Integrated Metabolomic and Transcriptomic Profiles Provide Insights into the Molecular Mechanisms in Modulating Female Flower of Coconut (Cocos nucifera L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Observing the Morphological Characteristics of Coconut Female Flowers
2.3. Nutrients and Enzymatic Activity Measurements
2.4. Endogenous Hormone Measurements
- (1)
- Sample Preparation: The experimental testing was conducted by Nanjing Ruiyuan Biotechnology Co., Ltd. (Nanjing, China). All samples were ground into powder in liquid nitrogen, accurately weighed into test tubes, and mixed with 10 mL of acetonitrile solution and 8 μL of internal standard mother liquor. The extract was stored overnight at 4 °C, then centrifuged at 12,000× g for 5 min at 4 °C, and the supernatant was collected. The precipitate was re-extracted twice with 5 mL of acetonitrile, and the supernatants were combined. The extract was purified by adding C18 and GCB to remove impurities, followed by centrifugation (12,000× g, 5 min, 4 °C). The supernatant was nitrogen-dried, reconstituted in 400 μL of methanol, filtered through a 0.22 μm organic-phase membrane, and stored at −20 °C for analysis.
- (2)
- Standard Solution Preparation: A 1.5 mL centrifuge tube was filled with 984 μL of methanol and 2 μL each of 500 μg/mL IAA, ABA, JA, and ZR, and then GA standard stock solutions (Sigma) were added to prepare a 1 μg/mL mother liquor. Similarly, a 1 μg/mL internal standard mother liquor was prepared by adding 2 μL of each 500 μg/mL internal standard stock solution to 990 μL of methanol. Standard curve solutions were prepared at concentrations of 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, 100, and 200 ng/mL, with each point containing a 20 ng/mL internal standard.
- (3)
- LC-MS/MS Analysis: The analysis was performed using a PE QSight 420 triple quadrupole mass spectrometer (PerkinElmer, Waltham, MA, USA) coupled with a high-performance liquid chromatography (HPLC) system. The mobile phase was delivered by a binary pump, and the sample was injected via an autosampler. Components were separated in the chromatographic column based on their retention times and then ionized via electrospray ionization (ESI). The ionized components were accelerated into the mass analyzer, where they were fragmented and detected in multiple reaction monitoring (MRM) mode. For each analyte, 2 or more fragment ions were monitored, and identification was confirmed by matching retention times and response ratios with standards. Quantification was achieved using the standard curve. Liquid phase conditions were as follows: A Poroshell 120 SB-C18 reverse-phase chromatography column (2.1 mm × 150 mm, 2.7 μm) was used at a column temperature of 30 °C. The mobile phase consisted of A (water/0.02% formic acid) and B (chromatographic methanol) with an elution gradient as follows: 0~1 min, 0.3 mL/min-A—95%; 1~9 min, 0.3 mL/min-A—95%~40%; 9~11 min, 0.3 mL/min-A—40%~5%; 11~13 min, 0.3 mL/min-A—5%; 13~13.2 min, 0.3 mL/min-A—5%~95%; 13.2~15 min, 0.3 mL/min-A—95%. The injection volume was 2 μL. Mass spectrometry parameters: Ionization was performed in ESI positive and negative ion modes separately. The scanning type was MRM. The air curtain pressure was 15 psi, with spray voltages of +5500 V (positive mode) and −5000 V (negative mode). The atomizing gas pressure was 65 psi, the auxiliary gas pressure was 70 psi, and the atomization temperature was 300 °C.
2.5. Nutritional Element Measurements
- (1)
- Nitrogen (N) detection: Sample preparation: 0.2 g of sample (accurate to 0.0001 g) was weighed into a 300 mL digestion tube, avoiding contact with the tube neck. A small amount of water was added to moisten the sample, followed by 5 mL sulfuric acid and 2 g accelerator. A curved-neck funnel was placed on the tube mouth and heated at 250 °C on a digestion furnace (timer started after temperature stabilization; duration ~30 min). After H2SO4 decomposition (white smoke emission), the temperature was raised to 400 °C. It was removed when the solution turned uniformly brownish-black (~3 h) and cooled before distillation. Blank Test: Reagent dosage and procedure were identical to sample analysis, except no sample was added. Distillation: Sodium hydroxide and sulfuric acid standard solutions were prepared, indicators mixed, and the nitrogen analyzer (SKD-1800, Shanghai, China) preheated. Air distillation was performed to clean the pipelines until the readings stabilized then the total nitrogen content calculated using formula [31].
- (2)
- Chlorine (Cl) determination: Sample preparation: Weigh 0.5000 g sample into a 100 mL stoppered colorimetric tube, add 25 mL water; if necessary, heat in a 70 °C water bath for 10 min to dissolve. Shake and sonicate for 20 min, cool to room temperature, shake again, and filter. Discard the initial filtrate. Titration: Transfer 5–20 mL filtrate to a 100 mL beaker, add 5 mL nitric acid solution and 25 mL acetone, immerse the glass and silver electrodes, and start the electromagnetic stirrer. Titrate with silver nitrate standard solution from an acid burette, recording the potential after each drop. Near the endpoint, add 0.1 mL per drop until the potential is stabilized. Use a potentiometric titrator (ZDJ-4D, Shanghai, China) to record the volume and potential automatically. Blank Test: Conduct simultaneously and record the silver nitrate consumption. Calculate the chloride ion content using formula [31].
- (3)
- Elemental analysis (P, K, Ca, Mg, S, Fe, Cu, Mn, Zn, B, Mo): Sample digestion: Weigh 0.1–0.4 g dry sample (or 0.5–5 mL, accurate to 0.0001 g) into a PTFE digestion tank and soak overnight in 5 mL nitric acid. Seal with inner lid and stainless steel jacket, then place in an oven: 80 °C for 2 h, 120 °C for 2 h, 160 °C for 4 h. Cool naturally to room temperature, open, and heat to near dryness. Solution preparation: Wash the digestion solution into a 25 mL volumetric flask. Rinse the tank and lid three times with 1% nitric acid, combining the rinses in a flask. Dilute to mark with 1% nitric acid, mix well, and set aside. Measurement: Conduct a reagent blank test and measure the element content in the test solution using inductively coupled plasma mass spectrometry (ICP-MS) (NexION® 5000, Woodbridge, CT, USA). Calculate the content of female flowers based on the dilution ratio.
2.6. RNA Extraction and RNA-Sequencing (RNA-Seq)
2.7. Metabolite Analysis
2.8. Integrated Metabolome and Transcriptome Analyses
2.9. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.10. Statistical Analysis
3. Results
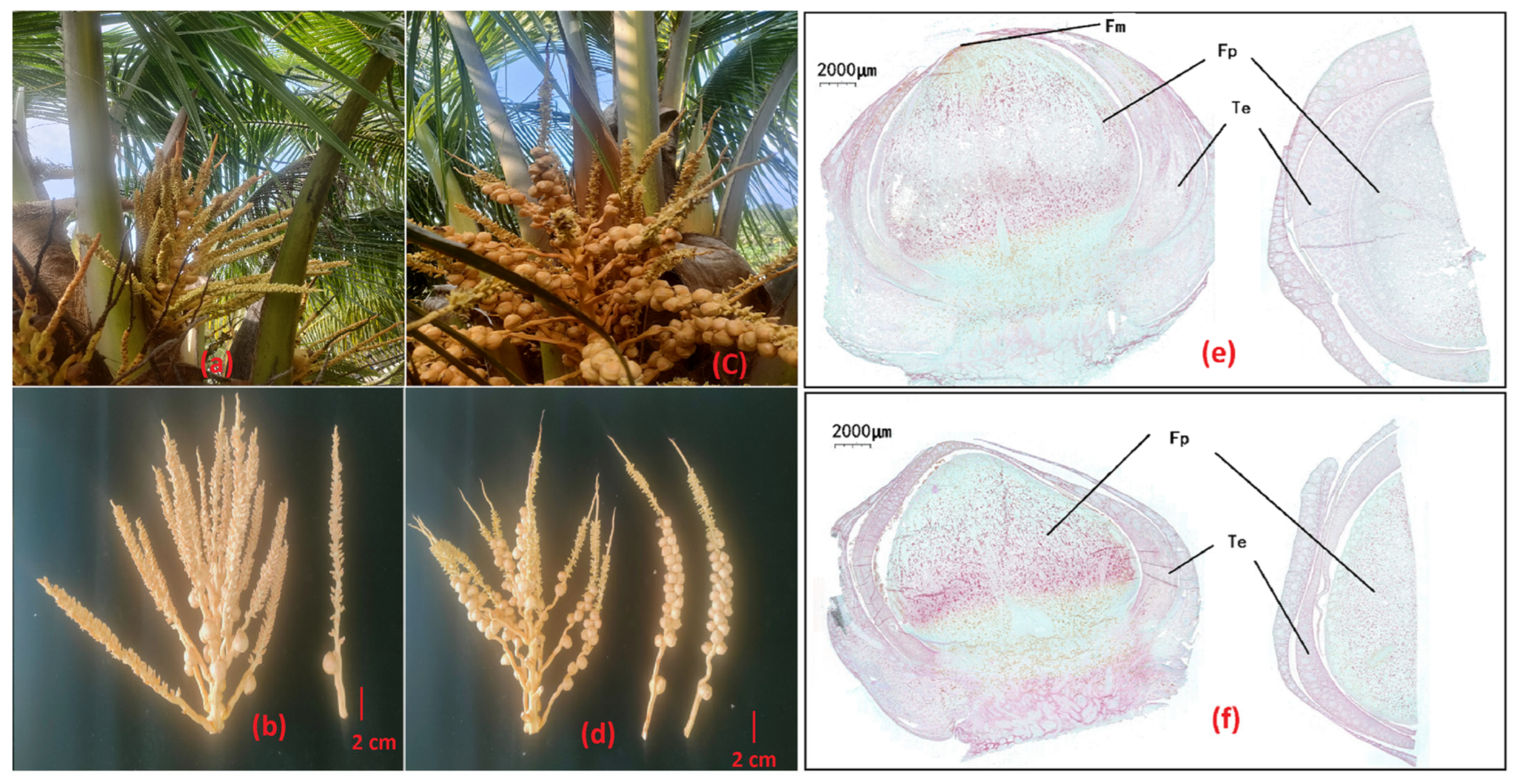
3.1. Morphological Comparison in Coconut Female Flowers of NFF and MFF
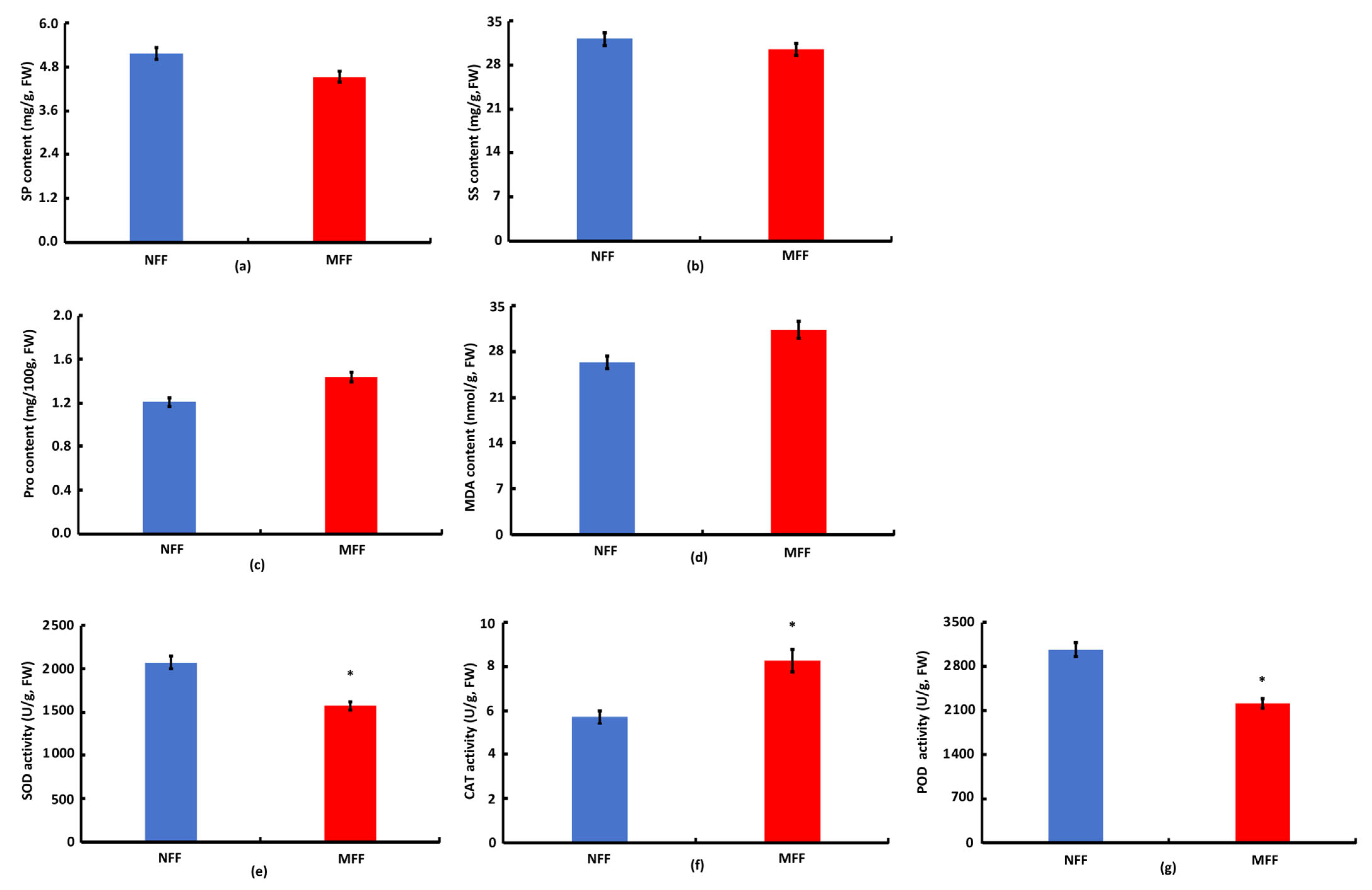
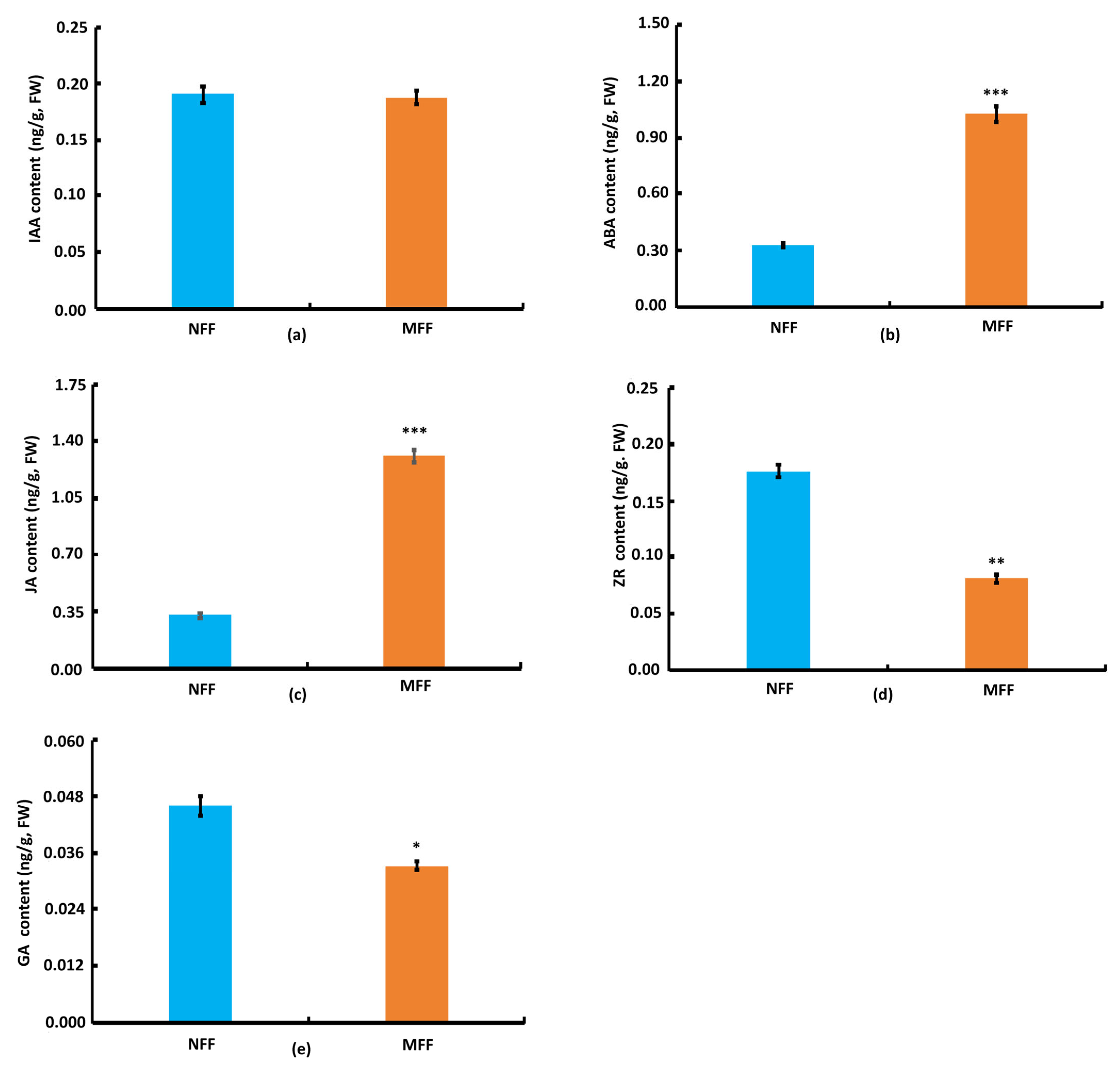
3.2. Nutrition Elements, Nutrients, Enzyme Activity, and Phytohormone Content in Coconut Female Flowers of NFF and MFF
3.3. Transcriptome Analysis in Coconut Female Flowers of NFF and MFF
3.3.1. Transcriptome Assessment
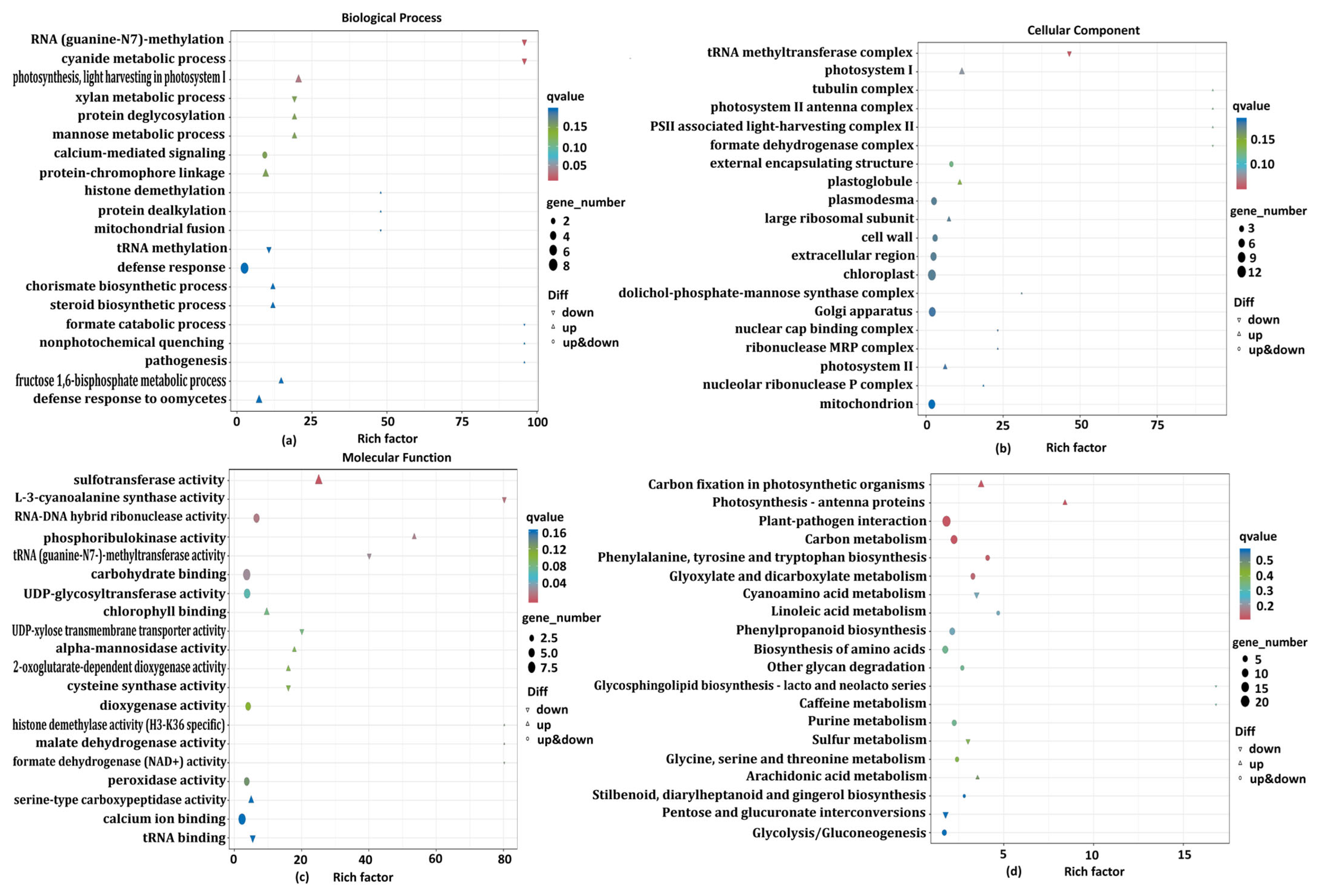
3.3.2. Gene Ontology (GO) Annotation and Enrichment Analysis of the DEGs
3.3.3. KEGG Annotation and Enrichment Analysis of DEGs
3.3.4. DEGs Related to Phytohormones
3.3.5. Transcription Factors
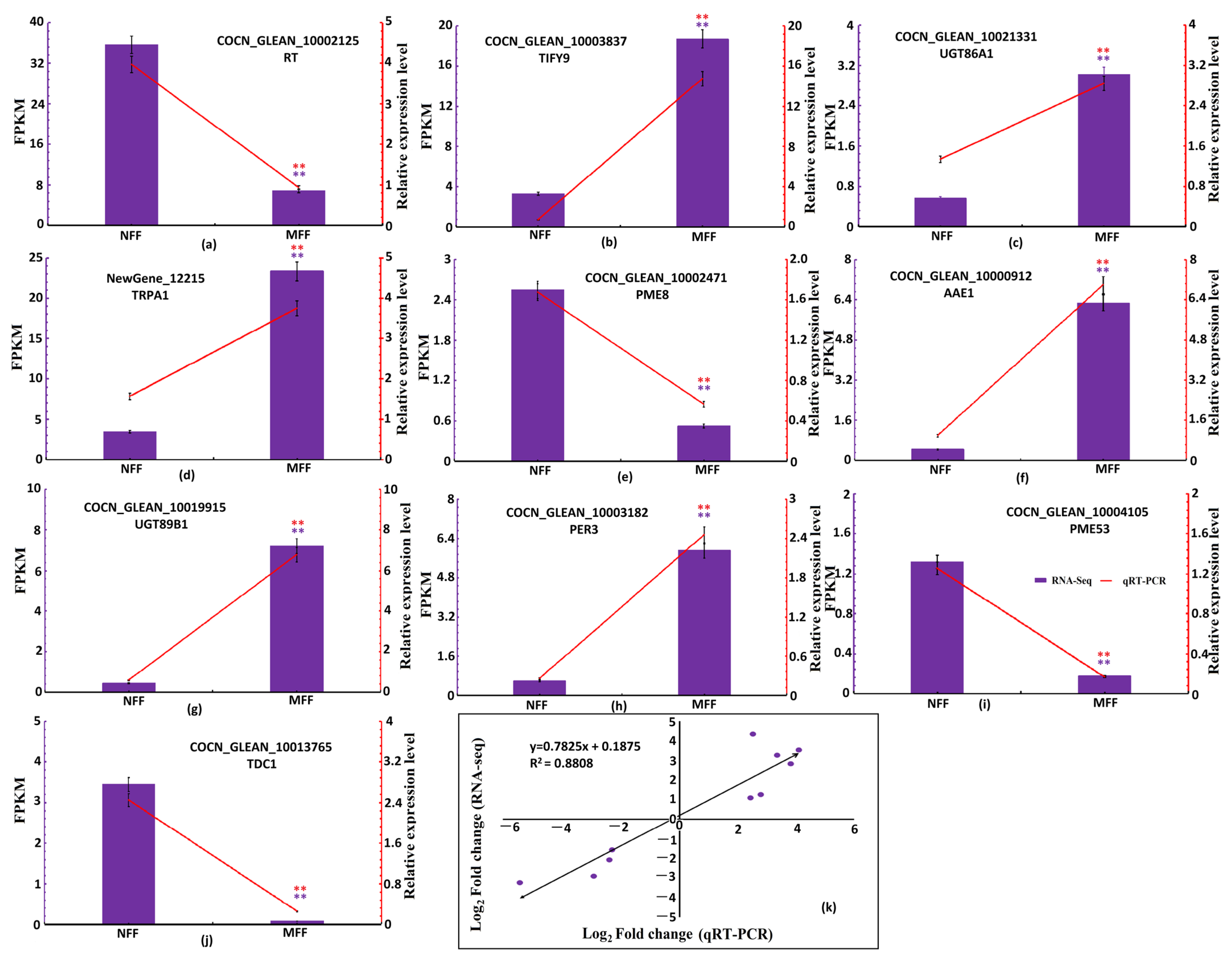
3.3.6. Validation of DEGs Through qRT-PCR Analysis
3.4. Metabolome Analysis in Coconut Female Flowers of NFF and MFF
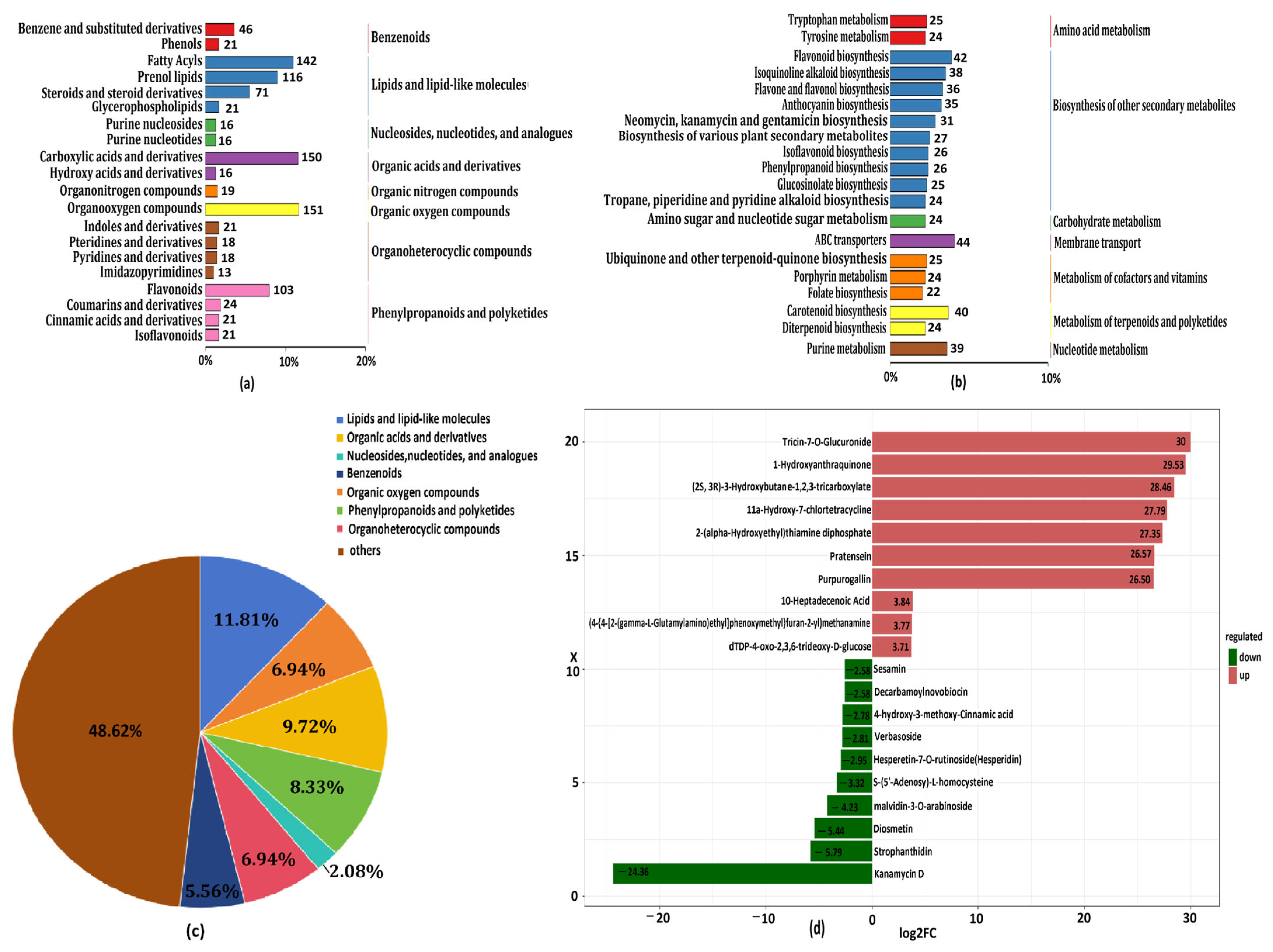
3.4.1. Metabolomics Characterization
3.4.2. Differentially Accumulated Metabolites (DAMs) Analysis
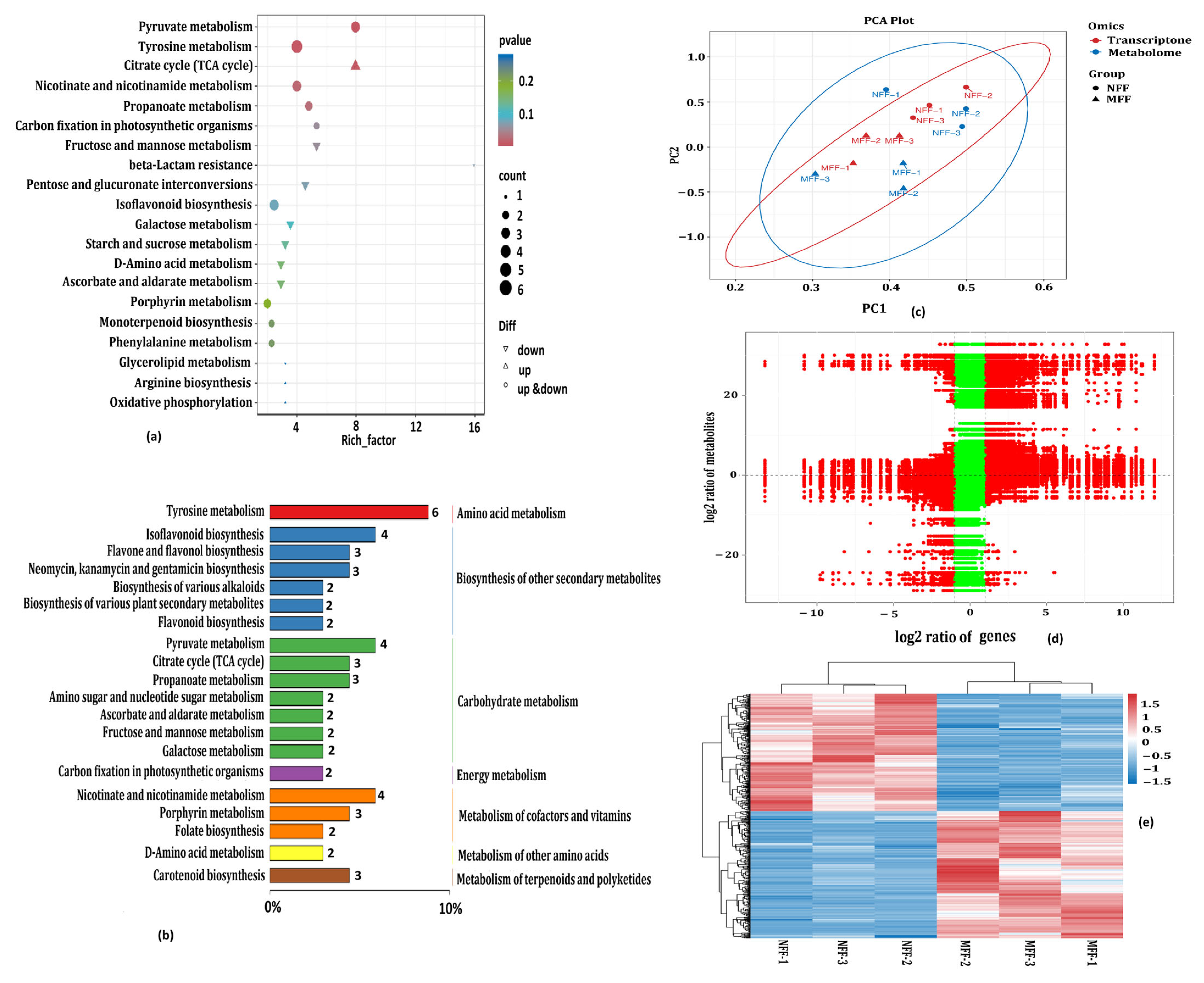
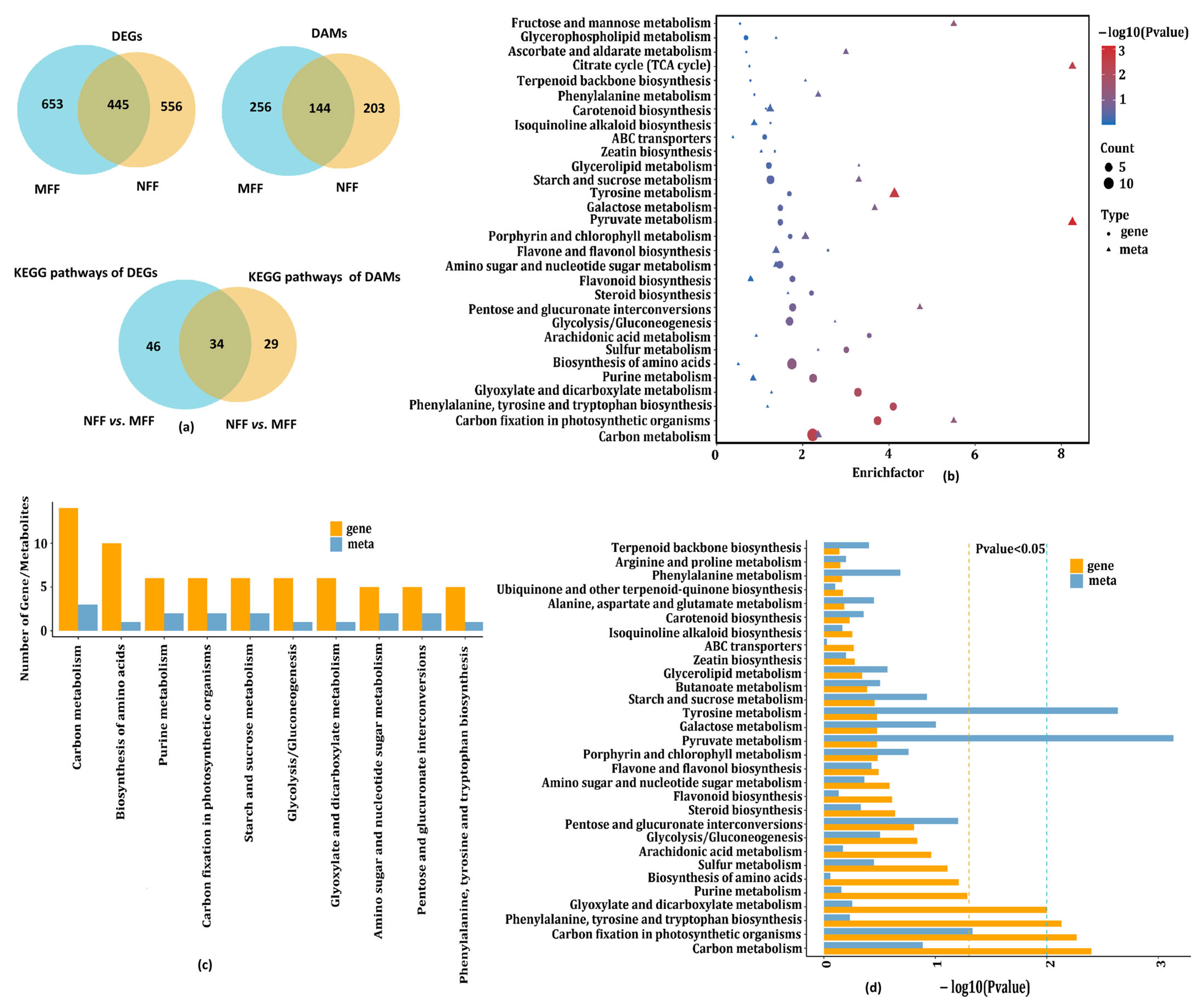
3.5. Integrated Metabolome and Transcriptome Analysis to Reveal Crucial Pathways Responsive to Coconut Female Flowers of NFF and MFF
3.5.1. Analysis of Soluble Sugars and Organic Acid Related to DAMs and DEGs
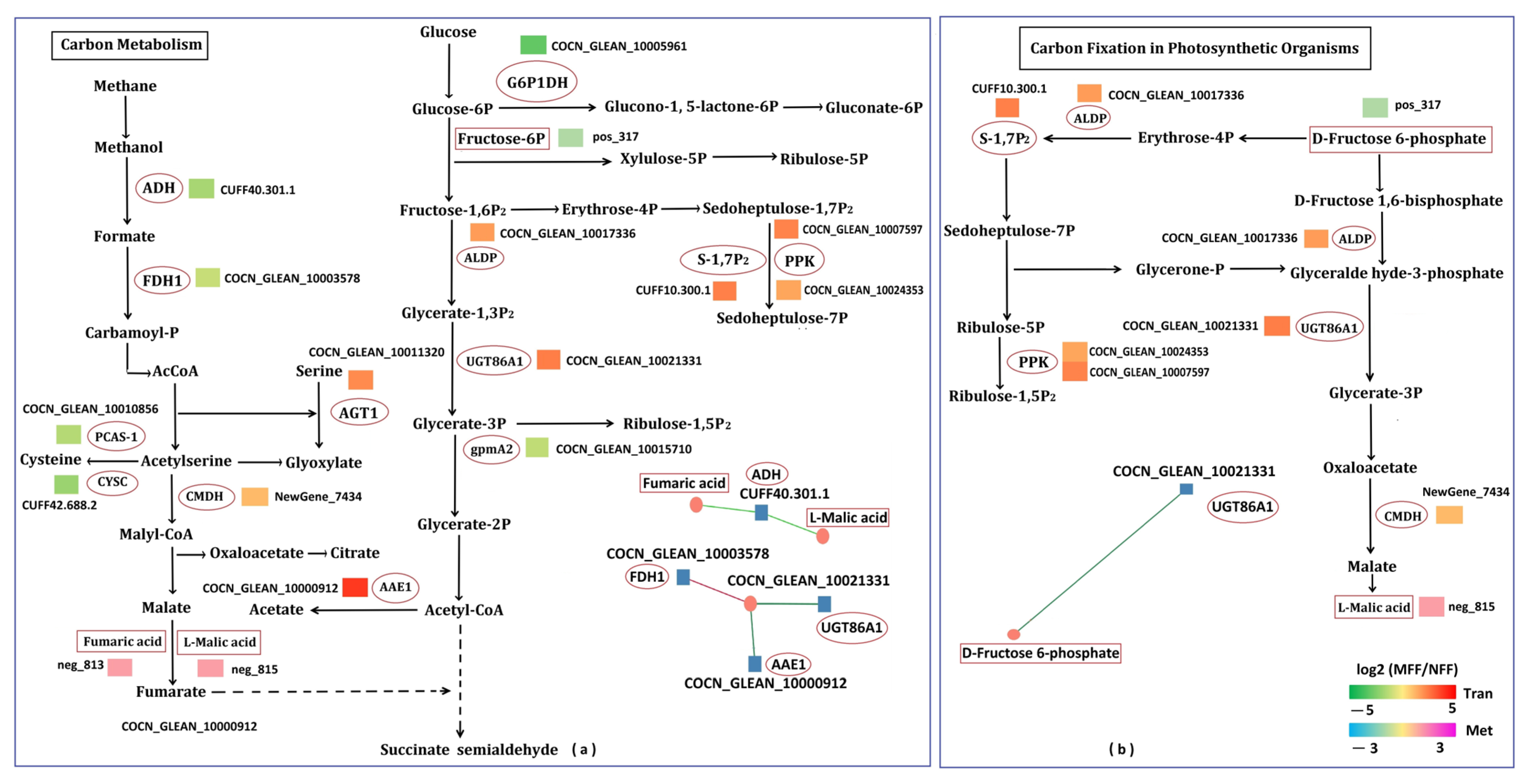
3.5.2. Analysis of Carbon Fixation in Photosynthetic Organisms Related to DAMs and DEGs
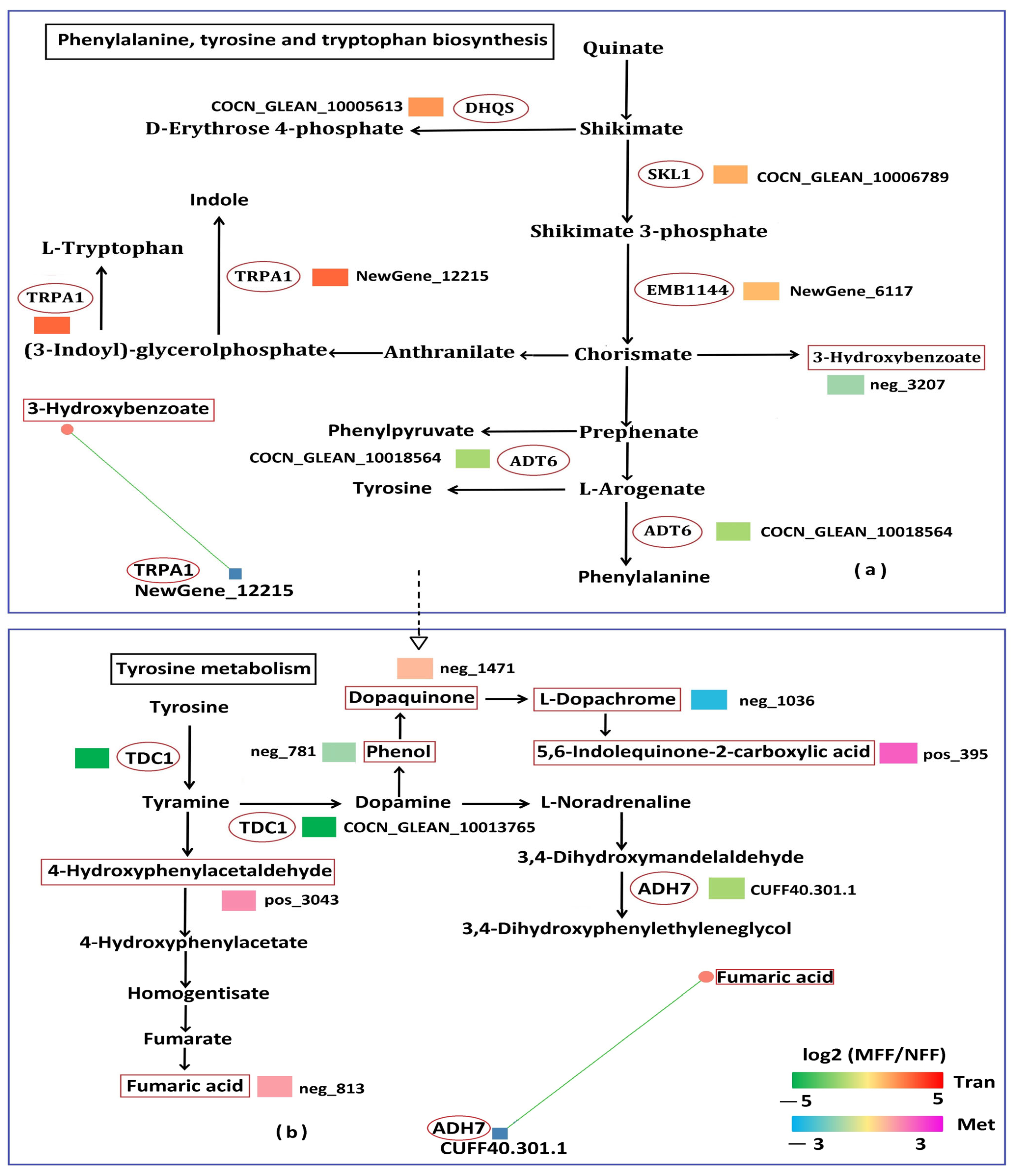
3.5.3. Analysis of Amino Acid Metabolism Related to DAMs and DEGs
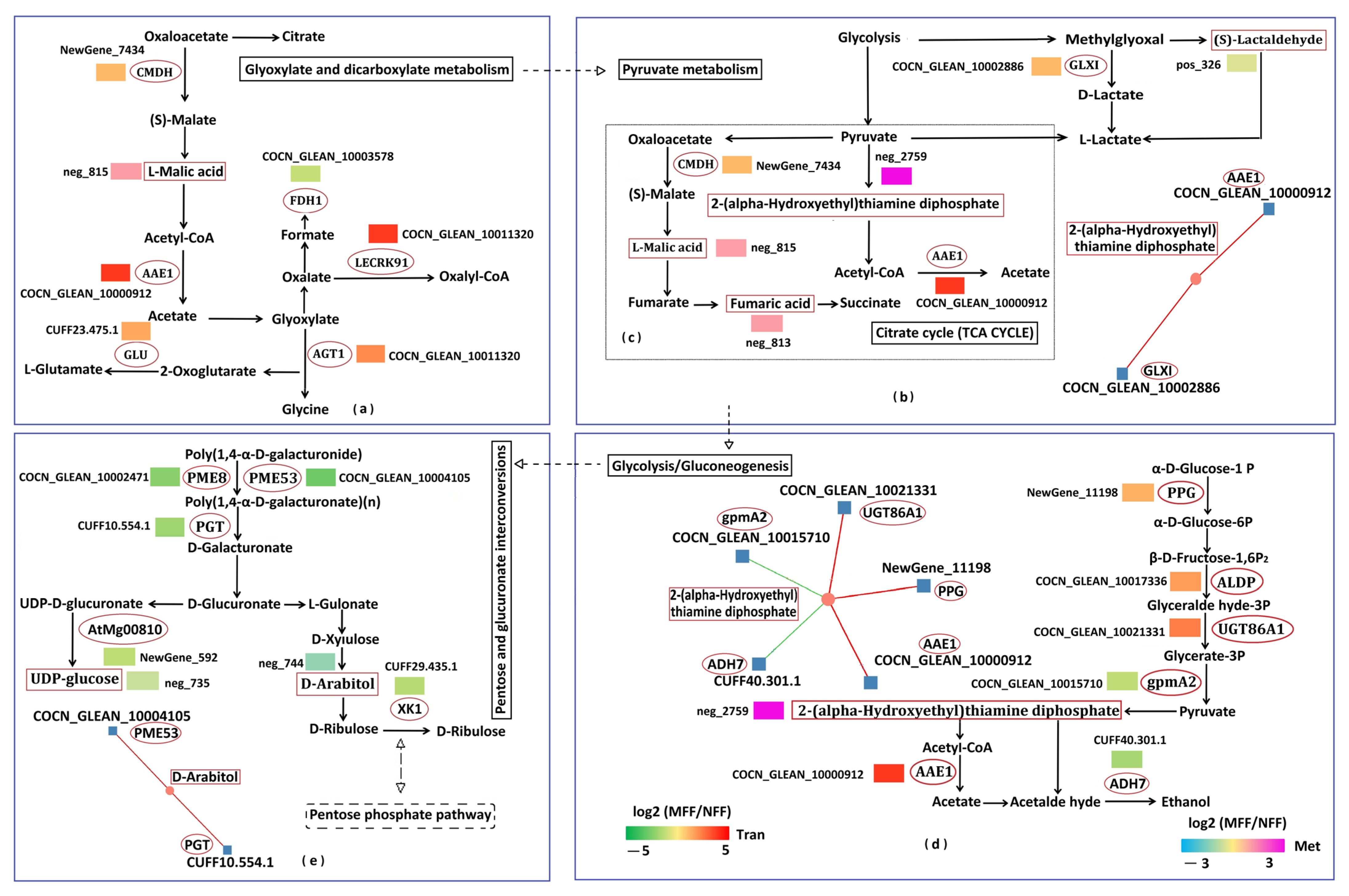
3.5.4. Analysis of Carbohydrate Metabolism Related to DAMs and DEGs
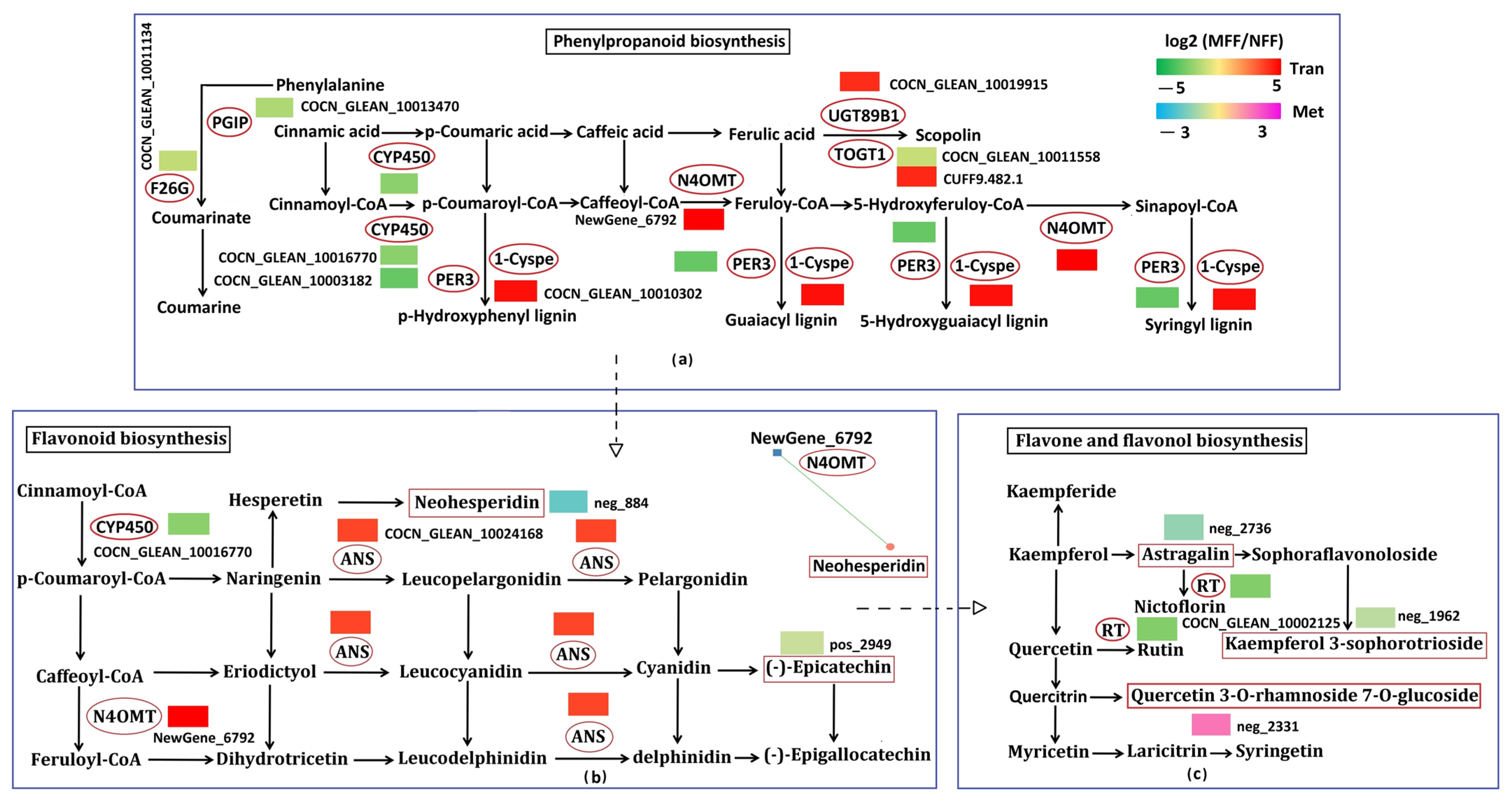
3.5.5. Analysis of Biosynthesis of Other Secondary Metabolites Related to DAMs and DEGs
4. Discussion
4.1. Phenoty, Morphology and Physiology Play an Important Role in Multiple Female Flowers of Coconut
4.2. DAMs and DEGs Involved in Crucial Pathways in Multiple Female Flowers of Coconut
4.3. Transcription Factor in Response to Multiple Female Flowers of Coconut
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zik, M.; Irish, V.F. Flower development: Initiation, differentiation, and diversification. Annu. Rev. Cell Dev. Biol. 2003, 19, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Meijón, M.; Jesús, C.M.; Valledor, L.; Rodríguez, R.; Feito, I. Epigenetic and physiological effects of gibberellin inhibitors and chemical pruners on the floral transition of azalea. Physiol. Plant. 2010, 141, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.; Zhang, W.; Li, C.; Wang, R.; Pan, X.; Peng, J. Combined transcriptional and metabolomic analysis of flavonoids in the regulation of female flower bud differentiation in Juglans sigillata Dode. BMC Plant Biol. 2025, 25, 168. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C. The evolution of plant sexual diversity. Nat. Rev. Genet. 2002, 3, 274–284. [Google Scholar] [CrossRef]
- Chen, S.T.; Liu, R.; Lu, L.L. Study on the Flower Blooming Law of Three Fruit Coconut Varieties. Chin. Agric. Sci. Bull. 2022, 38, 43–46. (In Chinese) [Google Scholar] [CrossRef]
- Broun, P. Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 2005, 8, 272–279. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Shan, X.T.; Li, Y.Q.; Yang, S.; Yang, Z.Z.; Qiu, M.; Gao, R.F.; Han, T.T.; Meng, X.Y.; Xu, Z.Y.; Wang, L.; et al. The spatio-temporal biosynthesis of floral flavonols is controlled by differential phylogenetic MYB regulators in Freesia hybrida. New Phytol. 2020, 228, 1864–1879. [Google Scholar] [CrossRef]
- Shi, Y.F.; Jiang, X.L.; Chen, L.B.; Xia, T. Functional analyses of flavonol synthase genes from camellia sinensis reveal their roles in Anther development. Front. Plant Sci. 2021, 12, 753131. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, B.; Wang, L.; Song, Z.; Niu, L.; Li, H.; Cao, H.; Meng, D.; Fu, Y. CDPK6 phosphorylates and stabilizes MYB30 to promote hyperoside biosynthesis that prolongs the duration of full-blooming in okra. J. Exp. Bot. 2020, 71, 4042–4056. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Chen, M.; Dong, B.; Wang, N.; Yu, Q.; Wang, X.; Xuan, L.; Wang, Y.; Zhang, S.; Shen, Y. Transcriptomic Analysis of Flower Bud Differentiation in Magnolia sinostellata. Genes 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zeng, Z.; Wang, Y.; Lyu, Y. Transcriptome Analysis of Carbohydrate Metabolism Genes and Molecular Regulation of Sucrose Transport Gene LoSUT on the Flowering Process of Developing Oriental Hybrid Lily ‘Sorbonne’ Bulb. Int. J. Mol. Sci. 2020, 21, 3092. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Fan, J.; Wei, X.; Zhang, D.; Ren, J.; Zhang, L. Differences in floral development between Lycoris radiata and Lycoris sprengeri. Scienceasia 2020, 46, 271–279. [Google Scholar] [CrossRef]
- Matsoukas, I.G. Interplay between sugar and hormone signaling pathways modulate floral signal transduction. Front. Genet. 2014, 5, 218. [Google Scholar] [CrossRef]
- Shang, C.; Cao, X.; Tian, T.; Hou, Q.; Wen, Z.; Qiao, G.; Wen, X. Cross-Talk between Transcriptome Analysis and Dynamic Changes of Carbohydrates Identifies Stage-Specific Genes during the Flower Bud Differentiation Process of Chinese Cherry (Prunus pseudocerasus L.). Int. J. Mol. Sci. 2022, 23, 15562. [Google Scholar] [CrossRef]
- Freytes, S.N.; Canelo, M.; Cerdán, P.D. Regulation of flowering time: When and where? Curr. Opin. Plant Biol. 2021, 63, 102049. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040–104059. [Google Scholar] [CrossRef]
- Hui, W.; Yang, Y.; Wu, G.; Peng, C.; Chen, X.; Zayed, M.Z. Transcriptome profile analysis reveals the regulation mechanism of floral sex differentiation in Jatropha curcas L. Sci. Rep. 2017, 7, 16421. [Google Scholar] [CrossRef]
- Ito, S.; Song, Y.H.; Josephson-Day, A.R.; Miller, R.J.; Breton, G.; Olmstead, R.G.; Imaizumi, T. FLOWERING BHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 3582–3587. [Google Scholar] [CrossRef]
- Abe, M.; Kaya, H.; Watanabe-Taneda, A.; Shibuta, M.; Yamaguchi, A.; Sakamoto, T.; Kurata, T.; Ausin, I.; Araki, T.; Alonso-Blanco, C. FE, a phloem-specific Myb-related protein, promotes flowering through transcriptional activation of FLOWERING LOCUS T and FLOWERING LOCUS T INTERACTING PROTEIN 1. Plant J. 2015, 83, 1059–1068. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, F.; Shu, X.; Wang, N.; Wang, T.; Zhuang, W.; Wang, Z. Identification of Differentially Expressed Genes Related to Floral Bud Differentiation and Flowering Time in Three Populations of Lycoris radiata. Int. J. Mol. Sci. 2022, 23, 14036. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, L.; Qiang, X.; Meng, Y.; Li, Z.; Huang, F. Integrative Metabolomic and Transcriptomic Analysis Elucidates That the Mechanism of Phytohormones Regulates Floral Bud Development in Alfalfa. Plants 2024, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, R.; Xiao, Y.; Li, J.; Shen, X.; Li, H.; Fan, H. Research Progress on Coconut Germplasm Resources, Cultivation and Utilization. Chin. J. Trop. Crops 2021, 42, 1795–1803. (In Chinese) [Google Scholar]
- Deng, B.; Li, X.; Yang, J.; He, X.; Qian, C.; Bao, Q.; Yang, H.; Jie, W. Research Progress in Deep Processing and High Value Utilisation of Coconut By-products. China Fruit Veg. 2023, 43, 25–30. (In Chinese) [Google Scholar] [CrossRef]
- Mao, Z.S.; Qiu, W.M. Coconut High-Yield Cultivation Technology; Hainan Press: Haikou, China, 2007; pp. 1–62. (In Chinese) [Google Scholar]
- Hassankhah, A.; Rahemi, M.; Ramshini, H.; Sarikhani, S.; Vahdati, K. Flowering in Persian walnut: Patterns of gene expression during flower development. BMC Plant Biol. 2020, 20, 136. [Google Scholar] [CrossRef]
- Ma, K.; Xu, R.; Zhao, Y.; Han, L.; Xu, Y.; Li, L.; Wang, J.; Li, N. Walnut N-Acetylserotonin Methyltransferase Gene Family Genome-Wide Identification and Diverse Functions Characterization During Flower Bud Development. Front. Plant Sci. 2022, 15, 861043. [Google Scholar] [CrossRef]
- Li, J.J.; Pan, X.J.; Zhang, W.E. Relationship between mineral nutrition, hormone content and flower bud differentiation of Juglans sigillata. Acta Bot. Boreali-Occiden. Sin. 2016, 36, 0971–0978. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiology and Biochemistry Experiment; Higher Education Press: Beijing, China, 2000; pp. 25–63. (In Chinese) [Google Scholar]
- Bao, S.D. Agrochemical Analysis of Soil, 3rd ed.; Agricultural Publishing House: Beijing, China, 2000; pp. 23–56. (In Chinese)
- Vennapusa, A.R.; Somayandal, C.J.; Jagadish, S.V.K. A universal method for highquality RNA extraction from plant tissues rich in starch, proteins and fiber. Sci. Rep. 2020, 10, 16887. [Google Scholar] [CrossRef]
- Sasi, S.; Krishnan, S.; Kodackattumannil, P.; Shamisi1, A.A.L.; Aldarmaki, M.; Lekshmi, G.; Kottackal, M.; Amiri, K.M.A. DNA-free high-quality RNA extraction from 39 difficult-to-extract plant species (representing seasonal tissues and tissue types) of 32 families, and its validation for downstream molecular applications. Plant Methods 2023, 19, 84. [Google Scholar] [CrossRef]
- Lu, L.; Wang, Y.; Sayed, M.A.; Iqbal, A.; Yang, Y. Exploring the Physiological and Molecular Mechanisms by Which Potassium Regulates Low-Temperature Tolerance of Coconut (Cocos nucifera L.) Seedlings. Agronomy 2024, 14, 2983. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xu, P.X.; Fan, H.K.; Luc, B.; Xia, W.; Stephanie, B.; Xu, J.Y.; Li, Q.; Guo, A.P.; Zhou, L.X.; et al. The genome draft of coconut (Cocos nucifera). GigaScience 2017, 6, gix095. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Penelope, C.; Eberhardt, R.Y.; Eddy, S.R.; Andreas, H.; Kirstie, H.; Liisa, H.; Jaina, M.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenfuhrer, J. topGO: Enrichment analysis for gene ontology. R package version 2.8. Cranio 2016, 2, 289–300. [Google Scholar]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, P.; Tan, H.; Budhu, A.; Gaedcke, J.; Ghadimi, B.M.; Ried, T.; Yfantis, H.G.; Lee, D.H.; Maitra, A.; et al. Integration of metabolomics and transcriptomics revealed a fatty acid network exerting growth inhibitory effects in human pancreatic cancer. Clin. Cancer Res. 2013, 15, 4983–4993. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.E.; Li, J.J.; Zhang, W.L.; Njie, A.; Pan, X.J. The changes in C/N, carbohydrate, and amino acid content in leaves during female flower bud differentiation of Juglans sigillata. Acta Physiol. Plant. 2022, 44, 19. [Google Scholar] [CrossRef]
- Xia, X.Q.; Xi, R.T. The periods of physiological and morphological differentiation of pistillate flower buds in walnut (Juglans regia L.). J. Hebei Agric. Univ. 1989, 12, 18–21. (In Chinese) [Google Scholar]
- Yu, S.; Tang, Z. Plant Physiology and Molecular Biology, 2nd ed.; Science Press: Beijing, China, 1998; pp. 556–562. (In Chinese) [Google Scholar]
- Qi, H.; Hao, J.; Wang, H. The Changes of Mineral Nutrient Content and C/N in Melon Leaves During Flower Bud Differentiation. J. Shenyang Agric. Univ. 2008, 39, 530–533. (In Chinese) [Google Scholar] [CrossRef]
- Chen, C.; Xia, C. Regulation techniques for the annual chrysanthemum season in Hainan region. Trop. Agric. Sci. 1998, 06, 59–65. (In Chinese) [Google Scholar]
- Dai, L.; Zhang, Q.; He, M. The Relationship between Mineral Nutrients and Flower Formation during the Flower Bud Differentiation Stage of Ponkan. Chin. Citrus 1995, 25, 20–21. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, X.; Wei, X.; Tian, H.; Han, L.; Xu, J. Changes in Protein and Amino Acid Content during Flower Bud Germination of “Gala” Apple. North. Hortic. 2013, 9, 5–7. (In Chinese) [Google Scholar]
- Fang, Y.; Geng, Y.; Sun, W.; Gao, C.; Zhang, A.; Zhao, X.; Meng, J.; Zhang, Z. Studies on the Nitrogen Metabolism in the Process of Dormancy and Germinating of Cabernet Sauvignon Grapevine. Sci. Agric. Sin. 2011, 44, 5041–5049. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Y.; Li, P.; Wu, Q. Effect of Carbohydrate and Protein Metabolism of Grapevine Leaves on Bud differentiation. J. Zhejiang Wanli Univ. 2002, 15, 54–57. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Wang, G.; Lv, J. Changes of Acid Invertase Activity and Correlated Sugar Level in Bayberry Leaves During Flower Bud Initiation Stage. J. Sichuan Agric. Univ. 2000, 18, 164–166. (In Chinese) [Google Scholar] [CrossRef]
- Guo, Y.Y.; An, L.Z.; Yu, H.Y.; Yang, M.M. Endogenous hormones and biochemical changes during flower development and florescence in the buds and leaves of Lycium ruthenicum Murr. Forests 2022, 13, 763. [Google Scholar] [CrossRef]
- Deng, C. Study on the Physiological Mechanism of Flower Bud Differentiation in Summer Shoots of Loquat Variety ‘Zaozhong 6’. Doctoral Thesis, Sichuan Agricultural University, Chengdu, China, 2016. (In Chinese). [Google Scholar]
- Niwa, T.; Suzuki, T.; Takebayashi, Y.; Ishiguro, R.; Higashiyama, T.; Sakakibara, H.; Ishiguro, S. Jasmonic Acid Facilitates Flower Opening and Floral Organ Development through the Upregulated Expression of SlMYB21 Transcription Factor in Tomato. Biosci. Biotechnol. Biochem. 2018, 82, 292–303. [Google Scholar] [CrossRef]
- Bernier, G.; Perilleux, C. A physiological overview of the genetics of flowering time control. Plant Biotechnol. J. 2005, 3, 3–16. [Google Scholar] [CrossRef]
- Chmielewski, F.M.; Goetz, K.P. Metabolites in cherry buds to detect winter dormancy. Metabolites 2022, 12, 247. [Google Scholar] [CrossRef]
- Naor, V.; Kigel, J.; Ben-Tal, Y.; Ziv, M. Variation in endogenous gibberellins, abscisic acid, and carbohydrate content during the growth cycle of colored Zantedeschia spp., a tuberous geophyte. J. Plant Growth Regul. 2008, 27, 211–220. [Google Scholar] [CrossRef]
- Wan, C.Y.; Mi, L.; Chen, B.Y.; Li, J.F.; Huo, H.Z.; Xu, J.T.; Chen, X.P. Effects of nitrogen during nursery stage on flower bud differentiation and early harvest after transplanting in strawberry. Braz. J. Bot. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Xing, L.B.; Zhang, D.; Li, Y.M.; Shen, Y.W.; Zhao, C.P.; Ma, J.J.; An, N.; Han, M.Y. Transcription profiles reveal sugar and hormone signaling pathways mediating flower induction in apple (Malus domestica Borkh.). Plant Cell Physiol. 2015, 56, 2052–2068. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.Y.; Zhu, C.X.; Zhou, Y.; Niu, X.L.; Miao, M.; Tang, X.F.; Chen, F.; Zhao, W.P.; Liu, Y.S. Transcriptome analysis of differentially expressed unigenes involved in flavonoid biosynthesis during flower development of Chrysanthemum morifolium ‘Chuju’. Sci. Rep. 2018, 8, 13414. [Google Scholar] [CrossRef] [PubMed]
- Bendahmane, M.; Dubois, A.; Raymond, O.; Bris, M.L. Genetics and Genomics of Flower Initiation and Development in Roses. J. Exp. Bot. 2013, 64, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tang, A.; Wan, H.; Zhang, T.; Cheng, T.; Wang, J.; Yang, W.; Pan, H.; Zhang, Q. An APETALA2 Homolog, RcAP2, Regulates the Number of Rose Petals Derived from Stamens and Response to Temperature Fluctuations. Front. Plant Sci. 2018, 9, 481. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB Transcription Factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Punwani, J.A.; Rabiger, D.S.; Lloyd, A.; Drews, G.N. The MYB98 Subcircuit of the Synergid Gene Regulatory Network Includes Genes Directly and Indirectly Regulated by MYB98. Plant J. 2008, 55, 406–414. [Google Scholar] [CrossRef]
- Chen, M.-K.; Lin, I.-C.; Yang, C.-H. Functional Analysis of Three Lily (Lilium longiflorum) APETALA1-like MADS Box Genes in Regulating Floral Transition and Formation. Plant Cell Physiol. 2008, 49, 704–717. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Li, K.; Liu, H.; Lin, C. Multiple bHLH Proteins Form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in Arabidopsis. PLoS Genet. 2013, 9, e1003861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Zhang, Y.; Dong, Z.; Yang, W.; Yu, R. Integrated Metabolomic and Transcriptomic Profiles Provide Insights into the Molecular Mechanisms in Modulating Female Flower of Coconut (Cocos nucifera L.). Agriculture 2025, 15, 2336. https://doi.org/10.3390/agriculture15222336
Lu L, Zhang Y, Dong Z, Yang W, Yu R. Integrated Metabolomic and Transcriptomic Profiles Provide Insights into the Molecular Mechanisms in Modulating Female Flower of Coconut (Cocos nucifera L.). Agriculture. 2025; 15(22):2336. https://doi.org/10.3390/agriculture15222336
Chicago/Turabian StyleLu, Lilan, Yuan Zhang, Zhiguo Dong, Weibo Yang, and Ruoyun Yu. 2025. "Integrated Metabolomic and Transcriptomic Profiles Provide Insights into the Molecular Mechanisms in Modulating Female Flower of Coconut (Cocos nucifera L.)" Agriculture 15, no. 22: 2336. https://doi.org/10.3390/agriculture15222336
APA StyleLu, L., Zhang, Y., Dong, Z., Yang, W., & Yu, R. (2025). Integrated Metabolomic and Transcriptomic Profiles Provide Insights into the Molecular Mechanisms in Modulating Female Flower of Coconut (Cocos nucifera L.). Agriculture, 15(22), 2336. https://doi.org/10.3390/agriculture15222336



