |
| 74 | 26 | Alperen | 72.50 | 0.740 | 0.718 | 0.729 | 0.772 | 0.741 | |
| 29 | 71 | Alınoğlu | - | 0.710 | 0.732 | 0.721 | 0.772 | 0.771 | |
| RF | Alperen | Alınoğlu | - | - | - | - | - | - | - |
| 74 | 26 | Alperen | 75.00 | 0.740 | 0.755 | 0.747 | 0.767 | 0.735 | |
| 24 | 76 | Alınoğlu | - | 0.760 | 0.750 | 0.750 | 0.767 | 0.730 | |
| SVM | Alperen | Alınoğlu | - | - | - | - | - | - | - |
| 79 | 21 | Alperen | 76.00 | 0.790 | 0.745 | 0.767 | 0.760 | 0.694 | |
| 27 | 73 | Alınoğlu | - | 0.790 | 0.777 | 0.753 | 0.760 | 0.702 | |
| Alperen vs. A. Moru | |||||||||
| MLP | Alperen | A. Moru | - | - | - | - | - | - | - |
| 74 | 26 | Alperen | 77.00 | 0.740 | 0.787 | 0.763 | 0.831 | 0.853 | |
| 20 | 80 | A. Moru | - | 0.800 | 0.755 | 0.777 | 0.831 | 0.779 | |
| RF | Alperen | A. Moru | - | - | - | - | - | - | - |
| 75 | 25 | Alperen | 77.00 | 0.750 | 0.781 | 0.765 | 0.859 | 0.869 | |
| 21 | 79 | A. Moru | - | 0.790 | 0.760 | 0.775 | 0.859 | 0.835 | |
| SVM | Alperen | A. Moru | - | - | - | - | - | - | - |
| 65 | 35 | Alperen | 79.50 | 0.650 | 0.915 | 0.760 | 0.795 | 0.770 | |
| 6 | 94 | A. Moru | - | 0.940 | 0.729 | 0.821 | 0.795 | 0.715 | |
| Alperen vs. Ayaz | |||||||||
| MLP | Alperen | Ayaz | - | - | - | - | - | - | - |
| 88 | 12 | Alperen | 86.00 | 0.880 | 0.846 | 0.863 | 0.942 | 0.948 | |
| 16 | 84 | Ayaz | - | 0.840 | 0.875 | 0.857 | 0.942 | 0.941 | |
| RF | Alperen | Ayaz | - | - | - | - | - | - | - |
| 89 | 11 | Alperen | 88.00 | 0.890 | 0.873 | 0.881 | 0.925 | 0.905 | |
| 13 | 87 | Ayaz | - | 0.870 | 0.880 | 0.880 | 0.925 | 0.935 | |
| SVM | Alperen | Ayaz | - | - | - | - | - | - | - |
| 89 | 11 | Alperen | 81.00 | 0.890 | 0.767 | 0.824 | 0.810 | 0.738 | |
| 27 | 73 | Ayaz | - | 0.730 | 0.869 | 0.793 | 0.810 | 0.769 | |
| Alperen vs. Doruk | |||||||||
| MLP | Alperen | Doruk | - | - | - | - | - | - | - |
| 79 | 21 | Alperen | 77.50 | 0.790 | 0.767 | 0.778 | 0.879 | 0.874 | |
| 24 | 76 | Doruk | - | 0.760 | 0.784 | 0.771 | 0.879 | 0.895 | |
| RF | Alperen | Doruk | - | - | - | - | - | - | - |
| 85 | 15 | Alperen | 82.00 | 0.850 | 0.802 | 0.825 | 0.900 | 0.903 | |
| 21 | 79 | Doruk | - | 0.790 | 0.840 | 0.814 | 0.900 | 0.896 | |
| SVM | Alperen | Doruk | - | - | - | - | - | - | - |
| 79 | 21 | Alperen | 80.50 | 0.790 | 0.814 | 0.802 | 0.805 | 0.748 | |
| 18 | 82 | Doruk | - | 0.820 | 0.796 | 0.808 | 0.805 | 0.743 | |
| Alperen vs. Kristal | |||||||||
| MLP | Alperen | Kristal | - | - | - | - | - | - | - |
| 62 | 38 | Alperen | 60.50 | 0.620 | 0.602 | 0.611 | 0.698 | 0.718 | |
| 41 | 59 | Kristal | 0.590 | 0.608 | 0.599 | 0.698 | 0.695 | ||
| RF | Alperen | Kristal | - | - | - | - | - | - | - |
| 62 | 38 | Alperen | 62.00 | 0.620 | 0.620 | 0.620 | 0.678 | 0.646 | |
| 38 | 62 | Kristal | - | 0.620 | 0.620 | 0.620 | 0.678 | 0.682 | |
| SVM | Alperen | Kristal | - | - | - | - | - | - | - |
| 58 | 42 | Alperen | 67.50 | 0.580 | 0.716 | 0.641 | 0.675 | 0.625 | |
| 23 | 77 | Kristal | - | 0.770 | 0.647 | 0.703 | 0.675 | 0.613 | |
| Alperen vs. Özveren | |||||||||
| MLP | Alperen | Özveren | - | - | - | - | - | - | - |
| 78 | 22 | Alperen | 76.50 | 0.780 | 0.757 | 0.768 | 0.849 | 0.842 | |
| 25 | 75 | Özveren | - | 0.750 | 0.773 | 0.761 | 0.849 | 0.870 | |
| RF | Alperen | Özveren | - | - | - | - | - | - | - |
| 74 | 26 | Alperen | 74.00 | 0.740 | 0.740 | 0.740 | 0.828 | 0.806 | |
| 26 | 74 | Özveren | - | 0.740 | 0.740 | 0.740 | 0.828 | 0.845 | |
| SVM | Alperen | Özveren | - | - | - | - | - | - | - |
| 81 | 19 | Alperen | 79.00 | 0.810 | 0.779 | 0.794 | 0.790 | 0.726 | |
| 23 | 77 | Özveren | - | 0.770 | 0.802 | 0.786 | 0.790 | 0.733 | |
| Alperen vs. Ürkmez | |||||||||
| MLP | Alperen | Ürkmez | - | - | - | - | - | - | - |
| 81 | 19 | Alperen | 80.00 | 0.810 | 0.794 | 0.802 | 0.904 | 0.913 | |
| 21 | 79 | Ürkmez | - | 0.790 | 0.806 | 0.798 | 0.904 | 0.910 | |
| RF | Alperen | Ürkmez | - | - | - | - | - | - | - |
| 80 | 20 | Alperen | 81.50 | 0.800 | 0.825 | 0.812 | 0.901 | 0.914 | |
| 17 | 83 | Ürkmez | - | 0.830 | 0.806 | 0.818 | 0.901 | 0.883 | |
| SVM | Alperen | Ürkmez | - | - | - | - | - | - | - |
| 76 | 24 | Alperen | 82.00 | 0.760 | 0.864 | 0.809 | 0.820 | 0.776 | |
| 12 | 88 | Ürkmez | - | 0.880 | 0.786 | 0.830 | 0.820 | 0.751 | |
| Classifiers | Predicted | Actual | Accuracy (%) | TPR | Precision | F1 | ROC | PRC | |
|---|---|---|---|---|---|---|---|---|---|
| Alınoğlu vs. A. Moru | |||||||||
| MLP | Alınoğlu | A. Moru | - | - | - | - | - | - | |
| 80 | 20 | Alınoğlu | 80.50 | 0.800 | 0.808 | 0.804 | 0.886 | 0.865 | |
| 19 | 81 | A. Moru | - | 0.810 | 0.802 | 0.806 | 0.886 | 0.880 | |
| RF | Alınoğlu | A. Moru | - | - | - | - | - | - | - |
| 82 | 18 | Alınoğlu | 80.00 | 0.820 | 0.788 | 0.804 | 0.867 | 0.848 | |
| 22 | 78 | A. Moru | - | 0.780 | 0.813 | 0.796 | 0.867 | 0.839 | |
| SVM | Alınoğlu | A. Moru | - | - | - | - | - | - | - |
| 83 | 17 | Alınoğlu | 83.00 | 0.830 | 0.830 | 0.830 | 0.830 | 0.774 | |
| 17 | 83 | A. Moru | - | 0.830 | 0.830 | 0.830 | 0.830 | 0.774 | |
| Alınoğlu vs. Ayaz | |||||||||
| MLP | Alınoğlu | Ayaz | - | - | - | - | - | - | - |
| 76 | 24 | Alınoğlu | 73.50 | 0.760 | 0.724 | 0.741 | 0.826 | 0.813 | |
| 29 | 71 | Ayaz | - | 0.710 | 0.747 | 0.728 | 0.826 | 0.834 | |
| RF | Alınoğlu | Ayaz | - | - | - | - | - | - | - |
| 78 | 22 | Alınoğlu | 75.00 | 0.780 | 0.736 | 0.757 | 0.832 | 0.800 | |
| 28 | 72 | Ayaz | - | 0.720 | 0.766 | 0.742 | 0.832 | 0.829 | |
| SVM | Alınoğlu | Ayaz | - | - | - | - | - | - | - |
| 78 | 22 | Alınoğlu | 71.00 | 0.780 | 0.684 | 0.729 | 0.710 | 0.644 | |
| 36 | 64 | Ayaz | - | 0.640 | 0.744 | 0.688 | 0.710 | 0.656 | |
| Alınoğlu vs. Doruk | |||||||||
| MLP | Alınoğlu | Doruk | - | - | - | - | - | - | - |
| 76 | 24 | Alınoğlu | 78.50 | 0.760 | 0.800 | 0.779 | 0.853 | 0.859 | |
| 19 | 81 | Doruk | - | 0.810 | 0.771 | 0.790 | 0.853 | 0.833 | |
| RF | Alınoğlu | Doruk | - | - | - | - | - | - | - |
| 75 | 25 | Alınoğlu | 77.00 | 0.750 | 0.781 | 0.765 | 0.840 | 0.833 | |
| 21 | 79 | Doruk | - | 0.790 | 0.760 | 0.775 | 0.840 | 0.832 | |
| SVM | Alınoğlu | Doruk | - | - | - | - | - | - | - |
| 71 | 29 | Alınoğlu | 77.50 | 0.710 | 0.816 | 0.759 | 0.775 | 0.724 | |
| 16 | 84 | Doruk | - | 0.840 | 0.743 | 0.789 | 0.775 | 0.704 | |
| Alınoğlu vs. Kristal | |||||||||
| MLP | Alınoğlu | Kristal | - | - | - | - | - | - | - |
| 84 | 16 | Alınoğlu | 79.50 | 0.840 | 0.771 | 0.804 | 0.860 | 0.846 | |
| 25 | 75 | Kristal | - | 0.750 | 0.824 | 0.785 | 0.860 | 0.854 | |
| RF | Alınoğlu | Kristal | - | - | - | - | - | - | - |
| 76 | 24 | Alınoğlu | 76.50 | 0.760 | 0.768 | 0.764 | 0.830 | 0.836 | |
| 23 | 77 | Kristal | - | 0.770 | 0.762 | 0.766 | 0.830 | 0.801 | |
| SVM | Alınoğlu | Kristal | - | - | - | - | - | - | - |
| 88 | 12 | Alınoğlu | 83.50 | 0.880 | 0.807 | 0.842 | 0.835 | 0.770 | |
| 21 | 79 | Kristal | - | 0.790 | 0.868 | 0.827 | 0.835 | 0.791 | |
| Alınoğlu vs. Özveren | |||||||||
| MLP | Alınoğlu | Özveren | - | - | - | - | - | - | - |
| 73 | 27 | Alınoğlu | 73.50 | 0.730 | 0.737 | 0.734 | 0.832 | 0.807 | |
| 26 | 74 | Özveren | - | 0.740 | 0.733 | 0.736 | 0.832 | 0.833 | |
| RF | Alınoğlu | Özveren | - | - | - | - | - | - | - |
| 75 | 25 | Alınoğlu | 76.00 | 0.750 | 0.765 | 0.758 | 0.832 | 0.823 | |
| 23 | 77 | Özveren | - | 0.770 | 0.755 | 0.762 | 0.832 | 0.836 | |
| SVM | Alınoğlu | Özveren | - | - | - | - | - | - | - |
| 69 | 31 | Alınoğlu | 73.00 | 0.690 | 0.750 | 0.719 | 0.730 | 0.673 | |
| 23 | 77 | Özveren | - | 0.770 | 0.713 | 0.740 | 0.730 | 0.664 | |
| Alınoğlu vs. Ürkmez | |||||||||
| MLP | Alınoğlu | Ürkmez | - | - | - | - | - | - | - |
| 73 | 27 | Alınoğlu | 75.50 | 0.730 | 0.768 | 0.749 | 0.843 | 0.865 | |
| 22 | 78 | Ürkmez | - | 0.780 | 0.743 | 0.761 | 0.843 | 0.821 | |
| RF | Alınoğlu | Ürkmez | - | - | - | - | - | - | - |
| 71 | 29 | Alınoğlu | 73.00 | 0.710 | 0.740 | 0.724 | 0.818 | 0.830 | |
| 25 | 75 | Ürkmez | - | 0.750 | 0.721 | 0.735 | 0.818 | 0.804 | |
| SVM | Alınoğlu | Ürkmez | - | - | - | - | - | - | - |
| 74 | 26 | Alınoğlu | 76.50 | 0.740 | 0.779 | 0.759 | 0.765 | 0.706 | |
| 21 | 79 | Ürkmez | - | 0.790 | 0.752 | 0.771 | 0.765 | 0.699 | |
| Classifiers | Predicted | Actual | Accuracy (%) | TPR | Precision | F1 | ROC | PRC | |
|---|---|---|---|---|---|---|---|---|---|
| A. Moru vs. Ayaz | |||||||||
| MLP | A. Moru | Ayaz | - | - | - | - | - | - | - |
| 93 | 7 | A. Moru | 89.00 | 0.930 | 0.861 | 0.894 | 0.909 | 0.891 | |
| 15 | 85 | Ayaz | - | 0.850 | 0.924 | 0.885 | 0.909 | 0.908 | |
| RF | A. Moru | Ayaz | - | - | - | - | - | - | - |
| 92 | 8 | A. Moru | 90.00 | 0.920 | 0.885 | 0.902 | 0.965 | 0.951 | |
| 12 | 88 | Ayaz | - | 0.880 | 0.917 | 0.898 | 0.965 | 0.969 | |
| SVM | A. Moru | Ayaz | - | - | - | - | - | - | - |
| 96 | 4 | A. Moru | 82.50 | 0.960 | 0.756 | 0.846 | 0.825 | 0.746 | |
| 31 | 69 | Ayaz | - | 0.690 | 0.945 | 0.798 | 0.825 | 0.807 | |
| A. Moru vs. Doruk | |||||||||
| MLP | A. Moru | Doruk | - | - | - | - | - | - | - |
| 93 | 7 | A. Moru | 87.50 | 0.930 | 0.838 | 0.882 | 0.911 | 0.875 | |
| 18 | 82 | Doruk | - | 0.820 | 0.921 | 0.868 | 0.911 | 0.918 | |
| RF | A. Moru | Doruk | - | - | - | - | - | - | - |
| 89 | 11 | A. Moru | 87.00 | 0.890 | 0.856 | 0.873 | 0.909 | 0.879 | |
| 15 | 85 | Doruk | - | 0.850 | 0.885 | 0.867 | 0.909 | 0.922 | |
| SVM | A. Moru | Doruk | - | - | - | - | - | - | - |
| 79 | 21 | A. Moru | 77.00 | 0.790 | 0.760 | 0.775 | 0.770 | 0.705 | |
| 25 | 75 | Doruk | - | 0.750 | 0.781 | 0.765 | 0.770 | 0.711 | |
| A. Moru vs. Kristal | |||||||||
| MLP | A. Moru | Kristal | - | - | - | - | - | - | - |
| 84 | 16 | A. Moru | 70.00 | 0.840 | 0.656 | 0.737 | 0.733 | 0.659 | |
| 44 | 56 | Kristal | - | 0.560 | 0.778 | 0.651 | 0.733 | 0.771 | |
| RF | A. Moru | Kristal | - | - | - | - | - | - | - |
| 79 | 21 | A. Moru | 73.50 | 0.790 | 0.712 | 0.749 | 0.799 | 0.769 | |
| 32 | 68 | Kristal | - | 0.680 | 0.764 | 0.720 | 0.799 | 0.810 | |
| SVM | A. Moru | Kristal | - | - | - | - | - | - | - |
| 83 | 17 | A. Moru | 71.50 | 0.830 | 0.675 | 0.744 | 0.715 | 0.645 | |
| 40 | 60 | Kristal | - | 0.600 | 0.779 | 0.678 | 0.715 | 0.668 | |
| A. Moru vs. Özveren | |||||||||
| MLP | A. Moru | Özveren | - | - | - | - | - | - | - |
| 89 | 11 | A. Moru | 88.00 | 0.890 | 0.873 | 0.881 | 0.931 | 0.912 | |
| 11 | 87 | Özveren | - | 0.870 | 0.888 | 0.879 | 0.931 | 0.931 | |
| RF | A. Moru | Özveren | - | - | - | - | - | - | - |
| 91 | 9 | A. Moru | 90.50 | 0.910 | 0.901 | 0.905 | 0.966 | 0.965 | |
| 10 | 90 | Özveren | - | 0.900 | 0.909 | 0.905 | 0.966 | 0.968 | |
| SVM | A. Moru | Özveren | - | - | - | - | - | - | - |
| 90 | 10 | A. Moru | 88.00 | 0.900 | 0.865 | 0.882 | 0.880 | 0.829 | |
| 14 | 86 | Özveren | - | 0.860 | 0.896 | 0.878 | 0.880 | 0.840 | |
| A. Moru vs. Ürkmez | |||||||||
| MLP | A. Moru | Ürkmez | - | - | - | - | - | - | - |
| 85 | 15 | A. Moru | 80.50 | 0.850 | 0.780 | 0.813 | 0.873 | 0.849 | |
| 24 | 76 | Ürkmez | - | 0.760 | 0.835 | 0.796 | 0.873 | 0.873 | |
| RF | A. Moru | Ürkmez | - | - | - | - | - | - | - |
| 83 | 17 | A. Moru | 80.50 | 0.830 | 0.790 | 0.810 | 0.878 | 0.853 | |
| 22 | 78 | Ürkmez | - | 0.780 | 0.821 | 0.800 | 0.878 | 0.852 | |
| SVM | A. Moru | Ürkmez | - | - | - | - | - | - | - |
| 67 | 33 | A. Moru | 75.00 | 0.670 | 0.798 | 0.728 | 0.750 | 0.699 | |
| 17 | 83 | Ürkmez | - | 0.830 | 0.716 | 0.769 | 0.750 | 0.679 | |
| Classifiers | Predicted | Actual | Accuracy (%) | TPR | Precision | F1 | ROC | PRC | |
|---|---|---|---|---|---|---|---|---|---|
| Ayaz vs. Doruk | |||||||||
| MLP | Ayaz | Doruk | - | - | - | - | - | - | - |
| 82 | 18 | Ayaz | 87.50 | 0.820 | 0.921 | 0.868 | 0.908 | 0.941 | |
| 7 | 93 | Doruk | - | 0.930 | 0.838 | 0.882 | 0.908 | 0.816 | |
| RF | Ayaz | Doruk | - | - | - | - | - | - | - |
| 86 | 14 | Ayaz | 89.50 | 0.860 | 0.925 | 0.860 | 0.938 | 0.954 | |
| 7 | 93 | Doruk | - | 0.930 | 0.869 | 0.899 | 0.938 | 0.908 | |
| SVM | Ayaz | Doruk | - | - | - | - | - | - | - |
| 69 | 31 | Ayaz | 81.00 | 0.690 | 0.908 | 0.784 | 0.810 | 0.781 | |
| 7 | 93 | Doruk | - | 0.930 | 0.750 | 0.830 | 0.810 | 0.732 | |
| Ayaz vs. Kristal | |||||||||
| MLP | Ayaz | Kristal | - | - | - | - | - | - | - |
| 80 | 20 | Ayaz | 85.50 | 0.800 | 0.899 | 0.847 | 0.901 | 0.885 | |
| 9 | 91 | Kristal | - | 0.910 | 0.820 | 0.863 | 0.901 | 0.874 | |
| RF | Ayaz | Kristal | - | - | - | - | - | - | - |
| 88 | 12 | Ayaz | 88.50 | 0.880 | 0.889 | 0.884 | 0.932 | 0.944 | |
| 11 | 89 | Kristal | - | 0.890 | 0.881 | 0.886 | 0.932 | 0.908 | |
| SVM | Ayaz | Kristal | - | - | - | - | - | - | - |
| 70 | 30 | Ayaz | 79.50 | 0.700 | 0.864 | 0.773 | 0.795 | 0.755 | |
| 11 | 89 | Kristal | - | 0.890 | 0.748 | 0.813 | 0.795 | 0.721 | |
| Ayaz vs. Özveren | |||||||||
| MLP | Ayaz | Özveren | - | - | - | - | - | - | - |
| 85 | 15 | Ayaz | 86.50 | 0.850 | 0.876 | 0.863 | 0.935 | 0.913 | |
| 12 | 88 | Özveren | - | 0.880 | 0.854 | 0.867 | 0.935 | 0.928 | |
| RF | Ayaz | Özveren | - | - | - | - | - | - | - |
| 87 | 13 | Ayaz | 89.50 | 0.870 | 0.916 | 0.892 | 0.944 | 0.946 | |
| 8 | 92 | Özveren | - | 0.920 | 0.876 | 0.898 | 0.944 | 0.911 | |
| SVM | Ayaz | Özveren | - | - | - | - | - | - | - |
| 61 | 39 | Ayaz | 73.00 | 0.610 | 0.803 | 0.693 | 0.730 | 0.685 | |
| 15 | 85 | Özveren | - | 0.850 | 0.685 | 0.759 | 0.730 | 0.658 | |
| Ayaz vs. Ürkmez | |||||||||
| MLP | Ayaz | Ürkmez | - | - | - | - | - | - | - |
| 85 | 15 | Ayaz | 88.50 | 0.850 | 0.914 | 0.881 | 0.916 | 0.943 | |
| 8 | 92 | Ürkmez | - | 0.920 | 0.860 | 0.889 | 0.916 | 0.873 | |
| RF | Ayaz | Ürkmez | - | - | - | - | - | - | - |
| 84 | 16 | Ayaz | 88.00 | 0.840 | 0.913 | 0.875 | 0.930 | 0.932 | |
| 8 | 92 | Ürkmez | - | 0.920 | 0.852 | 0.885 | 0.930 | 0.912 | |
| SVM | Ayaz | Ürkmez | - | - | - | - | - | - | - |
| 63 | 37 | Ayaz | 75.00 | 0.630 | 0.829 | 0.716 | 0.750 | 0.707 | |
| 13 | 87 | Ürkmez | - | 0.870 | 0.702 | 0.777 | 0.750 | 0.675 | |
| Classifiers | Predicted | Actual | Accuracy (%) | TPR | Precision | F1 | ROC | PRC | |
|---|---|---|---|---|---|---|---|---|---|
| Doruk vs. Kristal | |||||||||
| MLP | Doruk | Kristal | - | - | - | - | - | - | - |
| 82 | 18 | Doruk | 77.00 | 0.820 | 0.745 | 0.781 | 0.800 | 0.746 | |
| 28 | 72 | Kristal | - | 0.720 | 0.800 | 0.758 | 0.800 | 0.833 | |
| RF | Doruk | Kristal | - | - | - | - | - | - | - |
| 79 | 21 | Doruk | 77.00 | 0.790 | 0.760 | 0.775 | 0.846 | 0.833 | |
| 25 | 75 | Kristal | - | 0.750 | 0.781 | 0.765 | 0.846 | 0.847 | |
| SVM | Doruk | Kristal | - | - | - | - | - | - | - |
| 83 | 17 | Doruk | 78.50 | 0.830 | 0.761 | 0.794 | 0.785 | 0.717 | |
| 26 | 74 | Kristal | - | 0.740 | 0.813 | 0.775 | 0.785 | 0.732 | |
| Doruk vs. Özveren | |||||||||
| MLP | Doruk | Özveren | - | - | - | - | - | - | - |
| 83 | 17 | Doruk | 76.00 | 0.830 | 0.728 | 0.776 | 0.831 | 0.796 | |
| 31 | 69 | Özveren | - | 0.690 | 0.802 | 0.742 | 0.831 | 0.823 | |
| RF | Doruk | Özveren | - | - | - | - | - | - | - |
| 76 | 24 | Doruk | 76.5 | 0.760 | 0.768 | 0.764 | 0.835 | 0.821 | |
| 23 | 77 | Özveren | - | 0.770 | 0.762 | 0.766 | 0.835 | 0.802 | |
| SVM | Doruk | Özveren | - | - | - | - | - | - | - |
| 75 | 25 | Doruk | 78.50 | 0.750 | 0.806 | 0.77 | 0.785 | 0.730 | |
| 18 | 82 | Özveren | - | 0.820 | 0.766 | 0.792 | 0.785 | 0.718 | |
| Doruk vs. Ürkmez | |||||||||
| MLP | Doruk | Ürkmez | - | - | - | - | - | - | - |
| 64 | 36 | Doruk | 64.00 | 0.640 | 0.640 | 0.640 | 0.645 | 0.599 | |
| 36 | 64 | Ürkmez | - | 0.640 | 0.640 | 0.640 | 0.645 | 0.644 | |
| RF | Doruk | Ürkmez | - | - | - | - | - | - | - |
| 66 | 34 | Doruk | 68.00 | 0.660 | 0.688 | 0.673 | 0.706 | 0.647 | |
| 30 | 70 | Ürkmez | - | 0.700 | 0.673 | 0.686 | 0.706 | 0.690 | |
| SVM | Doruk | Ürkmez | - | - | - | - | - | - | - |
| 60 | 40 | Doruk | 64.00 | 0.600 | 0.630 | 0.619 | 0.660 | 0.583 | |
| 32 | 68 | Ürkmez | - | 0.680 | 0.650 | 0.661 | 0.660 | 0.581 | |
| Kristal vs. Özveren | |||||||||
| MLP | Kristal | Özveren | - | - | - | - | - | - | - |
| 80 | 20 | Kristal | 76.50 | 0.800 | 0.748 | 0.773 | 0.841 | 0.827 | |
| 27 | 73 | Özveren | - | 0.730 | 0.785 | 0.756 | 0.841 | 0.848 | |
| RF | Kristal | Özveren | - | - | - | - | - | - | - |
| 83 | 17 | Kristal | 80.50 | 0.830 | 0.790 | 0.810 | 0.911 | 0.910 | |
| 22 | 78 | Özveren | - | 0.780 | 0.821 | 0.800 | 0.911 | 0.917 | |
| SVM | Kristal | Özveren | - | - | - | - | - | - | - |
| 75 | 25 | Kristal | 75.50 | 0.750 | 0.758 | 0.754 | 0.755 | 0.693 | |
| 24 | 76 | Özveren | - | 0.760 | 0.752 | 0.756 | 0.755 | 0.692 | |
| Kristal vs. Ürkmez | |||||||||
| MLP | Kristal | Ürkmez | - | - | - | - | - | - | - |
| 66 | 34 | Kristal | 74.00 | 0.660 | 0.786 | 0.717 | 0.786 | 0.842 | |
| 18 | 82 | Ürkmez | - | 0.820 | 0.707 | 0.759 | 0.786 | 0.698 | |
| RF | Kristal | Ürkmez | - | - | - | - | - | - | - |
| 70 | 30 | Kristal | 77.00 | 0.700 | 0.814 | 0.753 | 0.851 | 0.862 | |
| 16 | 84 | Ürkmez | - | 0.840 | 0.737 | 0.785 | 0.851 | 0.832 | |
| SVM | Kristal | Ürkmez | - | - | - | - | - | - | - |
| 71 | 29 | Kristal | 78.00 | 0.710 | 0.826 | 0.763 | 0.780 | 0.731 | |
| 15 | 85 | Ürkmez | - | 0.850 | 0.746 | 0.794 | 0.780 | 0.709 | |
| Özveren vs. Ürkmez | |||||||||
| MLP | Özveren | Ürkmez | - | - | - | - | - | - | - |
| 79 | 21 | Özveren | 81.50 | 0.790 | 0.832 | 0.810 | 0.859 | 0.875 | |
| 16 | 84 | Ürkmez | - | 0.840 | 0.800 | 0.820 | 0.859 | 0.818 | |
| RF | Özveren | Ürkmez | - | - | - | - | - | - | - |
| 77 | 23 | Özveren | 79.00 | 0.770 | 0.802 | 0.786 | 0.879 | 0.866 | |
| 19 | 81 | Ürkmez | - | 0.810 | 0.779 | 0.794 | 0.879 | 0.865 | |
| SVM | Özveren | Ürkmez | - | - | - | - | - | - | - |
| 78 | 22 | Özveren | 79.50 | 0.780 | 0.804 | 0.792 | 0.795 | 0.737 | |
| 19 | 81 | Ürkmez | - | 0.810 | 0.786 | 0.798 | 0.795 | 0.732 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çetin, N.; Okumuş, O.; Uzun, S.; Kaplan, M.; Jahanbakhshi, A.; Niedbała, G. Comprehensive Quality Analysis of Common Vetch (Vicia sativa L.) Varieties Using Image Processing Techniques and Artificial Intelligence. Agriculture 2025, 15, 2411. https://doi.org/10.3390/agriculture15232411
Çetin N, Okumuş O, Uzun S, Kaplan M, Jahanbakhshi A, Niedbała G. Comprehensive Quality Analysis of Common Vetch (Vicia sativa L.) Varieties Using Image Processing Techniques and Artificial Intelligence. Agriculture. 2025; 15(23):2411. https://doi.org/10.3390/agriculture15232411
Chicago/Turabian StyleÇetin, Necati, Onur Okumuş, Satı Uzun, Mahmut Kaplan, Ahmad Jahanbakhshi, and Gniewko Niedbała. 2025. "Comprehensive Quality Analysis of Common Vetch (Vicia sativa L.) Varieties Using Image Processing Techniques and Artificial Intelligence" Agriculture 15, no. 23: 2411. https://doi.org/10.3390/agriculture15232411
APA StyleÇetin, N., Okumuş, O., Uzun, S., Kaplan, M., Jahanbakhshi, A., & Niedbała, G. (2025). Comprehensive Quality Analysis of Common Vetch (Vicia sativa L.) Varieties Using Image Processing Techniques and Artificial Intelligence. Agriculture, 15(23), 2411. https://doi.org/10.3390/agriculture15232411









