Abstract
The BRI1-EMS suppressor/Brassinazole-resistant (BES/BZR) transcription factors (TFs) act as regulators of the Brassinosteroid (BR) signaling pathway and play key roles in modulating plant growth, development, and abiotic stress tolerance. However, the function of BES/BZR TFs remains unknown in warm-season turfgrass species. In this study, ZjBZR2, a BES/BZR TF in Zoysia japonica was identified and shared the closest evolutionary relationship with OsBZR2 from Oryza sativa. ZjBZR2 was a nuclear-localized protein and had transcriptional activation activity. ZjBZR2 was predominantly expressed in roots, stems, and lamina joints, and could be significantly induced by BR treatment and osmotic stresses including PEG and salinity. ZjBZR2-overexpressing rice lines increased leaf angle compared with wild-type plants. Furthermore, overexpression of ZjBZR2 enhanced osmotic stress (PEG and salt) tolerance which is associated with the upregulation of stress-responsive and ROS-scavenging genes. These findings provide the first functional characterization of ZjBZR2 in rice and offer excellent genetic resources for the improvement of turfgrass cultivars.
1. Introduction
As the sixth category of plant hormones, brassinosteroids (BRs) have been demonstrated to play crucial roles in plant growth, development, and stress responses [1,2,3]. In Arabidopsis thaliana, the transcription factors (TFs) BRI1-EMS suppressor 1 (BES1/BZR2) and Brassinazole-resistant 1 (BZR1) are directly regulated by BRASSINOSTEROID INSENSITIVE 2 (BIN2). BIN2 mediates phosphorylation targets BES1/BZR2 and BZR1 for degradation and negatively regulates their abundance [2,4]. BR signaling inhibits BIN2 activity, resulting in BES1/BZR2 and BZR1 dephosphorylation and accumulation [4,5]. BES1/BZR2 and BZR1 binds to the promoters of BR-regulated target genes to regulate their expression level [6,7].
Furthermore, the key components of the BR signaling pathway, including glycogen synthase kinase 2 (GSK2/BIN2) and BZR1, show evolutionary conservation between rice (Oryza sativa) and Arabidopsis thaliana [8,9]. Research indicates that OsBZR1 plays a core regulatory role in the BR signaling pathway and mediates the metabolism of endosperm starch during seed germination [10]. Overexpression of OsBZR1 can also enhance carbohydrate accumulation in anthers and seeds, thereby increasing grain yield [3]. Rice histone deacetylase 1 (OsHDAC1) enhances lateral root growth in rice by blocking OsGSK2 activity. This stabilization of OsBZR1 prevents its phosphorylation-dependent breakdown. Thus, OsBZR1 acts as a positive regulator of lateral root primordia [11]. Previous studies have demonstrated that BR treatment significantly increases the leaf angle in rice, and this phenotypic response has been developed into a quantitative bioassay for BR activity [12]. Besides regulating leaf angle, suppression of OsBZR1 and OsBZR2 expression leads to dwarfism in rice plants. Notably, OsBZR2 may play a more critical role than OsBZR1 in regulating leaf angle, as the osbzr2 mutant displays a stronger erect- leaf phenotype [12,13].
Environmental stresses directly induce physicochemical alterations in plant biomolecules, triggering cellular stress responses [5]. Plants employ interconnected regulatory pathways to rapidly adapt to these challenges [14]. BES/BZR TFs are key downstream components of the BR signaling pathway. They coordinate plant developmental processes and abiotic stress responses through transcriptional regulation of target genes. Osmotic stresses are common abiotic stresses, such as salt stress, drought stress, and cold stress. In birch (Betula platyphylla), BpBZR1-6 enhances salt tolerance by modulating antioxidant enzyme gene expression and strengthening the reactive oxygen species (ROS) scavenging system [15]. In wheat (Triticum aestivum), TaBZR2 positively regulates drought stress response through transcriptional activation of Glutathione S-transferase 1 (TaGST1) to facilitate ROS detoxification [16]. In Arabidopsis thaliana, chilling stress converts BZR1 from an inactive phosphorylated form to an active dephosphorylated form which can enhance freezing tolerance by activating the expression of C-repeat Binding Factor (CBF1 and CBF2). Furthermore, studies demonstrate that AtWRKY6 serves as a direct downstream target of AtBZR1, contributing to freezing stress regulation [17]. These findings collectively highlight the multifaceted roles of BES/BZR TFs in orchestrating plant stress adaptation mechanisms.
Zoysia japonica, a perennial warm-season turfgrass, has been extensively utilized in practical applications due to its superior stress tolerance and low maintenance requirements [18,19,20]. This species serves as a preferred choice for constructing sports fields, ornamental landscaping, and erosion-prone slope stabilization [21,22]. Currently, genome annotation efforts in the genus Zoysia have led to the identification of 29,551, 32,925, 53,226, and 53,656 putative genes in Zoysia sinica, Z. japonica, Zoysia matrella, and Zoysia pacifica, respectively. Notably, re-annotation of the Z. japonica, Z. matrella, and Z. pacifica genomes has further improved the accuracy of their gene models [23,24]. These high-quality genomic resources provide a crucial foundation for elucidating gene function in Zoysia plants at the molecular level. In response to abiotic stresses such as osmotic stresses that severely impede growth, plants have developed highly sophisticated regulatory mechanisms. Although the role of BES/BZR transcription factors in plant stress responses has been extensively documented, their functional characterization in turfgrass species, including Z. japonica, is still largely unknown. Meanwhile, the genetic research in Z. japonica has lagged behind that of phylogenetically related species, primarily owing to its allotetraploid nature with high heterozygosity, and difficulties in establishing efficient regeneration and stable genetic transformation systems [18,19,20,23]. Given its short lifecycle and mature genetic transformation system, rice serves as a model organism for functional gene research in Poaceae plants. Therefore, to efficiently and rapidly achieve a preliminary functional analysis of Z. japonica BES/BZR transcription factors, this study employed a heterologous expression approach in the rice system, as detailed below. The objective of this study was mainly to explore the preliminary function of ZjBZR2 in rice to predict its potential molecular roles in Z. japonica. Salt- and PEG-induced stresses were selected to represent osmotic stresses in the present study. The function of ZjBZR2, a member of the BES/BZR TFs in Z. japonica was analyzed by ZjBZR2-overexpressing (OE) lines after treatments of BR, PEG, and salt stresses in rice. Our findings provide the first functional characterization of ZjBZR2 in rice and offer excellent genetic resources for the improvement of turfgrass cultivars.
2. Materials and Methods
2.1. Construction of Expression Vectors and Generation of Transgenic Rice
To generate a ZjBZR-OE construct, a full-length coding sequence was cloned into the plant binary vector pCambia1305.2--UbiP-NostT, enabling constitutive expression under the control of the maize ubiquitin (Ubi-1) promoter. The OE construct was transformed into wild-type (WT) Nipponbare using Agrobacterium transformation, and transgenic lines (L1 and L2) were selected for further analysis. An approximate 2000- bp promoter region of ZjBZR2 was cloned into the pCAMBIA1301 vector to drive the expression of the β-glucuronidase (GUS) gene. pCAMBIA1301-proZjBZR2:GUS transgenic rice was generated. All the specific primers are listed in Table S1.
2.2. Plant Materials and Growth Conditions
Wild-type Zoysia japonica seedlings were planted in pots containing mixed soil (1:2 vermiculite/humus) and cultured in a growth chamber XBQH-1 (Jinan Xubang, Jinan, China) under 16 h/8 h (light/dark), 65–75% relative humidity, and temperatures of 30 °C/25 °C (day/night). Sterilized WT and transgenic rice seeds were germinated on a moistened sterile filter paper under 16 h light/8 h dark and temperatures of 30 °C/25 °C (day/night). After 3–4 d, germinated seeds were transferred to culture boxes containing full-strength Hoagland medium solution and maintained in a plant growth chamber under 16 h/8 h (light/dark), 65–75% relative humidity, and temperatures of 30 °C/25 °C (day/night) for 7–9 d. A part of the seedlings were subsequently transplanted into pots filled with field soil.
2.3. Experimental Treatments
To analyze ZjBZR2 expression under BR, PEG, and salt treatments in Z. japonica, seedlings were sprayed with 1 µM 2, 4-epibrassinolide (EBL) and treated with a solution of 20% PEG 6000 (w/v) or 200 mM NaCl, respectively [15,25]. Leaf and root samples were collected at 0, 0.5, 1, 2, 4, 8, 12, and 24 h, respectively. Samples from the root, stem, and leaf of Z. japonica seedlings were collected for the analysis of ZjBZR2 expression pattern. All the samples were frozen in liquid nitrogen and stored at −80 °C for further measurements.
For PEG and salt treatments, four-week-old transgenic seedlings (16 plants in each line) were pre-cultured in 96-well plates containing full-strength Hoagland solution. The seedlings were then transferred to treatment solutions consisting of either 20% (w/v) PEG6000 or 200 mM NaCl in water for 5 d. After 7 d of rehydration, the survival rate was counted and photographed [26,27].
2.4. Phylogenetic Tree, Multiple Sequence Alignment, and Promoter Cis-Acting Element Analysis
The ZjBZR2 coding sequence was retrieved by a BLASTN search of OsBZR2 against the Zoysia Genome Portal (http://zoysia.kazusa.or.jp, accessed on 23 March 2021). ZjBZR2 amino acid sequence was used as a query for BLASTP searches against other species in the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/nuccore/, accessed on 14 April 2025). Target sequences from Arabidopsis thaliana, Oryza sativa, Zea mays, Sorghum bicolor, Brachypodium distachyon, Triticum aestivum, and Lolium perenne were subsequently downloaded. The selected sequences were aligned using ClustalW, and phylogenetic analysis was performed using the neighbor-joining method by MEGA11 (Mega Limited, Auckland, New Zealand). ESPript 3.0 (https://espript.ibcp.fr/ESPript/ESPript/, accessed on 16 April 2025) was used for visualizing multiple sequence alignment. The putative PEST motifs were predicted using the online tool (http://emboss.bioinformatics.nl/cgi-bin/emboss/epestfind accessed on 16 April 2025). The 2000- bp promoter sequence of ZjBZR2 was analyzed using the online PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 17 November 2024) to identify cis-acting elements and visualize their positional information through the GSDS 2.0 (http://gsds.gao-lab.org/, accessed on 30 May 2025).
2.5. Leaf Angle Measurement and BR Sensitivity Assays
The leaf angle was measured at the seedling stage (second leaf from the top) and heading stage (flag leaf and second leaf from the top) using ImageJ software (Version 1.54, National Institutes of Health, Bethesda, MD, USA) to measure the angle between the leaf midrib and the vertical stem axis [28]. For BR sensitivity assays, surface-sterilized seeds were sown on half-strength Murashige and Skoog (MS) medium, either without treatment or supplemented with 5 µM EBL [16]. After 7 d of growth at 30 °C under long-day conditions (16 h light/8 h dark cycle), seedlings were photographed, and root length was measured for each seedling.
2.6. GUS Staining and Subcellular Localization
Transgenic rice seedlings carrying proZjBZR2:GUS constructs were subjected to dehydration stress. Samples collected at 0, 4, and 12 h post-treatment underwent incubation in 1 mM GUS staining solution for 12 h (37 °C in darkness), followed by chlorophyll removal through ethanol destaining [29]. For subcellular localization analyses, the ZjBZR2 coding sequence was cloned into the pCAMBIA1305-GFP vector. The 1305-mCherry was used as a nuclear marker. The 1305-ZjBZR2:GFP and 1305-mCherry constructs were co-transformed into Nicotiana benthamiana leaves via Agrobacterium-mediated infiltration. Confocal microscopy was conducted on a Zeiss LSM 900 system (Carl Zeiss AG, Oberkochen, Germany) with the following settings: GFP 488 nm argon laser excitation, 500–550 nm bandpass emission filter; mCherry 561 nm diode laser excitation, 570–620 nm emission.
2.7. Transcriptional Activation Analysis
In the yeast system, the full-length coding region of ZjBZR2 was cloned into the pGBKT7 and then transformed into the AH109 strain to test for transcriptional activation. For the luciferase assay, ZjBZR2 was cloned into the effector vector pGreenII 62-SK-GAL4BD (pBD) to drive luciferase (LUC) expression. The reporter vector pGreenII-0800-LUC contained a Renilla luciferase (REN) gene under the control of the CaMV35S promoter and served as an internal control. These constructs were co-transfected into tobacco leaves through Agrobacterium-mediated transformation. Relative LUC activity was quantified using a dual-luciferase reporter assay system (Yeasen, Shanghai, China) with an Infinite M200 Pro microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
2.8. Water Loss Rate and Electrolyte Leakage Measurements
For the rate of water loss, the second fully expanded leaves (second leaves from the top) from four-week-old seedlings of WT and ZjBZR2-OE were detached and placed on dry filter paper under normal laboratory conditions. The fresh weight of each leaf was recorded every 30 min for 3 h, and the water loss rate was calculated as the percentage of total fresh weight lost compared with the initial weight at each time point [30].
Electrolyte leakage was examined in accordance with the methods described by the authors of [31]. The leaves of WT and transgenic seedlings were collected before treatments and 3 d after PEG/salt treatments, respectively. Samples were immersed in 30 mL of deionized water for 12 h, followed by measuring the initial solution conductivity (C1). After boiling the leaves for 15 min and cooling them completely to room temperature, the final conductivity (C2) was measured. Electrolyte leakage was calculated as the ratio of C1/C2 × 100.
2.9. RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated from samples stored at −80 °C using a Plant total RNA isolation Kit (Vazyme, Nanjing, China). First-strand cDNA was synthesized using a supermix for RT-PCR (Yeasen, Shanghai, China). Quantitative real-time PCR (qRT-PCR) was performed using Hieff qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) with a two-step amplification program: initial denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s, and 60 °C for 30 s. A melting curve analysis was subsequently performed using the default program of the Roche LightCycler 480 II system (Roche Diagnostic, Basel,, Switzerland). Relative expression levels were determined based on the 2−ΔΔCT method [32]. The OsActin1 and ZjActin genes were used as internal control [21,33]. All the specific primers are listed in Table S2 [34,35,36].
2.10. Data Analyses
All data were analyzed using SPSS (Version 13.0; IBM Corp., Armonk, NY, USA). Graphs were prepared using GraphPad Prism 9.5 (GraphPad Software, San Diego, CA, USA). Differences between WT and transgenic lines were analyzed by one-way ANOVA. Measured parameters are presented as mean ± standard error (SE). Significant differences (p < 0.05) are shown by different letters.
3. Results
3.1. Identification of ZjBZR2 in Zoysia japonica
Using the BES/BZR TFs from rice as query sequences, the most similar sequence to OsBZR2 (Os01g0203000) was identified in the Zoysia Genome Database and designated as ZjBZR2. The sequence length of ZjBZR2 was 1047 bp, encoding 348 amino acids. In the phylogenetic tree, ZjBZR2 exhibited the highest amino acid sequence homology (87.8%) with SbBZR2. Meanwhile, ZjBZR2 was grouped together with OsBZR2 (86.2%) (Figure 1A). Another BES/BZR TF in Zoysia japonica was identified and clustered with OsBZR1, designated as ZjBZR1.
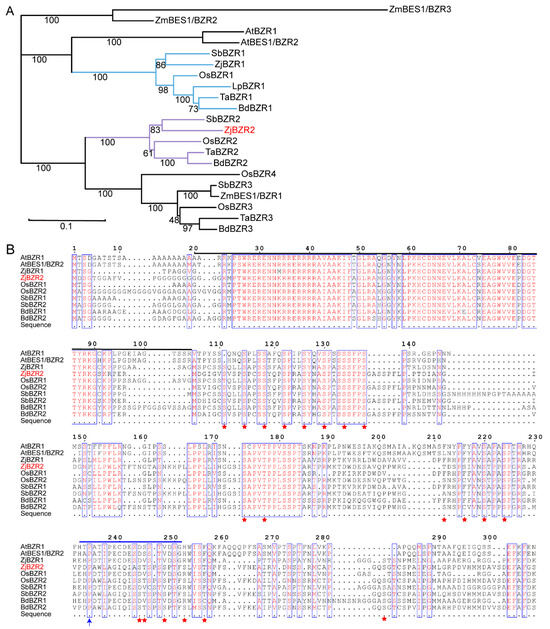
Figure 1.
Phylogenetic analysis and multiple sequence alignments of ZjBZR2. (A). Phylogenetic tree analysis of ZjBZR2 and BZR proteins in Arabidopsis thaliana, Oryza sativa, Zea mays, Sorghum bicolor, Brachypodium distachyon, Triticum aestivum, and Lolium perenne. The branches containing ZjBZR2 and ZjBZR1 were indicated by blue and purple lines, respectively, and the ZjBZR2 label was highlighted in red. (B). Sequence alignment of ZjBZR2 with BZR transcription factors in other plant species. The black lines mark the N-terminal DNA-binding domain. The blue arrow and blue line highlight the bzr1-1D and bes1-D mutations and the putative PEST sequence. The red star marks the putative glycogen synthase kinase 3 (GSK3) -like kinase phosphorylation sites (S/TXXXS/T).
Multiple sequence alignment exhibited that ZjBZR2 shared high sequence similarity with BES/BZR TFs from other species. Both protein groups contained the conserved N-terminal DNA-binding domain (Figure 1B). Protein structure analysis illustrated that the ZjBZR2 contained 20 putative glycogen synthase kinase 3 (GSK3) -like kinase phosphorylation sites (S/TXXXS/T). Seven putative PEST motifs (region rich in proline, glutamate, serine, and threonine) were identified. Significantly, one of the PEST motifs (amino acids 230–270) was found to contain the proline residue mutated in bzr1-1D and bes1-D mutants (P206 in OsBZR1). But the potential 14-3-3 binding site (RXXXpSXP, where X is any amino acid, R is Arg, pS is phospho-Ser, and P is Pro) was not identified.
3.2. Subcellular Localization and Transcriptional Activity of ZjBZR2
To determine the subcellular localization of ZjBZR2, the 1305-GFP and the 1305-ZjBZR2:GFP fusion proteins were transiently expressed under the control of the CaMV35S promoter in Nicotiana tabacum leaves. The GFP signals of ZjBZR2:GFP overlapped with the 1305-mCherry, and both localized to the nucleus (Figure 2A). To assess the transcriptional activity of ZjBZR2, a transcriptional activation assay was performed in yeasts. Yeast cells co-transformed with pGADT7 and pGBKT7-ZjBZR2 exhibited the enhanced growth on histidine- and adenine- deficient mediums compared to controls (pGADT7+pGBKT7) (Figure 2B). Furthermore, dual-luciferase assays were conducted to confirm that ZjBZR2 had transcriptional activation activity in tobacco leaves. The effector construct pBD-ZjBZR2 was generated by fusing ZjBZR2 to the GAL4 DNA-binding domain (GAL4-DBD). The reporter construct contained a firefly luciferase (LUC) gene driven by 5× GAL4 upstream activating sequences, with Renilla luciferase (REN) under the 35S promoter serving as an internal control (Figure 2C). Dual-luciferase assays exhibited that pBD-ZjBZR2 generated significantly higher LUC/REN ratios compared to the empty pBD vector control (Figure 2D).
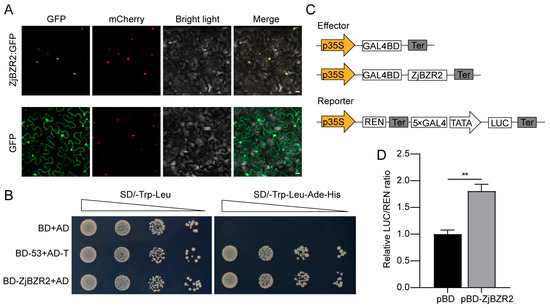
Figure 2.
Subcellular localization and transactivation activity of ZjBZR2. (A). Subcellular localization of ZjBZR2. The full length CDS of ZjBZR2 was cloned and fused to GFP for localization analysis. mCherry was used as a nuclear marker. Scale bar = 20 μm. (B). Transcriptional activity of ZjBZR2 protein in yeast. (C). Diagram of vector construction for detecting self-activation ability of ZjBZR2. (D). Transactivation activity of ZjBZR2 in Nicotiana benthamiana. Data are displayed by means ± SE of three biological replicates. Student’s t-test was used to compare the difference. ** p < 0.01.
3.3. Cis-Acting Element Analysis of ZjBZR2 Promoter
Predicted cis-acting elements included the following: ABRE, CGTCA/TGACG-motif, GARE-motif, TGA-element, and light-responsive elements (I-box/G-box) (Figure 3A). These cis-acting elements were associated with light regulation and hormone signaling, such as abscisic acid (ABA), methyl jasmonate (MeJA), gibberellins (GA), and auxin.
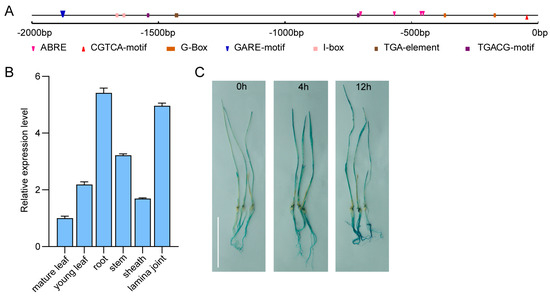
Figure 3.
Analysis of ZjBZR2 cis-acting elements and expression levels, and GUS staining of ProZjBZR2: GUS transgenic seedlings. (A). The cis-acting element analysis of ZjBZR2 promoter. (B). The ZjBZR2 expression level was determined by qRT-PCR in the old leaf, young leaf, root, stem, sheath, and lamina joint. Each data point represents the mean ± SE of three biological replicates. (C). GUS staining of ProZjBZR2: GUS seedlings in Oryza sativa under dehydration treatments at 0, 4, and 12 h, respectively. Scale bars = 5 cm.
3.4. The Expression Patterns of ZjBZR2
The results of qRT-PCR exhibited ubiquitous expression of ZjBZR2 in in roots, stems, and leaves (young leaves, mature leaves, leaf sheaths, and lamina joints) (Figure 3B), with particularly high transcript accumulation in roots, stems, and lamina joints. Higher expression of ZjBZR2 was observed in young leaves compared to mature leaves. Remarkably, dehydration treatment induced pronounced GUS staining in ProZjBZR2: GUS transgenic seedlings (Figure 3C). After BR, PEG, and salt treatments, the expression level of ZjBZR2 was significantly upregulated in both leaves and roots (Figure 4A–F).
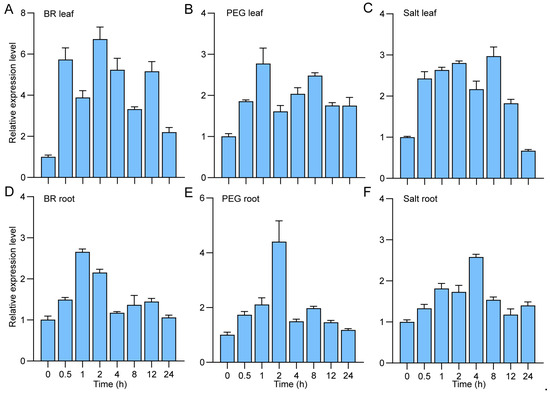
Figure 4.
The expression pattern analysis of ZjBZR2 in leaves and roots under BR, PEG, and salt treatments. (A–C). The expression analysis of ZjBZR2 in leaves after treatments with 1 µM 2,4-epibrassinolide (EBL), 20% PEG6000 (w/v), and 200 mM NaCl over 24 h, respectively. (D–F). The expression analysis of ZjBZR2 in roots after treatments with 1 µM 2,4-epibrassinolide (EBL), 20% PEG6000 (w/v), and 200 mM NaCl over 24 h, respectively. Data are displayed by means ± SE of three biological replicates.
3.5. The Function of ZjBZR2 in the BR Signaling Pathway
To study the function of ZjBZR2, ZjBZR2-overexpressing (OE) lines were generated in rice (Figure S1A,B). At the second leaves from the top, leaf angle was significantly increased in ZjBZR2-OE L1 and L2, being 1.41- and 1.56- fold greater than WT, respectively, at seedling stage (Figure 5A–C). At the heading stage, the phenotype of ZjBZR2-OE plants was consistent with seedling stage. ZjBZR2-OE lines (L1 and L2) showed a larger leaf angle than WT in both the flag leaves and the second leaves from the top (Figure 5D,E). ZjBZR1-OE lines were also generated in this study (Figure S1C,D). At the heading stage, ZjBZR1-OE plants showed only 63.0% and 46.4% increases in the leaf angles of the flag leaves and the second leaves from the top, respectively, compared to WT plants (Figure S2).
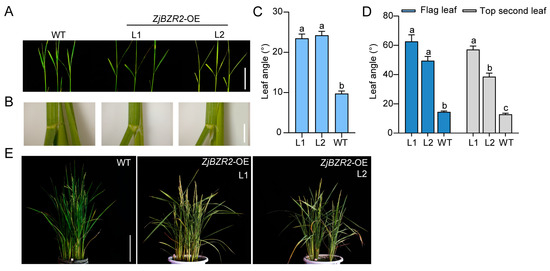
Figure 5.
The phenotypes of plants at seedling and heading stages. (A). The phenotype of the leaf angle of two-week-old WT and ZjBZR2-OE lines at seedling stage. Scale bar = 5 cm. (B). Stereomicroscopic images of the second leaf angle in two-week-old WT and ZjBZR2-OE lines. Scale bar = 5 mm. (C). The leaf angle at the second leaf in two-week-old WT and ZjBZR2-OE lines at seedling stage. (D). The leaf angle at the second leaf and flag leaf of two-week-old WT and ZjBZR2-OE lines at heading stage. (E). The phenotype of the leaf angle of WT and ZjBZR2-OE lines at heading stage. Scale bar = 20 cm. Data are displayed by means ± SE (n ≥ 15). One-way ANOVA was conducted and significant differences (p < 0.05) are shown by different letters.
To further elucidate the role of ZjBZR2 in the BR signaling pathway, BR sensitivity was analyzed in WT and ZjBZR2 transgenic seedlings. ZjBZR2-OE seedlings displayed different BR sensitivity, as shown by differential root growth in response to BR treatment (Figure 6). In the absence of BR, the root length of ZjBZR2-OE seedlings was significantly longer than that of WT seedlings. However, in the presence of BR, ZjBZR2-OE seedlings exhibited shorter root length compared to WT (Figure 6B).
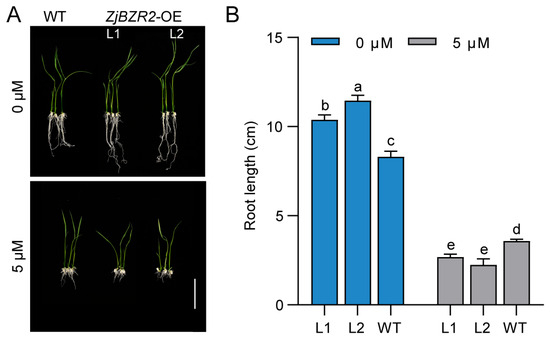
Figure 6.
BR sensitivity of ZjBZR2-OE transgenic lines. (A). Phenotypes of WT and ZjBZR2-OE seedlings grown in half-strength MS medium without 2, 4-epibrassinolide (EBL) (0 µM) and medium with 5 µM. Scale bar = 5 cm. (B). Root length of WT and ZjBZR2-OE lines grown in half-strength MS medium supplemented with 0 µM or 5 µM 2,4-epibrassinolide (EBL) under the light for 7 d. Data are displayed by means ± SE of three biological replicates. One-way ANOVA was conducted and significant differences (p < 0.05) are shown by different letters.
3.6. Overexpression of ZjBZR2 Enhanced Osmotic Stress Tolerance in Rice
To characterize the stress-response functions, WT and ZjBZR2-OE seedlings were subjected to osmotic stresses (20% PEG6000 and 200 mM NaCl), respectively. For the rate of leaf water loss, ZjBZR2-OE lines were significantly lower than that of WT (Figure 7B). The survival rate of ZjBZR2-OE lines (L1 and L2) was significantly increased by 28.1% and 17.7%, respectively, compared to WT plants after PEG treatments (Figure 7C). Similarly, the survival rate of ZjBZR2-OE lines (L1 and L2) was significantly increased by 43.8% and 19.8%, respectively, compared to WT plants after salt treatments (Figure 7D). Compared with WT seedlings, ZjBZR2-OE lines exhibited significantly lower electrolyte leakage after PEG or salt treatments (Figure 7E).
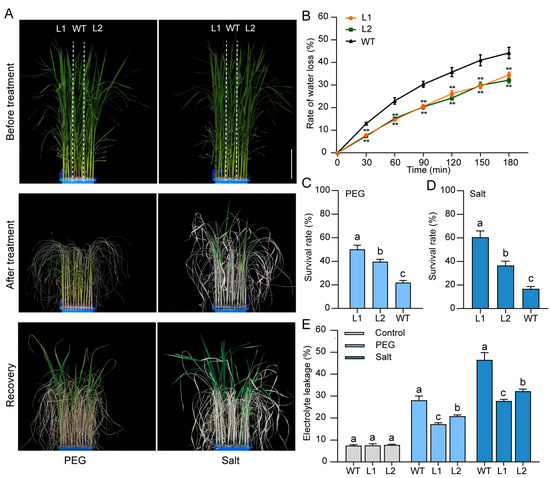
Figure 7.
Overexpression of ZjBZR2 enhanced PEG and salt tolerance in rice. (A). The phenotypes of four-week-old seedlings of ZjBZR2-OE (L1, L2) and WT after treatments with 20% PEG6000 and 200 mM NaCl for 5 d and then recovery for 7 d, respectively. The 12-row PCR plates were divided into three sections. Each set of four rows represents one line, designated from left to right as ZjBZR2-OE L1, WT, and ZjBZR2-OE L2. Scale bars = 10 cm. (B). Changes in water loss rate in detached leaves of WT and ZjBZR2-OE plants over 3 h. Data are displayed by means ± SE of three biological replicates. ** p < 0.01. (C,D). The survival rate of WT and ZjBZR2-OE seedlings after 5 d of PEG and salt treatments followed by 7 d of recovery, respectively. (E). Electrolyte leakage of leaves detached from WT and ZjBZR2-OE seedlings after 5 d of PEG and salt treatments, respectively. Data are displayed by means ± SE of three biological replicates. One-way ANOVA was conducted and significant differences (p < 0.05) are shown by different letters.
3.7. The Expression of Stress-Responsive and ROS-Scavenging-Related Genes in Rice
To explore the mechanism by which ZjBZR2 regulates osmotic stress tolerance, qRT-PCR was performed to evaluate the differential expression levels of genes associated with osmotic stress and ROS- scavenging in ZjBZR2-OE and WT plants under both normal and PEG-stressed conditions. The expression levels of osmotic stress tolerance-related genes including OsDREB1C, OsRREB16C, OsABCG5, and OsRAB21 were significantly upregulated in ZjBZR2-OE plants compared to WT (Figure 8A–D). ROS-scavenging genes including OsCATA, OsSODA1, OsPOD1, and OsPOD2 in ZjBZR2-OE lines were observed to be upregulated compared to WT under PEG treatment (Figure 8E–H).
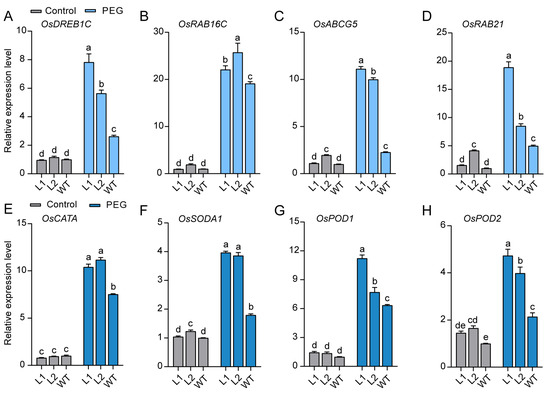
Figure 8.
The expression of stress-responsive and ROS-scavenging genes in WT and ZjBZR2-OE seedlings after 3 d of PEG treatments. (A). OsDREB1C, (B). OsRAB16C, (C). OsABCG5, (D). OsRAB21, (E). OsCATA, (F). OsSODA1, (G). OsPOD1, (H). OsPOD2. Data are displayed by means ± SE of three biological replicates. One-way ANOVA was conducted and significant differences (p < 0.05) are shown by different letters.
4. Discussion
Transcriptional factors play crucial roles in signal perception, signal transduction, and transcriptional regulation of gene expression [16,28,37]. Different BES/BZR TFs exhibit distinct regulatory mechanisms. In Arabidopsis thaliana, AtBZR1 functions as a transcriptional repressor [38], whereas AtBES1 acts as a transcriptional activator [39]. Both BRRE (BR response element) and E-box motifs are present in the promoters of BR-induced or BR-repressed downstream target genes, suggesting that different BES/BZR members selectively regulate downstream gene expression as either activators or repressors [17,38]. In this study, phylogenetic analysis showed that ZjBZR2 clustered with BZR2 proteins from other monocotyledonous plants (Figure 1A). Protein structure analysis revealed that the amino acid sequence of ZjBZR2 contained the N-terminal binding domain, GSK3-like kinase phosphorylation sites, and PEST motifs (Figure 1B). These characteristic structural features are also found in BES/BZR TFs of other species, such as Arabidopsis, wheat, and rice [9,16]. Additionally, our study confirmed that ZjBZR2 was a nuclear-localized transcriptional activator (Figure 2). However, the direct target genes of ZjBZR2 require further experimental validation in our next study.
Erect leaf angle is a valuable agronomic trait. It enhances light capture and biomass yield under high-density planting [40,41,42,43,44,45]. Therefore, it is a key target for breeding gramineous crops like maize, sorghum and rice [40,43,46]. In turfgrass, this trait also improves sod density, leading to superior turf quality [47]. Recent studies have observed extensive crosstalk among different plant hormone signaling pathways [48,49]. It has been reported that auxin, GA, MeJA, and ABA are able to directly or indirectly interact with key components of the BR pathway to regulate leaf angle [50,51,52,53,54,55]. In this study, the ZjBZR2 promoter contained multiple cis-acting elements associated with responses to ABA, auxin, GA, and MeJA (Figure 3A), suggesting that ZjBZR2, as a critical component of the BR signaling pathway, might mediate the crosstalk with other hormonal pathways to regulate plant growth and development. Expression pattern analysis revealed that ZjBZR2 was highly expressed in stems and lamina joints (Figure 3B), further supporting its role in regulating shoot height and leaf angle. ZjBZR2-OE plants showed an increased leaf angle phenotype (Figure 5) Combined with root length, assays under BR treatment suggested that ZjBZR2-OE seedlings showed enhanced BR sensitivity (Figure 6). These results illustrated that ZjBZR2, a member of the BES/BZR TFs family, acted as a positive regulator of the BR signaling pathway in rice and modulated leaf angle. Therefore, as a key regulator, controlling leaf angle in plants, ZjBZR2 was indicated to play a crucial role in breeding new varieties with high biomass and high-density tolerance.
Furthermore, multiple BES/BZR transcription factors are induced by osmotic stresses (drought, salt, cold, and heat), and BR signaling positively regulates plant tolerance to these stresses [16,25,56,57,58,59,60]. In the present study, the ZjBZR2 promoter could respond to dehydration stress (Figure 3C). Meanwhile, BR-induced expression patterns of ZjBZR2 in Zoysia japonica leaves and roots were consistent with those observed under PEG and salt stresses (Figure 4). These results indicated that ZjBZR2 played a conservative and crucial role in BR-mediated plant tolerance to osmotic stresses. In this study, overexpression of ZjBZR2 significantly enhanced osmotic stress (PEG and salt) tolerance in transgenic rice seedlings as shown by higher survival rate and lower water loss and electrolyte leakage, suggesting its positive regulatory role in osmotic stresses (Figure 7). The rice OsDREB1C, OsABCG5, OsRAB16C, and OsRAB21 genes were reported to be responsive to drought or salt stresses [61,62,63,64]. It has been previously reported that TaBZR2, the wheat ortholog of ZjBZR2, contributed to the BR-mediated drought tolerance partially by reducing O2- accumulation [16]. Notably, ZjBZR2 and TaBZR2 clustered into a group in the phylogenetic tree with an identity percentage of 83.79%. Therefore, the enhanced osmotic stress tolerance in this case was associated with the upregulation of stress-responsive genes, including OsDREB1C, OsABCG5, OsRAB16C, and OsRAB21, as well as genes encoding ROS-scavenging enzymes (OsCATA, OsSODA1, OsPOD1, and OsPOD2) in ZjBZR2-OE plants (Figure 8).
In conclusion, ZjBZR2 functioned as a positive regulator in the BR signaling pathway. The expression level of ZjBZR2 could be induced by BR, PEG, and salt stresses. ZjBZR2 positively regulated osmotic stresses (PEG and salt) tolerance in transgenic rice and modulated the expression of stress-responsive and ROS-scavenging genes, including OsDREB1C, OsRAB16C, OsABCG5, OsRAB21, OsCATA, OsSODA1, OsPOD1, and OsPOD2. Although this study characterized the function of ZjBZR2 primarily in rice, supplemented by bioinformatic analyses, its role in Z. japonica remains unexplored. Therefore, our conclusions primarily establish ZjBZR2 as a potential regulatory factor, and its practical potential in breeding awaits future functional confirmation in the native host.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture15192091/s1, Figure S1. Identification of ZjBZR2 and ZjBZR1-OE transgenic lines. A. Histochemical GUS staining of leaves in ZjBZR2-OE transgenic rice. B. Identification of ZjBZR2-OE transgenic lines using primers spanning the vector–gene junctions. C. Histochemical GUS staining of leaves in ZjBZR1-OE transgenic rice. D. Identification of ZjBZR1-OE transgenic lines. Figure S2. The phenotypes of WT and ZjBZR1-OE transgenic rice at heading stages. A. The phenotype of WT and ZjBZR1-OE lines at seedling stage. Scale bar = 20 cm. B. The leaf angle at the second leaf and flag leaf of WT andZjBZR1-OE lines at heading stage. Data are displayed by means ± SE (n ≥ 15). One-way ANOVA was conducted and significant differences (p < 0.05) are shown by different letters. Table S1. Primers used to construct expression vectors. Table S2. qPCR primer sequences used in this study.
Author Contributions
Q.Z.: methodology, data curation, writing original draft. J.Y.: supervision, writing—review and editing. Q.L.: resources, software, writing—review and editing. T.H.: data curation, visualization, writing—review and editing. Z.Y.: conceptualization, supervision, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Forestry Science and Technology Promotion Demonstration Funding Program of China (Su[2023]TG1) and the Breeding of New Varieties Evergreen Grass in Yangtze River Delta (ZA32303).
Data Availability Statement
The data were provided in the manuscript and the Supplementary Files. The sequence data supporting the findings of this study had been deposited in the NCBI database under accession numbers PV978106 (ZjBZR1) and PV978107 (ZjBZR2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shimada, S.; Komatsu, T.; Yamagami, A.; Nakazawa, M.; Matsui, M.; Kawaide, H.; Natsume, M.; Osada, H.; Asami, T.; Nakano, T. Formation and dissociation of the BSS1 protein complex regulates plant development via brassinosteroid signaling. Plant Cell 2015, 27, 375–390. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, W.; Cui, X.; Chen, M.; Yin, C.; Luo, Z.; Zhu, J.; Lucas, W.J.; Wang, Z.; Zhang, D. Brassinosteroids promote development of rice pollen grains and seeds by triggering expression of Carbon Starved Anther, a MYB domain protein. Plant J. 2015, 82, 570–581. [Google Scholar] [CrossRef] [PubMed]
- He, J.X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 10185–10190. [Google Scholar] [CrossRef]
- An, S.; Liu, Y.; Sang, K.; Wang, T.; Yu, J.; Zhou, Y.; Xia, X. Brassinosteroid signaling positively regulates abscisic acid biosynthesis in response to chilling stress in tomato. J. Integr. Plant Biol. 2023, 65, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Bai, M.Y.; Oh, E.; Zhu, J.Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu. Rev. Genet. 2012, 46, 701–724. [Google Scholar] [CrossRef]
- Tong, H.; Liu, L.; Jin, Y.; Du, L.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 2012, 24, 2562–2577. [Google Scholar] [CrossRef]
- Bai, M.Y.; Zhang, L.Y.; Gampala, S.S.; Zhu, S.W.; Song, W.Y.; Chong, K.; Wang, Z.Y. Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc. Natl. Acad. Sci. USA 2007, 104, 13839–13844. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Yu, J.; Wang, J.; Gao, Q.; Huang, L.; Chen, C.; Zhang, C.; Fan, X.; Zhao, D.; Liu, Q.Q.; et al. Brassinosteroids regulate rice seed germination through the BZR1-RAmy3D transcriptional module. Plant Physiol. 2022, 189, 402–418. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, X.; Ren, R.; Shi, Q.; Xiao, H.; Chen, Z.; Yue, M.; Wu, Y.; Hou, H.; Li, L. The histone deacetylase 1/GSK3/SHAGGY-like kinase 2/BRASSINAZOLE-RESISTANT 1 module controls lateral root formation in rice. Plant Physiol. 2022, 189, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, C.; Ihara, Y.; Wu, X.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 2000, 12, 1591–1606. [Google Scholar] [CrossRef][Green Version]
- Liu, D.; Yu, Z.; Zhang, G.; Yin, W.; Li, L.; Niu, M.; Meng, W.; Zhang, X.; Dong, N.; Liu, J.; et al. Diversification of plant agronomic traits by genome editing of brassinosteroid signaling family genes in rice. Plant Physiol. 2021, 187, 2563–2576. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Chi, Y.; Yu, M.; Wang, Z.; Zhou, M.; Zhao, L.; Shi, J.; Wang, F.; Wang, C. Birch (Betula platyphylla) BES/BZR transcription factor BpBZR1-6 improves salt tolerance in transgenic Arabidopsis thaliana. BMC Plant Biol. 2024, 24, 1136. [Google Scholar] [CrossRef]
- Cui, X.Y.; Gao, Y.; Guo, J.; Yu, T.F.; Zheng, W.J.; Liu, Y.W.; Chen, J.; Xu, Z.S.; Ma, Y.Z. BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol. 2019, 180, 605–620. [Google Scholar] [CrossRef]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef]
- Shen, L.; Qi, Z.; Dai, X.; Ai, Y.; Chen, J.; Chao, Y.; He, H.; Han, L.; Xu, L. Chromosome-scale genome assembly of Zoysia japonica uncovers cold tolerance candidate genes. Sci. Data 2025, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.X.; Norton, T.; Wang, Z.Y. Transgenic zoysiagrass (Zoysia japonica) plants obtained by Agrobacterium-mediated transformation. Plant Cell Rep. 2006, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Toyama, K.; Bae, C.H.; Kang, J.G.; Lim, Y.P.; Adachi, T.; Riu, K.Z.; Song, P.S.; Lee, H.Y. Production of Herbicide-tolerant Zoysiagrass by Agrobacterium-mediated Transformation. Mol. Cells 2003, 16, 19–27. [Google Scholar] [CrossRef]
- Zuo, Z.F.; Kang, H.G.; Park, M.Y.; Jeong, H.; Sun, H.J.; Yang, D.H.; Lee, Y.E.; Song, P.S.; Lee, H.Y. Overexpression of ICE1, a regulator of cold-induced transcriptome, confers cold tolerance to transgenic Zoysia japonica. J. Plant Biol. 2019, 62, 137–146. [Google Scholar] [CrossRef]
- Chen, J.; Lei, X.W.; Zhang, H.L.; Lin, Z.; Wang, H.; Hu, W. Laboratory model test study of the hydrological effect on granite residual soil slopes considering different vegetation types. Sci. Rep. 2021, 11, 14668. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirakawa, H.; Kosugi, S.; Nakayama, S.; Ono, A.; Watanabe, A.; Hashiguchi, M.; Gondo, T.; Ishigaki, G.; Muguerza, M.; et al. Sequencing and comparative analyses of the genomes of zoysiagrasses. DNA Res. 2016, 23, 171–180. [Google Scholar] [CrossRef]
- Park, H.; Bae, E.; Jung, J.G.; Kim, J.; Choi, B.Y.; Lee, G.J.; Kim, C.; Shim, D. Chromosome-level genome of Zoysia sinica in the intertidal zone reveals genomic insights into waterlogging stress adaptation. Plant Genome-Us 2025, 18, e70070. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, G.; Zhang, Z.; Wan, Z.; Liu, Z.; Lv, J.; Yu, J. Genome-wide identification and expression analysis of BZR gene family and associated responses to abiotic stresses in cucumber (Cucumis sativus L.). BMC Plant Biol. 2023, 23, 214. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Tan, Y.Q.; Qu, M.H.; Hong, K.; Zeng, L.J.; Wang, L.; Zhuang, C.X.; Qian, Q.; Hu, J.; Xiong, G.S. OsWR2 recruits HDA704 to regulate the deacetylation of H4K8ac in the promoter of OsABI5 in response to drought stress. J. Integr. Plant Biol. 2023, 65, 1651–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Zhao, R.R.; Wang, H.T.; Ren, S.L.; Shi, L.Y.; Huang, S.Z.; Wei, Z.Q.; Guo, B.Y.; Jin, J.Y.; Zhong, Y.; et al. OsWRKY28 positively regulates salinity tolerance by directly activating OsDREB1B expression in rice. Plant Cell Rep. 2023, 42, 223–234. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Wang, J.; Fan, Z.; Qu, X.; Yan, M.; Zhang, C.; Yang, K.; Zou, J.; Le, J. The rice R2R3 MYB transcription factor FOUR LIPS connects brassinosteroid signaling to lignin deposition and leaf angle. Plant Cell 2024, 36, 4768–4785. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Sun, X.; Khan, N.U.; Zhong, Q.; Zhang, Z.; Zhang, H.; Ming, F.; Li, Z.; Li, J. Variations in OsSPL10 confer drought tolerance by directly regulating OsNAC2 expression and ROS production in rice. J. Integr. Plant Biol. 2023, 65, 918–933. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Li, X.; Tan, W.; You, A.; Wu, S.; Tao, Y.; Chen, C.; Wang, J.; Zhang, D.; et al. OsPP2C09, a negative regulatory factor in abscisic acid signalling, plays an essential role in balancing plant growth and drought tolerance in rice. New Phytol. 2020, 227, 1417–1433. [Google Scholar] [CrossRef]
- Cao, W.H.; Liu, J.; He, X.J.; Mu, R.L.; Zhou, H.L.; Chen, S.Y.; Zhang, J.S. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007, 143, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Jin, L.; Sun, Y.; Zhan, C.; Tang, S.; Qin, T.; Liu, N.; Huang, J. OsNAC120 balances plant growth and drought tolerance by integrating GA and ABA signaling in rice. Plant Commun. 2024, 5, 100782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Z.; Wang, Y.; Wang, J.; Xiao, M.; Liu, H.; Quan, R.; Zhang, H.; Huang, R.; Zhu, L.; et al. Cellulose synthase-like protein OsCSLD4 plays an important role in the response of rice to salt stress by mediating abscisic acid biosynthesis to regulate osmotic stress tolerance. Plant Biotechnol. J. 2022, 20, 468–484. [Google Scholar] [CrossRef]
- Xiong, H.; Yu, J.; Miao, J.; Li, J.; Zhang, H.; Wang, X.; Liu, P.; Zhao, Y.; Jiang, C.; Yin, Z.; et al. Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol. 2018, 178, 451–467. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, L.; Zhang, L.; Zhang, Z.; Zhang, H.; Quan, R.; Zhou, S.; Huang, R. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2012, 78, 275–288. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukasinovic, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- He, J.X.; Gendron, J.M.; Sun, Y.; Gampala, S.S.; Gendron, N.; Sun, C.Q.; Wang, Z.Y. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 2005, 307, 1634–1638. [Google Scholar] [CrossRef]
- Yin, Y.; Vafeados, D.; Tao, Y.; Yoshida, S.; Asami, T.; Chory, J. A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 2005, 120, 249–259. [Google Scholar] [CrossRef]
- Cao, Y.; Zhong, Z.; Wang, H.; Shen, R. Leaf angle: A target of genetic improvement in cereal crops tailored for high-density planting. Plant Biotechnol. J. 2022, 20, 426–436. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, J.; Huang, R.; Huang, Y.; Wang, H.; Jiang, L.; Fang, X. Phytohormones signaling and crosstalk regulating leaf angle in rice. Plant Cell Rep. 2016, 35, 2423–2433. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Chen, F.; Qin, W.; Yang, H.; Zhao, S.; Xia, J.; Du, X.; Zhu, Y.; Wu, L.; et al. Maize smart-canopy architecture enhances yield at high densities. Nature 2024, 632, 576–584. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Y. Leaf angle regulation toward a maize smart canopy. Plant J. 2025, 121, e17208. [Google Scholar] [CrossRef]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 2006, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, Y.; Sakamoto, T.; Inukai, Y.; Agetsuma, M.; Kitano, H.; Ashikari, M.; Matsuoka, M. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 2006, 141, 924–931. [Google Scholar] [CrossRef]
- Truong, S.K.; McCormick, R.F.; Rooney, W.L.; Mullet, J.E. Harnessing genetic variation in leaf angle to increase productivity of Sorghum bicolor. Genetics 2015, 201, 1229–1238. [Google Scholar] [CrossRef]
- Kazemi, F.; Golzarian, M.R.; Rabbani Kheir Khah, S.M. Quality and establishment of some water-conserving turfgrass species for sustainable development and some ecosystem services in arid urban environments. Land 2024, 13, 721. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, Z.; Wang, X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 4543–4548. [Google Scholar] [CrossRef]
- Tong, H.; Xiao, Y.; Liu, D.; Gao, S.; Liu, L.; Yin, Y.; Jin, Y.; Qian, Q.; Chu, C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, F.; Chen, Z.; Teng, Z.; Sun, K.; Li, X.; Yu, J.; Zhang, G.; Liang, Y.; Huang, X.; et al. Synergistic interplay of ABA and BR signal in regulating plant growth and adaptation. Nat. Plants 2021, 7, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Plitsi, P.K.; Samakovli, D.; Roka, L.; Rampou, A.; Panagiotopoulos, K.; Koudounas, K.; Isaioglou, I.; Haralampidis, K.; Rigas, S.; Hatzopoulos, P.; et al. GA-mediated disruption of RGA/BZR1 complex requires HSP90 to promote hypocotyl elongation. Int. J. Mol. Sci. 2022, 24, 88. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Li, X.; Shi, H.; Lv, M.; He, L.; Bai, W.; Cheng, S.; Chu, J.; He, K.; et al. Jasmonates regulate apical hook development by repressing brassinosteroid biosynthesis and signaling. Plant Physiol. 2023, 193, 1561–1579. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, H.; Wu, D.; Zhang, Z.; Guo, Z.; Yang, N.; Xia, K.; Zhou, X.; Oh, K.; Matsuoka, M.; et al. Methyl jasmonate inhibits lamina joint inclination by repressing brassinosteroid biosynthesis and signaling in rice. Plant Sci. 2015, 241, 238–245. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Mockler, T.C.; Chory, J. Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol. 2004, 2, E258. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Xu, Y.; Yu, C.; Shen, C.; Qian, Q.; Geisler, M.; Jiang, D.A.; Qi, Y. The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.P.; Duan, X.B.; Luo, L.; Dai, S.J.; Ding, Z.J.; Xia, G.M. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Guo, G.; Yan, H.; Qiu, Z.; Liu, Q.; Zeng, B. Characterization of Brassinazole resistant (BZR) gene family and stress induced expression in Eucalyptus grandis. Physiol. Mol. Biol. Plants 2018, 24, 821–831. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Qiu, S.; Zheng, H.; Lu, Y.; Peng, J.; Wu, G.; Chen, J.; Rao, S.; Yan, F. Genome-wide identification and expression profiling of the BZR transcription factor gene family in Nicotiana benthamiana. Int. J. Mol. Sci. 2021, 22, 10379. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, J.; Hou, J.; Liu, C.; Geng, S.; Qin, M.; Li, W.; Dai, Z.; Wu, Z.; Lei, Z.; et al. Identification of the brassinazole-resistant (BZR) gene family in wheat (Triticum aestivum L.) and the molecular cloning and functional characterization of TaBZR2.1. Int. J. Mol. Sci. 2024, 25, 12545. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.Q.; Li, G.L.; Zhang, S.Y. Genome-wide identification, characterization, and expression patterns of the BZR transcription factor family in sugar beet (Beta vulgaris L.). BMC Plant Biol. 2019, 19, 191. [Google Scholar] [CrossRef]
- Mundy, J.; Chua, N.H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988, 7, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Takano, S.; Sato, M.; Furukawa, K.; Nagasawa, H.; Yoshikawa, S.; Kasuga, J.; Tokuji, Y.; Yazaki, K.; Nakazono, M.; et al. Rice stomatal closure requires guard cell plasma membrane ATP-binding cassette transporter RCN1/OsABCG5. Mol. Plant 2016, 9, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, S.; Guan, X.; Jiang, Y.; Wang, B.; Hua, J.; Zou, B. Dehydration-responsive element binding protein 1C, 1E, and 1G promote stress tolerance to chilling, heat, drought, and salt in rice. Front. Plant Sci. 2022, 13, 851731. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).