Impact of Grass Endophyte on Leaf Spot in Perennial Ryegrass Caused by Bipolaris sorokiniana and Subsequent Aphids’ Feeding Preference
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Fungi Used in the Study
2.2. Potting Medium
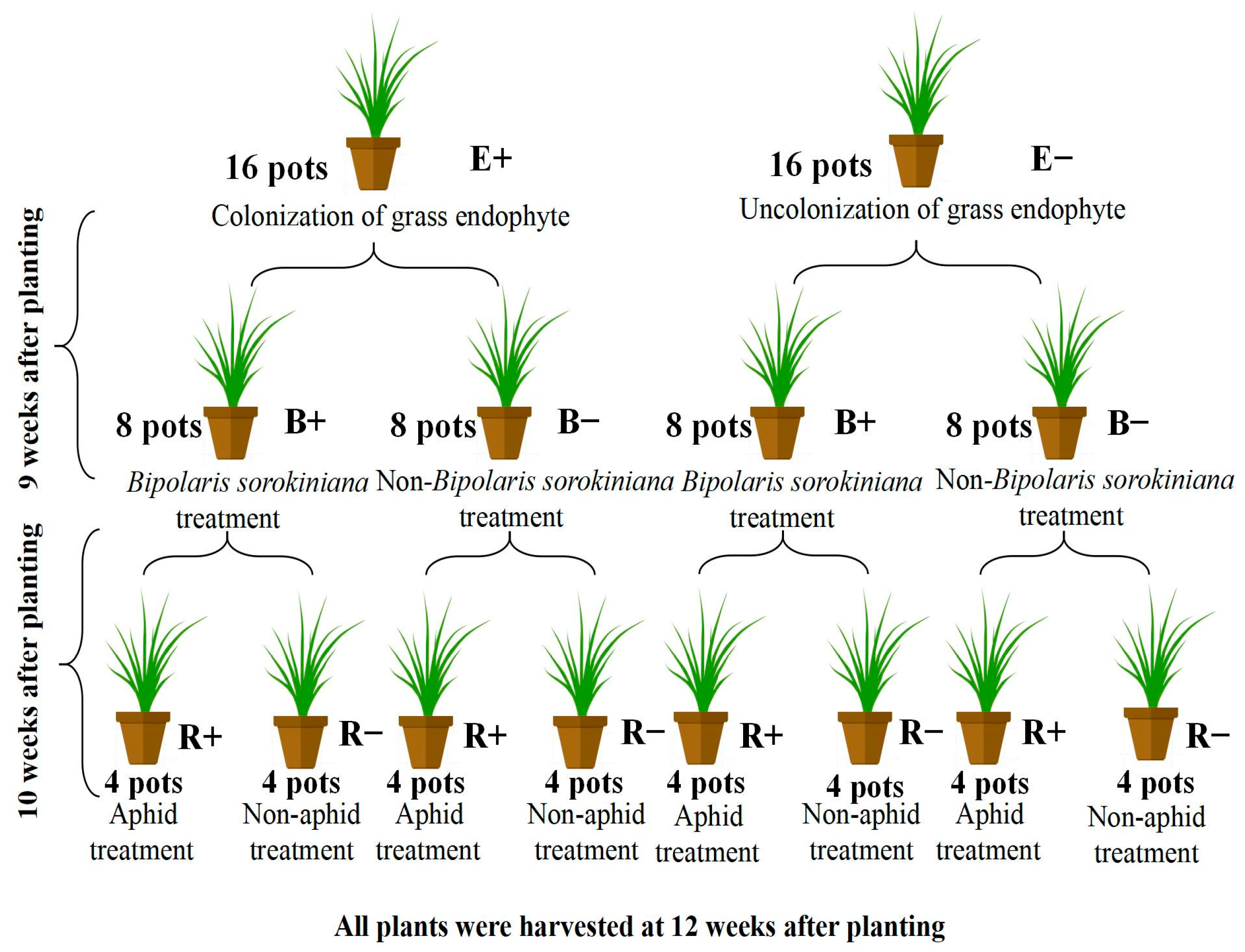
2.3. Experimental Design
2.4. Plant Harvest and Measurement
2.5. Olfactometer Test
2.6. Statistical Analysis
3. Results
3.1. Plant Growth
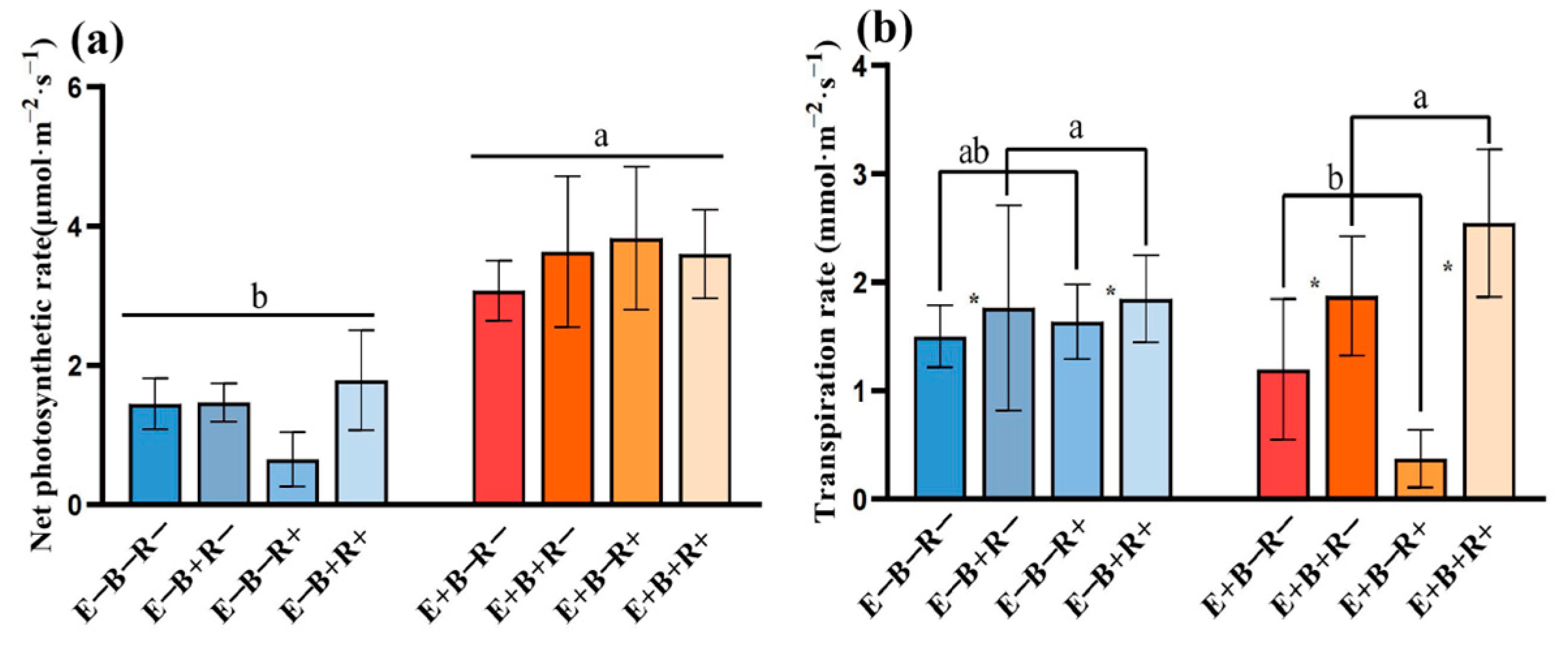
3.2. Plant Photosynthetic Parameters
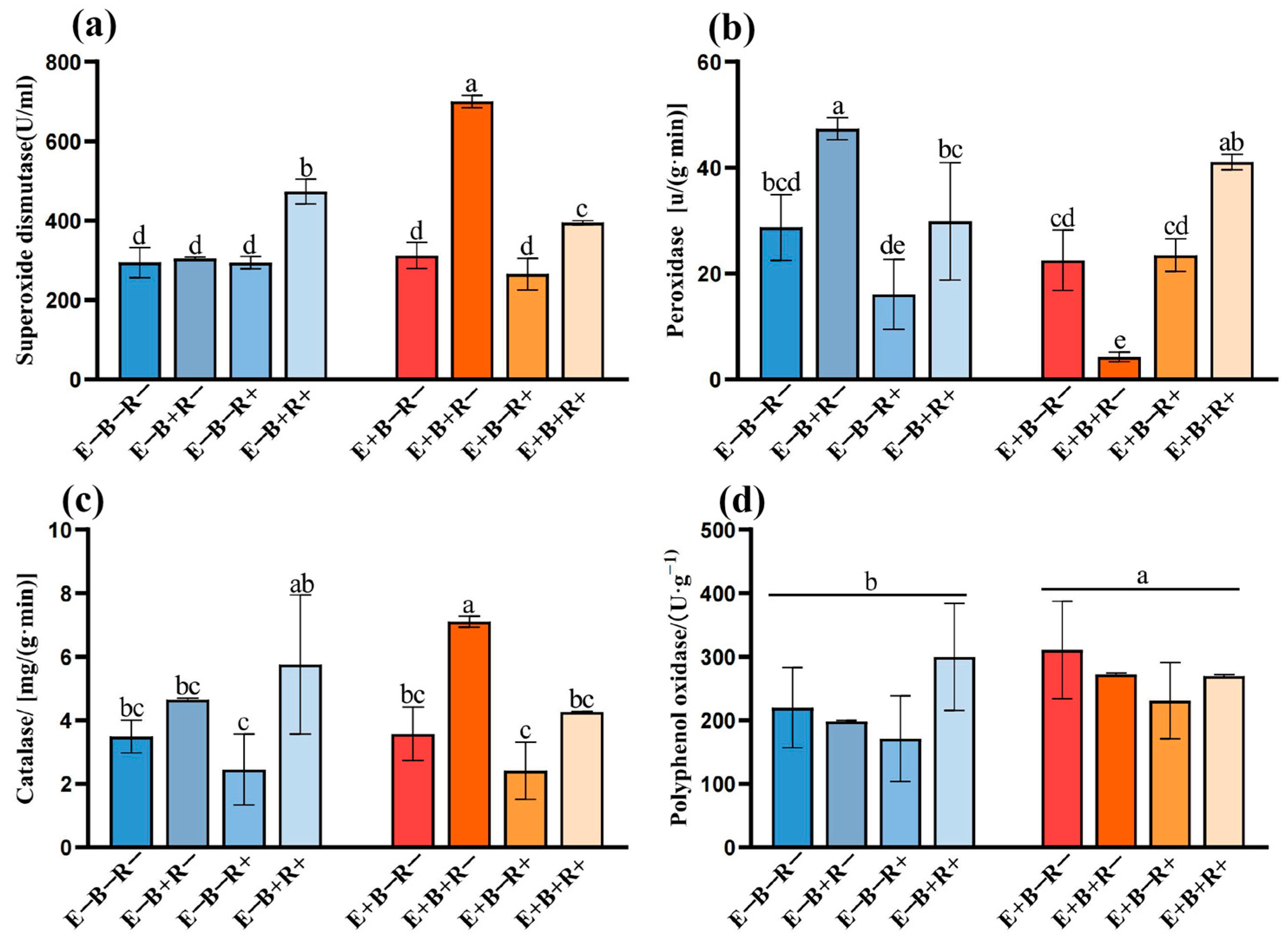
3.3. Plant Defense Enzymes Activities
3.4. Plant Hormone Signal Compounds
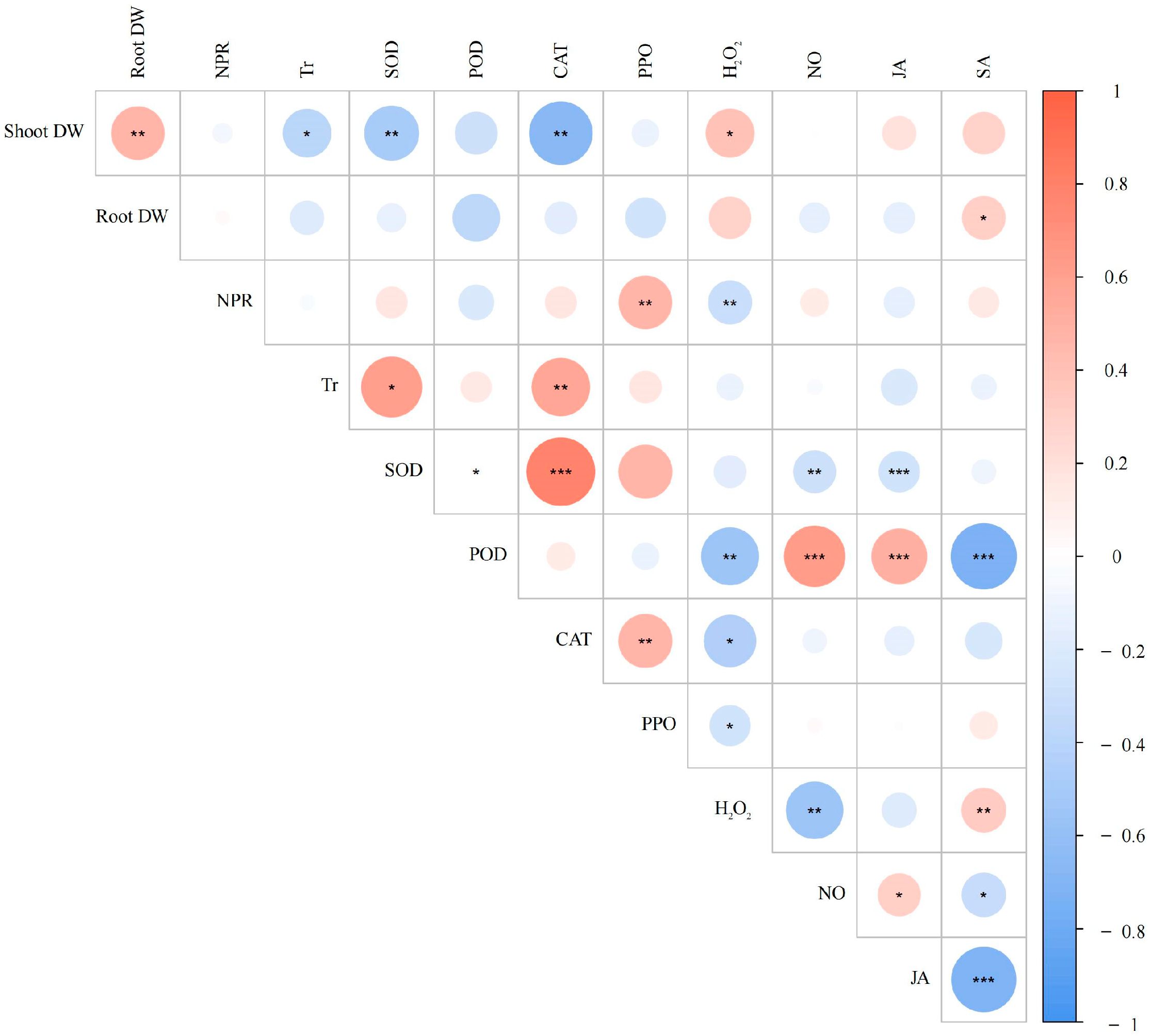
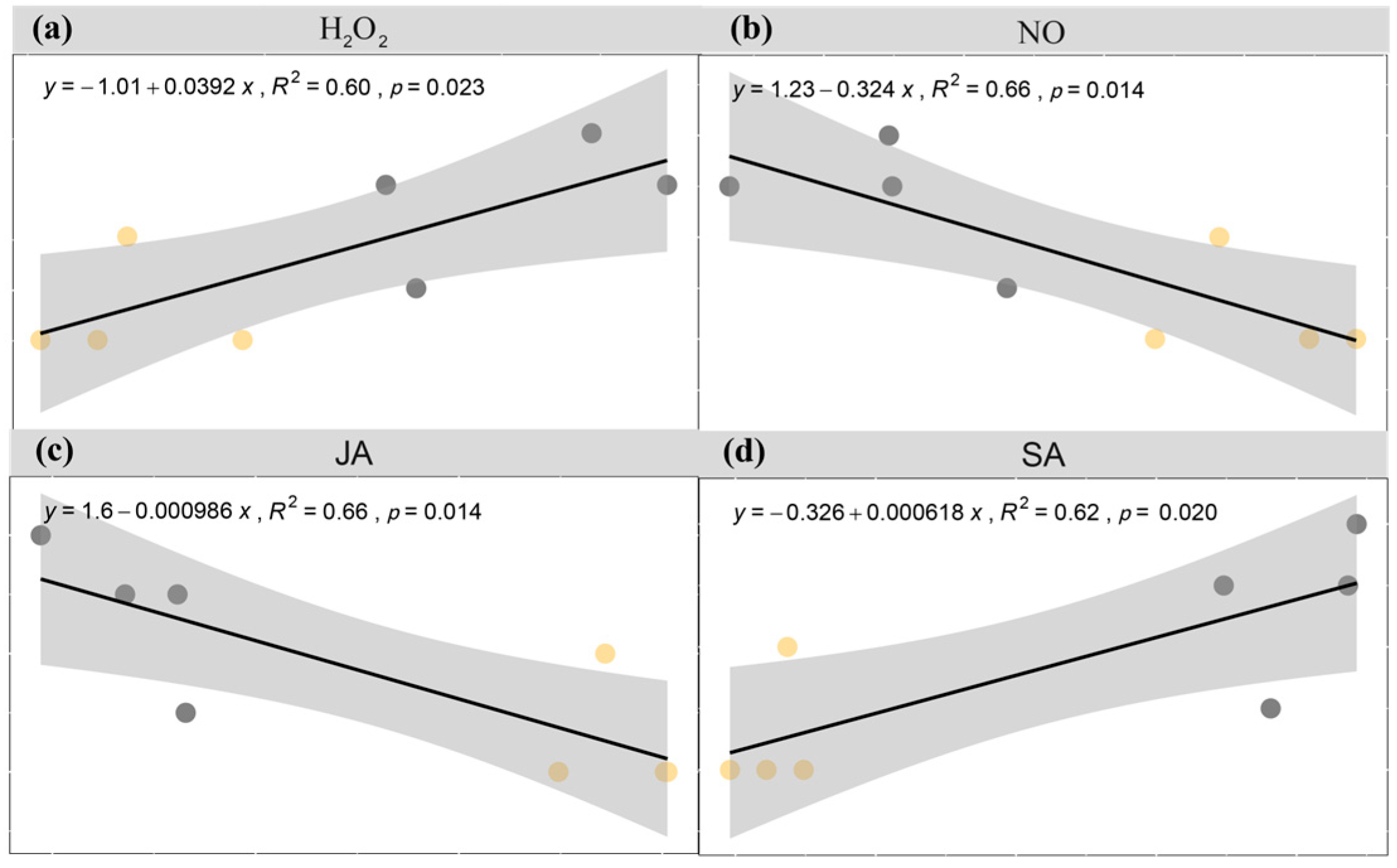
3.5. Correlation Analysis
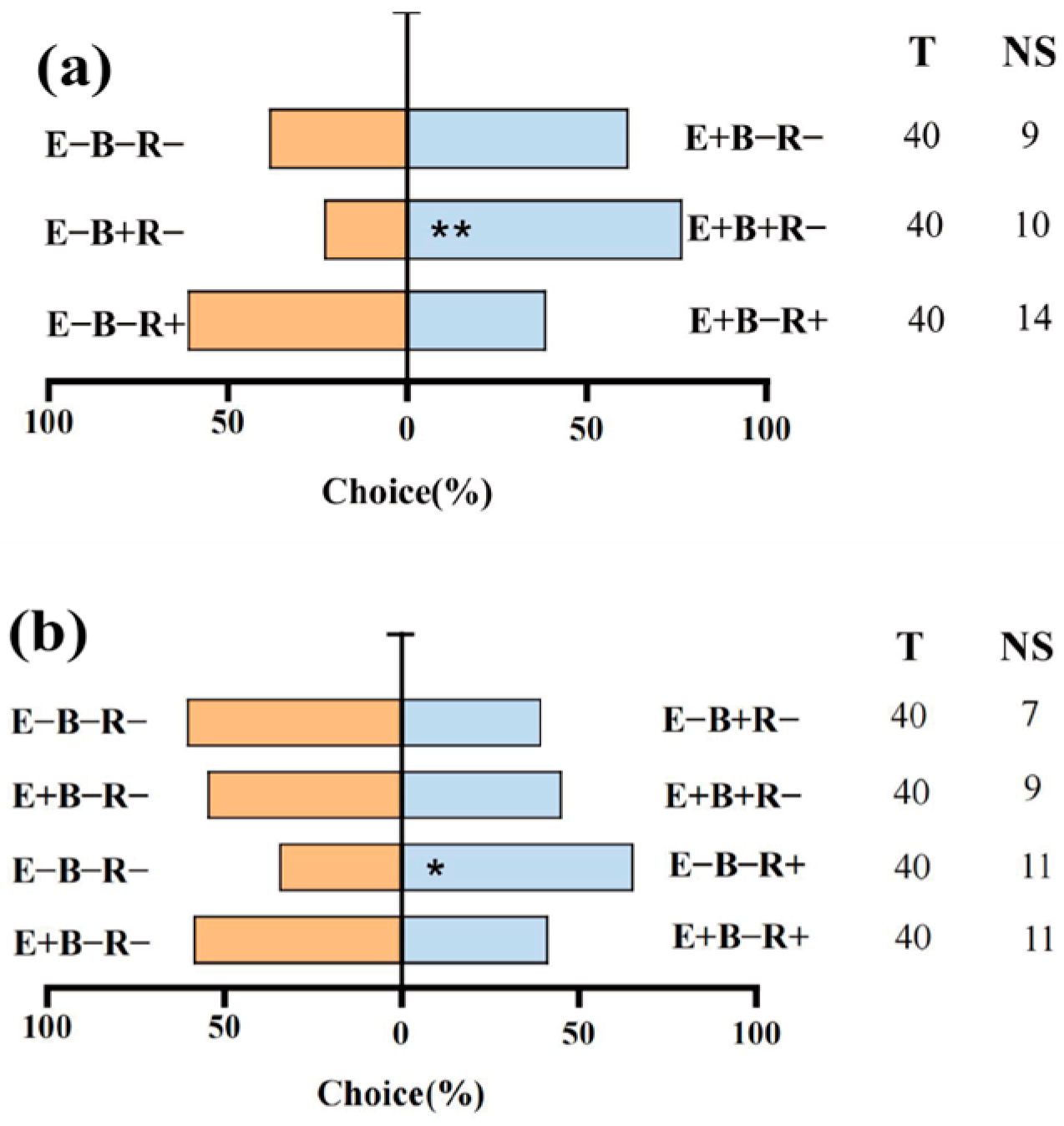
3.6. Aphid Selective Behavior
3.7. Correlation Analysis of Aphids Selective Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.Y.; Huang, X.; Liu, Y.R.; Ma, N.; Li, D.Y.; Hu, Q.N.; Zhang, W.J.; Wang, K.H. MicroRNA164 regulates perennial ryegrass (Lolium perenne L.) adaptation to changing light intensity. Agronomy 2024, 14, 1142. [Google Scholar] [CrossRef]
- Ahloowalia, B.S. Hybrids between tetraploid Italian and perennial ryegrass. Theor. Appl. Genet. 1977, 49, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.H.; Majidi, M.M.; Pirnajmedin, F.; Maibody, S.A.M.M. Plant functional trait responses to cope with drought in seven cool-season grasses. Sci. Rep. 2023, 13, 5285. [Google Scholar] [CrossRef] [PubMed]
- Kulczycki, G.; Sacała, E.; Koszelnik-Leszek, A.; Milo, Ł. Perennial Ryegrass (Lolium perenne L.) response to different forms of sulfur fertilizers. Agriculture 2023, 13, 1773. [Google Scholar] [CrossRef]
- Ying, C.Y.; Li, L.X.; Makeen, G.M.H.; Liu, Y.B. Erosion control of Chinese loess using polymer SH and ryegrass. J. Mt. Sci. 2024, 21, 2043–2058. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L. Perennial energy grasses: Resilient crops in a changing European agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Pawluśkiewicz, B.; Kalaji, H.M.; Baczewska, A.H. The effect of light availability on leaf area index, biomass production and plant species composition of park grasslands in Warsaw. Plant Soil. Environ. 2013, 59, 543–548. [Google Scholar] [CrossRef]
- Żurek, G.; Wiewiórka, B.; Rybka, K.; Prokopiuk, K. Different response of perennial ryegrasss–Epichloë endophyte symbiota to the elevated concentration of heavy metals in soil. J. Appl. Gen. 2022, 63, 47–59. [Google Scholar] [CrossRef]
- Altpeter, F. Perennial ryegrass (Lolium perenne L.). Agrobacterium Protoc. 2007, 2, 55–64. [Google Scholar]
- Jo, Y.K.; Barker, R.; Pfender, W.; Warnke, S.; Sim, S.C.; Jung, G.H. Comparative analysis of multipledisease resistance in ryegrass and cereal crops. Theor. Appl. Genet. 2008, 117, 531–543. [Google Scholar] [CrossRef]
- Boutaj, H.; Meddich, A.; Roche, J.; Mouzeyar, S.; Modafar, C.E. The effects of mycorrhizal fungi on vascular wilt diseases. Crop. Prot. 2022, 155, 105938. [Google Scholar] [CrossRef]
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A.; Patterson, L.M. Pathogenicity of fungi associated with the wheat crown rot complex in Oregon and Washington. Plant Dis. 2005, 89, 949–957. [Google Scholar] [CrossRef]
- Iftikhar, S.; Asad, S.; Munir, A.; Sultan, A.; Ahmad, I. Hosts of Bipolaris sorokiniana, the major pathogen of spot blotch of wheat in Pakistan. Pak. J. Bot. 2009, 41, 1433–1436. [Google Scholar]
- Hewitt, K.G.; Hofmann, R.W.; Ball, O.J.; Cox, N.; Bryant, R.H.; Finch, S.C.; Popay, A.J. Root aphid (Aploneura lentisci) population size on perennial ryegrass is determined by drought and endophyte strain. J. Pest. Sci. 2024, 97, 369–384. [Google Scholar] [CrossRef]
- Bell, N.L.; Townsend, R.J.; Popay, A.I.; Mercer, C.F.; Jackson, T.A. Black beetle: Lessons from the past and options for the future. Pasture Persistence Symp. 2011, 15, 119–124. [Google Scholar] [CrossRef]
- Ferguson, C.M.; Barratt, B.I.P.; Bell, N.; Goldson, S.L.; Hardwick, S.; Jackson, M.; Jackson, T.A.; Phillips, C.B.; Popay, A.J.; Rennie, G.; et al. Quantifying the economic cost of invertebrate pests to New Zealand’s pastoral industry. N. Z. J. Agric. Res. 2019, 62, 255–315. [Google Scholar] [CrossRef]
- Popay, A.J.; Gerard, P.J. Cultivar and endophyte effects on a root aphid Aploneura lentisci in perennial ryegrass. N. Z. Plant Prot. 2007, 60, 223–237. [Google Scholar] [CrossRef]
- Popay, A.J.; Cox, N.R. Aploneura lentisci (Homoptera: Aphididae) and its interactions with fungal endophytes in perennial ryegrass (Lolium perenne). Front. Plant Sci. 2016, 7, 1395. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.L. A Study of Root Aphid Aploneura lentisci Pass. Biology and Root Aphid-Host Interactions with Perennial Ryegrass/Endophyte Associations in New Zealand. Doctoral Dissertation, Massey University, Palmerston North, New Zealand, 2019. [Google Scholar]
- Popay, A.J.; Hume, D.E.; Mace, W.J.; Faville, M.J.; Finch, S.C.; Cave, V. A root aphid Aploneura lentisci is affected by Epichloë endophyte strain and impacts perennial ryegrass growth in the field. Crop Pasture Sci. 2021, 72, 155–164. [Google Scholar] [CrossRef]
- Cisternas, E. Plagas claves en la producción de praderas. In Seminario Praderas: Haciaunnuevo Estilo Productivo; Serie Actas; Instituto de Investigaciones Agropecuarias, Centro Regional de Investigación Remehue: Osorno, Chile, 2001; Volume 9, pp. 48–57. [Google Scholar]
- Siegel, M.R.; Latch, G.C.M.; Johnson, M.C. Fungal endophytes of grasses. Annu. Rev. Phytopathol. 1987, 25, 293–315. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, S99–S127. [Google Scholar] [CrossRef] [PubMed]
- West, C.P.; Izekor, E.; Turner, K.E.; Elmi, A.A. Endophyte effects on growth and persistence of tall fescue along a water-supply gradient. Agron. J. 1993, 85, 264–270. [Google Scholar] [CrossRef]
- Barker, G.M. Mycorrhizal infection influences Acremonium-induced resistance to argentine stem weevil inryegrass. In Proceedings of the New Zealand Weed and Pest Control Conference, Wellington, New Zealand, 1–13 August 1987; pp. 199–203. [Google Scholar]
- Vicari, M.; Hatcher, P.E.; Ayres, P.G. Combined effect of foliar and mycorrhizal endophytes on an insect herbivore. Ecology 2002, 83, 2452–2464. [Google Scholar] [CrossRef]
- Tian, Z. Effects of Epichloë Festucae Fungal Endophyte Infection in Strong Creeping Red Fescue on Abiotic and Biotic Stresses; Rutgers The State University of New Jersey, School of Graduate Studies: New Brunswick, NJ, USA, 2017. [Google Scholar]
- Ma, M.Z.; Christensen, M.J.; Nan, Z.B. Effects of the endophyte Epichloë festucae, var. lolii, of perennial ryegrass (Lolium perenne) on indicators of oxidative stress from pathogenic fungi during seed germination and seedling growth. Eur. J. Plant Pathol. 2015, 141, 571–583. [Google Scholar] [CrossRef]
- Scott, B. Epichloë endophytes: Fungal symbionts of grasses. Curr. Opin. Microbiol. 2001, 4, 393–398. [Google Scholar] [CrossRef]
- Shymanovich, T.; Saari, S.; Lovin, M.E.; Jarmusch, A.K.; Jarmusch, S.A.; Musso, A.M.; Charlton, N.D.; Young, C.A.; Cech, N.B.; Faeth, S.H. Alkaloid variation among epichloid endophytes of sleepygrass (Achnatherum robustum) and consequences for resistance to insect herbivores. J. Chem. Ecol. 2015, 41, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Saini, R.; Shukla, P.K.; Tiwari, K.N. Role of secondary metabolites in plant defense mechanisms: A molecular and biotechnological insights. Phytochem. Rev. 2024, 1–31. [Google Scholar] [CrossRef]
- Gogoi, K.; Gogoi, H.; Borgohain, M.; Saikia, R.; Chikkaputtaiah, C.; Hiremath, S.; Basu, U. The molecular dynamics between reactive oxygen species (ROS), reactive nitrogen species (RNS) and phytohormones in plant’s response to biotic stress. Plant Cell Rep. 2024, 43, 263. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, Y.E.; Christensen, M.J.; Gao, P.; Li, Y.Z.; Duan, T.Y. An arbuscular mycorrhizal fungus and Epichloë festucae var. lolii reduce Bipolaris sorokiniana disease incidence and improve perennial ryegrass growth. Mycorrhiza 2018, 28, 159–169. [Google Scholar] [CrossRef]
- Li, Y.D.; Duan, T.Y.; Li, Y.Z. Research progress in the interactions of fungal pathogens and insect pests during host plant colonization. J. Plant Dis. Protect. 2021, 128, 633–647. [Google Scholar] [CrossRef]
- Jindřichová, B.; Rubil, N.; Rezek, J.; Ourry, M.; Hauser, T.P.; Burketová, L. Does fungal infection increase the palatability of oilseed rape to insects? Pest. Manag. Sci. 2024, 80, 2480–2494. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, Y.J.; Tumlinson, J.H. Compatible and incompatible Xanthomonas infections differentially affect herbivore-induced volatile emission by pepper plants. J. Chem. Ecol. 2006, 32, 1755–1768. [Google Scholar] [CrossRef]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Alborn, H.T.; Stelinski, L.L. Induced release of a plant-defense volatile ‘deceptively’attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog. 2012, 8, e1002610. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Guo, S.S.; Zhang, W.J.; Wang, C.F.; HAN, J.; Geng, Z.F.; Wu, Y.; Du, S.S.; Deng, Z.W. Repellent activity of Glycosmis plant extracts against two stored product insects. In Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas; Universidad de Santiago de Chile: Santiago, Chile, 2015; Volume 14, pp. 462–469. [Google Scholar]
- Meyling, N.V.; Pell, J.K.; Eilenberg, J. Dispersal of Beauveria bassiana by the activity of nettle insects. J. Invertebr. Pathol. 2006, 93, 121–126. [Google Scholar] [CrossRef]
- Li, Y.D.; Nan, Z.B.; Matthew, C.; Wang, Y.J.; Duan, T.Y. Arbuscular mycorrhizal fungus changes alfalfa (Medicago sativa) metabolites in response to leaf spot (Phoma medicaginis) infection, with subsequent effects on pea aphid (Acyrthosiphon pisum) behavior. New Phytol. 2023, 239, 286–300. [Google Scholar] [CrossRef]
- Ellsbury, M.M.; Pratt, R.G.; Knight, W.E. Effects of single and combined infection of arrowleaf clover with bean yellow mosaic virus and a Phytophthora sp. on reproduction and colonization by pea aphids (Homoptera: Aphididae). Environ. Entomol. 1985, 14, 356–359. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.S.; Gao, X.F. A new method for accurate determination of peroxidase activity based on fluorescence decrease of guaiacol. Chin. J. Anal. Chem. 2015, 43, 1040–1046. [Google Scholar]
- Jiang, Z.; Weng, B.; Lei, J.; Wang, Y.; Tang, X.; Xiao, S. Effect of exogenous zinc addition on cell protective enzyme activities in the fruit bodies of Lentinus giganteus. Acta Microb. Sin. 2009, 49, 1121–1125. [Google Scholar]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Montagu, M.V.; Inzé, D.; Camp, W.V. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef] [PubMed]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and the control of its deleterious effects with melatonin and salicylic acid. Plants 2021, 10, 459. [Google Scholar] [CrossRef]
- Li, F.; Duan, T.Y.; Li, Y.Z. Effects of the fungal endophyte Epichloë festucae var. lolii on growth and physiological responses of perennial ryegrass cv. fairway to combined drought and pathogen stresses. Microorganisms 2020, 8, 1917. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, J.; Nzabanita, C.; Li, Y.Z.; Duan, T.Y. Growth and physiological responses of perennial ryegrass to an AMF and an Epichloë endophyte under different soil water contents. Symbiosis 2019, 79, 151–161. [Google Scholar] [CrossRef]
- Rozpądek, P.; Wężowicz, K.; Nosek, M.; Ważny, R.; Tokarz, K.; Lembicz, M.; Miszalski, Z.; Turnau, K. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata). Planta 2015, 242, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Nan, Z.B.; Li, C.J.; Spangenberg, G. Effect of the endophyte Neotyphodium lolii on susceptibility and host physiological response of perennial ryegrass to fungal pathogens. Eur. J. Plant Pathol. 2008, 122, 593–602. [Google Scholar] [CrossRef]
- Backman, P.A.; Sikora, R.A. Endophytes: An emerging tool for biological control. Biol. Control. 2008, 46, 1–3. [Google Scholar] [CrossRef]
- Hoye, A.T.; Davoren, J.E.; Wipf, P.; Fink, M.P.; Kagan, V.E. Targeting mitochondria. Acc. Chem. Res. 2008, 41, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Loake, G.J. Redox-based protein modifcations: The missing link in plant immune signalling. Curr. Opin. Plant Biol. 2011, 14, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Nassimi, Z.; Taheri, P. Endophytic fungus Piriformospora indica induced systemic resistance against rice sheath blight via affecting hydrogen peroxide and antioxidants. Biocontrol Sci. Technol. 2017, 27, 252–267. [Google Scholar] [CrossRef]
- Xie, X.G.; Fu, W.Q.; Zhang, F.M.; Shi, X.M.; Zeng, Y.T.; Li, H.; Zhang, W.; Dai, C.C. The endophytic fungus Phomopsis liquidambari increases nodulation and N2 fixation in Arachis hypogaea by enhancing hydrogen peroxide and nitric oxide signalling. Microb. Ecol. 2017, 74, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- He, Y.L.; Chen, T.X.; Zhang, H.J.; White, J.F.; Li, C.J. Fungal endophytes help grasses to tolerate sap-sucking herbivores through a hormone-signaling system. J. Plant Growth Regul. 2022, 41, 2122–2137. [Google Scholar] [CrossRef]
- Schwartzberg, E.G.; Tumlinson, J.H. Aphid honeydew alters plant defence responses. Funct. Ecol. 2014, 28, 386–394. [Google Scholar] [CrossRef]
- Schweiger, R.; Heise, A.M.; Persicke, M.; Müller, C. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 2014, 37, 1574–1585. [Google Scholar] [CrossRef]
- Kou, M.Z.; Bastías, D.A.; Bastías, M.J.; Zhong, R.; Nan, Z.B.; Zhang, X.X. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.N.; Rowe, R.C.; Hall, C.R. Aphid feeding induces phytohormonal cross-talk without affecting silicon defense against subsequent chewing herbivores. Plants 2020, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Gange, A.C. Positive effects of endophyte infection on sycamore aphids. Oikos 1996, 75, 500–510. [Google Scholar] [CrossRef][Green Version]
- Hammon, K.E.; Faeth, S.H. Ecology of plant-herbivore communities: A fungal component? Nat. Toxins 1993, 1, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P.; Steinebrunner, F.; Schulz, C.; Von Reuss, S.; Francke, W.; Weymuth, C.; Leuchtmann, A. Evolution of ‘pollinator’-attracting signals in fungi. Biol. Lett. 2006, 2, 401–404. [Google Scholar] [CrossRef]
- Yue, Q.; Wang, C.; Gianfagna, T.J.; Meyer, W.A. Volatile compounds of endophyte-free and infected tall fescue (Festuca arundinacea Schreb.). Phytochemistry 2001, 58, 935–941. [Google Scholar] [CrossRef]
- Hartley, S.E.; Gange, A.C. Impacts of plant symbiotic fungi on insect herbivores: Mutualism in a multitrophic context. Annu. Rev. Entomol. 2009, 54, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.; Dicke, M.; Pieterse, C.M.J.; Pozo, M.J. Beneficial microbes in a changing environment: Are they always helping plants to deal with insects? Funct. Ecol. 2013, 27, 574–586. [Google Scholar] [CrossRef]



| Source of Variation | Endophyte | B. Sorokiniana | R. Maidis | Interaction | |||
|---|---|---|---|---|---|---|---|
| (E) | (B) | (R) | E × B | E × R | B × R | E × B × R | |
| Aboveground biomass | ns * | <0.001 | 0.006 | ns | ns | ns | ns |
| Underground biomass | ns | 0.023 | ns | ns | ns | ns | ns |
| Net photosynthetic rate | <0.001 | ns | ns | ns | ns | ns | ns |
| Transpiration rate | ns | <0.001 | ns | 0.006 | ns | ns | ns |
| SOD | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.025 | <0.001 |
| POD | <0.001 | <0.001 | ns | <0.001 | <0.001 | <0.001 | <0.001 |
| CAT | ns | <0.001 | 0.010 | ns | 0.008 | ns | 0.012 |
| PPO | 0.021 | ns | ns | ns | ns | 0.008 | ns |
| H2O2 | <0.001 | <0.001 | 0.009 | <0.001 | <0.001 | 0.008 | ns |
| NO | 0.006 | ns | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| SA | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | <0.001 | 0.001 |
| JA | <0.001 | ns | 0.025 | <0.001 | <0.001 | <0.001 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; He, J.; Shen, Y.; Li, Y.; Wang, P.; Duan, T. Impact of Grass Endophyte on Leaf Spot in Perennial Ryegrass Caused by Bipolaris sorokiniana and Subsequent Aphids’ Feeding Preference. Agriculture 2025, 15, 116. https://doi.org/10.3390/agriculture15020116
Ma Z, He J, Shen Y, Li Y, Wang P, Duan T. Impact of Grass Endophyte on Leaf Spot in Perennial Ryegrass Caused by Bipolaris sorokiniana and Subsequent Aphids’ Feeding Preference. Agriculture. 2025; 15(2):116. https://doi.org/10.3390/agriculture15020116
Chicago/Turabian StyleMa, Ziyuan, Jia He, Youlei Shen, Yingde Li, Ping Wang, and Tingyu Duan. 2025. "Impact of Grass Endophyte on Leaf Spot in Perennial Ryegrass Caused by Bipolaris sorokiniana and Subsequent Aphids’ Feeding Preference" Agriculture 15, no. 2: 116. https://doi.org/10.3390/agriculture15020116
APA StyleMa, Z., He, J., Shen, Y., Li, Y., Wang, P., & Duan, T. (2025). Impact of Grass Endophyte on Leaf Spot in Perennial Ryegrass Caused by Bipolaris sorokiniana and Subsequent Aphids’ Feeding Preference. Agriculture, 15(2), 116. https://doi.org/10.3390/agriculture15020116







