Synergistic Effect of PGPR and Nutrient Complex on Soybean Seed Germination and Initial Seedling Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Bacterial Strains
2.3. Preparation of PGPR Biostimulants and Nutrient Complex
2.4. Seed Treatments
2.5. Germination Assay
2.5.1. Determination of Seed Germination Parameters
2.5.2. Determination of Seedling Growth Parameters
- SLS, RLS—shoot and root length (mm) at the start (5th day) of a measurement period;
- SLE, RLE—shoot and root length (mm) at the end (8th day) of a measurement period;
- TE − TS—time duration (days) between two measurement periods.
2.5.3. Determination of Seedling Biomass Accumulation
2.5.4. Determination of Seedling Vigor Index
- SL, RL—shoot and root length (cm) on the 8th day;
- FG—final germination (%) on the 8th day.
- All determinations were performed in three replications.
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agyenim-Boateng, K.G.; Zhang, S.; Zhangm, S.; Khattak, A.N.; Shaibu, A.; Abdelghany, A.M.; Qi, J.; Azam, M.; Ma, C.; Feng, Y.; et al. The nutritional composition of the vegetable soybean (maodou) and its potential in combatting malnutrition. Front. Nutr. 2023, 9, 1034115. [Google Scholar] [CrossRef]
- Sohidul Islam, M.; Muhyidiyn, I.; Rafiqul Islam, M.; Kamrul Hasan, M.; Golam Hafeez, A.S.M.; Moaz Hosen, M.; Saneoka, H.; Ueda, A.; Liu, L.; Naz, M.; et al. Soybean and Sustainable Agriculture for Food Security. In Soybean—Recent Advances in Research and Applications; Ohyama, T., Takahashi, Y., Ohtake, N., Sato, T., Tanabata, S., Eds.; IntechOpen: London, UK, 2022; Chapter 1. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Pan, Y.; Zhang, X.; Xu, K.; Qu, Y.; Li, H. Quantifying high-temperature and drought stress effects on soybean growth and yield in the Western Guanzhong Plain. Atmosphere 2024, 15, 392. [Google Scholar] [CrossRef]
- Poudel, S.; Vennam, R.R.; Shrestha, A.; Reddy, K.R.; Wijewardane, N.K.; Reddy, K.N.; Bheemanahalli, R. Resilience of soybean cultivars to drought stress during flowering and early-seed setting stages. Sci. Rep. 2023, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Dell’Olmo, E.; Tiberini, A.; Sigillo, L. Leguminous seedborne pathogens: Seed health and sustainable crop management. Plants 2023, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Shango, A.J.; N’Danikou, S.; Ramadhani, S.; Sumaye, S.; Nickas, J.; Daud, M.L. Prevalence of seed-borne fungi on soybean (Glycine max L. Merr.) seeds stored under medium-term cold room facilities: Implications for genebanks. Seeds 2024, 3, 589–607. [Google Scholar] [CrossRef]
- Rao, P.J.M.; Pallavi, M.; Bharathi, Y.; Priya, P.B.; Sujatha, P.; Prabhavathi, K. Insights into mechanisms of seed longevity in soybean: A review. Front. Plant Sci. 2023, 14, 1206318. [Google Scholar] [CrossRef] [PubMed]
- Tchonkouang, R.D.; Onyeaka, H.; Nkoutchou, H. Assessing the vulnerability of food supply chains to climate change-induced disruptions. Sci. Total Environ. 2024, 920, 171047. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J. Harnessing Plant Growth-Promoting Rhizobacteria: A Dual Approach as Biofertilizers and Biopesticides for Field and Vegetable Crop Production. In Soil Bacteria: Biofertilization and Soil Health; Dheeman, S., Islam, T., Egamberdieva, D., Siddiqui, N., Eds.; Springer Nature: Singapore, 2024; Chapter 15; pp. 391–450. [Google Scholar]
- dos Reis, G.A.; Martínez-Burgos, W.J.; Pozzan, R.; Pastrana Puche, Y.; Ocán-Torres, D.; de Queiroz Fonseca Mota, P.; Rodrigues, C.; Lima Serra, J.; Scapini, T.; Karp, S.G.; et al. Comprehensive review of microbial inoculants: Agricultural applications, technology trends in patents, and regulatory frameworks. Sustainability 2024, 16, 8720. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Machado, C.G.; Silva, G.Z.d.; Cruz, S.C.S.; Anjos, R.C.L.d.; Silva, C.L.; Matos, L.F.L.d.; Smaniotto, A.O. Germination and vigor of soybean and corn seeds treated with mixed mineral fertilizers. Plants 2023, 12, 338. [Google Scholar] [CrossRef]
- Staniak, M.; Szpunar-Krok, E.; Wilczewski, E.; Kocira, A.; Podleśny, J. The function of macronutrients in helping soybeans to overcome the negative effects of drought stress. Agronomy 2024, 14, 1744. [Google Scholar] [CrossRef]
- Houmani, H.; Ben Slimene Debez, I.; Turkan, I.; Mahmoudi, H.; Abdelly, C.; Koyro, H.-W.; Debez, A. Revisiting the Potential of Seed Nutri-Priming to Improve Stress Resilience and Nutritive Value of Cereals in the Context of Current Global Challenges. Agronomy 2024, 14, 1415. [Google Scholar] [CrossRef]
- Zewide, I.; Sherefu, A. Review Paper on Effect of Micronutrients for Crop Production. Nutr. Food Process 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Yeremko, L.; Czopek, K.; Staniak, M.; Marenych, M.; Hanhur, V. Role of environmental factors in legume-Rhizobium symbiosis: A review. Biomolecules 2025, 15, 118. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Wirth, S.J.; Alam, P.; Alyemeni, M.N.; Ahmad, P. Interactive effects of nutrients and Bradyrhizobium japonicum on the growth and root architecture of soybean (Glycine max L.). Front. Microbiol. 2018, 9, 1000. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Tintor, B.; Milošević, D.; Ignjatov, M.; Nikolić, Z. Bio-priming of soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to improve seed germination and the initial seedling growth. Plants 2022, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Zeffa, D.M.; Fantin, L.H.; Koltun, A.; de Oliveira, A.L.M.; Nunes, M.P.B.A.; Canteri, M.G.; Gonçalves, L.S.A. Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: A meta-analysis of studies from 1987 to 2018. PeerJ 2020, 8, e7905. [Google Scholar] [CrossRef] [PubMed]
- Tamindžić, G.; Miljaković, D.; Ignjatov, M.; Miladinović, J.; Đorđević, V.; Milošević, D.; Jovičić, D.; Vlajić, S.; Budakov, D.; Grahovac, M. Impact of simultaneous nutrient priming and biopriming on soybean seed quality and health. Plants 2024, 13, 2557. [Google Scholar] [CrossRef]
- Marinković, J.; Bjelić, D.; Tintor, B.; Ignjatov, M.; Nikolić, Z.; Đukić, V.; Balešević-Tubić, S. Molecular identification of Bradyrhizobium japonicum strains isolated from root nodules of soybean (Glycine max L.). Zb. Matice Srp. Za Prir. Nauk. 2017, 132, 49–56. [Google Scholar] [CrossRef]
- Marinković, J.; Miljaković, D.; Tintor, B.; Vasiljević, M.; Đorđević, V.; Miladinović, J.; Đukić, V. New Bradyrhizobium strains enhance soybean nodulation in soils of different fertility. In Book of Abstracts of the 4th International and 16th National Congress of the Serbian Society of Soil Science: The Soil Re-Union: Science for Healthy Soils, Serbian Society of Soil Science, Belgrade, Serbia, and the Institute of Field and Vegetable Crops, National Institute of the Republic of Serbia, Novi Sad, Serbia, Fruške Terme, Vrdnik, Republic of Serbia, 20–23 October 2025. (In Press); Serbian Society of Soil Science: Belgrade, Serbia; Institute of Field and Vegetable Crops: Novi Sad, Serbia, 2025.
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Ignjatov, M.; Milošević, D.; Nikolić, Z. Effect of plant growth promoting Bacillus spp. on germination and seedling growth of soybean. Legume Res. 2023, 45, 487–491. [Google Scholar] [CrossRef]
- Bjelić, D.; Marinković, J.; Tintor, B.; Mrkovački, N. Antifungal and plant growth promoting activities of indigenous rhizobacteria isolated from Maize (Zea mays L.) rhizosphere. Commun. Soil. Sci. Plant Anal. 2018, 49, 88–98. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing 2022; International Seed Testing Association: Wallisellen, Switzerland, 2022. [Google Scholar]
- Tamindžić, G.; Ignjatov, M.; Miljaković, D.; Červenski, J.; Milošević, D.; Nikolić, Z.; Vasiljević, S. Seed priming treatments to improve heat stress tolerance of garden pea (Pisum sativum L.). Agriculture 2023, 13, 439. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Santoyo, G.; Glick, B.R. Recent advances in the bacterial phytohormone modulation of plant growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Kozaki, A.; Aoyanagi, T. Molecular Aspects of Seed Development Controlled by Gibberellins and Abscisic Acids. Int. J. Mol. Sci. 2022, 23, 1876. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Smalle, J.A. Auxin/Cytokinin Antagonistic Control of the Shoot/Root Growth Ratio and Its Relevance for Adaptation to Drought and Nutrient Deficiency Stresses. Int. J. Mol. Sci. 2022, 23, 1933. [Google Scholar] [CrossRef]
- Rubio, V.; Bustos, R.; Irigoyen, M.L.; Cardona-López, X.; Rojas-Triana, M.; Paz-Ares, J. Plant hormones and nutrient signaling. Plant Mol. Biol. 2009, 69, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Deng, X.; Xiao, Q.; Han, Y.; Zhu, S.; Chen, J. IAA priming improves the germination and seedling growth in cotton (Gossypium hirsutum L.) via regulating the endogenous phytohormones and enhancing the sucrose metabolism. Ind. Crops Prod. 2020, 155, 112788. [Google Scholar] [CrossRef]
- Araujo, F.F.; Bonifacio, A.; Bavaresco, L.G.; Mendes, L.W.; Araujo, A.S.F. Bacillus subtilis changes the root architecture of soybean grown on nutrient-poor substrate. Rhizosphere 2021, 18, 100348. [Google Scholar] [CrossRef]
- Pérez-García, L.-A.; Sáenz-Mata, J.; Fortis-Hernández, M.; Navarro-Muñoz, C.E.; Palacio-Rodríguez, R.; Preciado-Rangel, P. plant-growth-promoting rhizobacteria improve germination and bioactive compounds in cucumber seedlings. Agronomy 2023, 13, 315. [Google Scholar] [CrossRef]
- Kangsopa, J.; Singsopa, A.; Thawong, N. Seed coating with bacteria-producing indole-3-acetic acid (IAA) on germination, seedling growth and nutrient contents of vegetable soybean [Glycine max (L.) Merrill]. Legume Res. 2025, 48, 1130–1138. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Đorđević, V.; Vasiljević, M.; Tintor, B.; Jaćimović, S.; Ristić, Ž. Integrated use of Consortia-based Microbial Inoculants and Nutrient Complex Stimulates the Rhizosphere Microbiome and Soybean Productivity. Legume Res. 2024, 47, 120–125. [Google Scholar] [CrossRef]
- Holz, M.; Zarebanadkouki, M.; Benard, P.; Hoffmann, M.; Dubbert, M. Root and rhizosphere traits for enhanced water and nutrients uptake efficiency in dynamic environments. Front. Plant Sci. 2024, 15, 1383373. [Google Scholar] [CrossRef]
- Gholami, A.; Shahsavani, S.; Nezarat, S. The Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Germination, Seedling Growth and Yield of Maize. Waset 2009, 49, 19–24. [Google Scholar]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, S.; Saxena, G.; Pradhan, N.; Koul, M.; Kharkwal, A.C.; Sayyed, R. A review on microbe–mineral transformations and their impact on plant growth. Front. Microbiol. 2025, 16, 1549022. [Google Scholar] [CrossRef] [PubMed]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Verma, N.; Tewari, R.K. Micronutrient deficiency-induced oxidative stress in plants. Plant Cell Rep. 2024, 43, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Z.; Shao, J.; Xu, Z.; Liu, Y.; Xun, W.; Miao, Y.; Shen, Q.; Zhang, R. Biocontrol mechanisms of Bacillus: Improving the efficiency of green agriculture. Microb. Biotechnol. 2023, 16, 2250–2263. [Google Scholar] [CrossRef]
- Villavicencio-Vasquez, M.; Espinoza-Lozano, F.; Espinoza-Lozano, L.; Coronel-Leon, J. Biological control agents: Mechanisms of action, selection, formulation and challenges in agriculture. Front. Agron. 2025, 7, 1578915. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Rossetim, M.F.T.; Motta, A.C.V.; Kondo, Y.R.; Ruthes, B.E.S.; Hungria, M.; Salles, J.F.; Kaschuk, G. Enhancing soybean yield through inoculation of multifunctional microbial consortia. Int. J. Agron. 2025, 2025, 9491715. [Google Scholar] [CrossRef]
- Kozieł, M.; Gebala, B.; Martyniuk, S. Response of soybean to seed inoculation with Bradyrhizobium japonicum and with mixed inoculants of B. japonicum and Azotobacter chroococcum. Pol. J. Microbiol. 2013, 62, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-I.; Teaumroong, N. Co-Inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Iutynska, H.; Goloborodko, S.; Tytova, L.; Dubynska, O. Effectiveness of endophytic-rhizobial seed inoculation of soybean (Glycine max (L.) Merr.) cultivated in irrigated soil. J. Cent. Eur. Agri. 2022, 23, 40–53. [Google Scholar] [CrossRef]
- Xing, P.; Zhao, Y.; Guan, D.; Li, L.; Zhao, B.; Ma, M.; Jiang, X.; Tian, C.; Cao, F.; Li, J. Effects of Bradyrhizobium co-inoculated with Bacillus and Paenibacillus on the structure and functional genes of soybean rhizobacteria community. Genes 2022, 13, 1922. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.d.C.; Egea-Gilabert, C.; Conesa, E.; Ochoa, J.; Vicente, M.J.; Franco, J.A.; Bañon, S.; Martínez, J.J.; Fernández, J.A. The importance of ion homeostasis and nutrient status in seed development and germination. Agronomy 2020, 10, 504. [Google Scholar] [CrossRef]
- Abulfaraj, A.A.; Jalal, R.S. Use of plant growth-promoting bacteria to enhance salinity stress in soybean (Glycine max L.) plants. Saudi J. Biol. Sci. 2021, 28, 3823–3834. [Google Scholar] [CrossRef]
- Kuzmicheva, Y.V.; Shaposhnikov, A.I.; Petrova, S.N.; Makarova, N.M.; Tychinksaya, I.L.; Puhalsky, J.V.; Parahin, N.V.; Tikhonovich, I.A.; Belimov, A.A. Variety specific relationships between effects of rhizobacteria on root exudation, growth and nutrient uptake of soybean. Plant Soil 2017, 419, 83–96. [Google Scholar] [CrossRef]
- Egamberdieva, D. Growth response of wheat cultivars to bacterial inoculation in calcareous soil. Plant Soil Environ. 2010, 56, 570–573. [Google Scholar] [CrossRef]
- Figiel, S.; Rusek, P.; Ryszko, U.; Brodowska, M.S. Microbially Enhanced Biofertilizers: Technologies, Mechanisms of Action, and Agricultural Applications. Agronomy 2025, 15, 1191. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.; Singh, K.; Katoch, K.; Zaeen, A.A.; Birhan, D.A.; Singh, A.; Sandhu, H.S.; Singh, H.; Sahrma, L.K. Smart fertilizer technologies: An environmental impact assessment for sustainable agriculture. Smart Agr. Technol. 2024, 8, 100504. [Google Scholar] [CrossRef]
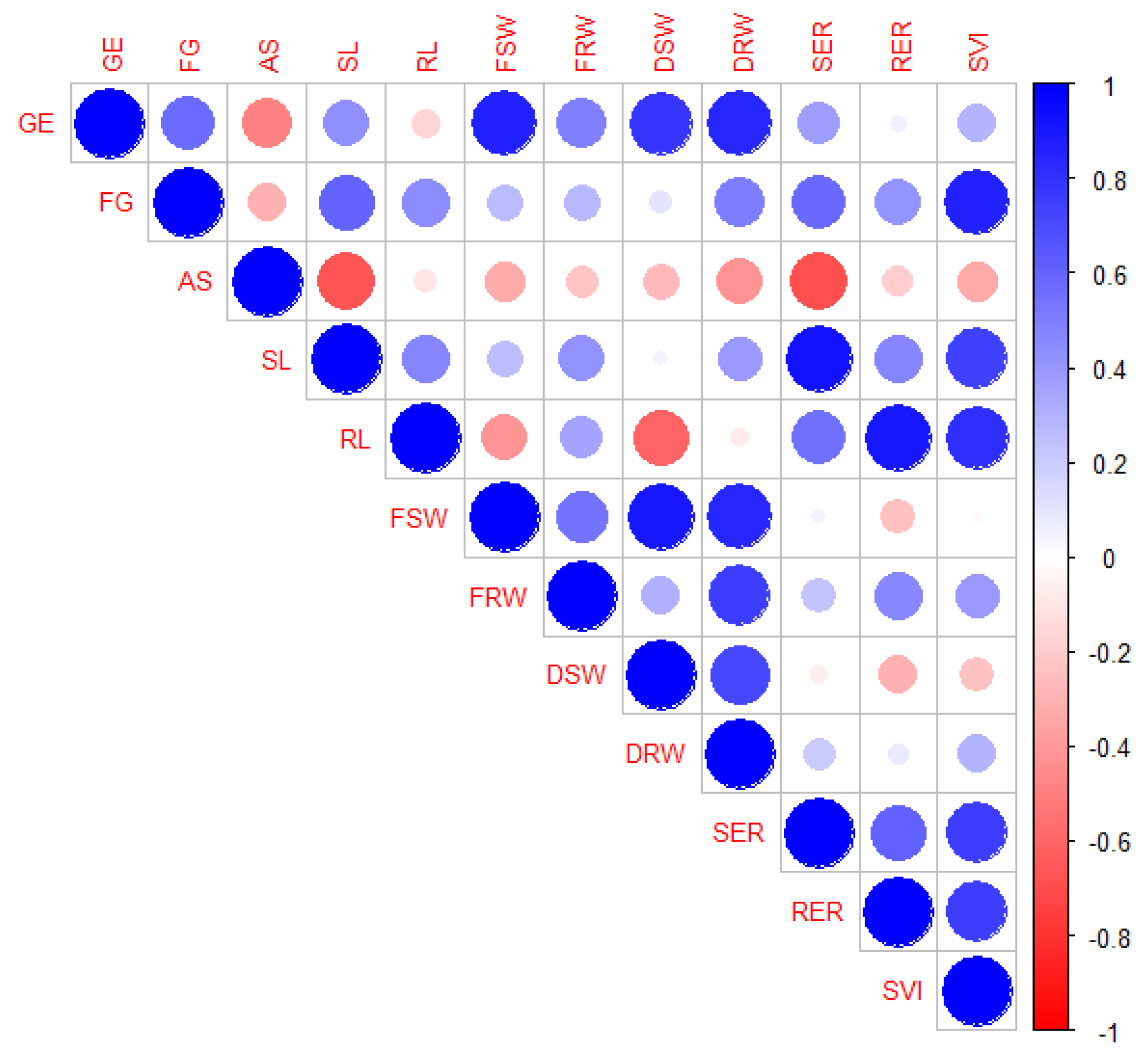
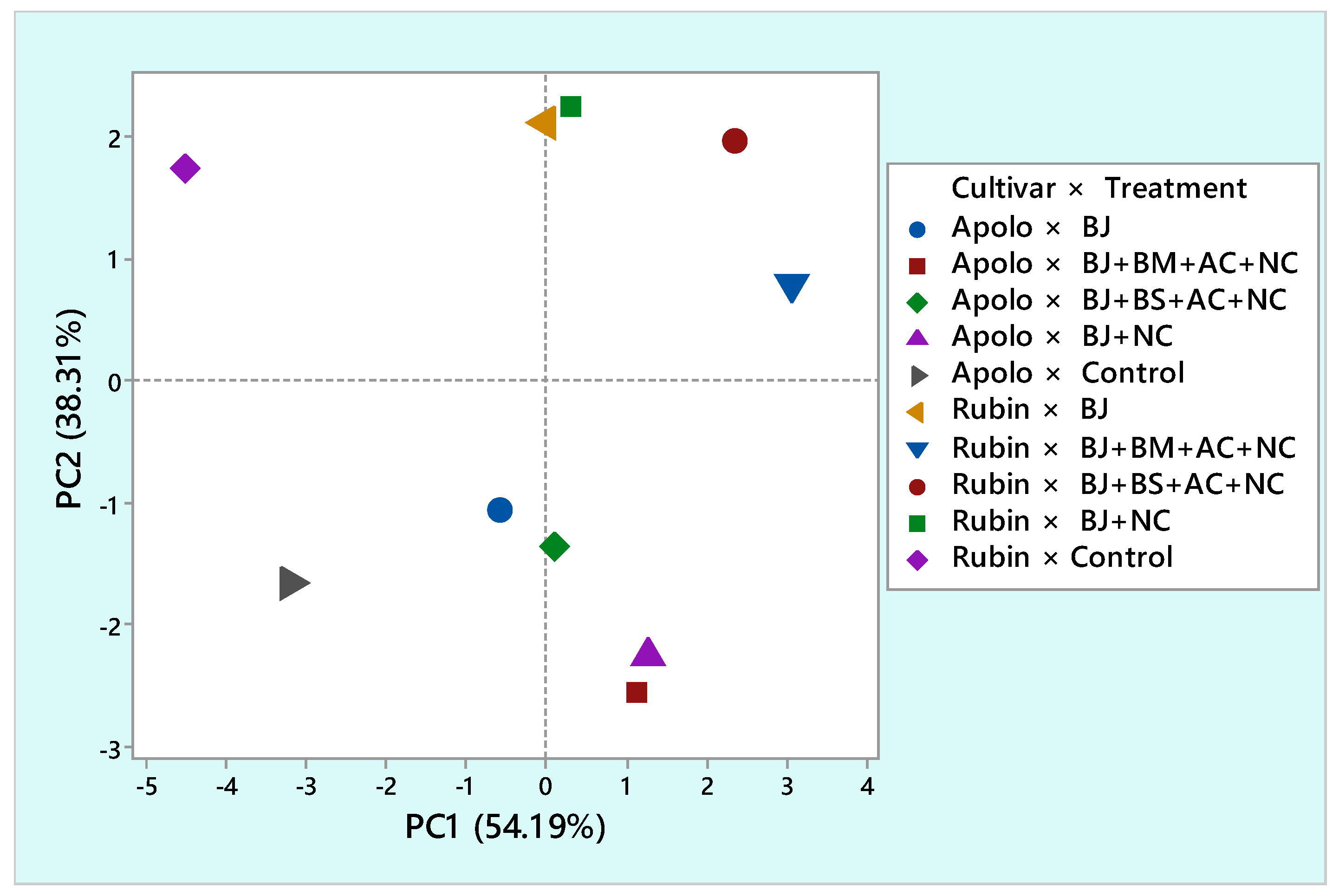
| Traits | Factor | ||
|---|---|---|---|
| Cultivar (C) | Treatment (T) | C × T | |
| Germination Energy | *** | *** | *** |
| Final Germination | ns | *** | *** |
| Abnormal Seedlings | ns | * | ns |
| Seedling Vigor Index | *** | *** | *** |
| Shoot Length | * | *** | *** |
| Root Length | *** | *** | *** |
| Shoot Elongation Rate | * | *** | *** |
| Root Elongation Rate | *** | *** | ** |
| Fresh Shoot Weight | *** | *** | ** |
| Fresh Root Weight | ns | *** | ** |
| Dry Shoot Weight | *** | *** | *** |
| Dry Root Weight | *** | *** | *** |
| Treatments | Germination Energy (%) | Final Germination (%) | Abnormal Seedlings (%) | Seedling Vigor Index |
|---|---|---|---|---|
| ‘NS Apolo’ | ||||
| Control | 57.7 g | 77.7 ce | 3.33 ab | 1923 c |
| BJ | 64.3 ef | 81.3 bcd | 3.67 ab | 2172 b |
| BJ + NC | 65.7 de | 93.7 a | 4.00 a | 2658 a |
| BJ + AC + BS + NC | 61.3 f | 81.3 bcd | 2.33 ab | 2199 b |
| BJ + AC + BM + NC | 65.0 e | 82.7 bc | 1.33 b | 2360 b |
| ‘NS Rubin’ | ||||
| Control | 63.3 ef | 77.3 e | 4.00 a | 1619 d |
| BJ | 70.3 c | 78.3 bcd | 3.67 ab | 1927 c |
| BJ + NC | 68.7 cd | 83.0 b | 1.67 ab | 2037 c |
| BJ + AC + BS + NC | 78.3 a | 89.3 a | 1.67 ab | 2264 ab |
| BJ + AC + BM + NC | 75.0 b | 89.7 a | 1.33 b | 2392 b |
| Average | ||||
| Control | 60.5 c | 77.5 b | 3.67 a | 1771 d |
| BJ | 67.3 b | 79.8 b | 3.67 a | 2049 c |
| BJ + NC | 67.2 b | 88.3 a | 2.83 ab | 2347 a |
| BJ + AC + BS + NC | 69.8 a | 85.3 a | 2.00 ab | 2232 b |
| BJ + AC + BM + NC | 70.0 a | 86.2 a | 1.33 b | 2376 a |
| Treatments | Shoot Length (cm) | Root Length (cm) | Shoot Elongation Rate | Root Elongation Rate |
|---|---|---|---|---|
| ‘NS Apolo’ | ||||
| Control | 120 de | 128 cd | 20.4 bcd | 15.2 c |
| BJ | 124 cd | 143 b | 20.7 bcd | 19.8 b |
| BJ + NC | 123 cd | 161 a | 20.4 bcd | 22.5 ab |
| BJ + AC + BS + NC | 127 bc | 143 b | 21.2 bc | 19.4 b |
| BJ + AC + BM + NC | 124 cd | 162 a | 22.6 ab | 25.4 a |
| ‘NS Rubin’ | ||||
| Control | 102 f | 107 e | 14.9 e | 12.6 c |
| BJ | 117 e | 129 cd | 18.3 d | 19.5 b |
| BJ + NC | 124 cd | 122 d | 19.1 cd | 13.6 c |
| BJ + AC + BS + NC | 129 b | 125 d | 22.3 ab | 15.9 c |
| BJ + AC + BM + NC | 133 a | 134. c | 24.4 a | 21.3 b |
| Average | ||||
| Control | 111 c | 118 d | 17.6 d | 13.9 c |
| BJ | 121 b | 136 c | 19.5 c | 19.6 b |
| BJ + NC | 123 b | 142 b | 19.8 c | 18.0 b |
| BJ + AC + BS + NC | 128 a | 134 c | 21.7 b | 17.7 b |
| BJ + AC + BM + NC | 128 a | 148 a | 23.5 a | 23.4 a |
| Treatments | Fresh Shoot Weight (g) | Fresh Root Weight (g) | Dry Shoot Weight (g) | Dry Root Weight (g) |
|---|---|---|---|---|
| ‘NS Apolo’ | ||||
| Control | 8.674 f | 1.587 c | 1.128 h | 0.115 h |
| BJ | 10.003 c | 1.782 b | 1.282 f | 0.130 g |
| BJ + NC | 9.498 de | 1.807 b | 1.180 g | 0.149 d |
| BJ + AC + BS + NC | 9.304 e | 1.836 b | 1.187 g | 0.147 d |
| BJ + AC + BM + NC | 9.133 e | 1.807 b | 1.171 g | 0.139 e |
| ‘NS Rubin’ | ||||
| Control | 9.705 cd | 1.621 c | 1.483 e | 0.134 f |
| BJ | 11.144 ab | 1.949 a | 1.651 b | 0.163 ab |
| BJ + NC | 11.316 a | 1.816 b | 1.585 c | 0.159 bc |
| BJ + AC + BS + NC | 11.358 a | 1.818 b | 1.561 d | 0.165 a |
| BJ + AC + BM + NC | 10.886 b | 1.766 b | 1.713 a | 0.158 c |
| Average | ||||
| Control | 9.190 c | 1.604 c | 1.305 d | 0.125 c |
| BJ | 10.573 a | 1.866 a | 1.466 a | 0.146 b |
| BJ + NC | 10.407 a | 1.811 b | 1.382 c | 0.154 a |
| BJ + AC + BS + NC | 10.331 a | 1.827 ab | 1.374 c | 0.156 a |
| BJ + AC + BM + NC | 10.009 b | 1.786 b | 1.442 b | 0.148 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinković, J.; Miljaković, D.; Červenski, J.; Vasiljević, M.; Đorđević, V.; Tamindžić, G.; Miladinović, J. Synergistic Effect of PGPR and Nutrient Complex on Soybean Seed Germination and Initial Seedling Growth. Agriculture 2025, 15, 2022. https://doi.org/10.3390/agriculture15192022
Marinković J, Miljaković D, Červenski J, Vasiljević M, Đorđević V, Tamindžić G, Miladinović J. Synergistic Effect of PGPR and Nutrient Complex on Soybean Seed Germination and Initial Seedling Growth. Agriculture. 2025; 15(19):2022. https://doi.org/10.3390/agriculture15192022
Chicago/Turabian StyleMarinković, Jelena, Dragana Miljaković, Janko Červenski, Marjana Vasiljević, Vuk Đorđević, Gordana Tamindžić, and Jegor Miladinović. 2025. "Synergistic Effect of PGPR and Nutrient Complex on Soybean Seed Germination and Initial Seedling Growth" Agriculture 15, no. 19: 2022. https://doi.org/10.3390/agriculture15192022
APA StyleMarinković, J., Miljaković, D., Červenski, J., Vasiljević, M., Đorđević, V., Tamindžić, G., & Miladinović, J. (2025). Synergistic Effect of PGPR and Nutrient Complex on Soybean Seed Germination and Initial Seedling Growth. Agriculture, 15(19), 2022. https://doi.org/10.3390/agriculture15192022







