Tannin-Based Strategies for Mitigating Greenhouse Gas Emissions Through Nitrogen and Carbon Metabolism in Ruminants
Abstract
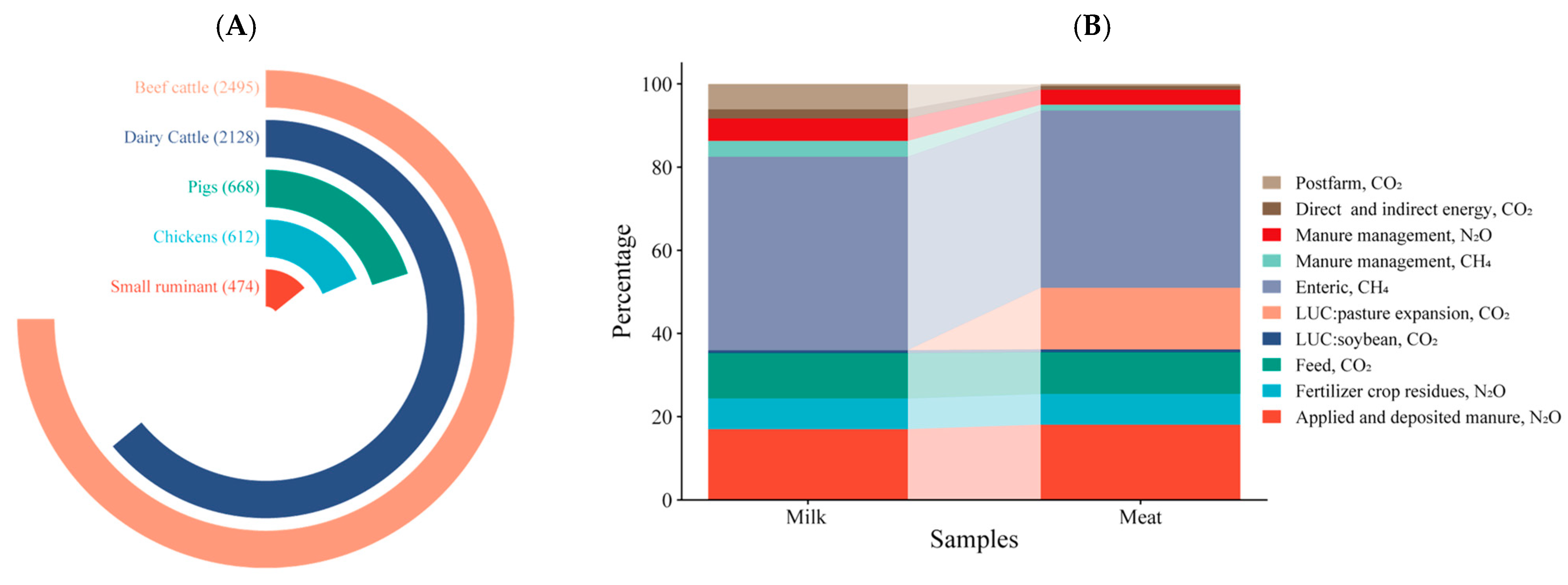
1. Introduction
2. Materials and Methods
3. Formation and Excretion of Greenhouse Gases in Ruminants
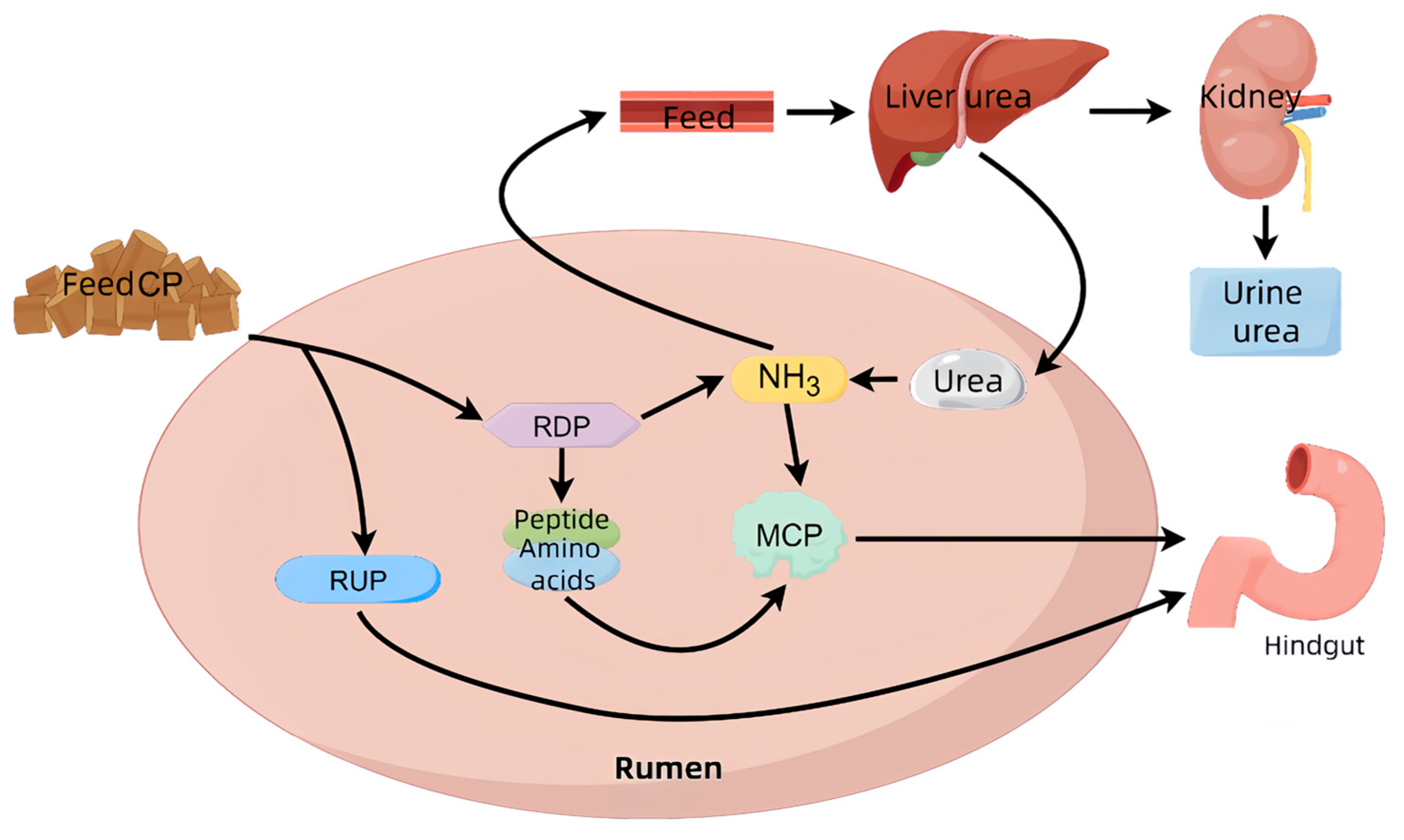
3.1. Nitrogen Metabolism and Nitrous Oxide Formation in Ruminants
3.1.1. Patterns of Nitrogen Metabolism in Ruminants
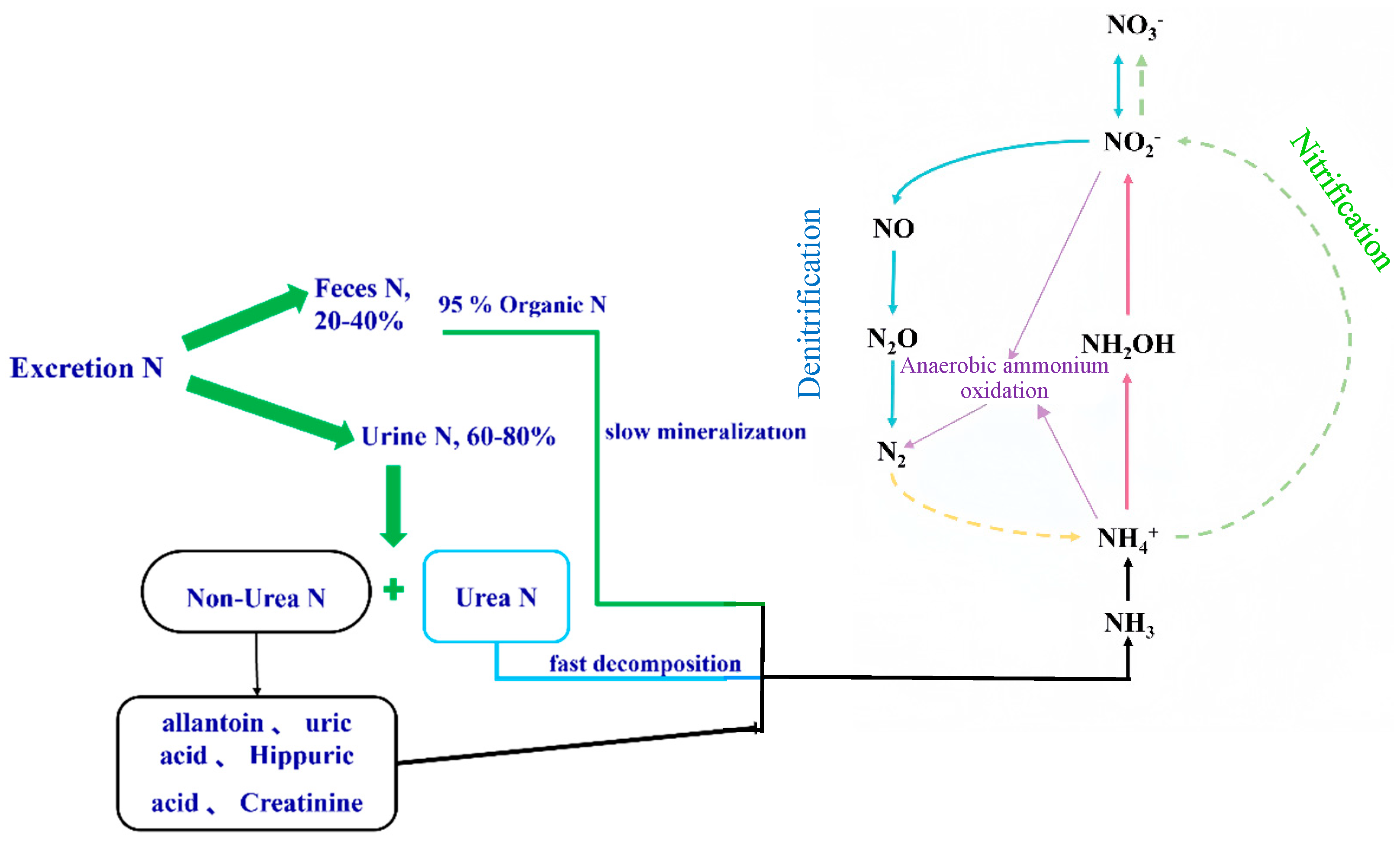
3.1.2. Nitrogen Excretion and Nitrous Oxide Production in Ruminants
Nitrification
Denitrification
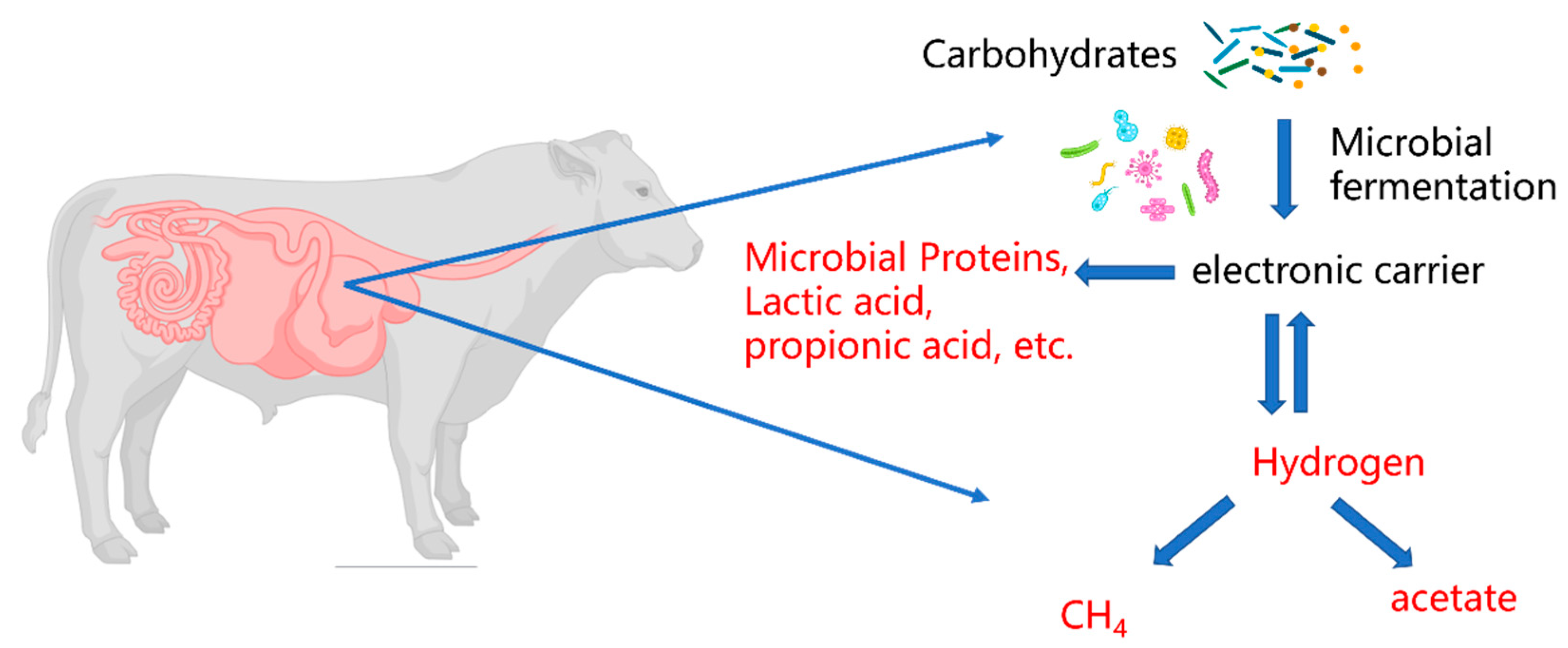
3.2. CH4 Formation in Ruminants
3.2.1. CH4 Formation in Rumen
3.2.2. CH4 Formation in Feces
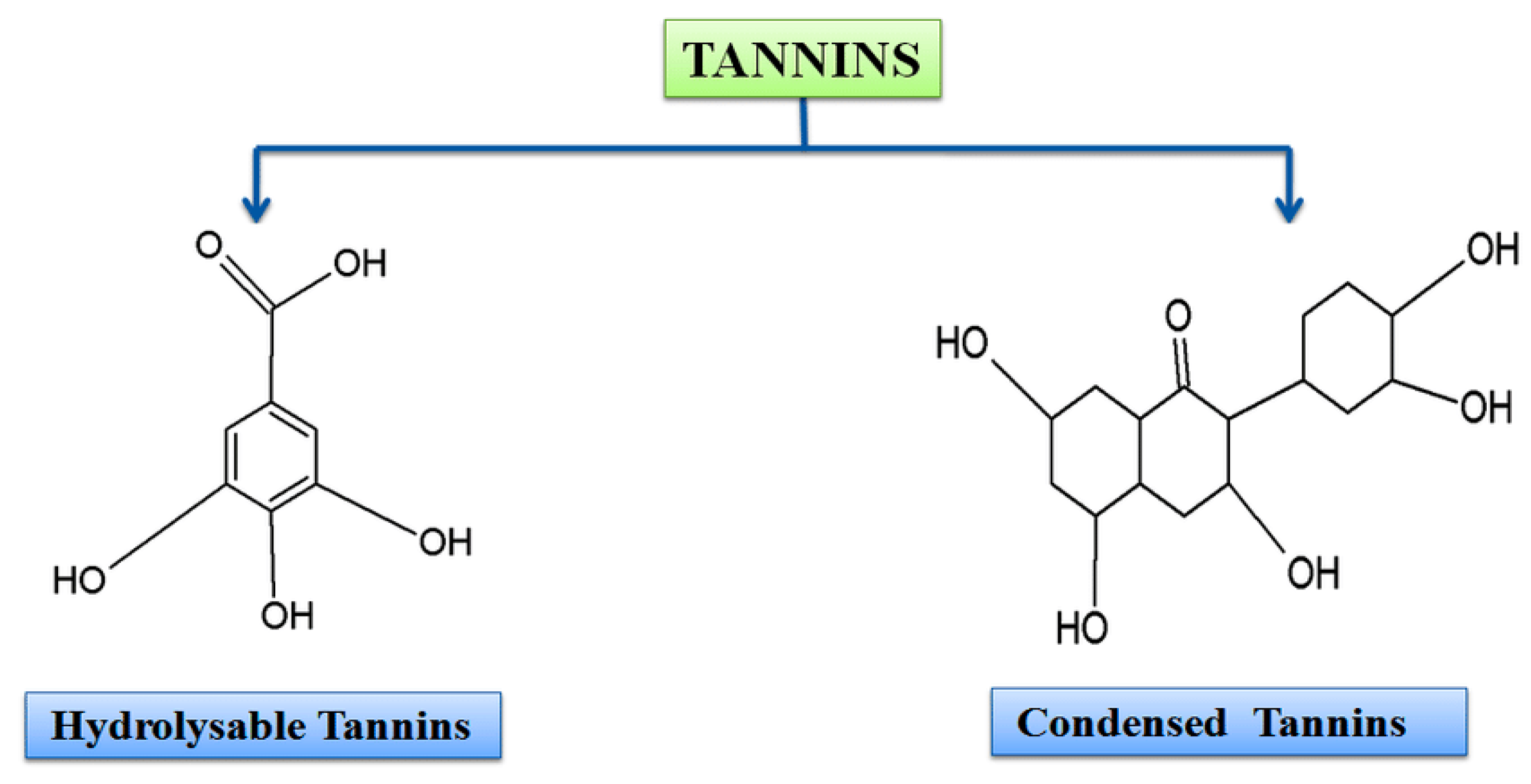
4. Sources and Classification of Tannins
4.1. Sources of Tannins
4.2. Types and Characteristics of Tannins
5. Impacts and Mechanisms of Tannins on Greenhouse Gas Mitigation in Ruminants
5.1. Influence of Tannins on Feed Intake and Digestive Efficiency in Ruminants
5.2. Regulation of Rumen GHG Production by Tannins
5.2.1. Tannins Alter the Type of Nitrogen Emission from Ruminants
5.2.2. Tannins Alter the CH4 Emission from Ruminants
| Types of Tannins | Sources | Experimental Animals | Additive Dosage | CH4 and N2O Production | Reference |
|---|---|---|---|---|---|
| Tannic acid | – | Castrated male cattle | 16.9 g/kg of DM | N2O production ↓ | [77] |
| CT | Acacia mearnsii | Sheep | 0.14%, 0.29% and 0.43% DM | N2O production ↑ | [78] |
| HT metabolite | – | Beef cattle | 15.2 g/kg | N2O production ↓ | [80] |
| HT metabolite | – | In vitro rumen fermentation | 5, 10, 15 and 20 mg | CH4 production ↓ | [86] |
| CT | L. pedunculatus | In vitro rumen fermentation | 0.5g DM | CH4 production ↓ | [87] |
| CT | Acacia mearnsii | Sheep | 41 g | CH4 production ↓ | [69] |
| CT | Acacia mearnsii | Dairy cattle | 163 and 244 g/d | CH4 production ↓ | [62] |
| Tannic acid | – | Beef cattle | 6.5, 13.0, or 26.0 g/kg of DM | CH4 production ↓ | [89] |
| CT | Chestnut | Beef cattle | 2% DM | Did not affect CH4 production | [90] |
6. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ripple, W.J.; Wolf, C.; Gregg, J.W.; Rockström, J.; Newsome, T.M.; Law, B.E.; Marques, L.; Lenton, T.M.; Xu, C.; Huq, S.; et al. The 2023 State of the Climate Report: Entering Uncharted Territory. BioScience 2023, 73, 841–850. [Google Scholar] [CrossRef]
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical Options for the Mitigation of Direct Methane and Nitrous Oxide Emissions from Livestock: A Review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Jantke, K.; Hartmann, M.J.; Rasche, L.; Blanz, B.; Schneider, U.A. Agricultural Greenhouse Gas Emissions: Knowledge and Positions of German Farmers. Land 2020, 9, 130. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Eshel, G.; Shepon, A.; Makov, T.; Milo, R. Land, Irrigation Water, Greenhouse Gas, and Reactive Nitrogen Burdens of Meat, Eggs, and Dairy Production in the United States. Proc. Natl. Acad. Sci. USA 2014, 111, 11996–12001. [Google Scholar] [CrossRef]
- Paul, S.S.; Chanu, Y.M.; Dey, A. Options for Improving Nitrogen Utilization Efficiency in Ruminants. Indian J. Anim. Nutr. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Tan, P.; Liu, H.; Zhao, J.; Gu, X.; Wei, X.; Zhang, X.; Ma, N.; Johnston, L.J.; Bai, Y.; Zhang, W. Amino Acids Metabolism by Rumen Microorganisms: Nutrition and Ecology Strategies to Reduce Nitrogen Emissions from the inside to the Outside. Sci. Total Environ. 2021, 800, 149596. [Google Scholar] [CrossRef]
- Ungerfeld, E.M.; Cancino-Padilla, N.; Vera-Aguilera, N. Fermentation in the Rumen. In Microbial Fermentations in Nature and as Designed Processes; Hurst, C.J., Ed.; Wiley: Hoboken, NJ, USA, 2023; pp. 133–165. ISBN 978-1-119-84997-1. [Google Scholar]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using Blood Urea Nitrogen to Predict Nitrogen Excretion and Efficiency of Nitrogen Utilization in Cattle, Sheep, Goats, Horses, Pigs, and Rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J. The Ruminant: Life History and Digestive Physiology of a Symbiotic Animal. In Sustainable and Environmentally Friendly Dairy Farms; SpringerBriefs in Applied Sciences and Technology; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–45. ISBN 978-3-030-46059-4. [Google Scholar]
- Klatt, B.J. Determining Rumen Degradable Protein Requirements in Growing Beef Cattle. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2019. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 2018. [Google Scholar]
- Tamminga, S. Protein Degradation in the Forestomachs of Ruminants. J. Anim. Sci. 1979, 49, 1615–1630. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Kristensen, N.B. Nitrogen Recycling through the Gut and the Nitrogen Economy of Ruminants: An Asynchronous Symbiosis. J. Anim. Sci. 2008, 86, E293–E305. [Google Scholar] [CrossRef]
- Pengpeng, W.; Tan, Z. Ammonia Assimilation in Rumen Bacteria: A Review. Anim. Biotechnol. 2013, 24, 107–128. [Google Scholar] [CrossRef]
- Reddy, P.R.K.; Hyder, I. Ruminant Digestion. In Textbook of Veterinary Physiology; Das, P.K., Sejian, V., Mukherjee, J., Banerjee, D., Eds.; Springer Nature: Singapore, 2023; pp. 353–366. ISBN 978-981-19-9410-4. [Google Scholar]
- Getahun, D.; Alemneh, T.; Akeberegn, D.; Getabalew, M.; Zewdie, D. Urea Metabolism and Recycling in Ruminants. Biomed. J. Sci. Tech. Res. 2019, 20, 14790–14796. [Google Scholar] [CrossRef]
- Li, Y.L.; Beauchemin, K.A.; McAllister, T.A.; Yang, W.Z. Intakes and Excretion Route of Nitrogen, Phosphorous and Sulfur by Finishing Beef Heifers Fed Increasing Levels of Wheat Dried Distillers Grains with Solubles to Substitute for Barley Grain and Barley Silage. Livest. Sci. 2014, 170, 43–52. [Google Scholar] [CrossRef]
- Harmeyer, J.; Martens, H. Aspects of Urea Metabolism in Ruminants with Reference to the Goat. J. Dairy Sci. 1980, 63, 1707–1728. [Google Scholar] [CrossRef]
- Dijkstra, J.; Oenema, O.; Van Groenigen, J.W.; Spek, J.W.; Van Vuuren, A.M.; Bannink, A. Diet Effects on Urine Composition of Cattle and N2O Emissions. Animal 2013, 7, 292–302. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia Oxidation Pathways and Nitrifier Denitrification Are Significant Sources of N2O and NO under Low Oxygen Availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Schils, R.L.M.; Eriksen, J.; Ledgard, S.F.; Vellinga, T.V.; Kuikman, P.J.; Luo, J.; Petersen, S.O.; Velthof, G.L. Strategies to Mitigate Nitrous Oxide Emissions from Herbivore Production Systems. Animal 2013, 7, 29–40. [Google Scholar] [CrossRef]
- Arp, D.J.; Stein, L.Y. Metabolism of Inorganic N Compounds by Ammonia-Oxidizing Bacteria. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 471–495. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, W.; Xu, Y.; Müller, C.; Rütting, T.; Yu, H.; Fan, J.; Zhang, J.; Zhu, T. Importance of Heterotrophic Nitrification and Dissimilatory Nitrate Reduction to Ammonium in a Cropland Soil: Evidences from a 15N Tracing Study to Literature Synthesis. Soil Biol. Biochem. 2015, 91, 65–75. [Google Scholar] [CrossRef]
- Philippot, L.; Čuhel, J.; Saby, N.P.A.; Chèneby, D.; Chroňáková, A.; Bru, D.; Arrouays, D.; Martin-Laurent, F.; Šimek, M. Mapping Field-scale Spatial Patterns of Size and Activity of the Denitrifier Community. Environ. Microbiol. 2009, 11, 1518–1526. [Google Scholar] [CrossRef]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef]
- Smith, M.S.; Zimmerman, K. Nitrous Oxide Production by Nondenitrifying Soil Nitrate Reducers. Soil Sci. Soc. Am. J. 1981, 45, 865–871. [Google Scholar] [CrossRef]
- Binnerup, S.J.; Jensen, K.; Revsbech, N.P.; Jensen, M.H.; Sørensen, J. Denitrification, Dissimilatory Reduction of Nitrate to Ammonium, and Nitrification in a Bioturbated Estuarine Sediment as Measured with 15N and Microsensor Techniques. Appl. Environ. Microbiol. 1992, 58, 303–313. [Google Scholar] [CrossRef]
- Van Cleemput, O.; Baert, L. Nitrite: A Key Compound in N Loss Processes under Acid Conditions? Plant Soil 1984, 76, 233–241. [Google Scholar] [CrossRef]
- Kappelmeyer, U.; Kuschk, P.; Stottmeister, U. Model Experiments on the Influence of Artificial Humic Compounds on Chemodenitrification. Water Air Soil Pollut. 2003, 147, 317–330. [Google Scholar] [CrossRef]
- Lan, W.; Yang, C. Ruminal Methane Production: Associated Microorganisms and the Potential of Applying Hydrogen-Utilizing Bacteria for Mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef]
- Hungate, R.E.; Smith, W.; Bauchop, T.; Yu, I.; Rabinowitz, J.C. Formate as an Intermediate in the Bovine Rumen Fermentation. J. Bacteriol. 1970, 102, 389–397. [Google Scholar] [CrossRef]
- Ferry, J.G. Methane: Small Molecule, Big Impact. Science 1997, 278, 1413–1414. [Google Scholar] [CrossRef]
- Janssen, P.H.; Kirs, M. Structure of the Archaeal Community of the Rumen. Appl. Environ. Microbiol. 2008, 74, 3619–3625. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen Microbial Community Composition Varies with Diet and Host, but a Core Microbiome Is Found across a Wide Geographical Range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whitman, W.B. Metabolic, Phylogenetic, and Ecological Diversity of the Methanogenic Archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic Archaea: Ecologically Relevant Differences in Energy Conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Ermler, U.; Grabarse, W.; Shima, S.; Goubeaud, M.; Thauer, R.K. Crystal Structure of Methyl-Coenzyme M Reductase: The Key Enzyme of Biological Methane Formation. Science 1997, 278, 1457–1462. [Google Scholar] [CrossRef]
- Ramin, M.; Chagas, J.C.; Pal, Y.; Danielsson, R.; Fant, P.; Krizsan, S.J. Reducing Methane Production from Stored Feces of Dairy Cows by Asparagopsis taxiformis. Front. Sustain. Food Syst. 2023, 7, 1187838. [Google Scholar] [CrossRef]
- Hindrichsen, I.K.; Wettstein, H.R.; Machmüller, A.; Jörg, B.; Kreuzer, M. Effect of the Carbohydrate Composition of Feed Concentratates on Methane Emission from Dairy Cows and Their Slurry. Environ. Monit. Assess. 2005, 107, 329–350. [Google Scholar] [CrossRef]
- Sutaryo, S.; Adiwinarti, R.; Ward, A.J.; Kurihara, M.; Purnomoadi, A. Effect of Different Feeding Management on the Respiratory Methane Emission and Feces-Derived Methane Yield of Goat. J. Adv. Vet. Anim. Res. 2019, 6, 431–437. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Z.P.; Liu, Y.F.; Guo, T.J.; Dong, H.M. Diversity and Abundance of the Rumen and Fecal Methanogens in Altay Sheep Native to Xinjiang and the Influence of Diversity on Methane Emissions. Arch. Microbiol. 2012, 194, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, J.M.; Correia, B.S.B.; Ceribeli, C.; Bruscadin, J.J.; Diniz, W.J.S.; Banerjee, P.; Vieira, D.d.S.; Cardoso, T.F.; Andrade, B.G.N.; Petrini, J.; et al. Ruminal and Feces Metabolites Associated with Feed Efficiency, Water Intake and Methane Emission in Nelore Bulls. Sci. Rep. 2023, 13, 18001. [Google Scholar] [CrossRef] [PubMed]
- Lileikis, T.; Nainienė, R.; Bliznikas, S.; Uchockis, V. Dietary Ruminant Enteric Methane Mitigation Strategies: Current Findings, Potential Risks and Applicability. Animals 2023, 13, 2586. [Google Scholar] [CrossRef]
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited; CUP Archive: Cambridge, UK, 1989. [Google Scholar]
- Reed, J.D. Nutritional Toxicology of Tannins and Related Polyphenols in Forage Legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Kluge, R.A.; Appezzato-da-Glória, B. The Accumulation of Tannins during the Development of ‘Giombo’ and ‘Fuyu’ Persimmon Fruits. Sci. Hortic. 2014, 172, 292–299. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Phenolic Compounds in Cereal Grains and Their Health Benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of Condensed Tannins: A Review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial Mechanisms to Overcome Inhibitory Effects of Dietary Tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef]
- Ghosh, D. Tannins from Foods to Combat Diseases. Int. J. Pharm. Res. Rev. 2015, 4, 40–44. [Google Scholar]
- Patra, A.K.; Saxena, J. Exploitation of Dietary Tannins to Improve Rumen Metabolism and Ruminant Nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Ajeet, S. Isolation, Characterization, and Therapeutic Applications of Natural Bioactive Compounds; IGI Global: Palmdale, PA, USA, 2022; ISBN 978-1-6684-7338-2. [Google Scholar]
- Singh, B.; Bhat, T.K.; Sharma, O.P. Biodegradation of Tannic Acid in an in Vitro Ruminal System. Livest. Prod. Sci. 2001, 68, 259–262. [Google Scholar] [CrossRef]
- Jones, W.T.; Mangan, J.L. Complexes of the Condensed Tannins of Sainfoin (Onobrychis viciifolia Scop.) with Fraction 1 Leaf Protein and with Submaxillary Mucoprotein, and Their Reversal by Polyethylene Glycol and pH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- Kumar, K.; Chaudhary, L.C.; Kumar, S. Exploitation of Tannins to Modulate Rumen Ecosystem and Ruminants Performance: A Review. Indian J. Anim. Sci 2014, 84, 609–618. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Cao, Z.; Wang, Y.; Yang, H.; Azarfar, A.; Li, S. Effect of Different Tannin Sources on Nutrient Intake, Digestibility, Performance, Nitrogen Utilization, and Blood Parameters in Dairy Cows. Animals 2019, 9, 507. [Google Scholar] [CrossRef]
- Aguiar, F.d.S.; Bezerra, L.R.; Cordão, M.A.; Cavalcante, I.T.R.; de Oliveira, J.P.F.; do Nascimento, R.R.; de Souza, B.B.; Oliveira, R.L.; Pereira, E.S.; Filho, J.M.P. Effects of Increasing Levels of Total Tannins on Intake, Digestibility, and Balance of Nitrogen, Water, and Energy in Hair Lambs. Animals 2023, 13, 2497. [Google Scholar] [CrossRef] [PubMed]
- Tseu, R.J.; Junior, F.P.; Carvalho, R.F.; Sene, G.A.; Tropaldi, C.B.; Peres, A.H.; Rodrigues, P.H.M. Effect of Tannins and Monensin on Feeding Behaviour, Feed Intake, Digestive Parameters and Microbial Efficiency of Nellore Cows. Ital. J. Anim. Sci. 2020, 19, 262–273. [Google Scholar] [CrossRef]
- Costa, E.I.d.S.; Ribiero, C.V.D.M.; Silva, T.M.; Ribeiro, R.D.X.; Vieira, J.F.; Lima, A.G.V.d.O.; Barbosa, A.M.; da Silva Júnior, J.M.; Bezerra, L.R.; Oliveira, R.L. Intake, Nutrient Digestibility, Nitrogen Balance, Serum Metabolites and Growth Performance of Lambs Supplemented with Acacia mearnsii Condensed Tannin Extract. Anim. Feed Sci. Technol. 2021, 272, 114744. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential Use of Acacia mearnsii Condensed Tannins to Reduce Methane Emissions and Nitrogen Excretion from Grazing Dairy Cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef]
- Piñeiro-Vázquez, A.T.; Jiménez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effects of Quebracho Tannin Extract on Intake, Digestibility, Rumen Fermentation, and Methane Production in Crossbred Heifers Fed Low-Quality Tropical Grass. Trop. Anim. Health Prod. 2018, 50, 29–36. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The Effect of Condensed Tannins on the Nutrition and Health of Ruminants Fed Fresh Temperate Forages: A Review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Frutos, P.; Raso, M.; Hervás, G.; Mantecón, Á.R.; Pérez, V.; Giráldez, F.J. Is There Any Detrimental Effect When a Chestnut Hydrolysable Tannin Extract Is Included in the Diet of Finishing Lambs? Anim. Res. 2004, 53, 127–136. [Google Scholar] [CrossRef]
- Puchala, R.; Min, B.R.; Goetsch, A.L.; Sahlu, T. The Effect of a Condensed Tannin-Containing Forage on Methane Emission by Goats. J. Anim. Sci. 2005, 83, 182–186. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the Conundrum of Tannins in Animal Nutrition and Health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Wischer, G.; Greiling, A.M.; Boguhn, J.; Steingass, H.; Schollenberger, M.; Hartung, K.; Rodehutscord, M. Effects of Long-Term Supplementation of Chestnut and Valonea Extracts on Methane Release, Digestibility and Nitrogen Excretion in Sheep. Animal 2014, 8, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii Tannins Decreases Methanogenesis and Urinary Nitrogen in Forage-Fed Sheep. Aust. J. Agric. Res. 2005, 56, 961–970. [Google Scholar] [CrossRef]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.-S.; Young, A.J.; Min, B.R. Effects of Supplementing Condensed Tannin Extract on Intake, Digestion, Ruminal Fermentation, and Milk Production of Lactating Dairy Cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; McAllister, T.A.; Chouinard, P.Y. Digestion, Ruminal Fermentation, Ciliate Protozoal Populations, and Milk Production from Dairy Cows Fed Cinnamaldehyde, Quebracho Condensed Tannin, or Yucca schidigera Saponin Extracts. J. Dairy Sci. 2008, 91, 4765–4777. [Google Scholar] [CrossRef] [PubMed]
- Baah, J.; Ivan, M.; Hristov, A.N.; Koenig, K.M.; Rode, L.M.; McAllister, T.A. Effects of Potential Dietary Antiprotozoal Supplements on Rumen Fermentation and Digestibility in Heifers. Anim. Feed Sci. Technol. 2007, 137, 126–137. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial Interactions with Tannins: Nutritional Consequences for Ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Al-Dobaib, S.N. Effect of Different Levels of Quebracho Tannin on Nitrogen Utilization and Growth Performance of Najdi Sheep Fed Alfalfa (Medicago sativa) Hay as a Sole Diet. Anim. Sci. J. 2009, 80, 532–541. [Google Scholar] [CrossRef]
- Getachew, G.; Pittroff, W.; Putnam, D.H.; Dandekar, A.; Goyal, S.; DePeters, E.J. The Influence of Addition of Gallic Acid, Tannic Acid, or Quebracho Tannins to Alfalfa Hay on in Vitro Rumen Fermentation and Microbial Protein Synthesis. Anim. Feed Sci. Technol. 2008, 140, 444–461. [Google Scholar] [CrossRef]
- Yang, K.; Wei, C.; Zhao, G.; Xu, Z.; Lin, S. Dietary Supplementation of Tannic Acid Modulates Nitrogen Excretion Pattern and Urinary Nitrogenous Constituents of Beef Cattle. Livest. Sci. 2016, 191, 148–152. [Google Scholar] [CrossRef]
- Zhou, K.; Bao, Y.; Zhao, G. Effects of Dietary Crude Protein and Tannic Acid on Nitrogen Excretion, Urinary Nitrogenous Composition and Urine Nitrous Oxide Emissions in Beef Cattle. Anim. Physiol. Nutr. 2019, 103, 1675–1683. [Google Scholar] [CrossRef]
- de Souza, M.N.; Bayer, C.; Lassalas, M.; Michelon, G.M.; Schaitz, L.H.; Biasiolo, R.; Civiero, M.; Ribeiro-Filho, H.M.N. Effects of Ground Corn and Acacia mearnsii Tannin Extract Supplementation on Nitrogen Excretion and Nitrous Oxide Emissions from Sheep. Livest. Sci. 2021, 246, 104458. [Google Scholar] [CrossRef]
- Eckard, R.J.; Grainger, C.; De Klein, C.A.M. Options for the Abatement of Methane and Nitrous Oxide from Ruminant Production: A Review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, K.; Zhao, G. Nitrous Oxide Emissions from the Urine of Beef Cattle as Regulated by Dietary Crude Protein and Gallic Acid. J. Anim. Sci. 2018, 96, 3699–3711. [Google Scholar] [CrossRef]
- Alves, T.P.; Dall-Orsoletta, A.C.; Ribeiro-Filho, H.M.N. The Effects of Supplementing Acacia mearnsii Tannin Extract on Dairy Cow Dry Matter Intake, Milk Production, and Methane Emission in a Tropical Pasture. Trop. Anim. Health Prod. 2017, 49, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, B.; Ban, Z.; Liang, H.; Li, L.; Zhao, W.; Yan, X. Evaluation of Origanum Oil, Hydrolysable Tannins and Tea Saponin in Mitigating Ruminant Methane: In Vitro and in Vivo Methods. J. Anim. Physiol. Anim. Nutr. 2021, 105, 630–638. [Google Scholar] [CrossRef]
- Bhatta, R.; Enishi, O.; Yabumoto, Y.; Nonaka, I.; Takusari, N.; Higuchi, K.; Tajima, K.; Takenaka, A.; Kurihara, M. Methane Reduction and Energy Partitioning in Goats Fed Two Concentrations of Tannin from Mimosa spp. J. Agric. Sci. 2013, 151, 119–128. [Google Scholar] [CrossRef]
- Pathak, A.K.; Dutta, N.; Pattanaik, A.K.; Chaturvedi, V.B.; Sharma, K. Effect of Condensed Tannins from Ficus Infectoria and Psidium Guajava Leaf Meal Mixture on Nutrient Metabolism, Methane Emission and Performance of Lambs. Asian-Australas. J. Anim. Sci. 2017, 30, 1702–1710. [Google Scholar] [CrossRef]
- Roth, S.; Steingass, H.; Drochner, W.; Pen, A. Wirkungen von Tanninextrakten Auf Die Parameter Der Pansenfermentation in Vitro. In Proceedings of the 10th Conference on Nutrition of Domestic Animals-Adolf Pen Zadravec-Erjavec Days, Radenci, Slovenia, 8–9 November 2001; pp. 64–70. [Google Scholar]
- Geerkens, C.H.; Schweiggert, R.M.; Steingass, H.; Boguhn, J.; Rodehutscord, M.; Carle, R. Influence of Apple and Citrus Pectins, Processed Mango Peels, a Phenolic Mango Peel Extract, and Gallic Acid as Potential Feed Supplements on in Vitro Total Gas Production and Rumen Methanogenesis. J. Agric. Food Chem. 2013, 61, 5727–5737. [Google Scholar] [CrossRef] [PubMed]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane Production from in Vitro Rumen Incubations with Lotus pedunculatus and Medicago sativa, and Effects of Extractable Condensed Tannin Fractions on Methanogenesis. Anim. Feed Sci. Technol. 2005, 123, 403–419. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.; Becker, K. Divergence between Purified Hydrolysable and Condensed Tannin Effects on Methane Emission, Rumen Fermentation and Microbial Population in Vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Yang, K.; Wei, C.; Zhao, G.Y.; Xu, Z.W.; Lin, S.X. Effects of Dietary Supplementing Tannic Acid in the Ration of Beef Cattle on Rumen Fermentation, Methane Emission, Microbial Flora and Nutrient Digestibility. J. Anim. Physiol. Anim. Nutr. 2017, 101, 302–310. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of Condensed Tannin Extract from Quebracho Trees to Reduce Methane Emissions from Cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Dietary Phytochemicals as Rumen Modifiers: A Review of the Effects on Microbial Populations. Antonie Van Leeuwenhoek 2009, 96, 363–375. [Google Scholar] [CrossRef] [PubMed]
| Types of Tannins | Sources | Experimental Animals | Additive Dosage | Digestive Efficiency | Reference |
|---|---|---|---|---|---|
| CT | Acacia mearnsii | Dairy cow | 163 and 326 g/kg DM | Dry matter intake ↓ | [62] |
| CT | Chestnut | Heifers | 4% | Dry matter intake, DM, OM and NDF digestibility ↓ | [63] |
| CT | – | Sheep | 55 g/kg DM | Feed intake and nutrient digestibility ↓ | [64] |
| HT | Chestnut | Lamb | 20.8 g/kg DM | = | [65] |
| CT | Lespedeza cuneata | Does | 17.7% | Dry matter intake ↑ | [66] |
| CT | Acacia mangium | Cows | 3% | Nutrient digestibility ↓ | [58] |
| CT | Mimosa tenuiflora hay | Lamb | 26.2 and 52.4 g/kg DM | Nutrient intake and digestibility ↑ | [59] |
| CT | Mimosa tenuiflora hay | Lamb | 78.6 g/kg DM | Nutrient intake and digestibility ↓ | [59] |
| CT | bark of A. mearnsii | Cow | 0.00, 0.75, 1.50, and 2.25% of DM | Nutrient digestibility ↓ | [60] |
| CT | Acacia mearnsii | Lamb | 20, 40, 60, and 80 g/kg DM | Nutrient digestibility ↓, Nutrient intake ↑ | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, S.; Zhang, Y. Tannin-Based Strategies for Mitigating Greenhouse Gas Emissions Through Nitrogen and Carbon Metabolism in Ruminants. Agriculture 2025, 15, 2234. https://doi.org/10.3390/agriculture15212234
Zhao X, Zhang S, Zhang Y. Tannin-Based Strategies for Mitigating Greenhouse Gas Emissions Through Nitrogen and Carbon Metabolism in Ruminants. Agriculture. 2025; 15(21):2234. https://doi.org/10.3390/agriculture15212234
Chicago/Turabian StyleZhao, Xiaoqiang, Shuo Zhang, and Yuanqing Zhang. 2025. "Tannin-Based Strategies for Mitigating Greenhouse Gas Emissions Through Nitrogen and Carbon Metabolism in Ruminants" Agriculture 15, no. 21: 2234. https://doi.org/10.3390/agriculture15212234
APA StyleZhao, X., Zhang, S., & Zhang, Y. (2025). Tannin-Based Strategies for Mitigating Greenhouse Gas Emissions Through Nitrogen and Carbon Metabolism in Ruminants. Agriculture, 15(21), 2234. https://doi.org/10.3390/agriculture15212234




