Improving Rice Seed Quality Through the Combined Application of DBD Plasma and CuO NPs
Abstract
1. Introduction
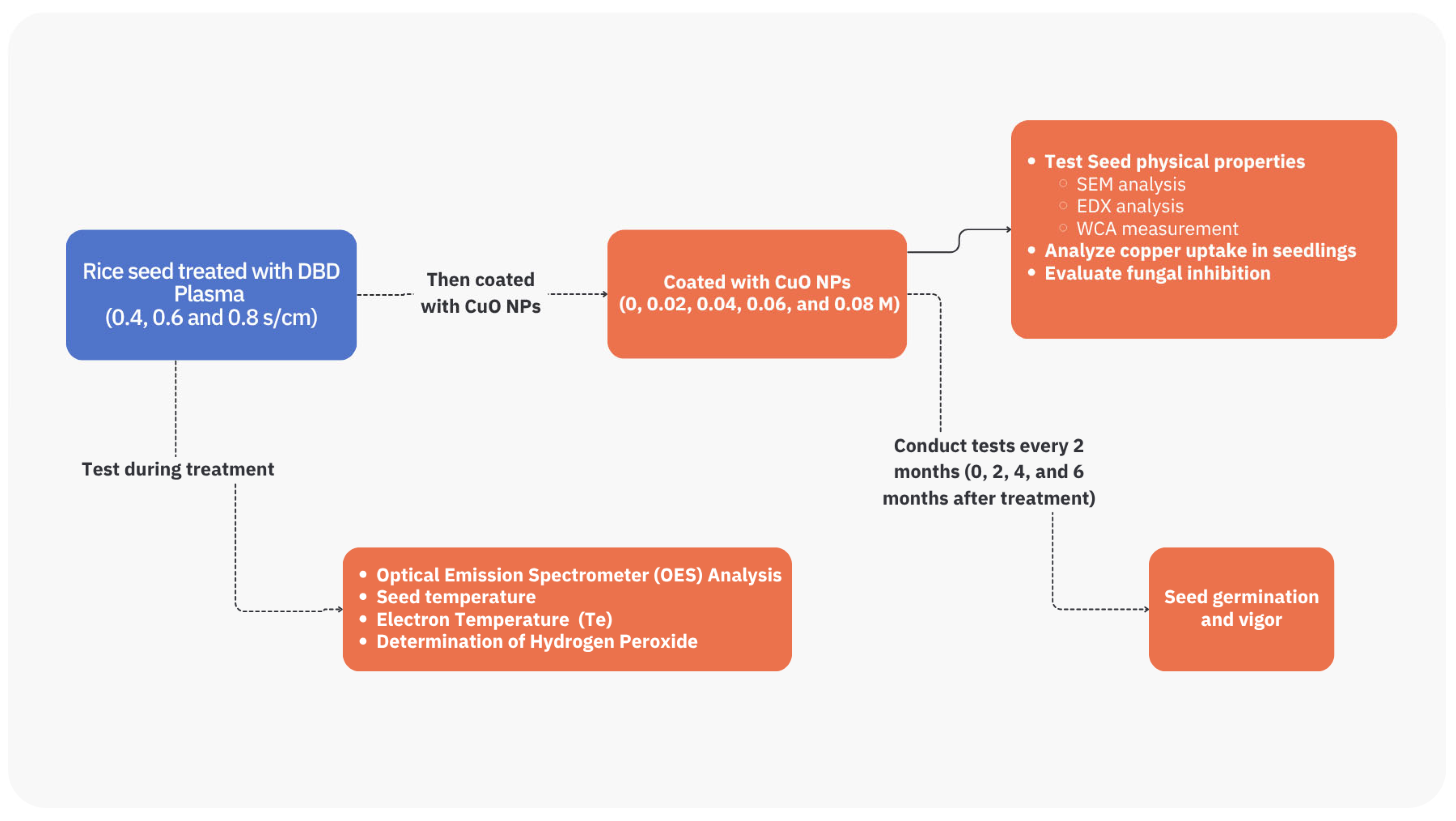
2. Materials and Methods
2.1. Rice Seed Sample
2.2. Experimental Design
2.3. Dielectric Barrier Discharge
2.4. CuO NPs Preparation
2.5. Seed Determination
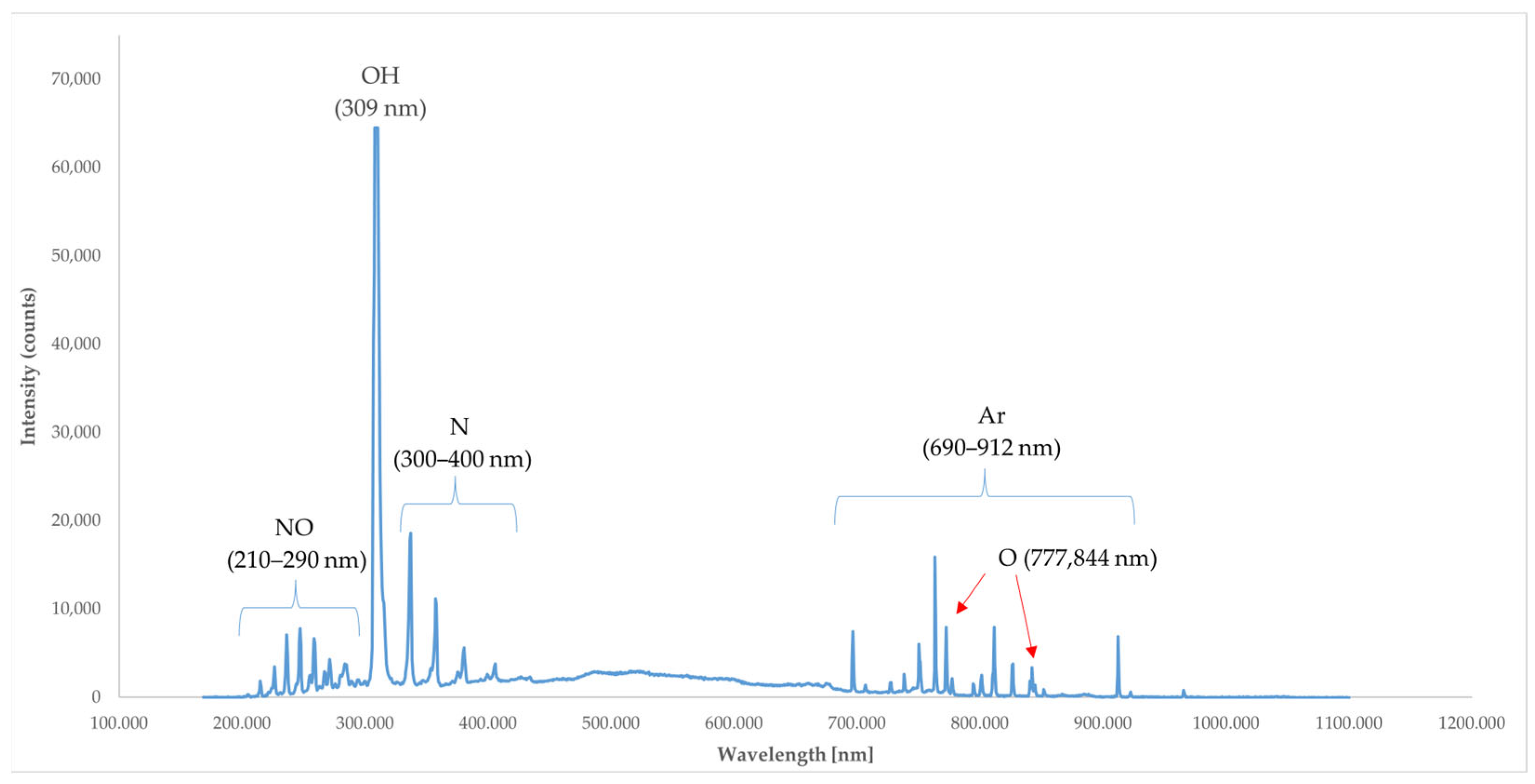
2.5.1. Optical Emission Spectrometer (OES) Analysis
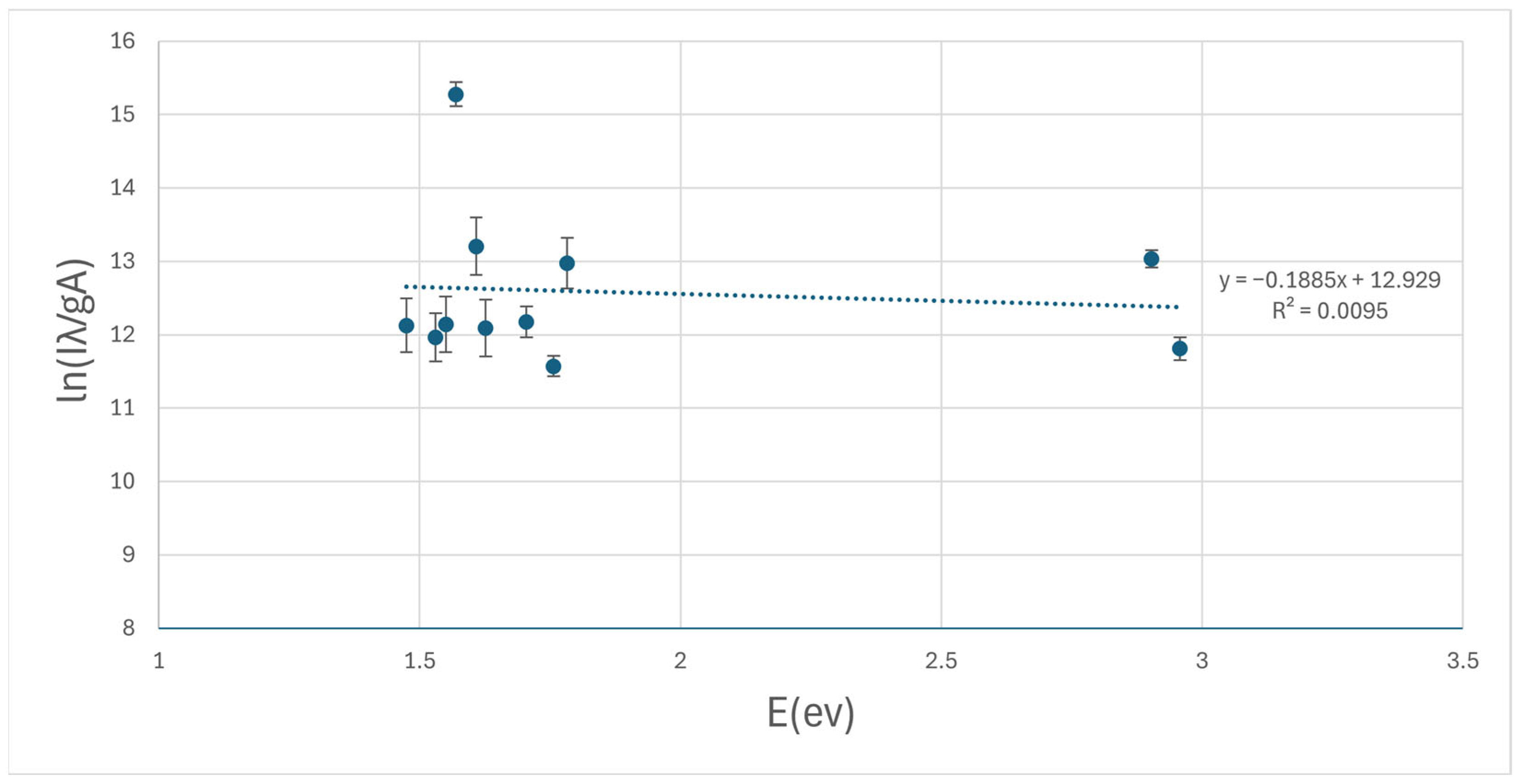
2.5.2. Determination of Electron Temperature by the Boltzmann Plot Method
2.5.3. Determination of Hydrogen Peroxide Concentration
2.5.4. Seed Physical Properties Test
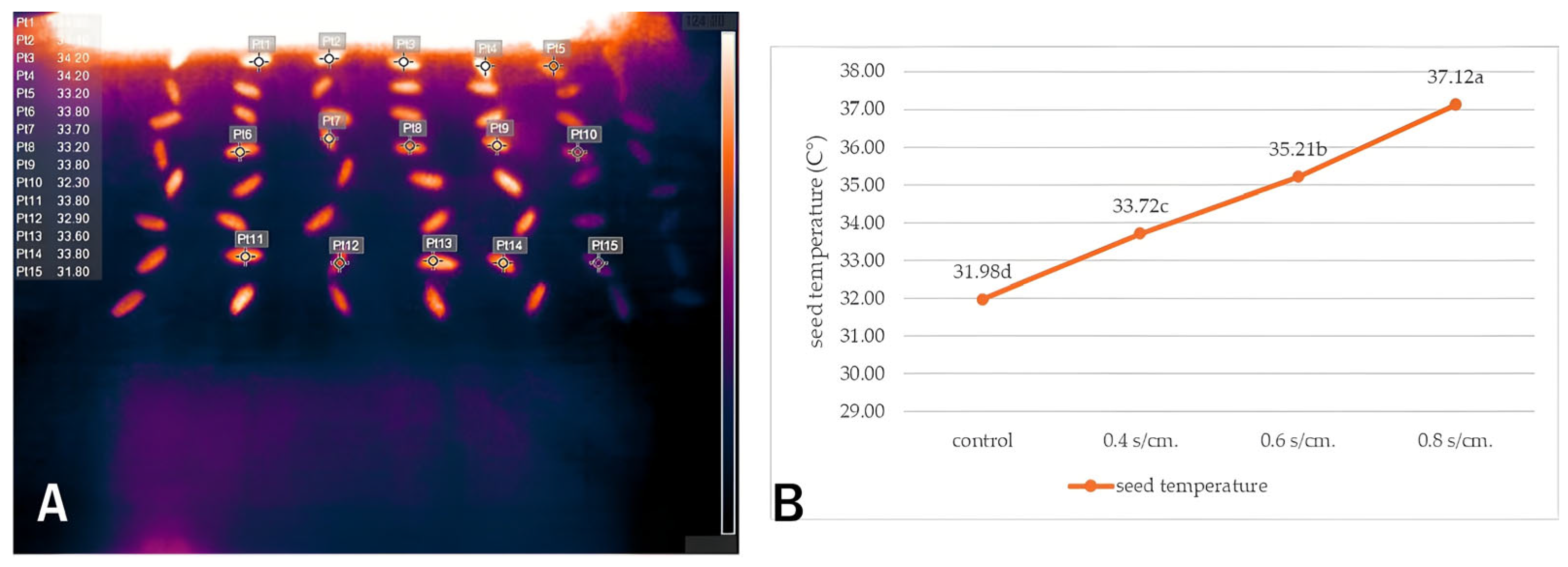
Seed Temperature
Scanning Electron Microscopy (SEM)
Water Contact Angle Measurement
2.6. Copper Uptake in Seedlings
2.7. Fungal Inhibition
2.8. Seed Germination and Vigor
2.8.1. Germination (G)
2.8.2. Radicle Emergence (RE)
2.8.3. Germination Index (GI)
2.8.4. Root and Shoot Length (RL and SL)
2.8.5. Seedling Dry Weight
2.9. Statistical Analysis
3. Results
3.1. Optical Emission Spectrometer (OES) Analysis
3.2. Determination of Electron Temperature by the Boltzmann Plot Method
3.3. Determination of Hydrogen Peroxide Concentration
3.4. Seed Physical Properties Test
3.4.1. Seed Temperature
3.4.2. Scanning Electron Microscopy
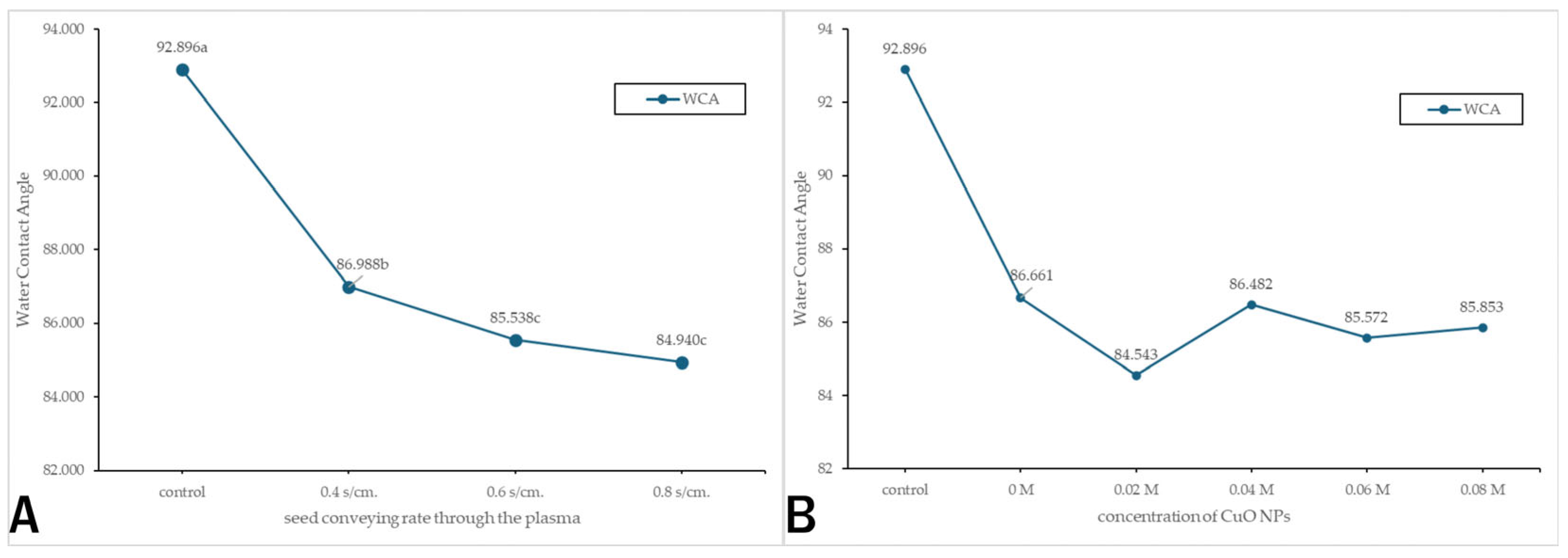
3.4.3. Contact Angle Measurement
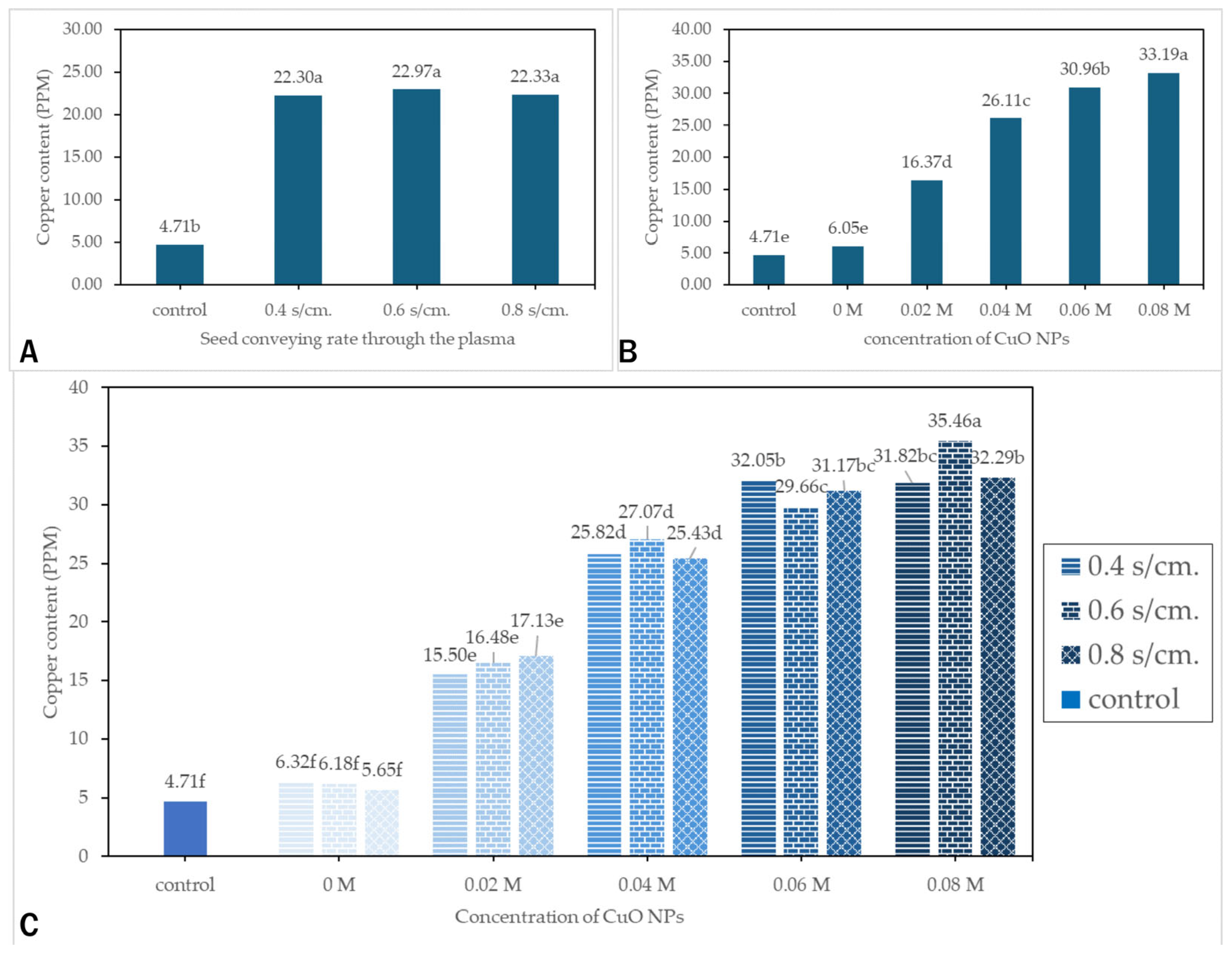
3.5. Copper Uptake in Seedlings
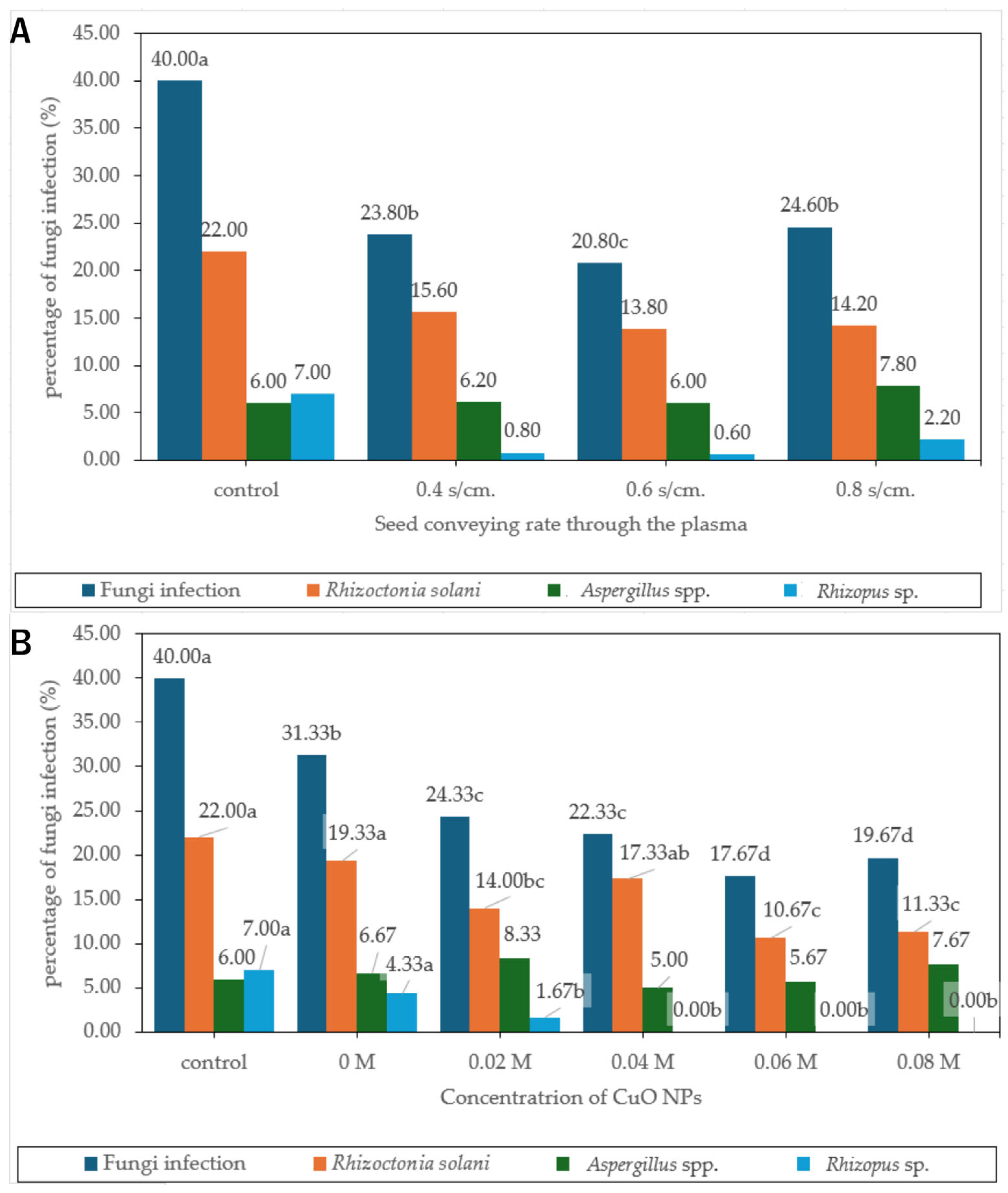
3.6. Fungal Inhibition
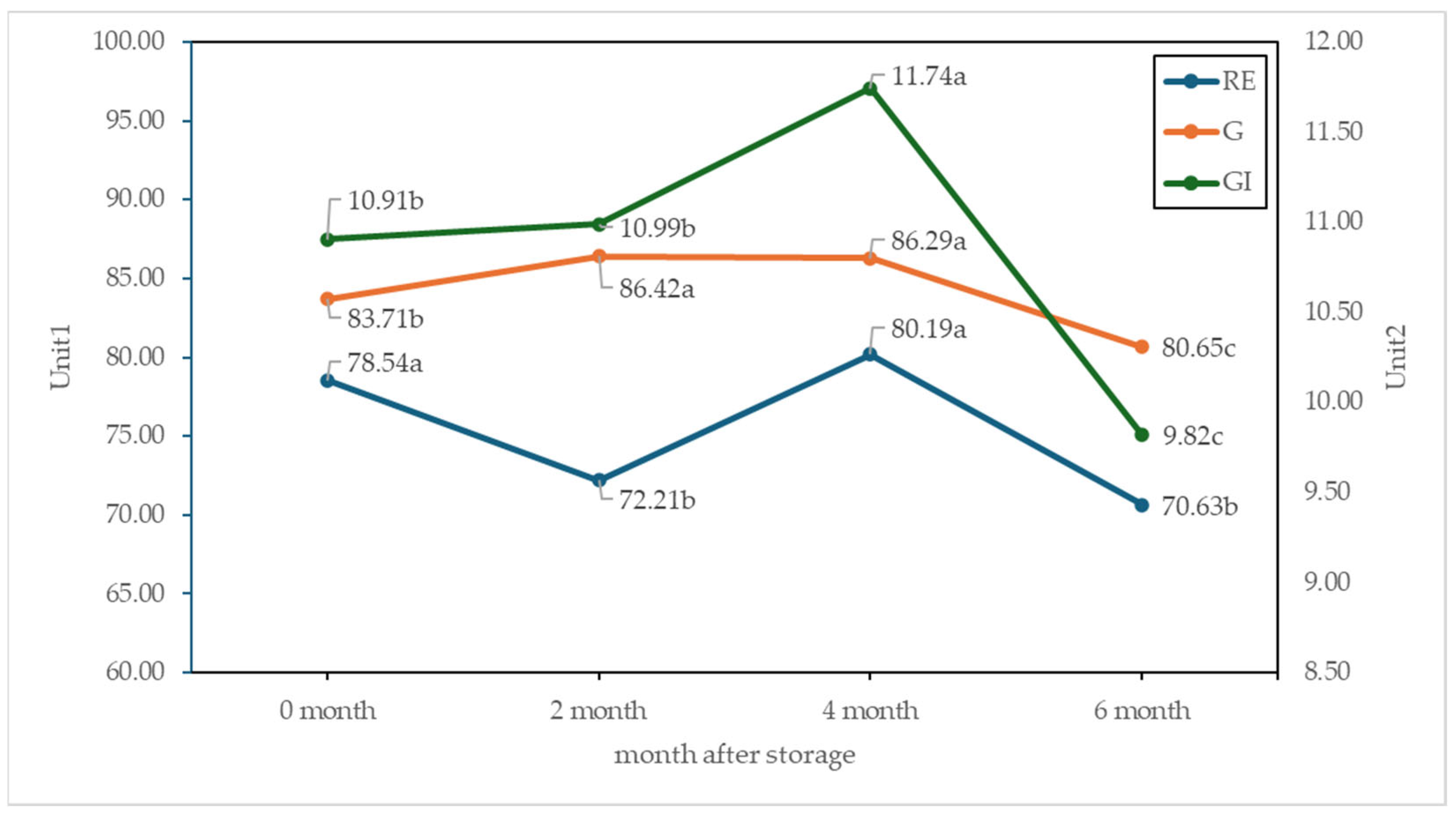
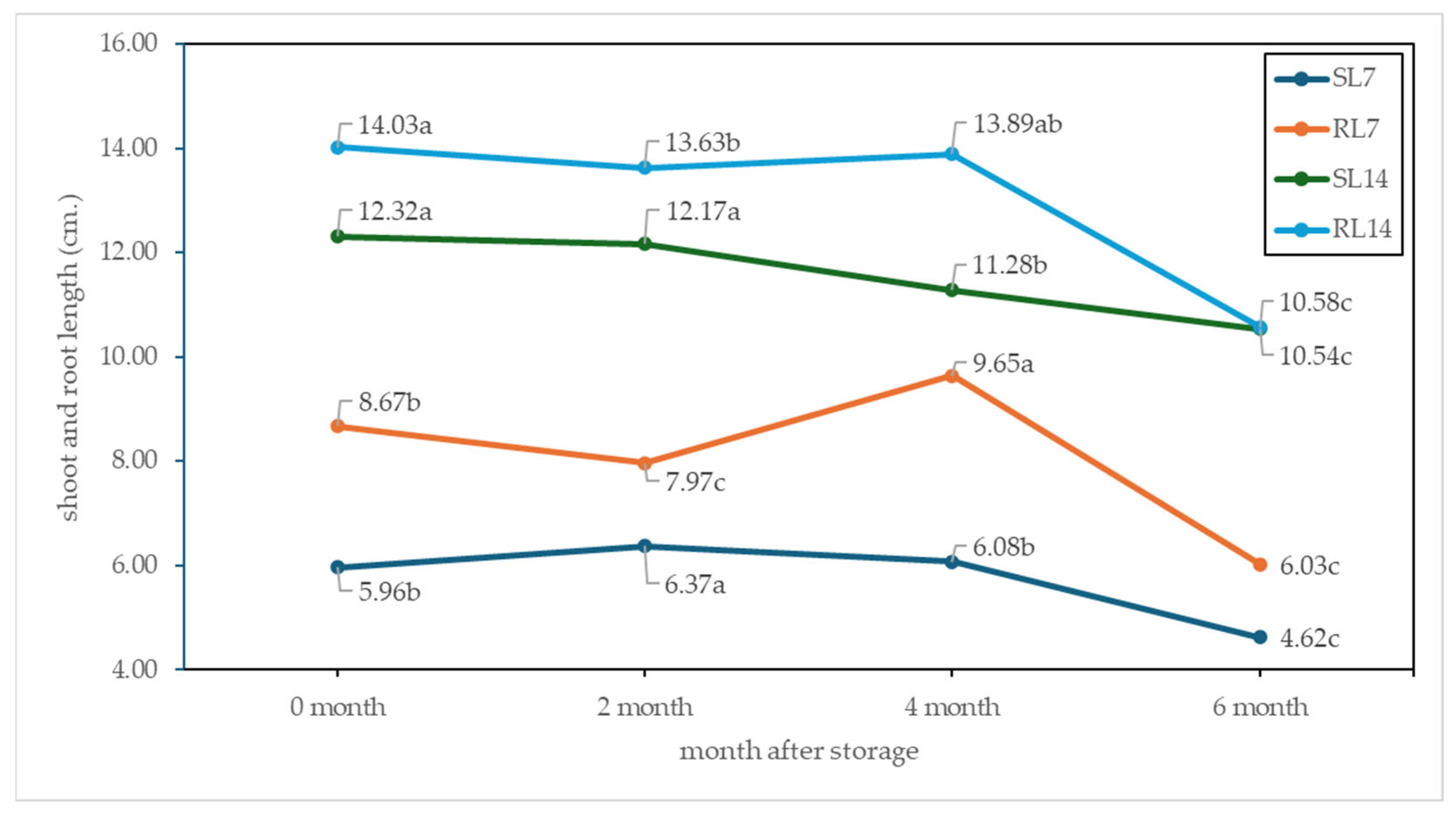
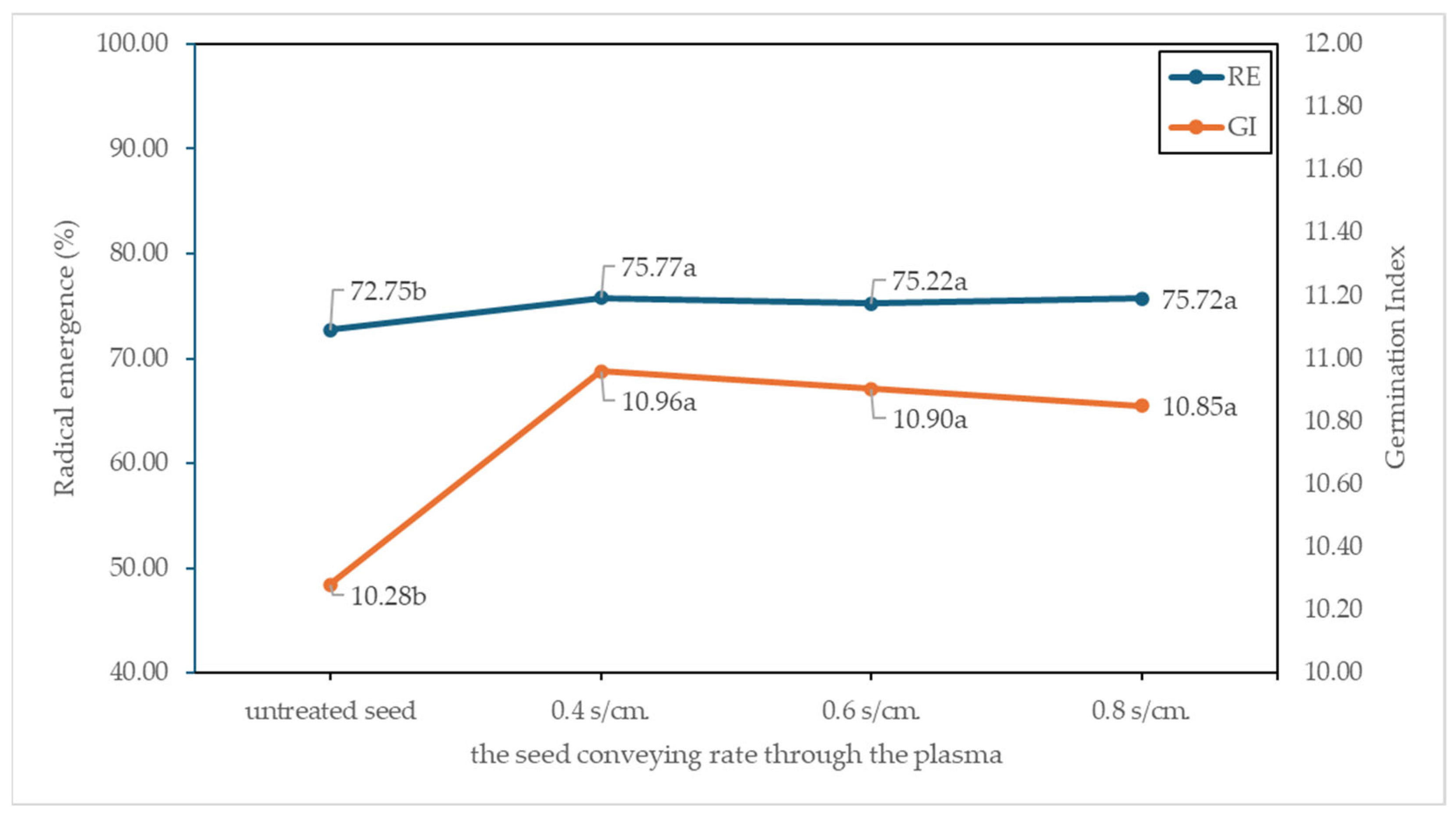
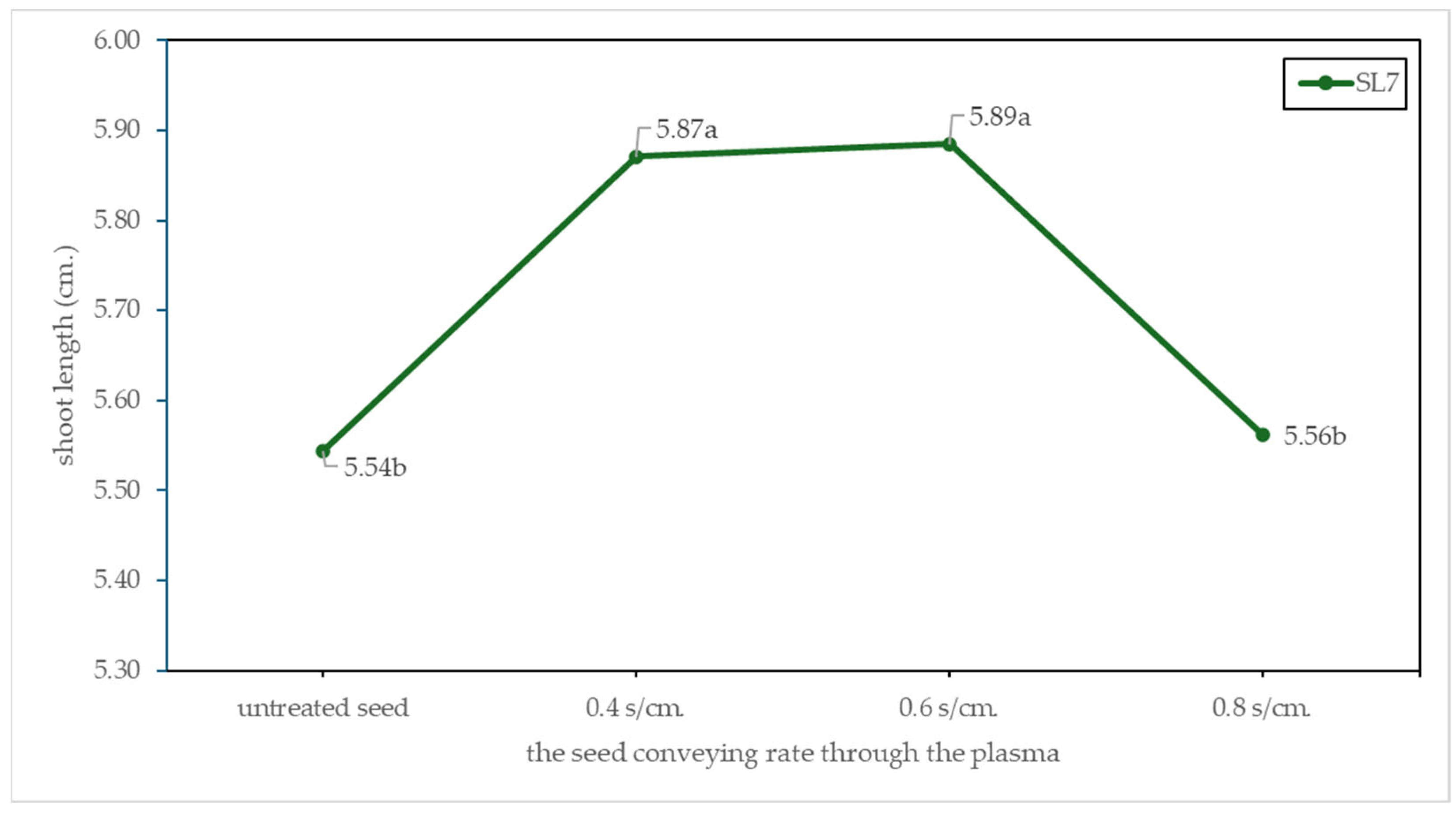
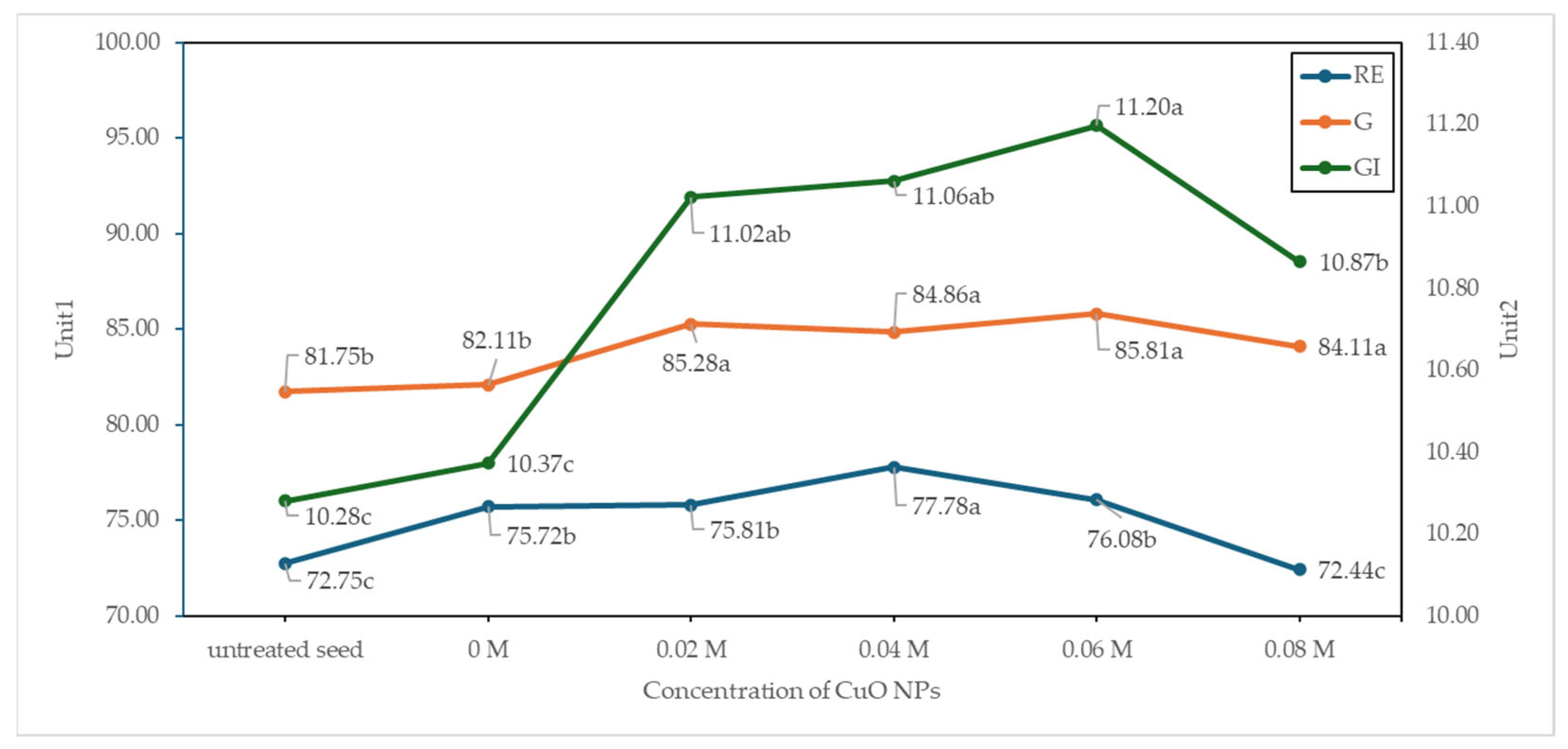
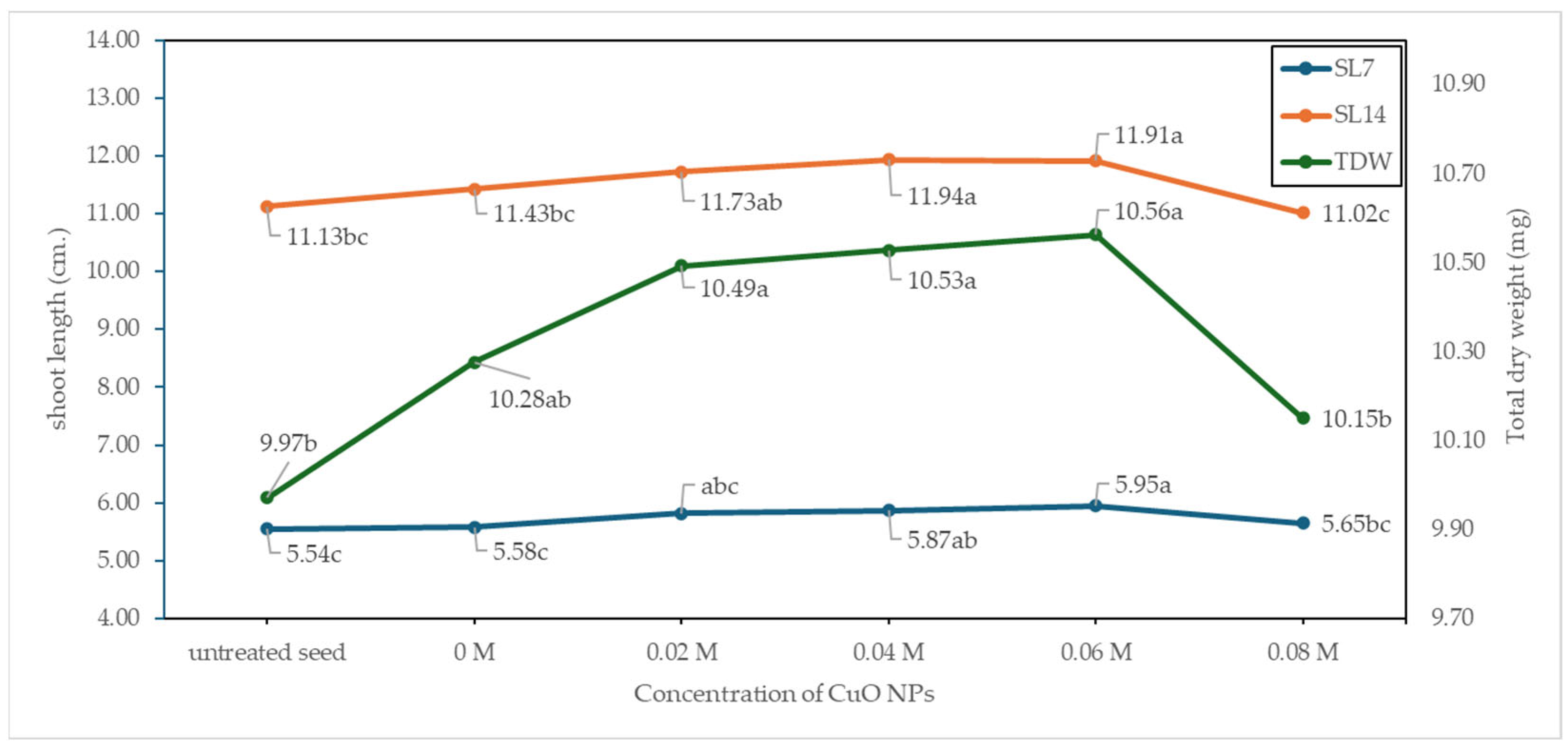
3.7. Seed Germination and Vigor
4. Discussion
4.1. Seed Physical Properties
4.2. Fungal Inhibition
4.3. Seed Quality and Seed Storage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, Y.; Zhu, X.; Qi, H.; Wang, Y.; Zhang, H.; He, J. Rice Seed Storability: From Molecular Mechanisms to Agricultural Practices. Plant Sci. 2024, 348, 112215. [Google Scholar] [CrossRef] [PubMed]
- Prasertsak, A. Rice Seed Multiplication System. Thai Rice Res. J. 2022, 13, 106–117. [Google Scholar]
- Kumar, R. Effectiveness of Seed Processing Machinery on Seed Quality Improvement in Paddy (Oryza sativa L.). J. AgriSearch 2016, 3, 187–190. [Google Scholar] [CrossRef]
- Mazandarani, A.; Goudarzi, S.; Ghafoorifard, H.; Eskandari, A. Evaluation of DBD Plasma Effects on Barley Seed Germination and Seedling Growth. IEEE Trans. Plasma Sci. 2020, 48, 3115–3121. [Google Scholar] [CrossRef]
- El Shaer, M.; Abdel-azim, M.; El-welily, H.; Hussein, Y.; Abdelghani, A.; Zaki, A.; Mobasher, M. Effects of DBD Direct Air Plasma and Gliding Arc Indirect Plasma Activated Mist on Germination, and Physiological Parameters of Rice Seed. Plasma Chem. Plasma Process. 2023, 43, 1169–1193. [Google Scholar] [CrossRef]
- Veerana, M.; Yu, N.; Ketya, W.; Park, G. Application of Non-Thermal Plasma to Fungal Resources. J. Fungi 2022, 8, 102. [Google Scholar] [CrossRef]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. (Eds.) Cold Plasma in Food and Agriculture: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2016; ISBN 978-0-12-801365-6. [Google Scholar]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, Y.Y.; Kim, Y.S.; Balaraju, K.; Mok, Y.S.; Yoo, S.J.; Jeon, Y. Enhancement of Seed Germination and Microbial Disinfection on Ginseng by Cold Plasma Treatment. J. Ginseng Res. 2021, 45, 519–526. [Google Scholar] [CrossRef]
- Varnagiris, S.; Vilimaite, S.; Mikelionyte, I.; Urbonavicius, M.; Tuckute, S.; Milcius, D. The Combination of Simultaneous Plasma Treatment with Mg Nanoparticles Deposition Technique for Better Mung Bean Seeds Germination. Processes 2020, 8, 1575. [Google Scholar] [CrossRef]
- Yawirach, S.; Sarapirom, S.; Janpong, K. The Effects of Dielectric Barrier Discharge Atmospheric Air Plasma Treatment to Germination and Enhancement Growth of Sunflower Seeds. J. Phys. Conf. Ser. 2019, 1380, 012148. [Google Scholar] [CrossRef]
- Park, J.; Cha, M.S. Successive Multi-Microdischarges Occurring in Pin-to-Line Geometry of Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2023, 43, 1435–1452. [Google Scholar] [CrossRef]
- Rongsangchaicharean, T.; Srisonphan, S.; Onwimol, D. Responses of Rice Seed Quality to Large-Scale Atmospheric Nonthermal Plasmas. Plasma Chem. Plasma Process. 2022, 42, 1127–1141. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Starič, P.; Mozetič, M.; Vogel-Mikuš, K. Cold Plasma Affects Germination and Fungal Community Structure of Buckwheat Seeds. Plants 2021, 10, 851. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Cho, J.; Tsai, T.-C.; Staack, D.; Kang, M.-H.; Roh, J.-H.; Shin, D.-B.; Cromwell, W.; Gross, D. A Non-Thermal Plasma Seed Treatment Method for Management of a Seedborne Fungal Pathogen on Rice Seed. Crop Sci. 2014, 54, 796–803. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Investigation of Mechanisms Involved in Germination Enhancement of Wheat (Triticum aestivum) by Cold Plasma: Effects on Seed Surface Chemistry and Characteristics. Plasma Process. Polym. 2019, 16, 1800148. [Google Scholar] [CrossRef]
- Shorstkii, I.; Mounassar, E.H. Atmospheric Microplasma Treatment Based on Magnetically Controlled Fe–Al Dynamic Platform for Organic and Biomaterials Surface Modification. Coatings 2023, 13, 1362. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Dong, Y. Seed Priming with Cold Plasma Mitigated the Negative Influence of Drought Stress on Growth and Yield of Rapeseed (Brassica napus L.). Ind. Crops Prod. 2025, 228, 120899. [Google Scholar] [CrossRef]
- Dilip, D.; Modupalli, N.; Rahman, M.M.; Kariyat, R. Atmospheric Cold Plasma Alters Plant Traits and Negatively Affects the Growth and Development of Fall Armyworm in Rice. Sci. Rep. 2025, 15, 3680. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P.; et al. Technology Readiness and Overcoming Barriers to Sustainably Implement Nanotechnology-Enabled Plant Agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Saritha, G.N.G.; Anju, T.; Kumar, A. Nanotechnology—Big Impact: How Nanotechnology Is Changing the Future of Agriculture? J. Agric. Food Res. 2022, 10, 100457. [Google Scholar] [CrossRef]
- Zakharova, O.; Kolesnikov, E.; Shatrova, N.; Gusev, A. The Effects of CuO Nanoparticles on Wheat Seeds and Seedlings and Alternaria Solani Fungi: In Vitro Study. IOP Conf. Ser. Earth Environ. Sci. 2019, 226, 012036. [Google Scholar] [CrossRef]
- Gaber, S.E.; Hashem, A.H.; El-Sayyad, G.S.; Attia, M.S. Antifungal Activity of Myco-Synthesized Bimetallic ZnO-CuO Nanoparticles against Fungal Plant Pathogen Fusarium Oxysporum. Biomass Convers. Biorefinery 2023, 14, 25395–25409. [Google Scholar] [CrossRef]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal Activity of Copper Oxide Nanoparticles against Root Rot Disease in Cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Bhat, A.H.; Kavitha, K.; Ramanan, A.; Rajagopal, K.; Srinivasan, R.; Manikandan, E. Enhanced Biosynthesis Synthesis of Copper Oxide Nanoparticles (CuO-NPs) for Their Antifungal Activity Toxicity against Major Phyto-Pathogens of Apple Orchards. Pharm. Res. 2020, 37, 246. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Rajput, V.D.; Minkina, T.; Hayat, S.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. CuO Nanoparticle-Mediated Seed Priming Improves Physio-Biochemical and Enzymatic Activities of Brassica Juncea. Plants 2023, 12, 803. [Google Scholar] [CrossRef]
- Kadri, O.; Karmous, I.; Kharbech, O.; Arfaoui, H.; Chaoui, A. Cu and CuO Nanoparticles Affected the Germination and the Growth of Barley (Hordeum vulgare L.) Seedling. Bull. Environ. Contam. Toxicol. 2022, 108, 585–593. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Ali, G.A.M.; Hassanein, A.; Attia, A.M.; Marzouk, E.R. Toxicity and Uptake of CuO Nanoparticles: Evaluation of an Emerging Nanofertilizer on Wheat (Triticum aestivum L.) Plant. Sustainability 2022, 14, 4914. [Google Scholar] [CrossRef]
- Feigl, G. The Impact of Copper Oxide Nanoparticles on Plant Growth: A Comprehensive Review. J. Plant Interact. 2023, 18, 2243098. [Google Scholar] [CrossRef]
- Billah, M.; Karmakar, S.; Mina, F.B.; Haque, M.d.N.; Rashid, M.d.M.; Hasan, M.d.F.; Acharjee, U.K.; Talukder, M.R. Investigation of Mechanisms Involved in Seed Germination Enhancement, Enzymatic Activity and Seedling Growth of Rice (Oryza sativa L.) Using LPDBD (Ar+Air) Plasma. Arch. Biochem. Biophys. 2021, 698, 108726. [Google Scholar] [CrossRef] [PubMed]
- Tanakaran, Y.; Matra, K. The Influence of Atmospheric Non-Thermal Plasma on Jasmine Rice Seed Enhancements. J. Plant Growth Regul. 2022, 41, 178–187. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Pradhan, S.P.; Pandey, B.P.; Shrestha, B.; Fronczak, M.; Kierzkowska-Pawlak, H.; Subedi, D.P. Growth Enhancement of Radish Seed Induced by Low-Temperature Argon Plasma. Plasma Chem. Plasma Process. 2023, 43, 111–137. [Google Scholar] [CrossRef]
- Stetzler, J.; Tang, S.; Chinni, R.C. Plasma Temperature and Electron Density Determination Using Laser-Induced Breakdown Spectroscopy (LIBS) in Earth’s and Mars’s Atmospheres. Atoms 2020, 8, 50. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Zheng, Q.; Miao, J.; Alvarez, P.J.; Long, M. Quantification of Photocatalytically-Generated Hydrogen Peroxide in the Presence of Organic Electron Donors: Interference and Reliability Considerations. Chemosphere 2021, 279, 130556. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association. Detection of Trichoconiella padwickii in Oryza sativa (Rice) Seed (Method 7-012). In International Rules for Seed Testing 2024: Validated Seed Health Testing Methods; International Seed Testing Association: Wallisellen, Switzerland, 2024; pp. 7-012-3–7-012-4. [Google Scholar]
- International Seed Testing Association. International Rules for Seed Testing; International Seed Testing Association: Zurich, Switzerland, 2019; p. i-19-8. [Google Scholar] [CrossRef]
- Shahin, N.; Islam, M.Z.; Zohura, F.; Mahmud, M.d.N.; Rahman, M.; Biswas, B. Prediction and Standardization of Relative Emergence and Seed Vigour in Rice (Oryza sativa L.) Through Radicle Emergence Analysis. J. Agric. Food Environ. 2022, 3, 39–44. [Google Scholar] [CrossRef]
- Falahat, A.; Ganjovi, A.; Taraz, M.; Ravari, M.N.R.; Shahedi, A. Optical Characteristics of a RF DBD Plasma Jet in Various Ar/O2 mixtures. Pramana 2018, 90, 27. [Google Scholar] [CrossRef]
- Song, W.S.; Kang, J.E.; Hong, S.J. Spectroscopic Analysis of CF4/O2 Plasma Mixed with N2 for Si3N4 Dry Etching. Coatings 2022, 12, 1064. [Google Scholar] [CrossRef]
- Qusnudin, A.; Kusumandari, K.; Saraswati, T.E. OH Radical Intensity of Dielectric Barrier Discharge (DBD) Plasma Measured by Optical Emission Spectroscopy (OES). J. Phys. Conf. Ser. 2021, 1825, 012072. [Google Scholar] [CrossRef]
- Yao, S.; Weng, S.; Tang, Y.; Zhao, C.; Wu, Z.; Zhang, X.; Yamamoto, S.; Kodama, S. Characteristics of OH Production by O2/H2O Pulsed Dielectric Barrier Discharge. Vacuum 2016, 126, 16–23. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X.; Keidar, M. Perspective: The Physics, Diagnostics, and Applications of Atmospheric Pressure Low Temperature Plasma Sources Used in Plasma Medicine. J. Appl. Phys. 2017, 122, 020901. [Google Scholar] [CrossRef]
- Khan, T.M.; Khan, S.U.; Raffi, M.; Khan, R. Theoretical–Computational Study of Atmospheric DBD Plasma and Its Utility for Nanoscale Biocompatible Plasmonic Coating. Molecules 2021, 26, 5106. [Google Scholar] [CrossRef]
- Wong, K.S.; Chew, N.S.L.; Low, M.; Tan, M.K. Plasma-Activated Water: Physicochemical Properties, Generation Techniques, and Applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Zambon, Y.; Contaldo, N.; Laurita, R.; Várallyay, E.; Canel, A.; Gherardi, M.; Colombo, V.; Bertaccini, A. Plasma Activated Water Triggers Plant Defence Responses. Sci. Rep. 2020, 10, 19211. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Pascoa, J.; Trancossi, M. Heat Generation Mechanisms of DBD Plasma Actuators. Exp. Therm. Fluid Sci. 2018, 90, 55–65. [Google Scholar] [CrossRef]
- Boonyawan, D. Physics of Plasma; Chiang Mai University, Ed.; Science CMU Printing Service: Chiang Mai, Thailand, 2020; ISBN 974-672-036-8. [Google Scholar]
- Holc, M.; Mozetič, M.; Recek, N.; Primc, G.; Vesel, A.; Zaplotnik, R.; Gselman, P. Wettability Increase in Plasma-Treated Agricultural Seeds and Its Relation to Germination Improvement. Agronomy 2021, 11, 1467. [Google Scholar] [CrossRef]
- Hoppanová, L.; Medvecká, V.; Dylíková, J.; Hudecová, D.; Kaliňáková, B.; Kryštofová, S.; Zahoranová, A. Low-Temperature Plasma Applications in Chemical Fungicide Treatment Reduction. Acta Chim. Slovaca 2020, 13, 26–33. [Google Scholar] [CrossRef]
- Holc, M.; Gselman, P.; Primc, G.; Vesel, A.; Mozetič, M.; Recek, N. Wettability and Water Uptake Improvement in Plasma-Treated Alfalfa Seeds. Agriculture 2022, 12, 96. [Google Scholar] [CrossRef]
- Shelar, A.; Singh, A.V.; Dietrich, P.; Maharjan, R.S.; Thissen, A.; Didwal, P.N.; Shinde, M.; Laux, P.; Luch, A.; Mathe, V.; et al. Emerging Cold Plasma Treatment and Machine Learning Prospects for Seed Priming: A Step towards Sustainable Food Production. RSC Adv. 2022, 12, 10467–10488. [Google Scholar] [CrossRef]
- Samsatly, J.; Chamoun, R.; Gluck-Thaler, E.; Jabaji, S. Genes of the de Novo and Salvage Biosynthesis Pathways of Vitamin B6 Are Regulated under Oxidative Stress in the Plant Pathogen Rhizoctonia solani. Front. Microbiol. 2016, 6, 1429. [Google Scholar] [CrossRef]
- Zakaria, L. An Overview of Aspergillus Species Associated with Plant Diseases. Pathogens 2024, 13, 813. [Google Scholar] [CrossRef]
- Datta, S.; Sarkar, M.; Chowdhury, A.; Rakwal, R.; Agrawal, G.K.; Sarkar, A. A Comprehensive Insight into the Biology of Rhizoctonia solani AG1-IA Kühn, the Causal Organism of the Sheath Blight Disease of Rice. J. Plant Pathol. 2022, 104, 79–98. [Google Scholar] [CrossRef]
- Senapati, M.; Tiwari, A.; Sharma, N.; Chandra, P.; Bashyal, B.M.; Ellur, R.K.; Bhowmick, P.K.; Bollinedi, H.; Vinod, K.K.; Singh, A.K.; et al. Rhizoctonia solani Kühn Pathophysiology: Status and Prospects of Sheath Blight Disease Management in Rice. Front. Plant Sci. 2022, 13, 881116. [Google Scholar] [CrossRef] [PubMed]
- Copley, T.R.; Duggavathi, R.; Jabaji, S. The Transcriptional Landscape of Rhizoctonia solani AG1-IA during Infection of Soybean as Defined by RNA-Seq. PLoS ONE 2017, 12, e0184095. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Active Oxygen Species and Antioxidants in Seed Biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Ren, Y.; Zhang, L.; Xue, Y.; Zhang, L.; He, J. Phytotoxicity Assessment of Copper Oxide Nanoparticles on the Germination, Early Seedling Growth, and Physiological Responses in Oryza sativa L. Bull. Environ. Contam. Toxicol. 2020, 104, 770–777. [Google Scholar] [CrossRef]
- Shaw, A.K.; Hossain, Z. Impact of Nano-CuO Stress on Rice (Oryza sativa L.) Seedlings. Chemosphere 2013, 93, 906–915. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, Y.; Jiao, T.; Zhang, Y.; Chen, J.; Gao, Y. Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza sativa L.) Seedlings and the Relevant Physiological Responses. Int. J. Environ. Res. Public Health 2020, 17, 1260. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.; Afzal, I.; Khalid, E.; Razzaq, M.; Arif, M.A.R. Rice Seed Longevity in the Context of Seed Moisture Contents and Hypoxic Conditions in the Storage Environment. Seed Sci. Res. 2023, 33, 39–49. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, Z.; Yu, Y.; Yu, S.; He, H. OsCSD2 and OsCSD3 Enhance Seed Storability by Modulating Antioxidant Enzymes and Abscisic Acid in Rice. Plants 2024, 13, 310. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Recek, N.; Holc, M.; Vesel, A.; Zaplotnik, R.; Gselman, P.; Mozetič, M.; Primc, G. Germination of Phaseolus vulgaris L. Seeds after a Short Treatment with a Powerful RF Plasma. Int. J. Mol. Sci. 2021, 22, 6672. [Google Scholar] [CrossRef]
- Arif, N.; Yadav, V.; Singh, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K.; Giorgetti, L. Chapter 13—Interaction of Copper Oxide Nanoparticles with Plants: Uptake, Accumulation, and Toxicity. In Nanomaterials in Plants, Algae, and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 297–310. ISBN 978-0-12-811487-2. [Google Scholar]




| Treatment No. | (M) | (mL) | (mL) | (mL) | (mL) | (mg L−1) |
|---|---|---|---|---|---|---|
| 1 | 0.0005 | 0.50 | 0.167 | 0.33 | 20 | 1.36 |
| 2 | 0.0005 | 0.55 | 0.167 | 0.38 | 20 | 1.56 |
| 3 | 0.0005 | 0.50 | 0.167 | 0.33 | 20 | 1.36 |
| Mean ± SD | 0.35 ± 0.03 | 1.49 ± 0.12 |
| Concentration/Speed | 0.4 s/cm. | 0.6 s/cm. | 0.8 s/cm. |
|---|---|---|---|
| 0 M | NA | NA | NA |
| 0.02 M | 0.02 | 0.02 | 0.03 |
| 0.04 M | 0.05 | 0.03 | 0.03 |
| 0.06 M | 0.03 | 0.03 | 0.03 |
| 0.08 M | 0.02 | 0.02 | 0.02 |
| SOV | df | RE | G | GI | SL7 | RL7 | SL14 | RL14 |
|---|---|---|---|---|---|---|---|---|
| Rep | 2 | 47.36 * | 5.64 | 0.05 | 0.06 | 0.58 | 2.42 | 0.43 |
| Storage (A) | 3 | 1052.44 * | 354.32 * | 30.09 * | 28.87 * | 112.20 * | 33.00 * | 129.48 * |
| Whole-plot Error | 6 | 12.39 | 15.67 | 0.19 | 0.18 | 0.49 | 0.60 | 0.43 |
| Seed conveying rate through the plasma (B) | 3 | 33.45 * | 36.15 | 1.57 * | 1.53 * | 2.24 | 0.94 | 4.83 |
| A × B | 9 | 78.91 * | 25.18 | 0.74 * | 0.27 | 1.26 | 0.33 | 3.95 |
| Sub-plot Error | 24 | 10.60 | 13.17 | 0.24 | 0.31 | 1.20 | 1.20 | 2.96 |
| Concentration (C) | 4 | 134.87 * | 74.48 * | 3.67 * | 0.83 * | 1.72 | 5.35 * | 2.39 |
| C × A | 12 | 63.87 * | 18.95 | 0.36 | 0.62 * | 1.09 | 1.25 | 1.71 |
| C × B | 8 | 77.47 * | 9.96 | 0.12 | 0.48 | 0.58 | 1.43 | 2.60 |
| C × A × B | 24 | 59.23 * | 22.69 | 0.64 * | 0.56 * | 1.18 | 0.99 | 1.78 |
| Sub-sub-plot Error | 96 | 12.64 | 13.92 | 0.24 | 0.29 | 1.13 | 0.99 | 2.64 |
| SOV | df | SW | RW | TDW |
|---|---|---|---|---|
| Rep | 2 | 0.09 | 0.01 | 0.14 |
| Storage (A) | 3 | 5.93 * | 2.20 * | 8.86 * |
| Whole-plot Error | 6 | 0.08 | 0.07 | 0.18 |
| Seed conveying rate through the plasma (B) | 3 | 0.19 | 0.21 | 0.72 |
| A × B | 9 | 0.12 | 0.13 | 0.36 |
| Sub-plot Error | 24 | 0.19 | 0.11 | 0.31 |
| Concentration (C) | 4 | 0.44 | 0.31 * | 1.16 * |
| C × A | 12 | 0.15 | 0.21 | 0.52 |
| C × B | 8 | 0.29 | 0.14 | 0.62 |
| C × A × B | 24 | 0.28 | 0.15 | 0.44 |
| Sub-sub-plot Error | 96 | 0.20 | 0.11 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praditwanich, J.; Chimupala, Y.; Thapanapongworakul, P.; Sawangrat, C.; Boonyawan, D.; Sawadeemit, C.; Thanapornpoonpong, S.-n. Improving Rice Seed Quality Through the Combined Application of DBD Plasma and CuO NPs. Agriculture 2025, 15, 2280. https://doi.org/10.3390/agriculture15212280
Praditwanich J, Chimupala Y, Thapanapongworakul P, Sawangrat C, Boonyawan D, Sawadeemit C, Thanapornpoonpong S-n. Improving Rice Seed Quality Through the Combined Application of DBD Plasma and CuO NPs. Agriculture. 2025; 15(21):2280. https://doi.org/10.3390/agriculture15212280
Chicago/Turabian StylePraditwanich, Jira, Yothin Chimupala, Pilunthana Thapanapongworakul, Choncharoen Sawangrat, Dheerawan Boonyawan, Chommanad Sawadeemit, and Sa-nguansak Thanapornpoonpong. 2025. "Improving Rice Seed Quality Through the Combined Application of DBD Plasma and CuO NPs" Agriculture 15, no. 21: 2280. https://doi.org/10.3390/agriculture15212280
APA StylePraditwanich, J., Chimupala, Y., Thapanapongworakul, P., Sawangrat, C., Boonyawan, D., Sawadeemit, C., & Thanapornpoonpong, S.-n. (2025). Improving Rice Seed Quality Through the Combined Application of DBD Plasma and CuO NPs. Agriculture, 15(21), 2280. https://doi.org/10.3390/agriculture15212280






