Effect of Pollination Methods on Fruit Development in Greenhouse Watermelon: Physiological and Molecular Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Bee Colonies
2.2. Experimental Design
2.3. Yield-Related and Physiological Property Measurement
2.4. RNA Extraction and Sequencing
2.5. Quality Control and Read Mapping
2.6. Protein Digestion, iTRAQ Labeling, and LC-MS/MS Analysis
2.7. Identification of Differentially Expressed Genes (DEGs) and Differentially Expressed Proteins (DEPs) Between Groups
2.8. Enrichment Analysis of DEGs and DEPs
3. Results
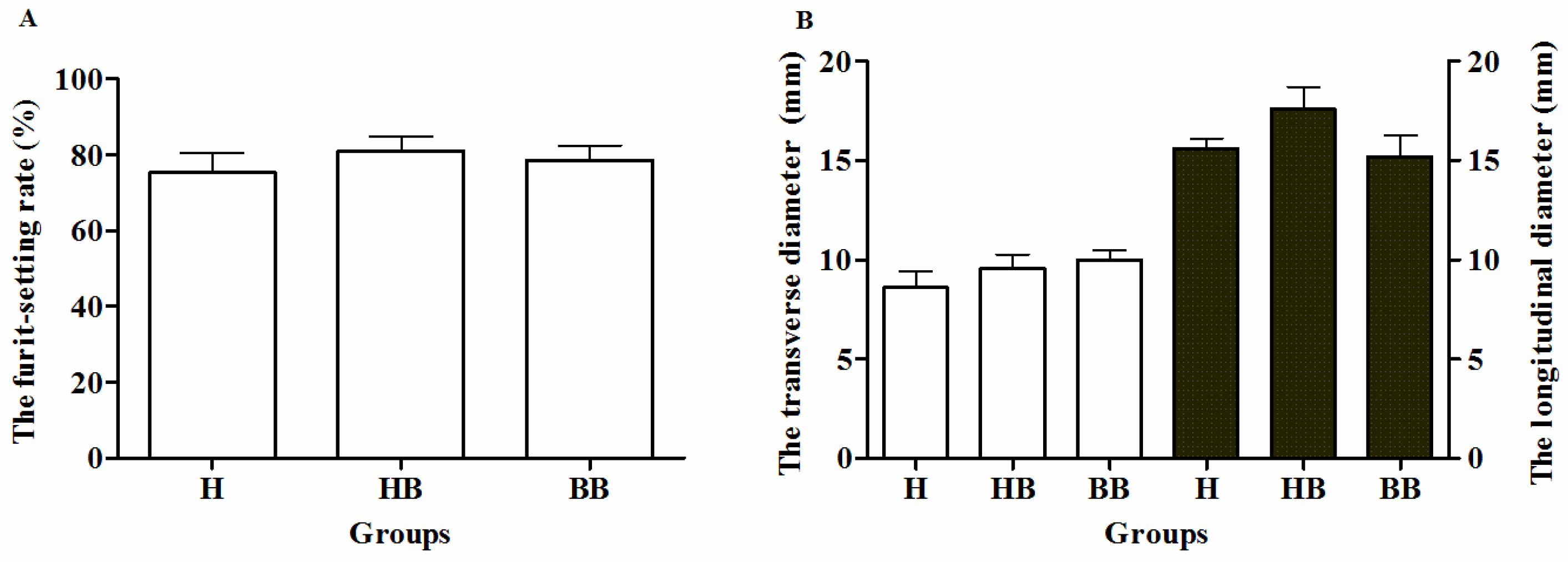
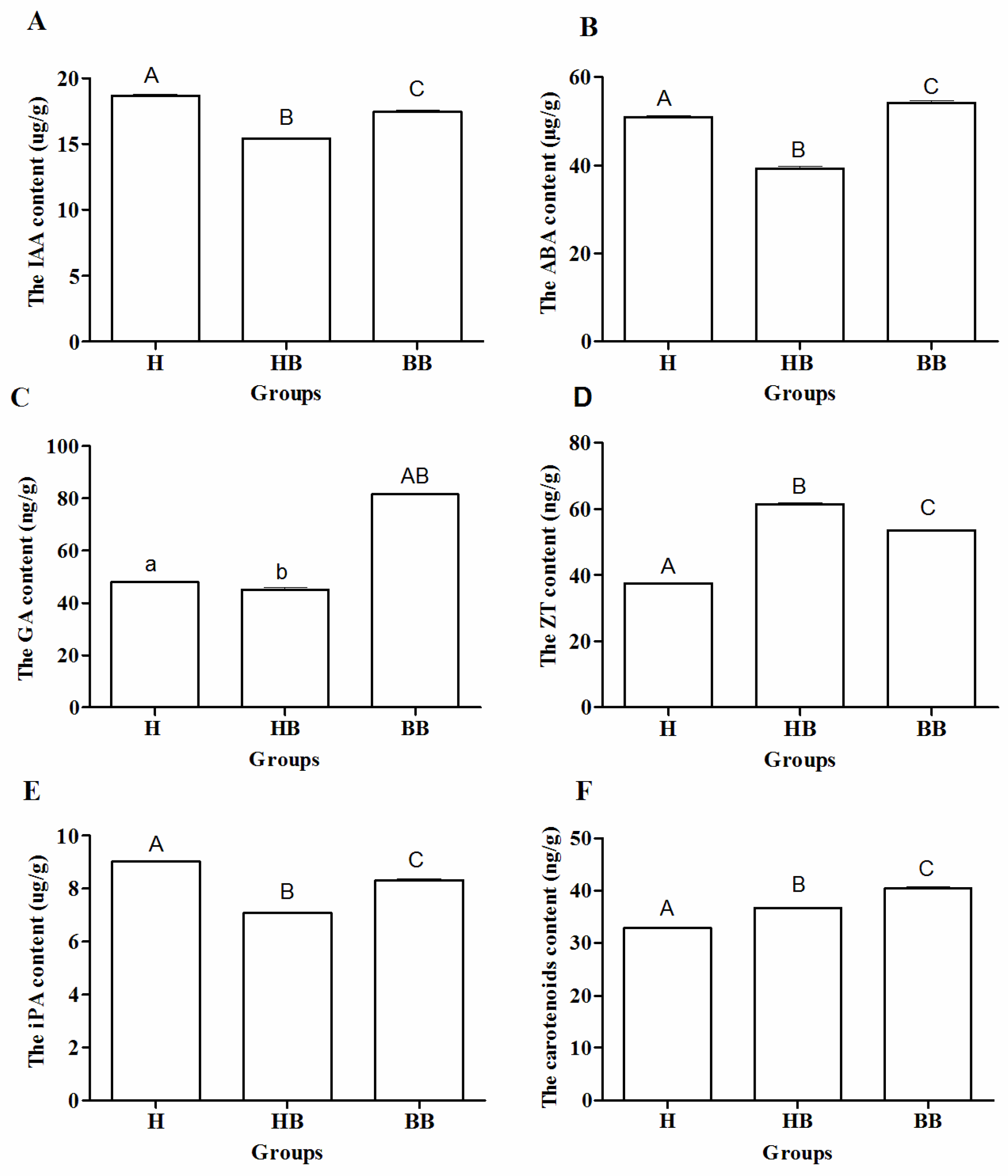
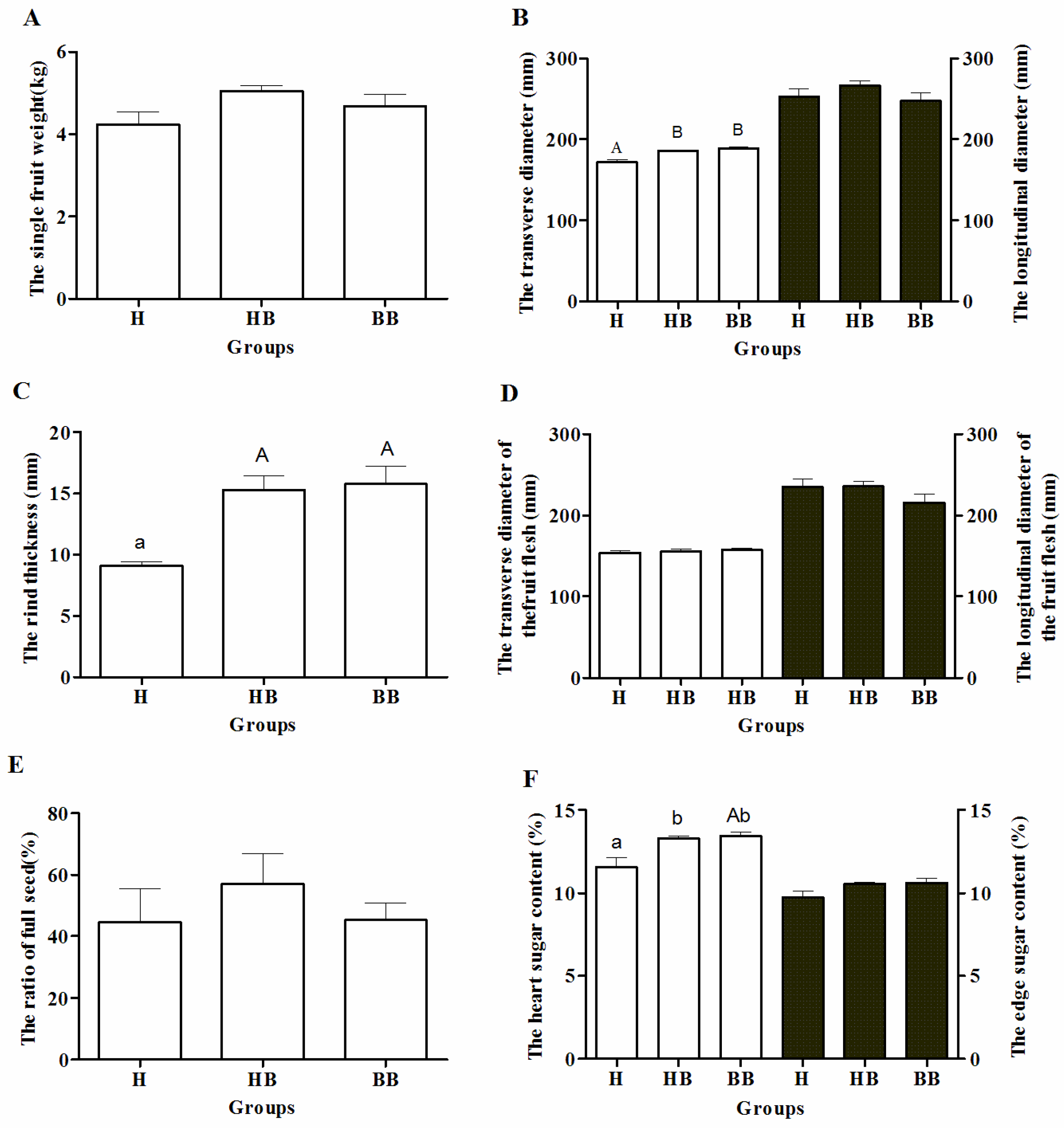
3.1. Yield-Related and Physiological Characteristics
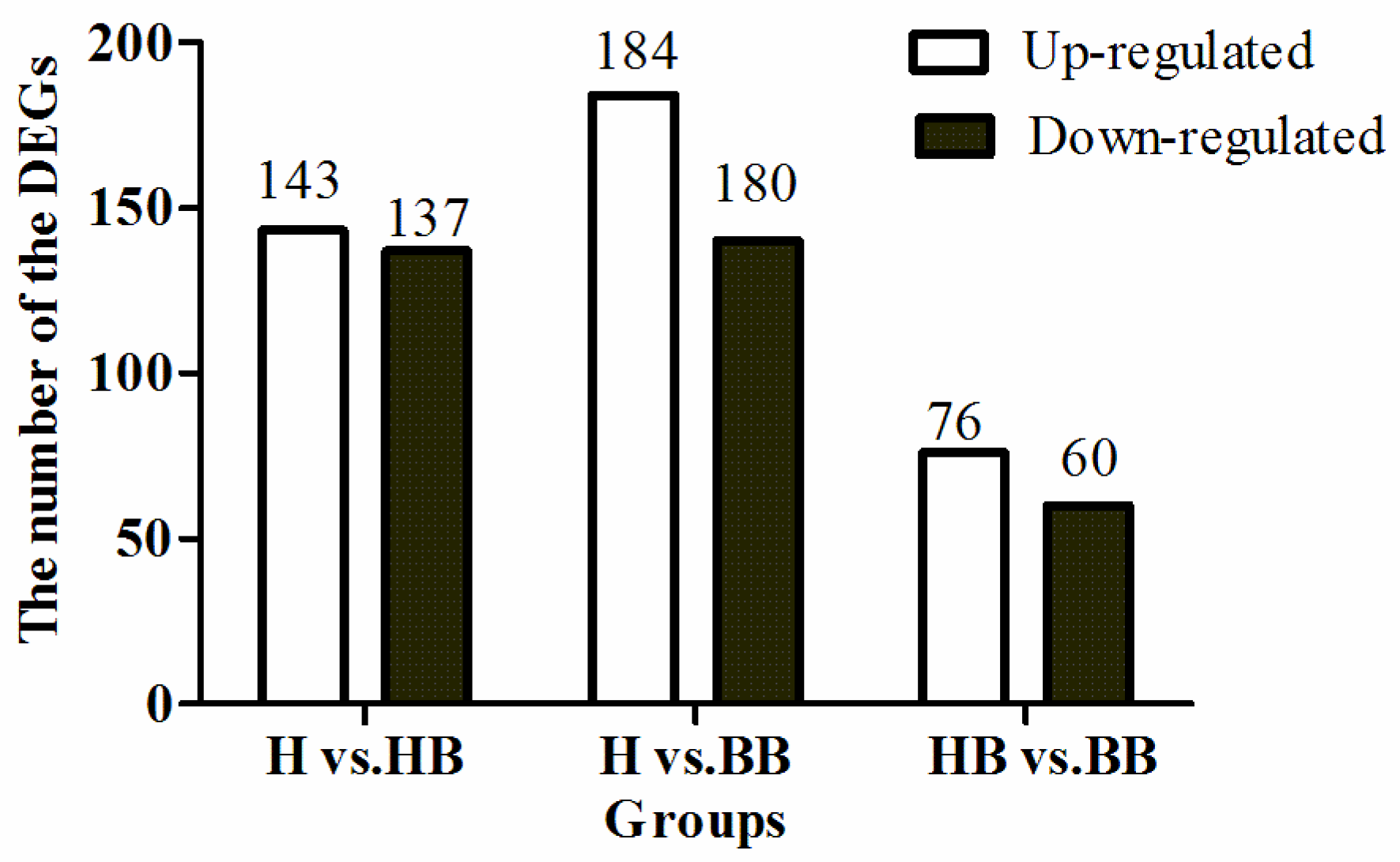
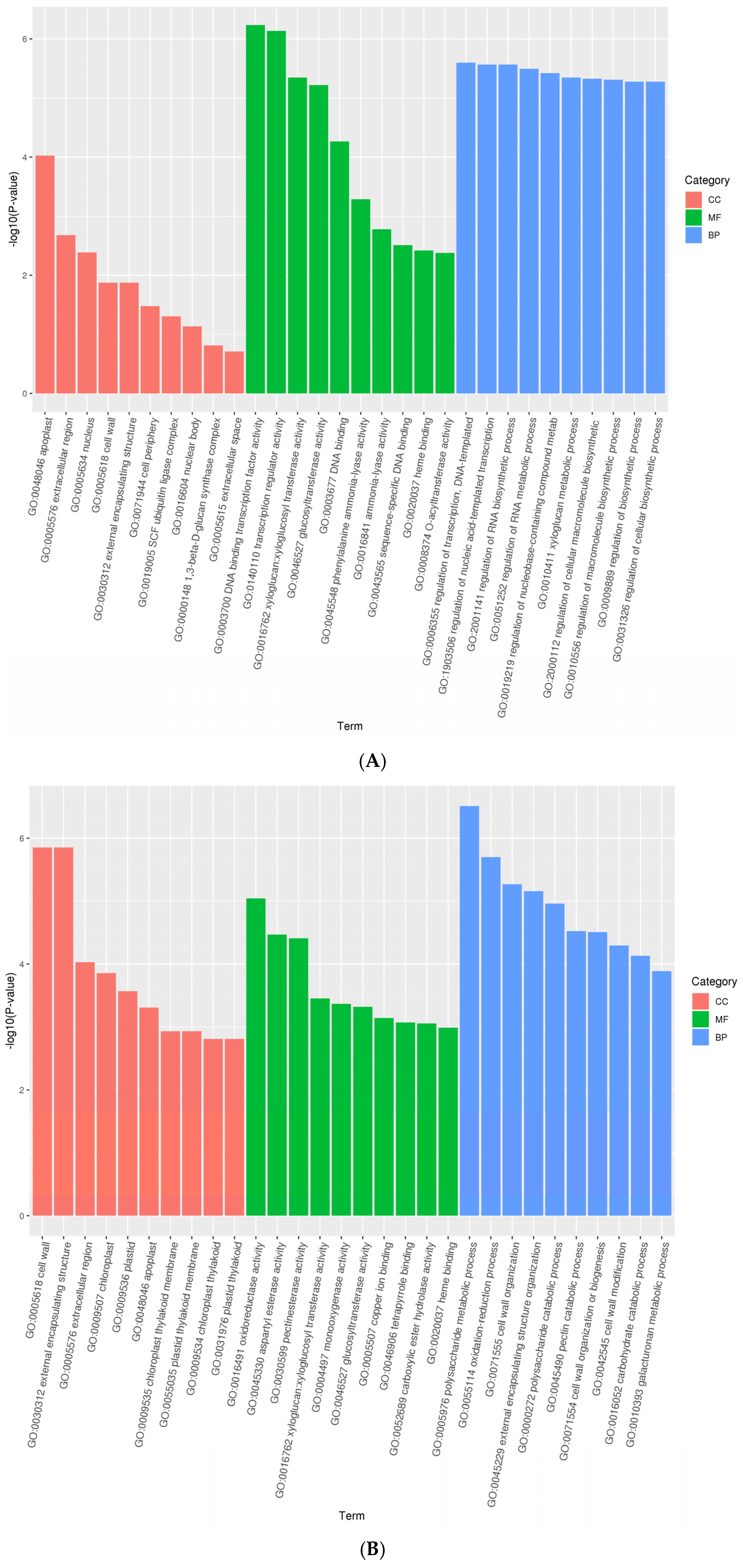
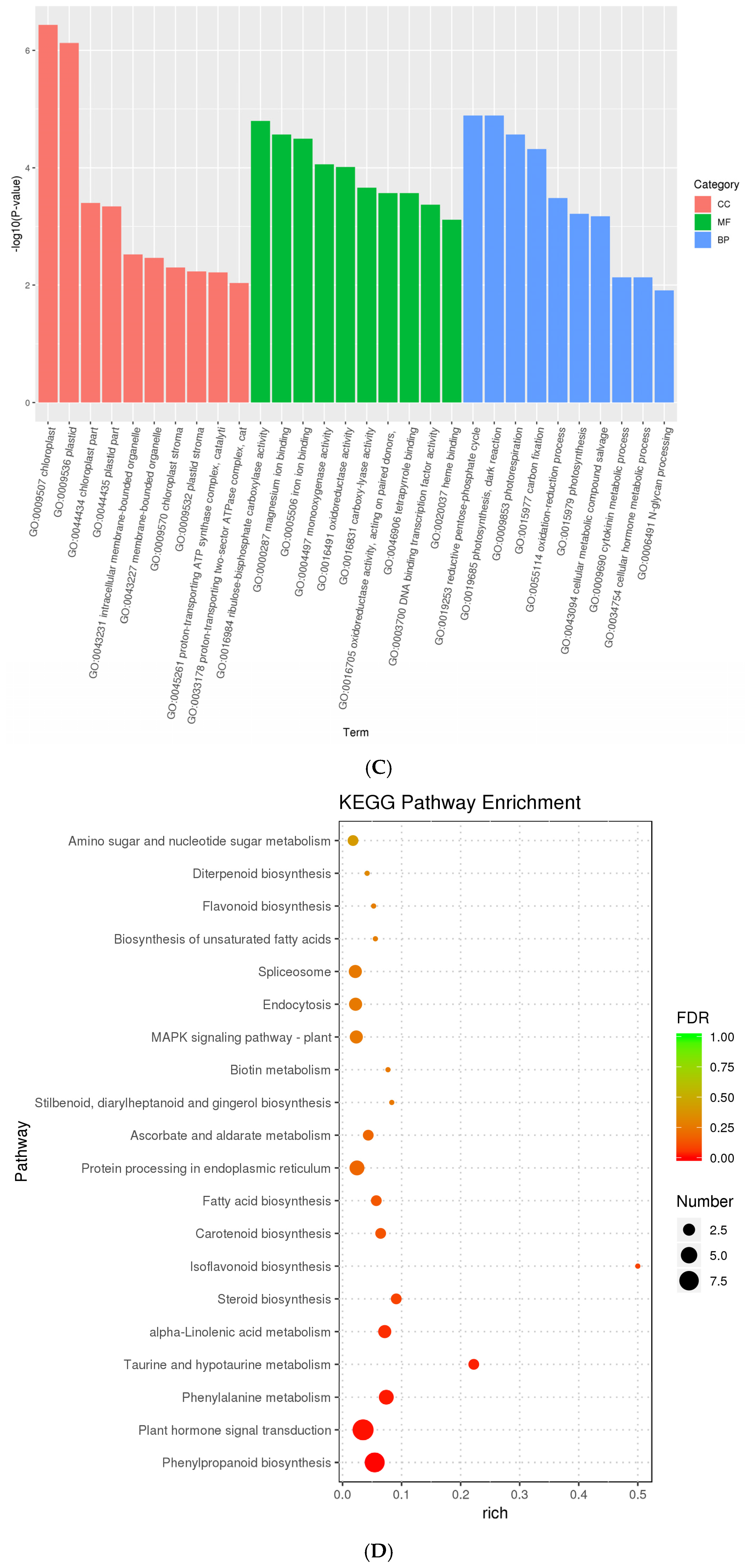
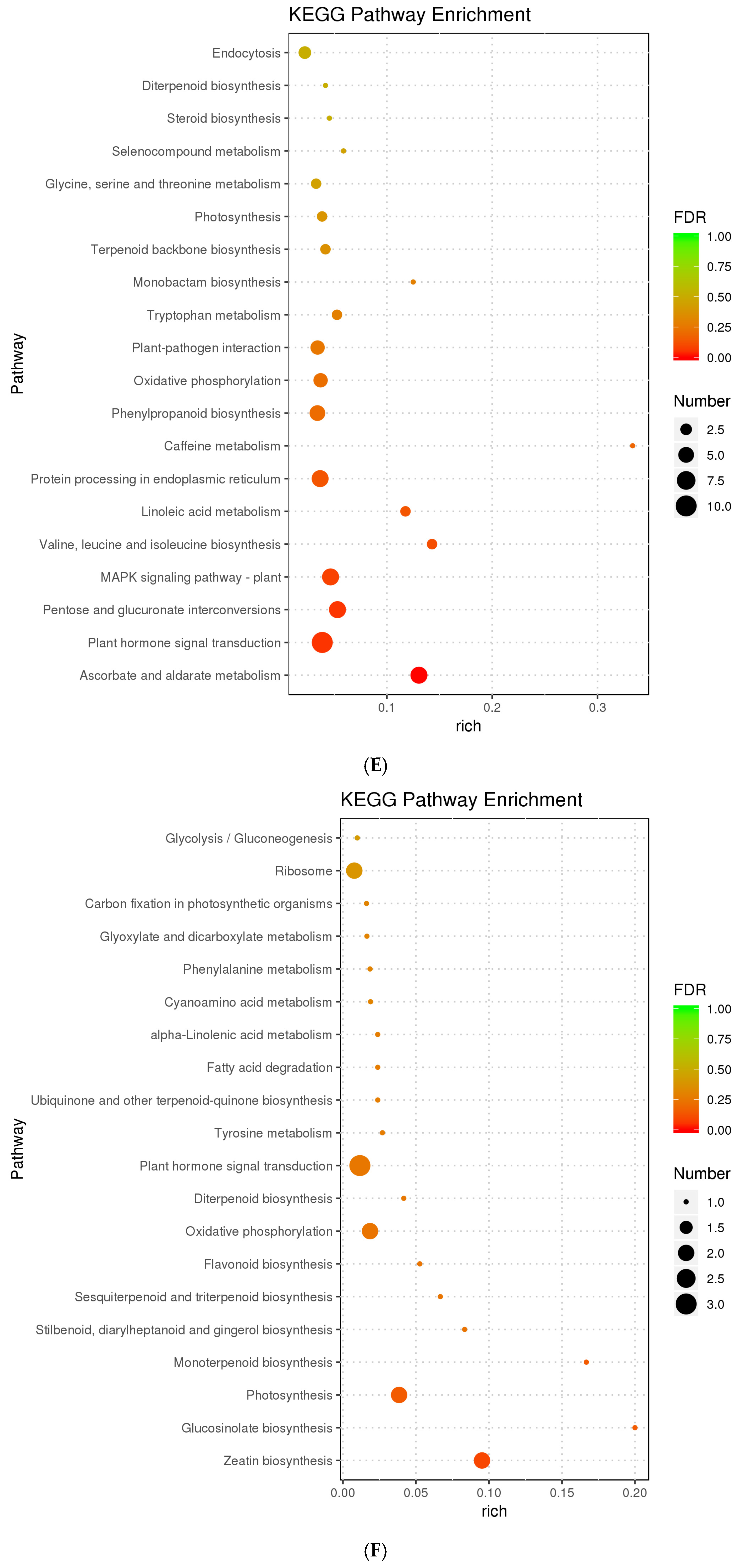
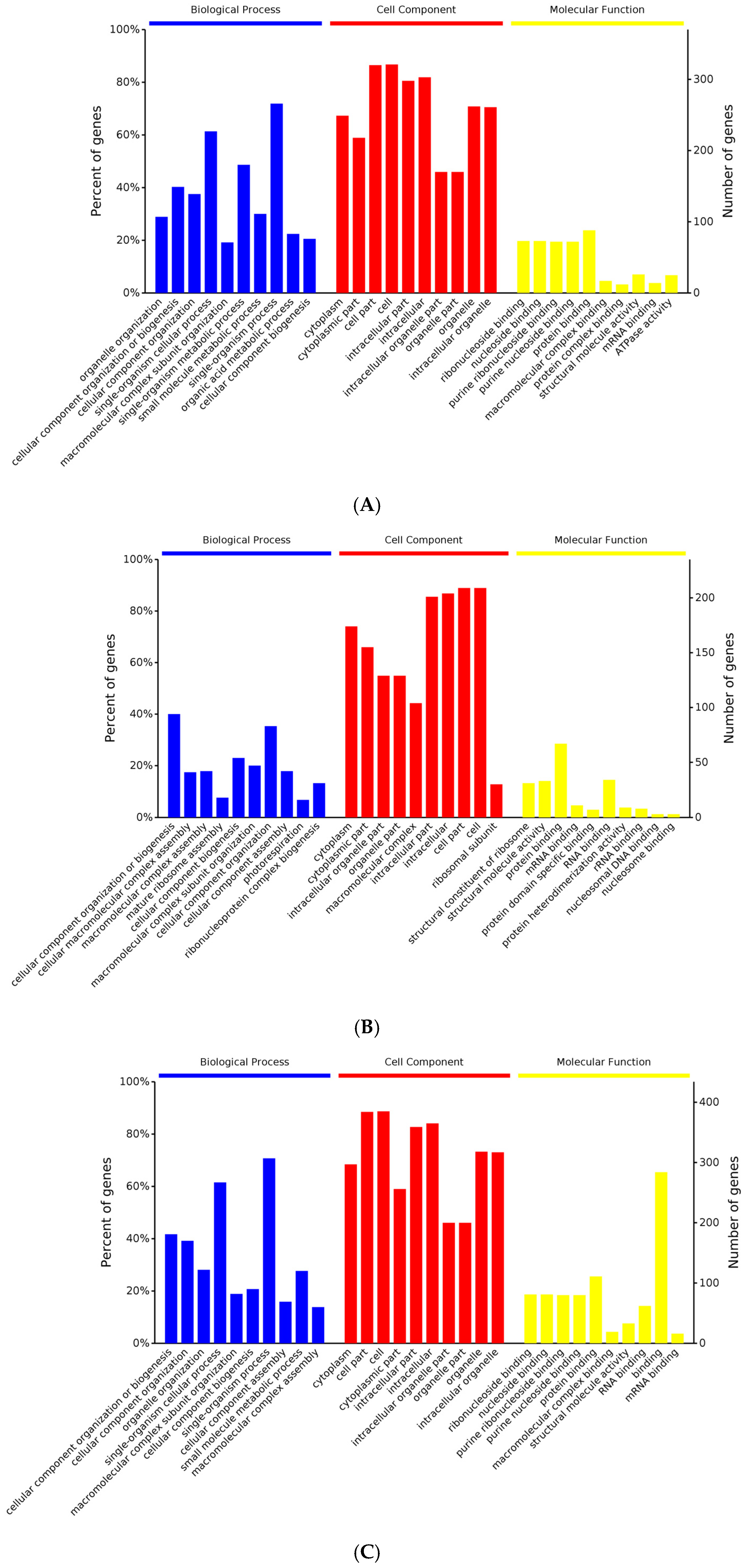
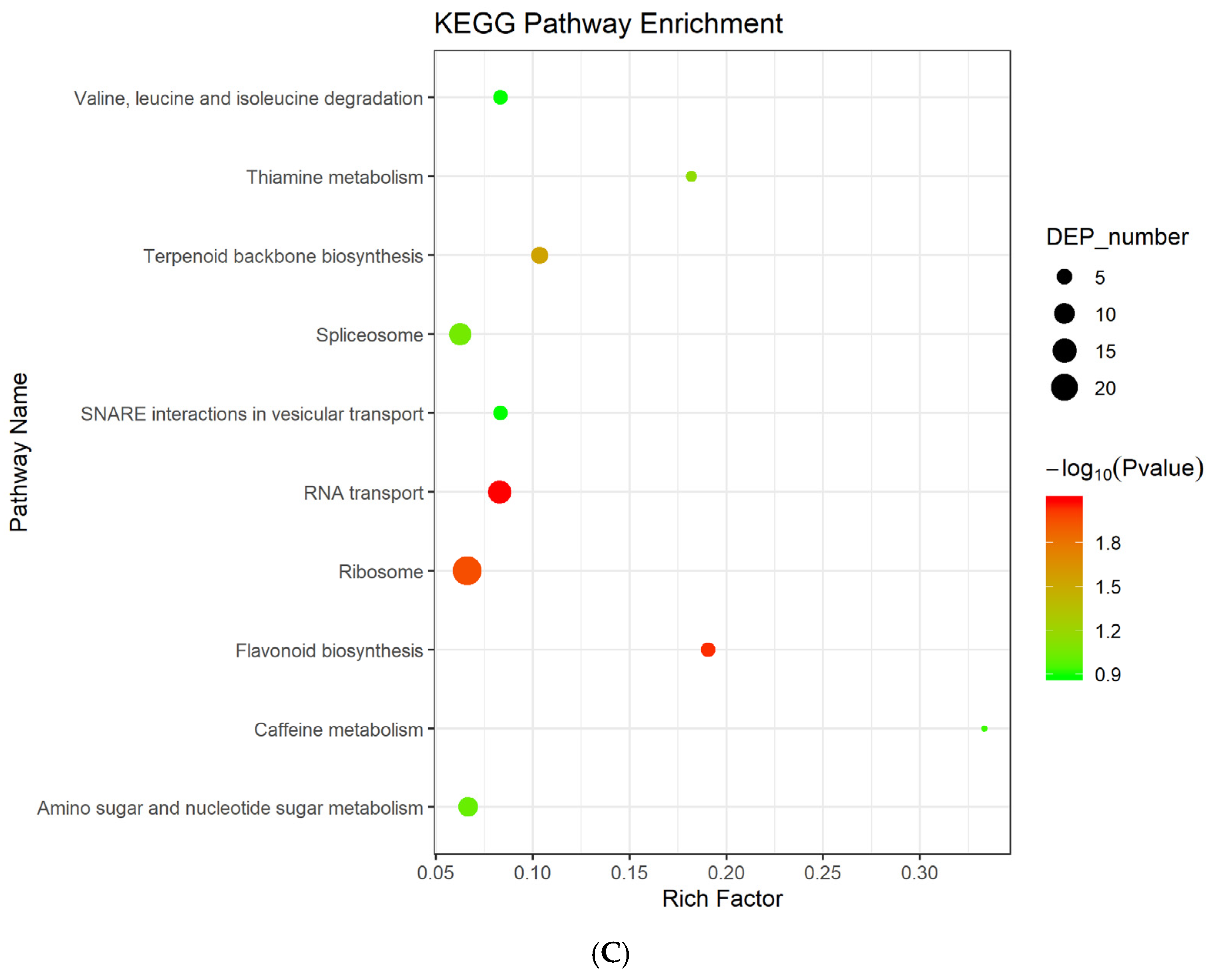
3.2. Differentially Expressed Genes Enrichment
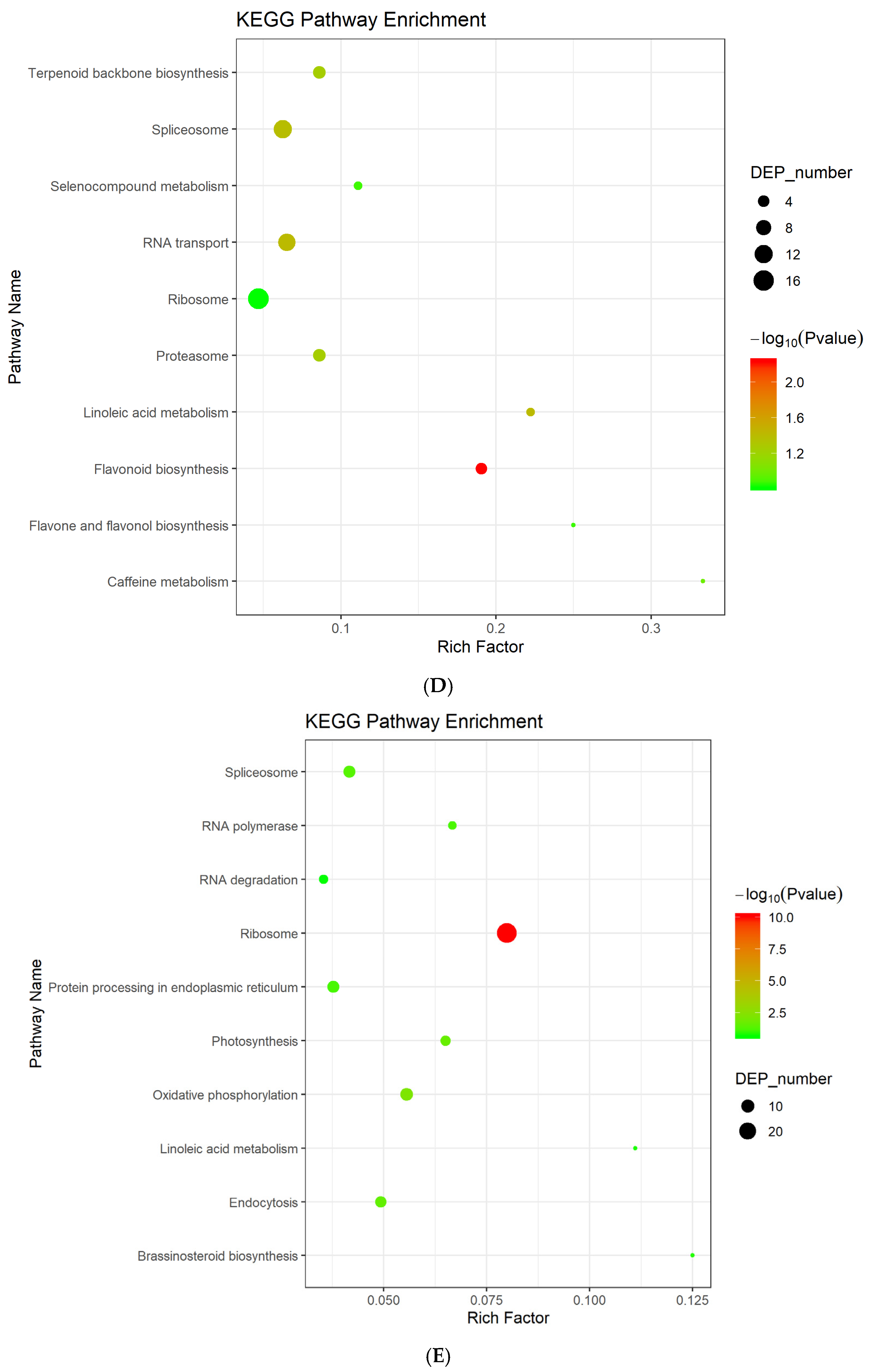
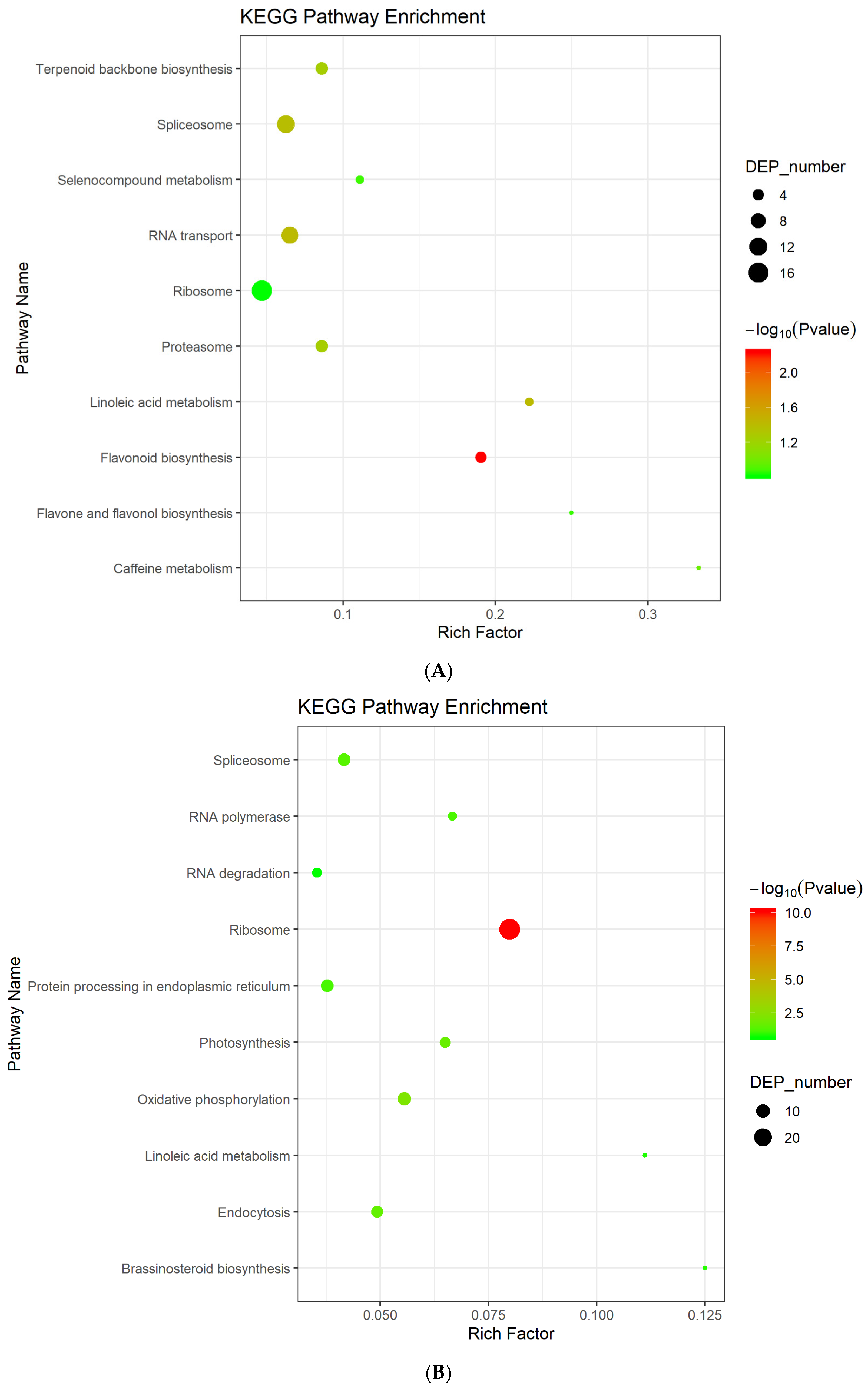
3.3. Differentially Expressed Proteins Enrichment
3.4. Association Analysis of DEGs and DEPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grumet, R.; McCreight, J.D.; McGregor, C.; Weng, Y.; Mazourek, M.; Reitsma, K.; Labate, J.; Davis, A.; Fei, Z. Genetic resources and vulnerabilities of major cucurbit Crops. Genes 2021, 12, 1222. [Google Scholar] [CrossRef]
- Ellis, A.C.; Dudenbostel, T.; Crowe-White, K. Watermelon juice: A novel functional food to increase circulating lycopene in older adult women. Plant Food Hum. Nutr. 2019, 74, 200–203. [Google Scholar] [CrossRef]
- Pereira, L.D.S.; Silva, E.M.D.; Ferreira, J.O.P.; Santos, V.L.G.; Lima, C.J.G.D.S.; Junior, G.B.D.S. Watermelon yield and efficiency of use of water and nitrogen. Rev. Caatinga 2019, 32, 769–777. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Garcés-Claver, A. Citrullus spp. germplasm diversity in Tunisia: An overview. Cucurbit Genet. Coop. Rep. 2021, 55, 1272–1279. [Google Scholar]
- Kumari, M.; Deltsidis, A.; Luo, X.L.; Mcavoy, C.; Mcavoy, T. Assessment of Triploid Watermelon Cultivars Grown in Georgia for Yield and Quality Parameters. HortTechnology 2025, 35, 81–89. [Google Scholar] [CrossRef]
- Chang, J.; Wu, X.; Liu, A.; Wang, Y.; Xu, B.; Yang, W.; Meyerson, L.A.; Gu, B.; Peng, C.; Ge, Y. Assessment of net ecosystem services of plastic greenhouse vegetable cultivation in China. Ecol. Econ. 2011, 70, 740–748. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.H.; Zhao, L.; Shen, T.; Chen, H.W.; Kong, Q.S.; Bie, A.L. Effects of different fruit setting methods on yield and quality of watermelon under protected cultivation. Chin. Cucurbits Veg. 2017, 30, 15–17. [Google Scholar]
- Sánchez, M.; Velásquez, Y.; González, M.; Cuevas, J. Pollination effectiveness of the Hoverfly Eristalinus aeneus (Scopoli, 1763) in diploid and triploid associated watermelon Crop. Insects 2022, 13, 1021. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M.; Weinbaum, S.A. High relative humidity reduces anther dehiscence in apricot, peach, and almond. HortScience 1999, 34, 322–325. [Google Scholar] [CrossRef]
- Bomfim, I.G.A.; de Melo Bezerra, A.D.; Nunes, A.C.; de Aragão, F.A.S.; Freitas, B.M. Adaptive and Foraging Behavior of Two Stingless Bee Species (Apidae: Meliponini) in Greenhouse Mini Watermelon Pollination. Sociobiology 2014, 61, 502–509. [Google Scholar]
- Zhang, H.; Huang, J.; Williams, P.H.; Vaissière, B.E.; Zhou, Z.; Gai, Q.; Dong, J.; An, J. Managed bumblebees outperform honeybees in increasing peach fruit set in China: Different limiting processes with different pollinators. PLoS ONE 2015, 10, e0121143. [Google Scholar] [CrossRef] [PubMed]
- Wurz, A.; Grass, I.; Tscharntke, T. Hand pollination of global crops—A systematic review. Basic Appl. Ecol. 2021, 56, 299–321. [Google Scholar] [CrossRef]
- Adlerz, W.C. Honey bee visit numbers and watermelon pollination. J. Econ. Entomol. 1966, 59, 28–30. [Google Scholar] [CrossRef]
- Stanghellini, M.S.; Ambrose, J.T.; Schultheis, J.R. The effects of honey bee and bumble bee pollination on fruit set and abortion of cucumber and watermelon. Am. Bee J. 1997, 137, 386–391. [Google Scholar]
- Walters, S.A. Influence of honey bee pollination on triploid watermelon fruit set and quality. HortScience 2005, 40, 1118C. [Google Scholar] [CrossRef]
- Rucker, R.R.; Thurman, W.N.; Burgett, M. Honey bee pollination markets and the internalization of reciprocal benefits. Am. J. Agric. Econ. 2012, 94, 956–977. [Google Scholar] [CrossRef]
- Campbell, J.W.; Stanley-Stahr, C.; Bammer, M.; Daniels, J.C.; Ellis, J.D. Contribution of bees and other pollinators to watermelon (Citrullus lanatus Thunb.) pollination. J. Apicult. Res. 2019, 58, 597–603. [Google Scholar] [CrossRef]
- Tan, S.S.; Wu, C.D.; Wang, D.S.; Liu, Q.Y.; Zhu, Y.Y.; Wu, H.; Li, W.M. Effects of different pollination methods on growth and quality of watermelon in greenhouse. Spec. Econ. Anim. Plants 2021, 24, 8–9. [Google Scholar]
- Wijesinghe, S.A.E.C.; Evans, L.J.; Kirkland, L.; Rader, R. A global review of watermelon pollination biology and ecology: The increasing importance of seedless cultivars. Sci. Hortic. 2020, 271, 109493. [Google Scholar] [CrossRef]
- Kasahara, R.D.; Notaguchi, M.; Nagahara, S.; Suzuki, T.; Susaki, T.; Honma, Y.; Maruyama, D.; Higashiyama, T. Pollen tube contents initiate ovule enlargement and enhance seed coat development without fertilization. Sci. Adv. 2016, 2, e1600554. [Google Scholar] [CrossRef]
- Kasahara, R.D.; Notaguchi, M.; Honma, Y. Discovery of pollen tube-dependent ovule enlargement morphology phenomenon, a new step in plant reproduction. Commun. Integr. Biol. 2017, 10, e1338989. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, R.X.; Liang, S.K.; Zhou, D.W.; Su, T.; Qihuang, L.; Liangyu, M.; Yuming, L. Effects of different pollination methods on expansion of bitter gourd. J. South Agric. 2018, 49, 768–772. [Google Scholar] [CrossRef]
- Rodger, J.G.; Bennett, J.M.; Razanajatovo, M.; Knight, T.M.; van Kleunen, M.; Ashman, T.-L.; Steets, J.A.; Hui, C.; Arceo-Gómez, G.; Burd, M.; et al. Widespread vulnerability of flowering plant seed production to pollinator declines. Sci. Adv. 2021, 7, eabd3524. [Google Scholar] [CrossRef]
- Du, X.; Liu, N.; Lu, P.L.; Wang, Y.; Lu, B.; Tian, S.B.; Zhang, Z.H. RNA-seq-based transcriptome profiling of early fruit development in Chiehqua and analysis of related transcription factors. Sci. Rep. 2024, 14, 13489. [Google Scholar] [CrossRef]
- Parry, C.; Turnbull, C.; Gill, R.J. Tracking pollen tube and ovule development in vivo reveals rapid responses to pollination in Brassica napus. AoB Plants 2025, 17, plaf002. [Google Scholar] [CrossRef]
- Layek, U.; Kundu, A.; Bisui, S.; Karmakar, P. Impact of managed stingless bee and western honey bee colonies on native pollinators and yield of watermelon: A comparative study. Ann. Agricult. Sci. 2021, 66, 38–45. [Google Scholar] [CrossRef]
- Kiatoko, N.; Pozo, M.I.; Oystaeyen, A.V.; Langevelde, F.V.; Wäckers, F.; Kumar, R.S.; Hundt, B.; Jaramillo, J. Effective pollination of greenhouse Galia musk melon (Cucumis melon L. var. reticulatus ser.) by afrotropical stingless bee species. J. Apicult. Res. 2022, 61, 664–674. [Google Scholar] [CrossRef]
- Dou, J.L.; Liu, W.G.; Zhao, S.J.; Lu, X.Q.; He, N.; Zhu, H.J.; Gao, L. Change of Endogenous Hormone Content during Watermelon Fruit Development. Chin. Cucurbits Veg. 2014, 27, 12–15. [Google Scholar]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Bangerth, F.; Li, C.J.; Gruber, J. Mutual interaction of auxin and cytokinins in regulating correlative dominance. Plant Growth Regul. 2000, 32, 205–217. [Google Scholar] [CrossRef]
- Hayata, Y.; Niimi, Y.; Inoue, K.; Kondo, S. CPPU and BA, with and without Pollination, Affect Set, Growth, and Quality of Muskmelon Fruit. Hortscience 2000, 35, 868–870. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Guo, H.X.; Lv, B.S.; Feng, J.; Wang, H.H.; Zhang, Z.H.; Chai, S. Gibberellin biosynthesis is required for CPPU-induced parthenocarpy in melon. Hortic. Res. 2023, 10, uhad084. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylenebiosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588. [Google Scholar] [CrossRef]
- Hermanns, A.S.; Zhou, X.S.; Xu, Q.; Tadmor, Y.; Li, L. Carotenoid Pigment Accumulation in Horticultural Plants. Hortic. Plant J. 2020, 6, 343–360. [Google Scholar] [CrossRef]
- Lobaton, J.; Andrew, R.; Duitama, J.; Kirkland, L.; Rader, R. Using RNA-seq to characterize pollen–stigma interactions for pollination studies. Sci. Rep. 2021, 11, 6635. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Rampitsch, C.; Bykova, N.V. Advances in plant proteomics toward improvement of crop productivity and stress resistancex. Front. Plant Sci. 2015, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.T.; Guo, S.G.; Ren, Y.; Zhang, J.; Li, M.Y.; Yian, S.; Wang, J.; Sun, H.; Zuo, Y.; Chen, Y.; et al. Quantitative Transcriptomic and Proteomic Analysis of Fruit Development and Ripening in Watermelon (Citrullus lanatus). Front. Plant Sci. 2022, 13, 818392. [Google Scholar] [CrossRef]
- Wu, B.; Ji, Y.J.; Li, P.P.; Fan, S.X.; Hao, J.H. Cytological observation and changes of endogenous hormones in melon fruit during early development. J. Beijing Univ. Agric. 2016, 31, 64–68. [Google Scholar]
- Pan, C.T.; Hu, X.J.; Chen, X.S.; Chang, Z.M.; Smagghe, G.; Long, J.K. Effects of different pollination treatments on the fertilization physiology and fruit production in passion fruit. Sci. Hortic. 2025, 343, 114070. [Google Scholar] [CrossRef]
- Li, J.L.; Wu, J.; An, J.D.; Guo, Z.B.; Tong, Y.M.; Wang, X.Q. Honeybee pollination technology on watermelon in greenhouse in Sanya area. Y Comparison of bumblebees and honeybees pollinating watermelon in plastic greenhouse. J. Bee 2006, 10, 7–9. [Google Scholar]
- Jiang, J.; Lu, J.S.; Zhang, B.D. Analysis on pollination effect of honeybee on small fruit watermelon in greenhouse. Chin. Cucurbits Veg. 2014, 27, 33–36. [Google Scholar]
- Su, X.L.; Hua, Q.Y.; Zhao, D.X.; Cao, C.X.; Bie, Z.L. Effects of different fruit setting methods on yield and quality of long-season protected cultivation of watermelon. Chin. Cucurbits Veg. 2016, 29, 11–13. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Sawe, T.; Nielsen, A.; Totland, Ø.; Macriced, S.; Eldegard, K. Inadequate pollination services limit watermelon yields in northern Tanzania. Basic. Appl. Ecol. 2020, 44, 35–45. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Yang, J.; Xiao, F.; Wang, X.R. Study on the physiological mechanism and transcriptional regulatory network of early fruit development in Gleditsia sinensis Lam. (Fabaceae). BMC Plant Biol. 2024, 24, 1213. [Google Scholar] [CrossRef]
- Kou, X.H.; Yang, S.; Chai, L.P.; Wu, C.; Zhou, J.Q.; Li, Y.F.; Xue, Z.H. Abscisic acid and fruit ripening: Multifaceted analysis of the effect of abscisic acid on fleshy fruit ripening. Sci. Hortic. 2021, 281, 109999. [Google Scholar] [CrossRef]
- Kishor, K.; Polavarapu, B.; Tiozon, R.N.; Fernie, A.R.; Sreenivasulu, N. Abscisic acid and its role in the modulation of plant growth, development, and yield stability. Trends Plant Sci. 2022, 27, 1283–1295. [Google Scholar] [CrossRef]
- Collin, A.; Matkowski, H.; Sybilska, E.; Biantari, A.; Kró, O.; Daszkowska-Golec, A. ABA-induced alternative splicing drives transcriptomic reprogramming for drought tolerance in barley. BMC Plant Biol. 2025, 25, 445. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Dong, T.Y.; Haider, M.S.; Jin, H.C.; Jia, H.F.; Fang, J.G. Brassinosteroid regulates 3-Hydroxy-3-methylglutaryl CoA reductase to promote grape fruit development. J. Agric. Food Chem. 2020, 68, 11987–11996. [Google Scholar] [CrossRef]
- Hui, B.D.; Li, J. Content and composition comparisons of carotenoids between red-flesh watermelon and yellow-flesh watermelon. Food Sci. 2008, 29, 587–591. [Google Scholar]
- Yuan, P.L.; Umer, M.J.; He, N.; Zhao, S.J.; Lu, X.Q.; Zhu, H.J.; Gong, C.S.; Diao, W.; Gebremeskel, H.; Kuang, H.; et al. Transcriptome regulation of carotenoids in five flesh-colored watermelons (Citrullus lanatus). BMC Plant Biol. 2021, 21, 203. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S.; et al. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2021, 335, 1348–1351. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic acid synthesis and response. Arab.-Sis Book 2013, 11, e0166. [Google Scholar] [CrossRef]
- Jia, K.P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef]
- Luo, Y.S.; Wang, C.J.; Wang, M.M.; Wang, Y.L.; Xu, W.L.; Han, H.; Wang, Z.; Zhong, Y.; Huang, H.; Qu, S. Accumulation of carotenoids and expression of carotenoid biosynthesis genes in fruit flesh during fruit development in two Cucurbita maxima inbred lines. Hortic. Plant J. 2021, 7, 529–538. [Google Scholar] [CrossRef]
- Li, J.; Berger, F. Endosperm: Food for humankind and fodder for scientific discoveries. New Phytol. 2012, 195, 290–305. [Google Scholar] [CrossRef]
- Dadlani, M.; Yadava, D.K. (Eds.) Seed Science and Technology: Biology, Production, Quality; Springer: Singapore, 2023. [Google Scholar]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biologicalfunctions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xu, F.; Li, Y.T.; Yu, L.; Fu, M.Y.; Liao, Y.L.; Yang, X.; Zhang, W.; Ye, J. Genome-wide characterization of bZIP gene family identifies potential members involved in flavonoids biosynthesis in Ginkgo biloba L. Sci. Rep. 2021, 11, 23420. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Pellny, T.; Goddijn, O. Enhancing photosynthesis with sugar signals. Trends Plant Sci. 2001, 6, 197–200. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; Breeze, T.D.; Jenner, N.; Polce, C.; Biesmeijer, J.C.; Potts, S.G. Avoiding a bad apple: Insect pollination enhances fruit quality and economic value. Agric. Ecosyst. Environ. 2014, 184, 34–40. [Google Scholar] [CrossRef]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. Biol. Sci. 2014, 281, 20132440. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; Abd El-Wahed, A.A.; Musharraf, S.G.; Algethami, A.F.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Singh, G.M.; Mozaffarian, D.; Myers, S.S. Effects of decreases of animal pollinators on human nutrition and global health: A modelling analysis. Lancet 2015, 386, 1964–1972. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.H.; Shen, T.; Chen, H.W.; Kong, Q.S.; Nawaz, M.A.; Bie, A.L. Sucrose metabolism in watermelon fruit is affected by fruit-setting method under plastic film greenhouses. J. Hortic. Sci. Biotechnol. 2018, 93, 585–594. [Google Scholar] [CrossRef]
- Tadeo, F.R.; Terola, J.; Rodrigo, M.J.; Licciardello, C.; Sadkad, A. Fruit growth and development. In The Genus Citrus; Tollon, M., Caruso, M., Gmiter, G.F., Jr., Eds.; Elsevier: Duxford, UK, 2020; pp. 245–269. [Google Scholar] [CrossRef]
- Yang, T.T.; Amanullah, S.; Pan, J.H.; Chen, G.X.; Liu, S.; Ma, S.W.; Wang, J.; Gao, P.; Wang, X. Identification of putative genetic regions for watermelon rind hardness and related traits by BSA-seq and QTL mapping. Euphytica 2021, 217, 19. [Google Scholar] [CrossRef]
- Fallik, E.; Ziv, C. How rootstock/scion combinations affect watermelon fruit quality after harvest? J. Sci.Food Agr. 2020, 100, 3275–3282. [Google Scholar] [CrossRef]
- Soteriou, G.A.; Rouphael, Y.; Emmanouilidou, M.G.; Antoniou, C.; Kyratzis, A.C.; Kyriacou, M.C. Biostimulatory action of vegetal protein hydrolysate and the configuration of fruit physicochemical characteristics in grafted watermelon. Horticulturae 2021, 7, 313. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Ma, W.; Li, L.; Lei, J.; Song, H.; Zhi, H.; Shen, J. Effect of Pollination Methods on Fruit Development in Greenhouse Watermelon: Physiological and Molecular Perspectives. Agriculture 2025, 15, 2291. https://doi.org/10.3390/agriculture15212291
Wu W, Ma W, Li L, Lei J, Song H, Zhi H, Shen J. Effect of Pollination Methods on Fruit Development in Greenhouse Watermelon: Physiological and Molecular Perspectives. Agriculture. 2025; 15(21):2291. https://doi.org/10.3390/agriculture15212291
Chicago/Turabian StyleWu, Wenqin, Weihua Ma, Lixin Li, Jia Lei, Huailei Song, Haiying Zhi, and Jinshan Shen. 2025. "Effect of Pollination Methods on Fruit Development in Greenhouse Watermelon: Physiological and Molecular Perspectives" Agriculture 15, no. 21: 2291. https://doi.org/10.3390/agriculture15212291
APA StyleWu, W., Ma, W., Li, L., Lei, J., Song, H., Zhi, H., & Shen, J. (2025). Effect of Pollination Methods on Fruit Development in Greenhouse Watermelon: Physiological and Molecular Perspectives. Agriculture, 15(21), 2291. https://doi.org/10.3390/agriculture15212291




