Preliminary Study of the Genetic Response of Grapevine Buds to a Preventive Natural Polysaccharide-Based Biogel Under Simulated Late Frost Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Product Characteristics, Experimental Treatments, and Cold Stress Protocol
2.3. RNA Extraction and Sequencing
2.4. RNA-Seq Analysis and Identification of Transcriptionally Altered Genes
2.5. Gene Ontology and Enrichment Analyses
2.6. Primer Design and qRT-PCR Analyses
3. Results
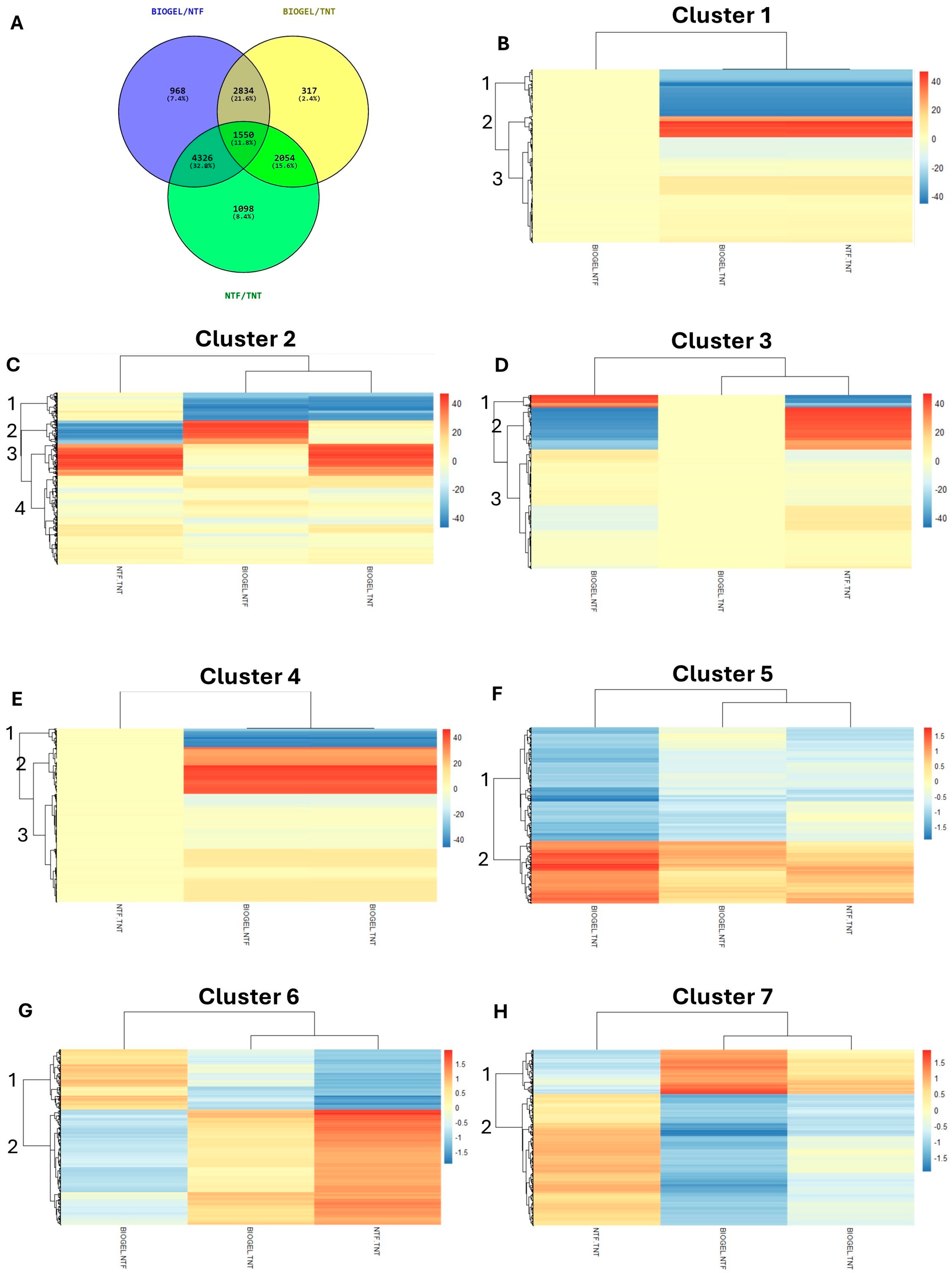
3.1. Preliminary RNA-Seq Analysis Allowed to Identify Candidate Genes Underlying the Protective Effect of Biogel
- Cluster 1_1 (939 loci, 45.7% of Cluster 1) included genes strongly repressed under cold stress in both treatments (log2FCs from −36.16 to −27.90);
- Cluster 1_2 (535 genes, 26.1%) comprised highly upregulated genes in response to cold (log2FCs from 28.90 to 34.50 in BIOGEL vs. TNT and from 28.89 to 34.07 in NTF vs. TNT);
- Cluster 1_3 (580 loci, 28.2%) included moderately regulated genes (log2FCs from −3 to 3.59 in BIOGEL vs. TNT and from −3.59 to 4.32 in NTF vs. TNT).
- Cluster 2_1 (325 genes, 20.9% of Cluster 2) comprises genes strongly repressed by biogel treatment, regardless of cold exposure (BIOGEL vs. NTF: log2FCs from −36.55 to −29.89; BIOGEL vs. TNT: log2FCs from −34.65 to −28.89), while showing weak modulation in NTF vs. TNT (log2FCs from −3.17 to 4.64);
- Cluster 2_2 (289 TAGs, 18.6%) showed a strong repression under cold stress in NTF samples (log2FCs from −34.96 to −28.83), but a much milder response in BIOGEL-treated samples (log2FCs from −3.92 to 5.26), resulting in significantly higher expression in BIOGEL vs. NTF (log2FCs from 27.58 to 37.51);
- Cluster 2_3 (493 loci, 31.81%) included genes induced by frost regardless of treatment (log2FCs: 28.90–37.91 in BIOGEL vs. TNT; 28.90–36.56 in NTF vs. TNT) and only slightly modulated in BIOGEL vs. NTF (log2FCs: −6.47 to 7.80);
- Cluster 2_4 includes 443 (28.58%) TAGs with moderate regulation in the three comparisons.
- Cluster 3_1: 387 (8.95% of Cluster 3) TAGs strongly induced in BIOGEL vs. NTF (log2FCs: 28.93–37.34), whereas strongly repressed in NTF vs. TNT (log2FCs from −37.92 to −28.86);
- Cluster 3_2: 1391 (32.15%) TAGs strongly repressed in BIOGEL vs. NTF (log2FCs from −37.76 to −28.90), while strongly activated in NTF vs. TNT (log2FCs: 28.90–37.76);
- Cluster 3_3: 2548 (58.90%) TAGs with slighter regulation in BIOGEL vs. NTF and NTF vs. TNT (log2FCs ranging, respectively, from −5.76 to 6.31 and from −5.65 to 5.13).
- Cluster 4_1, represented by 468 (16.51%) TAGs strongly repressed by the biogel application with log2FCs ranging from −36.93 to −28.90 in BIOGEL vs. NTF and from −36.45 to −28.90 in BIOGEL vs. TNT;
- Cluster 4_2 contained 1644 (58%) TAGs strongly induced by the biogel treatment with log2FCs from 27.57 and 36.64 in both comparisons;
- Cluster 4_3, including 722 (25.48%) TAGs showing lower regulation than the other two subgroups, with log2FCs from −4.17 to 7.54 in BIOGEL vs. NTF and from −3.69 to 6.96 in NTF vs. TNT.
3.2. Gene Ontology (GO) Enrichment Analysis
3.3. qRT-PCR Validation of Candidate Genes Underlying the Protective Effects of Biogel
4. Discussion
4.1. Biogel Apparently Reduces the Transcriptional Disruption Caused by Severe Cold Stress
4.2. Biogel Seems to Be Mainly Involved in Modulating Membrane-Related Gene Expression, Preventing Dehydration Under Severe Cold Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate Change and Global Wine Quality. Clim. Change 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; De Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Vautard, R.; van Oldenborgh, G.J.; Bonnet, R.; Li, S.; Robin, Y.; Kew, S.; Philip, S.; Soubeyroux, J.-M.; Dubuisson, B.; Viovy, N. Human Influence on Growing-Period Frosts like the Early April 2021 in Central France. Nat. Hazards Earth Syst. Sci. Discuss. 2022, 2022, 1045–1058. [Google Scholar] [CrossRef]
- Poni, S.; Sabbatini, P.; Palliotti, A. Facing Spring Frost Damage in Grapevine: Recent Developments and the Role of Delayed Winter Pruning—A Review. Am. J. Enol. Vitic. 2022, 73, 210–225. [Google Scholar] [CrossRef]
- Del Zozzo, F.; Canavera, G.; Pagani, S.; Gatti, M.; Poni, S.; Frioni, T. Post-Spring Frost Canopy Recovery, Vine Balance, and Fruit Composition in Cv. Barbera Grapevines. Aust. J. Grape Wine Res. 2022, 2022, 6596021. [Google Scholar] [CrossRef]
- Liles, C.; Verdon-Kidd, D.C. Assessment of Spatial and Temporal Trends Influencing the Occurrence of Frost After Budburst. Aust. J. Grape Wine Res. 2025, 2025, 5507651. [Google Scholar] [CrossRef]
- Leolini, L.; Moriondo, M.; Fila, G.; Costafreda-Aumedes, S.; Ferrise, R.; Bindi, M. Late Spring Frost Impacts on Future Grapevine Distribution in Europe. F. Crop. Res. 2018, 222, 197–208. [Google Scholar] [CrossRef]
- Peterson, A.G.; Abatzoglou, J.T. Observed Changes in False Springs over the Contiguous United States. Geophys. Res. Lett. 2014, 41, 2156–2162. [Google Scholar] [CrossRef]
- Schultze, S.R.; Sabbatini, P.; Luo, L. Interannual Effects of Early Season Growing Degree Day Accumulation and Frost in the Cool Climate Viticulture of Michigan. Ann. Am. Assoc. Geogr. 2016, 106, 975–989. [Google Scholar] [CrossRef]
- Evans, K.J.; Bricher, P.K.; Foster, S.D. Impact of Frost Injury Incidence at Nodes of Pinot Noir on Fruitfulness and Growth-stage Lag. Aust. J. Grape Wine Res. 2019, 25, 201–211. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Wilson, R.J.; Maclean, I.M.D. Climate Change and Crop Exposure to Adverse Weather: Changes to Frost Risk and Grapevine Flowering Conditions. PLoS ONE 2015, 10, e0141218. [Google Scholar] [CrossRef]
- Poling, E.B. Spring Cold Injury to Winegrapes and Protection Strategies and Methods. HortScience 2008, 43, 1652–1662. [Google Scholar] [CrossRef]
- Striegler, R.K. Passive Freeze Prevention Methods. In Proceedings of the Understanding and Preventing Freeze Damage in the Vineyards Workshop Proceedings, Missouri, CO, USA, 5–6 December 2007; pp. 39–46. [Google Scholar]
- Gutiérrez-Gamboa, G.; Mucalo, A. Adaptive Viticulture Strategies to Enhance Resilience and Grape Quality in Cold Climate Regions in Response to Climate Warming. Horticulturae 2025, 11, 394. [Google Scholar] [CrossRef]
- Snyder, R.L.; de Melo-Abreu, J.P. Frost Protection: Fundamentals, Practice and Economics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; Volume 1, pp. 1–240. [Google Scholar]
- Cervilieri, V.; Dami, I.; Signorini, G. Growers’ Perceptions and Strategies to Mitigate Spring Frost Injury in Grapevines. J. Ext. 2024, 62, 7. [Google Scholar] [CrossRef]
- Centinari, M.; Smith, M.S.; Londo, J.P. Assessment of Freeze Injury of Grapevine Green Tissues in Response to Cultivars and a Cryoprotectant Product. HortScience 2016, 51, 856–860. [Google Scholar] [CrossRef]
- Fuller, M.P.; Hamed, F.; Wisniewski, M.; Glenn, D.M. Protection of Plants from Frost Using Hydrophobic Particle Film and Acrylic Polymer. Ann. Appl. Biol. 2003, 143, 93–98. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Chapman, P.; Collier-Christian, L. Endogenous Levels and Exogenous Application of Glycinebetaine to Grapevines. Sci. Hortic. 2006, 111, 7–16. [Google Scholar] [CrossRef]
- Shu, L.; Wang, Z.; Zhang, X.-F.; Yao, J. Highly Conductive and Anti-Freezing Cellulose Hydrogel for Flexible Sensors. Int. J. Biol. Macromol. 2023, 230, 123425. [Google Scholar] [CrossRef]
- Antunes, D.R.; Forini, M.M.L.H.; Biscalchim, É.R.; Lima, P.H.C.; Cavalcante, L.A.F.; Teixeira Filho, M.C.M.; Tripathi, D.K.; Caballero, J.P.; Grillo, R. Polysaccharide-Based Sustainable Hydrogel Spheres for Controlled Release of Agricultural Inputs. Int. J. Biol. Macromol. 2024, 279, 135202. [Google Scholar] [CrossRef]
- Berradi, A.; Aziz, F.; Achaby, M.E.; Ouazzani, N.; Mandi, L. A Comprehensive Review of Polysaccharide-Based Hydrogels as Promising Biomaterials. Polymers 2023, 15, 2908. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Kennedy, J.F.; Rabiei, A.; Riseh, R.S.; Taheri, A. Nature’s Coatings: Sodium Alginate as a Novel Coating in Safeguarding Plants from Frost Damages. Int. J. Biol. Macromol. 2024, 267, 131203. [Google Scholar] [CrossRef]
- Pathak, V.; Ambrose, R.P.K. Starch-based Biodegradable Hydrogel as Seed Coating for Corn to Improve Early Growth under Water Shortage. J. Appl. Polym. Sci. 2020, 137, 48523. [Google Scholar] [CrossRef]
- Srivastava, N.; Choudhury, A.R. Stimuli-Responsive Polysaccharide-Based Smart Hydrogels and Their Emerging Applications. Ind. Eng. Chem. Res. 2022, 62, 841–866. [Google Scholar] [CrossRef]
- Llompart, B.; Dalmau, E.; Umaña, M.; Femenia, A. Physicochemical Characterization and Antioxidant Properties of Cellulose-Rich Extracts Obtained from Carob (Ceratonia siliqua L.) Pulp for Preparation of Cellulose-Rich Gels. Gels 2025, 11, 145. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological Growth Stages of the Grapevine (Vitis vinifera L. ssp. vinifera)—Codes and Descriptions According to the Extended BBCH Scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Velt, A.; Frommer, B.; Blanc, S.; Holtgräwe, D.; Duchêne, É.; Dumas, V.; Grimplet, J.; Hugueney, P.; Kim, C.; Lahaye, M.; et al. An Improved Reference of the Grapevine Genome Reasserts the Origin of the PN40024 Highly Homozygous Genotype. G3 Genes|Genomes|Genet. 2023, 13, jkad067. [Google Scholar] [CrossRef] [PubMed]
- Alexa, A.; Rahnenfuhrer, J. TopGO: Enrichment Analysis for Gene Ontology, R Package version 2.59.0; Bioconductor: Seattle, WA, USA, 2024. [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics (Version 3.5.1). 2024. Available online: https://ggplot2.tidyverse.org/reference/ggplot2-package.html (accessed on 14 November 2024).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zombardo, A.; Crosatti, C.; Bagnaresi, P.; Bassolino, L.; Reshef, N.; Puccioni, S.; Faccioli, P.; Tafuri, A.; Delledonne, M.; Fait, A. Transcriptomic and Biochemical Investigations Support the Role of Rootstock-Scion Interaction in Grapevine Berry Quality. BMC Genom. 2020, 21, 468. [Google Scholar] [CrossRef]
- Ko, B.; Van Raamsdonk, J.M. RNA Sequencing of Pooled Samples Effectively Identifies Differentially Expressed Genes. Biology 2023, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Nakano, S.; Tamoi, M.; Sakuda, S.; Fukamizo, T. Chitinase Gene Expression in Response to Environmental Stresses in Arabidopsis Thaliana: Chitinase Inhibitor Allosamidin Enhances Stress Tolerance. Biosci. Biotechnol. Biochem. 2009, 73, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Bilkova, A.; Jackson, P.; Dabravolski, S.; Riber, W.; Didi, V.; Houser, J.; Gigli-Bisceglia, N.; Wimmerova, M.; Budínská, E.; et al. Dirigent Proteins in Plants: Modulating Cell Wall Metabolism during Abiotic and Biotic Stress Exposure. J. Exp. Bot. 2017, 68, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Bosman, R.N.; Lashbrooke, J.G. Grapevine Mono- and Sesquiterpenes: Genetics, Metabolism, and Ecophysiology. Front. Plant Sci. 2023, 14, 1111392. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Gong, J.; Zhang, Z.; Wang, S.; Sun, J.; Li, Q.; Gu, X.; Jiang, J.; Qi, S. The Arabidopsis Thaliana Trehalose-6-Phosphate Phosphatase Gene AtTPPI Improve Chilling Tolerance through Accumulating Soluble Sugar and JA. Environ. Exp. Bot. 2023, 205, 105117. [Google Scholar] [CrossRef]
- Sasaki, K.; Ohara, K.; Yazaki, K. Gene Expression and Characterization of Isoprene Synthase from Populus Alba. FEBS Lett. 2005, 579, 2514–2518. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Z.; Zhang, L.; Fang, L.; Zhang, J.; Wang, Q.; Li, S.; Liang, Z.; Xin, H. Overexpression of Ethylene Response Factors VaERF080 and VaERF087 from Vitis Amurensis Enhances Cold Tolerance in Arabidopsis. Sci. Hortic. 2019, 243, 320–326. [Google Scholar] [CrossRef]
- de Oliveira, L.F.V.; Christoff, A.P.; de Lima, J.C.; de Ross, B.C.F.; Sachetto-Martins, G.; Margis-Pinheiro, M.; Margis, R. The Wall-Associated Kinase Gene Family in Rice Genomes. Plant Sci. 2014, 229, 181–192. [Google Scholar] [CrossRef]
- Slawinski, L.; Israel, A.; Paillot, C.; Thibault, F.; Cordaux, R.; Atanassova, R.; Dédaldéchamp, F.; Laloi, M. Early Response to Dehydration Six-Like Transporter Family: Early Origin in Streptophytes and Evolution in Land Plants. Front. Plant Sci. 2021, 12, 681929. [Google Scholar] [CrossRef]
- Peng, X.; Li, M.; Wu, H.; Chen, H.; Zhang, Z. Co-Regulation Role of Endogenous Hormones and Transcriptomics Profiling Under Cold Stress in Tetrastigma Hemsleyanum. J. Plant Growth Regul. 2021, 40, 1992–2006. [Google Scholar] [CrossRef]
- Li, F.; Liu, B.; Zhang, H.; Zhang, J.; Cai, J.; Cui, J. Integrative Multi-Omics Analysis of Chilling Stress in Pumpkin (Cucurbita moschata). BMC Genom. 2024, 25, 1042. [Google Scholar] [CrossRef]
- Baek, K.; Seo, P.J.; Park, C.M. Activation of a Mitochondrial ATPase Gene Induces Abnormal Seed Development in Arabidopsis. Mol. Cells 2011, 31, 361–369. [Google Scholar] [CrossRef]
- Londo, J.P.; Kovaleski, A.P.; Lillis, J.A. Divergence in the Transcriptional Landscape between Low Temperature and Freeze Shock in Cultivated Grapevine (Vitis vinifera). Hortic. Res. 2018, 5, 10. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Wong, D.C.J.; Wang, Y.; Zhu, Z.; Xu, G.; Wang, Q.; Li, S.; Liang, Z.; Xin, H. The Ethylene Response Factor VaERF092 from Amur Grape Regulates the Transcription Factor VaWRKY33, Improving Cold Tolerance. Plant J. 2019, 99, 988–1002. [Google Scholar] [CrossRef] [PubMed]
- Lissarre, M.; Ohta, M.; Sato, A.; Miura, K. Cold-Responsive Gene Regulation during Cold Acclimation in Plants. Plant Signal. Behav. 2010, 5, 948–952. [Google Scholar] [CrossRef]
- Fennell, A. Freezing Tolerance and Injury in Grapevines. J. Crop Improv. 2004, 10, 201–235. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, S.; García-Caparros, P.; Wang, L.; Liang, Z. Grapevine Adaptation to Cold and Heat Stress. J. Exp. Bot. 2025, 5, 40. [Google Scholar] [CrossRef]
- Ren, C.; Fan, P.; Li, S.; Liang, Z. Advances in Understanding Cold Tolerance in Grapevine. Plant Physiol. 2023, 192, 1733–1746. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Poverenov, E. Natural Biopolymer-based Hydrogels for Use in Food and Agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Gamage, A.; Liyanapathiranage, A.; Manamperi, A.; Gunathilake, C.; Mani, S.; Merah, O.; Madhujith, T. Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability 2022, 14, 6085. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Ding, P.; Johnson, K.; Li, X.; Zhang, Y. The Ankyrin-Repeat Transmembrane Protein BDA1 Functions Downstream of the Receptor-like Protein SNC2 to Regulate Plant Immunity. Plant Physiol. 2012, 159, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, M.; Zhu, Z.; Li, S.; Xu, Y.; Zhang, C.; Singer, S.D.; Wang, Y. Identification of the Dehydrin Gene Family from Grapevine Species and Analysis of Their Responsiveness to Various Forms of Abiotic and Biotic Stress. BMC Plant Biol. 2012, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.J.; Wood, A.J.; Lightfoot, D.A. Plant Receptor-like Serine Threonine Kinases: Roles in Signaling and Plant Defense. Mol. Plant-Microbe Interact. 2008, 21, 507–517. [Google Scholar] [CrossRef]
- Ye, Y.; Ding, Y.; Jiang, Q.; Wang, F.; Sun, J.; Zhu, C. The Role of Receptor-like Protein Kinases (RLKs) in Abiotic Stress Response in Plants. Plant Cell Rep. 2017, 36, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Roppolo, D.; De Rybel, B.; Tendon, V.D.; Pfister, A.; Alassimone, J.; Vermeer, J.E.M.; Yamazaki, M.; Stierhof, Y.-D.; Beeckman, T.; Geldner, N. A Novel Protein Family Mediates Casparian Strip Formation in the Endodermis. Nature 2011, 473, 380–383. [Google Scholar] [CrossRef]
- Yang, J.; Ding, C.; Xu, B.; Chen, C.; Narsai, R.; Whelan, J.; Hu, Z.; Zhang, M. A Casparian Strip Domain-like Gene, CASPL, Negatively Alters Growth and Cold Tolerance. Sci. Rep. 2015, 5, 14299. [Google Scholar] [CrossRef]
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| AtChi | AGCTTATTGGTGTTGCCAGTAGT | TGCCCTTAACACTGGCCTATT |
| IspS | CACACATGCATGGCTCAGGAAGA | CAGCCAGCGGCTTGGAGCTA |
| DIR-6 | ATGCGGTAAGTGGCATCCGC | GCCTTTGCTCGTGGTCTTGCT |
| ERF80 | CGGAGAGGATCGAGGGTATG | CAGCTTCAAGGGGGAAATTG |
| WAK | ACGCAACAAAGGAAAACTCAAGCCA | GGGTGAACATGAGGGAGACATTGGG |
| TPP | TGATCTGCTGTTCTTCAGGATTCA | ATCGGGTACCCCTCTCCTCG |
| ICE4 | GCTCCTTGAAGATGCCCATT | TGAAAGAGCTCCTAGAGAAAATCAA |
| COR78 | GAAGGTGGCAGAAGCAGGAA | CTTTCCGAACCAGTGCCTTG |
| WRKY33 | AGCCCCAACTTCAGTCACCA | AGGATCCAGCGGGAAACTGT |
| ERD6 | TGAGCCGGGAGTCCTCATGC | GACTGGGCCAAACACGCTGC |
| 4CL | TCATTGGAGGTTTACCCGATCGTT | GGAGTGGGTTTTTAAATAACTGGGC |
| AAA | CCACTCTGACTTTTTGCGCCC | TCAGAAATCGGCAGCGGAAGC |
| Cluster | Comparisons | Subcluster | n. of TAGs | Modulation |
|---|---|---|---|---|
| 1 | BIOGEL vs. TNT NTF vs. TNT | 1_1 | 939 (45.7%) | Strongly induced in both comparisons |
| 1_2 | 535 (26.1%) | Strongly repressed in both comparisons | ||
| 1_3 | 580 (28.2%) | Weakly regulated in both comparisons | ||
| 2 | BIOGEL vs. NTF BIOGEL vs. TNT NTF vs. TNT | 2_1 | 325 (20.9%) | Strongly repressed in BIOGEL vs. NTF and BIOGEL vs. TNT; weakly modulated in NTF vs. TNT |
| 2_2 | 289 (18.6%) | Strongly induced in BIOGEL vs. NTF; strongly repressed in NTF vs. TNT; weakly modulated in BIOGEL vs. TNT | ||
| 2_3 | 493 (31.81%) | Strongly induced in BIOGEL vs. TNT and BIOGEL vs. TNT; weakly modulated in BIOGEL vs. NTF | ||
| 2_4 | 443 (28.58%) | Weakly modulated in the three comparisons | ||
| 3 | BIOGEL vs. NTF NTF vs. TNT | 3_1 | 387 (8.95%) | Strongly induced in BIOGEL vs. NTF; strongly repressed in NTF vs. TNT |
| 3_2 | 1391 (32.15%) | Strongly repressed in BIOGEL vs. NTF; strongly induced in NTF vs. TNT | ||
| 3_3 | 2548 (58.90%) | Weakly modulated in both comparisons | ||
| 4 | BIOGEL vs. NTF BIOGEL vs. TNT | 4_1 | 468 (16.51%) | Strongly repressed in both comparisons |
| 4_2 | 1644 (58%) | Strongly induced in both comparisons | ||
| 4_3 | 722 (25.48%) | Weakly modulated in both comparisons |
| Gene/Protein | Gene ID (Vitis vinifera) | Function/Role | Stress Conditions | Mechanism/ Molecular Targets | Supporting References |
|---|---|---|---|---|---|
| AtChi Chitinase | Vitvi16g01978 | Defense protein involved in stress responses | Environmental stresses | Targeting of glycolipids; possible role in signaling | [35] |
| DIR6 Dirigent protein 6 | Vitvi18g00895 | Hormonal regulation and membrane integrity under stress | Various stresses | Membrane integrity maintenance | [36] |
| TPP Trehalose-6-phosphate phosphatase | Vitvi15g00992 | Stabilizes membranes and promotes isoprene accumulation | Abiotic stress | Membrane stabilization; involved in terpene metabolism | [37,38] |
| IspS Isoprene Synthase | Vitvi12g00576 | Isoprene biosynthesis pathway | Cold stress | Downregulation of isoprene production | [39] |
| ERF80 Ethylene-Responsive Factor 80 | Vitvi18g00895 | Transcription factor regulating hormone signaling | Cold stress | Synthesis of ethylene | [40] |
| WAKs Wall-Associated Kinases | Vitvi10g00955 | Hormone signaling, development, and stress responses | Biotic and abiotic stress, including cold | Links cell wall to cytoplasm | [41] |
| ERD6 Early Response to Dehydration Six-Like Transporter | Vitvi10g02222 | Sugar (hexoses) transporter across the tonoplast | Drought, cold stress | Upregulated under stress | [42] |
| 4CL 4-coumarate-CoA ligase | Vitvi14g01588 | Activation of phenylpropanoid pathway | Cold stress (chilling) | Flavonoids, lignin, coumarin production and accumulation | [43,44] |
| AAA AAA-type ATPase | Vitvi14g03042 | Core cellular functions (proteolysis, secretion, mitochondrial activity, etc.) | Cold, drought, salt stress | Upregulated under stress | [45] |
| ICE4 Inducer of CBF expression 4 | Vitvi07g02613 | Transcription factor regulating cold stress genes | Cold stress (freezing) | Hormone-mediated signaling, sugar accumulation | [46] |
| COR78 Cold-regulated gene 78 | Vitvi16g01022 | Signaling pathway (ICECBFCOR cascade) | Cold stress (freezing) | Stabilization of cell membranes | [46] |
| WRKY33 WRKY DNA-binding protein 33 | Vitvi15g01003 | Transcription factor regulating hormone signaling | Cold stress | Synthesis of ethylene, calcium and receptor kinase signaling, co-expressed with ERF family genes | [47] |
| Cluster | Gene | RNA-Seq | qRT-PCR | ||||
|---|---|---|---|---|---|---|---|
| BIOGEL/NTF | BIOGEL/TNT | NTF/TNT | BIOGEL/NTF | BIOGEL/TNT | NTF/TNT | ||
| 2_1 | AtChi | −33.7144 | −29.8986 | 3.8158 | −8.40 | −2.82 | 3.07 |
| 2_1 | DIR6 | −31.8976 | −29.8986 | 1.9990 | −5.64 | −3.47 | 1.63 |
| 2_1 | TPP | −31.4827 | −29.8984 | 1.5843 | −5.48 | −2.20 | 2.50 |
| 2_1 | IspS | −31.8978 | −29.8984 | 1.9994 | −3.95 | −5.56 | −1.41 |
| 2_1 | ERF80 | −31.8976 | −29.8986 | 1.9990 | −3.24 | −5.78 | −1.79 |
| 2_1 | WAK | −31.4828 | −29.8983 | 1.5846 | −2.56 | −3.18 | −1.24 |
| 3_1 | ERD6 | 29.8963 | −1.0013 | −30.8976 | 2.20 | −3.28 | −7.14 |
| 3_1 | 4CL | 31.8964 | 1.9988 | −29.8976 | 5.10 | −1.20 | −6.17 |
| 3_1 | AAA | 32.3049 | 2.3308 | −29.9740 | 3.20 | −1.14 | −3.68 |
| 5_1 | ICE4 | −1.6471 | −0.2440 | 1.4032 | 2.03 | 1.42 | −1.42 |
| 5_2 | COR78 | 1.1811 | −0.6344 | −1.8155 | 2.17 | 1.30 | −1.67 |
| 5_2 | WRKY33 | 1.5295 | 0.2214 | −1.3082 | −1.12 | −1.25 | −1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zombardo, A.; Garavelloni, S.; Biselli, C.; Fricano, A.; Bagnaresi, P.; Ammoniaci, M.; D’Arcangelo, M.E.M. Preliminary Study of the Genetic Response of Grapevine Buds to a Preventive Natural Polysaccharide-Based Biogel Under Simulated Late Frost Conditions. Agriculture 2025, 15, 2219. https://doi.org/10.3390/agriculture15212219
Zombardo A, Garavelloni S, Biselli C, Fricano A, Bagnaresi P, Ammoniaci M, D’Arcangelo MEM. Preliminary Study of the Genetic Response of Grapevine Buds to a Preventive Natural Polysaccharide-Based Biogel Under Simulated Late Frost Conditions. Agriculture. 2025; 15(21):2219. https://doi.org/10.3390/agriculture15212219
Chicago/Turabian StyleZombardo, Alessandra, Simone Garavelloni, Chiara Biselli, Agostino Fricano, Paolo Bagnaresi, Marco Ammoniaci, and Mauro Eugenio Maria D’Arcangelo. 2025. "Preliminary Study of the Genetic Response of Grapevine Buds to a Preventive Natural Polysaccharide-Based Biogel Under Simulated Late Frost Conditions" Agriculture 15, no. 21: 2219. https://doi.org/10.3390/agriculture15212219
APA StyleZombardo, A., Garavelloni, S., Biselli, C., Fricano, A., Bagnaresi, P., Ammoniaci, M., & D’Arcangelo, M. E. M. (2025). Preliminary Study of the Genetic Response of Grapevine Buds to a Preventive Natural Polysaccharide-Based Biogel Under Simulated Late Frost Conditions. Agriculture, 15(21), 2219. https://doi.org/10.3390/agriculture15212219







