Abstract
Addressing damage inflicted by environmental stress is difficult post-occurrence. The use of externally delivered gamma-aminobutyric acid (GABA) priming to healthy plants may serve as an effective preventive measure by stimulating plant defense pathways. A genome-wide transcriptional investigation was performed on tomato plants following GABA priming, with extended data about the stress memory of previously primed plants subjected to drought stress. GABA significantly stimulates starch and sucrose metabolism, amino sugar and nucleotide sugar metabolism, porphyrin metabolism, glycerolipid metabolism, biosynthesis of phenylalanine, tyrosine, and tryptophan, phenylalanine metabolism, ascorbate and aldarate metabolism, pantothenate and CoA biosynthesis, and plant hormone signal transduction pathways. The initial priming effect could be remembered when subsequent environmental stress arose, but its influence intensified in plants that had previously undergone priming. The application of GABA can establish a novel form of preventative defense against the detrimental effects of stresses. It can effectively enhance long-term plant defense by facilitating the development of plant stress memory.
1. Introduction
Global climate change is prompting researchers worldwide to seek environmentally friendly and practical solutions to mitigate the damage caused by stress. Since the discovery by Jones and Dangl (2006), which demonstrated that plants possess a two-layer immune system, increasing attention has been paid to prevention and the potential of stimulating (priming) the plant immune system [1]. As more bioactive compounds come into focus, their impact is constantly being studied by researchers. Such bioactive compounds include non-protein amino acids (NPAA), of which the alpha-aminobutyric acid (AABA), beta-aminobutyric acid (BABA), and gamma-aminobutyric acid (GABA) family of compounds is of particular interest. Over time, researchers have discovered that these compounds are naturally present in plants and that their levels begin to increase under stress. The question has arisen as to whether they play a role in the defense responses of plants.
Each of the three related compounds and their effects is a highly researched field of science, and a summary of the results achieved in this field is included in the review by Decsi et al. (2024) [2]. Focusing on one member of the compound family, GABA, it was found that plants produce GABA through the so-called GABA shunt, but it can also be produced through the polyamine biosynthesis pathway [3]. When stress occurs, rapidly changing abiotic stressors, such as temperature and wind, cause a sudden increase in Ca2+ levels, which indirectly affects the increase in GABA levels by forming a complex with calmodulin [4,5].
In the case of other, slower-changing stressors (such as water shortage or salt), a substantial pH decrease is observed in the cytoplasm, which indirectly increases the level of GABA in the cell. However, a common feature is that the activation of the enzyme glutamate decarboxylase (GAD) acts as a catalyst in both pathways, inducing the synthesis of GABA [6]. GABA induction then induces significant positive changes in the physiology and other life processes of the plant, which are further detailed in the literature.
It has a beneficial effect on chlorophyll levels, proline content, catalase enzyme activity [7], other antioxidant enzymes such as superoxide dismutase, peroxidase, glutathione reductase, glutathione-S-transferase [8], and photosynthesis [9]. Seifikalhor et al. (2019) demonstrated that GABA has a positive effect on the physiological properties of plants in the presence of salinity, hypoxia/anoxia, drought, temperature, heavy metals, plant–insect interactions, plant–microbe interactions, and reactive oxygen species (ROS)-related reactions [10].
Researchers turned their attention to the potential of stimulating the immune system of plants, which can help the plant’s organism to prepare in advance for later stress effects, creating a kind of hardened state. Several studies have also been conducted on the immunostimulatory effects of GABA, which were summarized by Tarkowski et al. (2020) regarding the priming of GABA in relation to biotic stressors [11] and Dabravolski and Isayenkov (2023) in relation to salt stress [12]. Some research groups have investigated the role of GABA in stress memory, primarily explaining the long-term positive effects through the regulation of stomatal closure [13] and the increase in endogenous GABA production [14]. Xu et al. (2019) demonstrated the contribution of GABA in stress memory during water deficit stress in Arabidopsis thaliana [15].
Lukić et al. (2020) were the first to demonstrate that the antioxidant enzyme system plays a role in the long-term maintenance of plant stress memory after activation [16]. Their results indicate that drought stress defense enhances the activity of enzymes known as antioxidants, which is essential for mitigating oxidative damage and preserving resistance to future drought stress (stress memory). Dabravolski and Isayenkov (2023) demonstrated the role of GABA in stress memory in plant cultures subjected to salt stress [12]. They showed that the application of exogenous GABA effectively reduces ROS levels, enhances membrane stability and modulates the interaction of phytohormones, thereby improving tolerance even to multiple stresses, and also plays a role in the reactivation of long-term stress memory.
In the present study, gene expression changes associated with the external application of GABA in tomato plants were investigated, using a novel next-generation sequencing (NGS) technique. Genome-wide analyses were conducted to examine whether the external application of GABA has an immunostimulatory and activating effect, and if so, which genes and biochemical processes are involved in this process. In addition, plants previously activated by GABA treatment were exposed to prolonged drought stress to examine the presence of a possible stress memory. Also individual gene expression studies were subjected to some stress-inducible genes (superoxide dismutase—SOD, catalase—CAT, peroxidase—POX, glutathione reductase—GR, glutathione-S-transferase—GST), in addition to which laboratory antioxidant enzyme activity measurements were performed to validate our in silico genome-wide studies, since according to the aforementioned literature data, the use of priming techniques with GABA can have a beneficial effect on the activity of the antioxidant enzyme system of plants, which is one of the best ways to verify the priming effect.
Such a comprehensive and deep exploratory study focusing on gene-level changes has not yet been conducted on tomato plants, examining both the short-term gene expression changes in plants before stress, under the influence of GABA treatment (primed), and the stress-induced long-term (stress memory), revealing and analyzing the role of the biochemical pathways involved in the effects of the treatments.
2. Materials and Methods
2.1. Experimental Design
The pot experiment utilized tomato seeds from the Kecskeméti 549 (K-549) variety of Solanum lycopersicum, cultivated in Hungary. The seeds underwent sterilization by immersion in 70% ethanol for two minutes, followed by three rinses with deionized water. The seeds were initially sown in seedling trays. And, when they developed 3–4 leaves, they were individually transferred into plastic pots measuring 28 cm in width and depth. Two kilograms of washed and dried pebbles were placed at the bottom of the pots and thereafter covered with a plastic mesh. The pot features three 8 mm drainage holes at the bottom for irrigation and aeration. They filled the pots with an equal mixture of soil and peat moss in a 1:1 ratio. A ventilation system and an automated window opening mechanism maintained the greenhouse temperature at 25/20 ± 5 °C both day and night, with a relative humidity of 60–65%. Four treatments were used, with four replicates per treatment. The treatments were as follows: (1)—Control (non-stressed tomato), (2)—Drought-stressed tomato, (3)—Non-stressed and GABA-treated tomato, and (4)—Drought-stressed and GABA-treated tomato. GABA treatment was applied by foliar spraying with a 0.02 w/v% GABA-containing solution.
A gravimetric approach was employed to determine the water retention capacity of the soil-peat mixture and its moisture content [17] to measure the amount of irrigation applied to the control and drought-stressed pots. Under drought stress, plants received 50% less water than control plants. The first sampling was performed 5 days after GABA treatment, which is sufficient time for the plant to produce defense responses in response to the stimulatory eustress effect induced by GABA, thereby proving or disproving the priming effect of GABA. Half of the plants were then subjected to drought stress and sampled after 10 days of treatment to determine whether stress memory existed and whether GABA could induce it. The 10-day sampling period was chosen based on the recommendations of GABA-containing plant conditioner companies, which recommend repeating the treatment every 14 days.
For transcriptomic analyses, 25–50 mg of healthy, young, dividing leaf tissue was collected from each treatment in four replicates per treatment. The samples were then bulked by the sequencing company per treatment, as samples from the same treatment can be considered replicates of each other. To measure the enzymatic activity of the plants, 4 × 100 g of healthy, fresh, mature leaves were collected from all the treatments.
2.2. In Silico Genome-Wide Analyses
Each treatment was sampled for bioinformatics analysis in sterile Eppendorf tubes. For leaf tissue preservation, 1 mL of RNALater (Invitrogen by Thermo Fisher Scientific Inc., Waltham, MA, USA) was used. The treatment prevented sensitive RNA degradation between collection and sequencing. Paramagnetic NEXTFLEX® Poly(A) Beads 2.0, HiSS Diagnostics, Freiburg, Germany, were used to isolate mRNA from high-quality samples (≥7 total RNA RIN value). Strand-specific NGS library preparation was performed with the NEXTFLEX® Rapid Directional RNA-Seq 2.0 kit, HiSS Diagnostics, Freiburg, Germany, after fragmentation. Shallow sequencing produced pooled libraries on the Illumina NovaSeq 6000 NGS platform, Illumina, Inc., San Diego, CA, USA. Each sample averaged 19–23 million 151-base-pair single-end reads [16]. The authors used FastQC version 0.12.0 (https://timkahlke.github.io/LongRead_tutorials/QC_F.html) (accessed on 12 August 2025) to assess raw reading quality, eliminate low-quality segments, and trim reads. Trimmomatic 0.39 (http://www.usadellab.org/cms/index.php?page=trimmomatic, accessed on 12 August 2025) [18]. The mean read length was 139 bp after preprocessing. Trinity 2.15.2 (http://TrinityRNASeq.sourceforge.net (accessed on 13 August 2025)) combined quality-controlled, preprocessed data into a de novo transcriptome [19]. Trinity sequencing assembles short nucleotide sequences into longer contigs and has the unique feature of not requiring a reference genome.
Utilizing the CloudBlast sequence alignment technique integrated into OmicsBox BioBam software version 3.4, we identified the de novo transcriptome contigs characterized by extended, unidentified sequences [20]. Gene ontology (GO) concepts were correlated (i.e., mapped) to the blasted sequences utilizing Blast2GO, a component of OmicsBox BioBam software version 3.4. (retrieved 13 August 2025) [21]. Functional annotation was subsequently conducted using the EggNOG mapper, a feature of OmicsBox BioBam software version 3.4, which is adept at the functional identification of novel, previously uncharacterized sequences [22].
To do differential expression analysis, one must examine the individual expression levels of the contigs within the de novo transcriptome. The software generates a transcript map in the absence of a reference genome. After mapping, the software utilizes the gene loci to infer the expression values of each read [23]. The software produces a summary of the results in a tabulated output file. Utilizing the provided count table and the NOIseq software (https://bioconductor.org/packages/release/bioc/html/NOISeq.html) (accessed on 13 August 2025), pairwise differential analysis was conducted on the contigs of the samples utilized for de novo transcriptome assembly to identify genes exhibiting statistically significant variations in expression (probability > 0.9) in response to various treatments [24,25,26].
Genes that were down-regulated, up-regulated, under-represented, or over-represented—indicating significant deviations from the average—were selected from the expressed sequences of the treated samples by comparing de novo blast, mapping, and annotation of transcriptome data [27]. The chosen genes, which showed considerable variations across treatments, were grouped by their molecular roles. This helped us better understand how treatments affect biochemical functions [28]. A comprehensive pathway analysis of the Plant Reactome [29] and KEGG [30] databases was conducted to identify biochemical pathways that responded positively or negatively to the treatments.
2.3. Analysis of Particular Genes
According to the literature sources, secondary oxidative stress effects were reduced after external application of GABA, which is due to the active neutralizing effect of the antioxidant enzyme system. Pretreatment of healthy plants with biostimulants can be considered as a kind of weak, stimulatory eustress, which activates the plant’s defense responses. Such a response is the activation of the antioxidant enzyme system, the detection of which is generally accepted as a means of monitoring priming effects.
Five tomato antioxidant enzyme sequences (CAT, SOD, POX, GST, and GR, as indicated in the literature) were identified in the de novo transcriptome and quantified in each sample using the NOISeq program subunit of the OmicsBox BioBam software version 3.4 [24]. The software settings used raw counts in in silico analysis, revealing sequence expression values.
The individual gene analysis performed was multi-purpose, as it is comparable to laboratory enzyme activity measurements and is thus suitable for validating genome-wide in silico analysis, on the other hand it is suitable for confirming or rejecting the existence of a priming reaction, and thirdly it shows a correlation with the possible long-term effects of treatments, thus it can also verify the existence of plant stress memory and its inducibility with the active substance GABA.
2.4. Laboratory Measurements of Antioxidant Enzyme Activity
For enzyme activity measurements, 10 g of healthy leaf samples were collected from each treatment, in four replicates, which were pre-cleaned and analyzed using suitable protocols as shown in Table 1.

Table 1.
A summary of the protocols used to determine the activities of the antioxidative enzymes.
2.5. Measurement of Plant Chlorophyll and Growth-Attributive Parameters
The chlorophyll content was determined using a (SPAD) 502 Plus device manufactured by Konica Minolta (Osaka, Japan), and the measurements were taken every 10 days with the growth attributive recordings; plant height, stem diameter, and the leaf surface are according to the protocol by Ahmed et al. (2024) [38].
3. Results
3.1. Analysis of Transcriptomes Across the Genome
After preprocessing the raw reads, a de novo transcriptome was reconstructed from 5,969,174 transcripts using the combined sequenced libraries from the four biological samples. The de novo transcriptome yielded 16,211 longer contigs representing 13,450 unique genes. The de novo supertranscriptome is available in Table S1. The de novo supertranscriptome, also known as TSA (Transcriptome Shootgun Assembly), is a computer-assembled archive of transcript sequences derived from primary data, such as raw reads (Sequence Read Archive—SRA) (see Data Availability Statement). The supertranscriptome was blasted, mapped, and annotated to identify individual contigs and associate actual biological functions with genes expressed during the treatments. Then, in the RNA-sequencing read quantification step, the gene expression levels per treatment were determined individually, yielding a count table (Table S1). Based on the data, approximately 70% of the raw reads aligned to the de novo transcriptome (Figure 1).
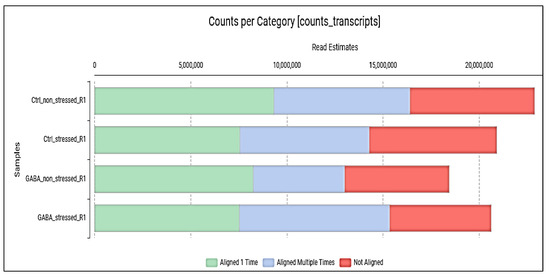
Figure 1.
Number of single and multiple aligned reads to the de novo transcriptome from different treatments.
Quantification step was necessary because performing pairwise differential expression analyses requires a count table containing the individual gene expression levels. Pairwise differential expression analyses were performed in all relevant pairings, for example:
- -
- Ctrl_non_stressed_R1 vs. Ctrl_stressed_R1
- -
- Ctrl_non_stressed_R1 vs. GABA_non_stressed_R1
- -
- Ctrl_stressed_R1 vs. GABA_stressed_R1
The comparison of control and drought-stressed stands identified 7837 differentially expressed sequences (likelihood > 0.9), including 3030 that were upregulated and 4807 that were downregulated (Table S2). Seven thousand four hundred ninety-four differentially expressed sequences were identified between the control and GABA-primed treatments (likelihood > 0.9). Among them, 3394 were upregulated and 4100 were downregulated (Table S3). The analysis of drought-stressed and GABA-treated drought-stressed datasets indicated that 4148 out of 7996 differentially expressed sequences were upregulated, whereas 3848 were downregulated (Table S4). The data suggest that drought stress causes a significant downregulation of gene expression compared to the control stand, thus the quality of life of the plants shows a deteriorating tendency.
Compared to the control but non-stressed stands, the intensified expression of upregulated genes starts in the GABA-treated but non-stressed stands, which can be attributed to the stimulating effect of eustress induced by the treatment. The increase in the number of upregulated, overexpressed genes may predict that the plant’s immune system initiates defensive responses as a result of the priming GABA treatment. The most spectacular expression changes are shown by the difference between the datasets of untreated stressed and GABA-primed and later stressed plants. The number of upregulated, overexpressed genes shows an increase compared to the previous ones, which seems to support the assumption that primed plants respond better to later stress effects than unprimed individuals. In other words, prior GABA treatment induces the stress memory of plants and provides more effective protection than the immune system of plants that have not been previously treated/primed.
Gene enrichment analyses identified 1360 over- and 2354 under-represented genes between the control and drought stress treatments, 1630 over- and 1946 under-represented genes between the control and GABA primed treatments, and 2088 over- and 1803 under-represented genes in the drought stress and GABA primed + drought stress datasets. Pairwise differential expression analysis of control and drought-stressed plant populations showed that drought stress activated not only basic biological metabolic processes but also processes involved in stress responses (Figure 2A). In the case of GABA-priming, the highest activation was realized in the cellular response to stimulus in addition to basic metabolic pathways (Figure 2B). It is also noteworthy that in the case of plants treated with the combined treatment, stress responses, signal transduction processes, and responses to abiotic stimuli were activated in addition to metabolic processes (Figure 2C).
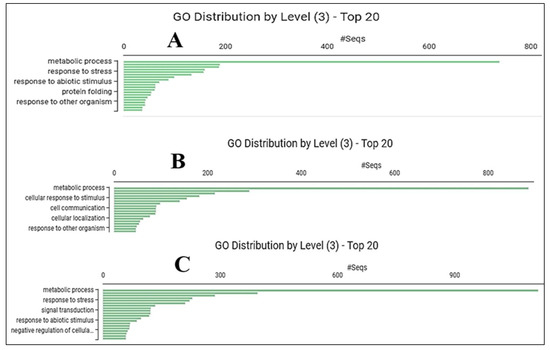
Figure 2.
Distribution of biological processes (BP) induced by drought stress (A), GABA priming (B), and combined drought + GABA (C) treatments.
Overexpressed, up- and downregulated genes from the pairwise compared treatments were identified by blast, mapping, and annotation steps, and their roles in biological processes were determined. Next, a combined pathway analysis was performed to place the genes in question on the biochemical pathways in which they play a role. The first comparison showed that 418 upregulated genes, differently expressed across control and drought-stressed plant populations, were connected to 211 metabolic pathways by KEGG. In contrast, the Plant Reactome database linked 376 genes to 381 pathways. For downregulated and overexpressed genes in the same dataset, KEGG related 631 genes to 294 pathways, while Plant Reactome linked 687 genes to 797 pathways.
When comparing control and GABA-treated plants, combined pathway analysis showed that 442 elevated genes were related to 243 KEGG pathways and 450 to 448 Plant Reactome pathways. In the context of downregulated and overexpressed genes in the same two datasets, 582 genes were connected to 261 KEGG pathways and 590 to 717 Plant Reactome pathways.
Comparing the data of the untreated but stressed stock and the GABA-treated and stressed stock, the pathway analysis linked 560 genes to 266 pathways in the case of the upregulated overexpressed genes to the KEGG database, while 585 sequences to 540 pathways in the Plant Reactome database. In the case of the overexpressed, downregulated genes of the same data, KEGG linked 587 genes to 256 pathways, while Plant Reactome linked 550 genes to 691 pathways. Further analysis of the datasets revealed the most activated biochemical pathways following the treatments. The pathways most induced by the upregulated genes were metabolisms of starch and sucrose, amino sugar and nucleotide sugar, porphyrin, phenylalanine, ascorbate and aldarate, and glycerolipid, and phenylalanine, and biosyntheses of tyrosine, tryptophan, pantothenate, and CoA, and plant hormone signal transduction. Comparing the three groups with each other, it was found that 47 enzymes in the above nine biochemical pathways were activated during drought stress, 65 enzymes during GABA priming, and 85 enzymes in GABA-primed and later drought-stressed plants (Tables S5–S7).
It can also be stated that in the case of the mentioned pathways, 11 enzymes were activated exclusively under drought stress, 7 exclusively under the influence of weaker, stimulating GABA-priming, and 31 exclusively in plants previously treated with priming and then exposed to drought for a longer period of time. In addition, activation of 22 enzymes was observed in all three treatments. In addition, 5, 9 and 27 enzymes overlapped between one, the other or the third treatment pairs (Figure 3). The results suggest that the same biochemical pathways are activated in the perception of the two different abiotic stress and in plant responses to it, but within the pathways, there are unique differences between the genes involved in the processes and the metabolic products they activate.
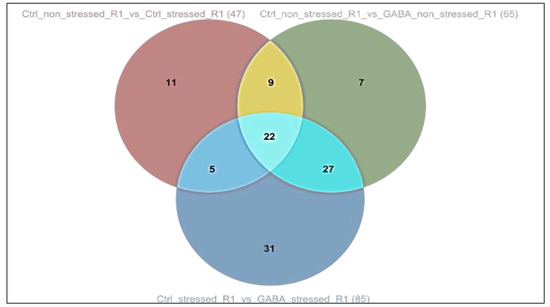
Figure 3.
Venn diagram of the enzymes activated by the treatments.
In addition, it can be stated that the GABA priming treatment can indeed be perceived as a mild eustress, which causes the plant to make acclimation (short-term) responses, which responses are preserved in the long-term in the plant stress memory and can be reactivated during a possible later stress. During the later activation, a stronger immune response from the plants can be observed, which is presumably due to the help of the prior priming treatment. The most active biochemical pathway was starch and sucrose metabolism, in which 16 upregulated genes activated a total of 15 enzymes under drought stress (Table S8); 25 genes activated 19 enzymes under GABA priming (Table S9), while 28 upregulated genes transcribed in GABA primed and subsequently drought-stressed plants activated 22 enzymes (Figure 4).
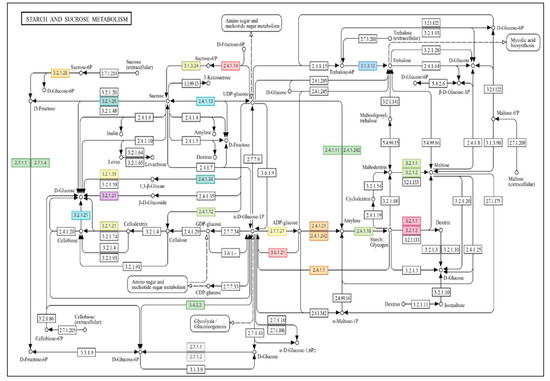
Figure 4.
Drought-induced enzymes and GABA priming affect starch and sucrose production. The figure was created using OmicsBox 3.4′s Combined Pathway Analysis tool (https://www.biobam.com/omicsbox/) and accessed on 22 August 2025, in its original version. The graphic shows gene-initiated subprocesses. Table 2 lists all of the relevant biochemical processes’ genes and enzymes (EC code classification). Significant treatment-induced changes are noted in red.
The trend is true for all nine pathways most activated by treatments, i.e., both the number of up-regulated, overexpressed genes and the number of enzymes activated by them are lowest in drought-stressed plants only, then increase in GABA-primed plants and are highest in GABA-primed and then drought-stressed plants (Tables S10–S17). The major enzymes activated in the nine most active biochemical pathways are listed in Table 2.

Table 2.
The major enzymes activated in the nine most active biochemical pathways.
According to our observations, in addition to the upregulated biochemical processes, several downregulated genes suppressed quite a few other biochemical processes, but the trend in these cases also showed that the most repressed pathways occurred under drought stress, but the same affected pathways were less repressed following GABA priming and the downregulation decreased even further following the combined long-term stress effects. The most active downregulated biochemical pathways based on these were the spliceosome function, glycerophospholipid metabolism (Tables S10 and S11), the MAPK signaling pathway, the steroid biosynthesis and the mRNA surveillance pathway.
The increase in the activity of the five mentioned pathways indicates that priming treatment, on the one hand, promotes the removal of nonsensical information-containing sections (introns) of transcribed mRNAs (splicing) through spliceosomes, on the other hand, the detection and degradation of erroneous transcripts through mRNA surveillance pathways, and thirdly, it activates the MAPK cascade, thus promoting signal transduction. In addition, it initiates glycerophospholipid biosynthesis, most of which serve as substrates in the formation of signal transduction molecules and are also the main components of membranes.
Furthermore, it activates steroid biosynthesis, which enhances plant stress tolerance, which may help in improving plant tolerance. All of these biochemical pathways suppressed during drought stress show even greater activity in the long term, in plants previously primed and then later exposed to stress, thereby confirming the positive effects of GABA priming on stress memory. Regarding the downregulated, overexpressed genes, using the example of the glycerophospholipid metabolism pathway, it can be stated that in response to drought stress, 19 sequences suppressed the activity of 14 enzymes (Table S18). In contrast, in the same pathway, under the stimulating effect of GABA priming, 16 sequences harmed the activity of 9 enzymes (Table S19), while following long-term, combined stress, 7 sequences downregulated the function of 4 enzymes (Figure 5). The same trend can be observed in the other most actively changing downregulated biochemical processes mentioned above.
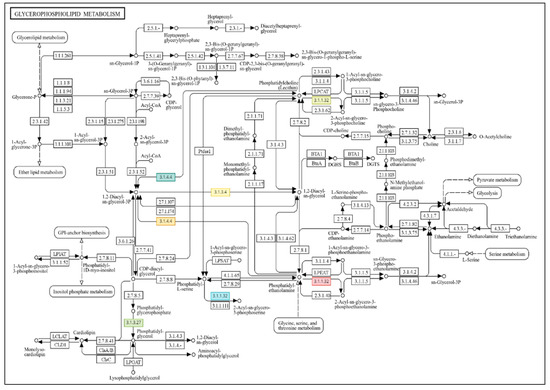
Figure 5.
Drought stress treatments and GABA priming on glycerophospholipid metabolism enzymes. The figure was created using OmicsBox 3.4′s Combined Pathway Analysis tool (https://www.biobam.com/omicsbox/) and accessed on 22 August 2025 as-is. Genes initiate subprocesses, as seen in the picture. The names represent all EC coded genes and enzymes (Table 2) that operate the biochemical pathway. Red items indicate substantial treatment modifications.
In summary, based on genome-wide transcriptomic analyses, drought stress, as an abiotic stressor, negatively affects most of the biochemical processes at the cellular level, as expected, but GABA-priming positively influences the negative effects. GABA activates several biochemical pathways that may contribute to making the plant immune system more resistant and increasing the level of individual stress tolerance (acclimatization).
The same research also showed that the effect of GABA-priming persists in the long term and that it reactivates certain plant physiological processes—previously activated by priming—that are involved in defense, proving the existence and active functioning of plant stress memory.
3.2. Individual Gene Analyses
Since most of the literature data refer to the strong antioxidant effect of GABA as a bioactive substance, but we did not find evidence for this during genome-wide transcriptional analyses, we individually examined the changes in the activities of the main antioxidant enzymes in our samples (Figure 6).
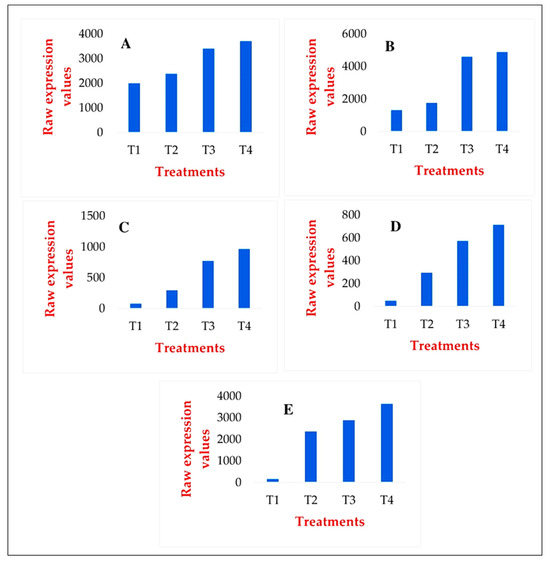
Figure 6.
Changes in the activities of the main antioxidant enzymes: SOD (A), CAT (B), POX (C), GR (D), GST (E), as a result of the treatments, expressed as gene expression levels based on in silico analyses. T1: Ctrl_non_stressed. T2: Ctrl_stressed. T3: GABA_non_stressed. T4: GABA_stressed.
Although the genes activating antioxidant enzymes examined in the genome-wide transcriptomic analysis were not among the most actively expressed, individual gene-level analyses showed that the increased enzyme activity during drought stress was consistent with the enzyme activity induced by GABA-priming. For all analyzed enzymes, the most significant increase in activity was observed in plants exposed to long-term combined treatments.
3.3. Estimation of the Enzymatic Activities
To verify the results of the in silico analyses, we also measured the activities of the antioxidant enzymes under conventional laboratory conditions. The measurement results supported the in silico results and showed the same trend (Figure 7).
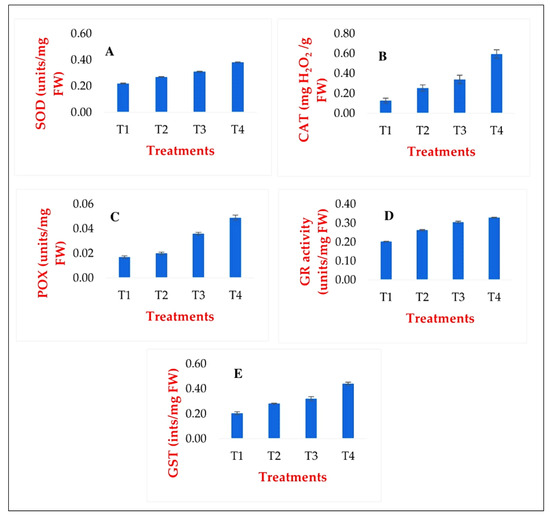
Figure 7.
Changes in the activities of the main antioxidant enzymes: SOD (A), CAT (B), POX (C), GR (D), GST (E), as a result of the treatments, based on laboratory analyses. T1: Ctrl_non_stressed. T2: Ctrl_stressed. T3: GABA_non_stressed. T4: GABA_stressed.
The activity of the antioxidant enzyme system is important due to its prominent role in plant stress responses. The experiment demonstrated that stimulatory priming treatment could activate individual members of the antioxidant enzyme system as a eustressor, contributing to the active cellular defense of plants. It was also established that the activity of antioxidant enzymes increases when previously primed plants are subsequently exposed to a stress effect.
3.4. Plant Chlorophyll and Growth Parameters
The GABA treatment had a positive effect on the development of chlorophyll content during the growing season, with the chlorophyll content of the treated plants increasing significantly (SD: 5%) compared to the control plants. The average chlorophyll content of the plants exposed to drought stress decreased, but the degree of the decrease was not significant compared to either the control or the GABA-primed plant group (Figure 8A).
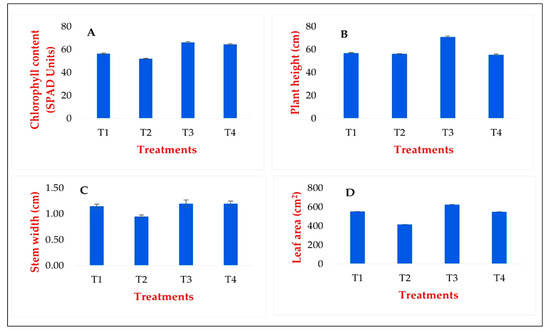
Figure 8.
Determination of chlorophyll content (SPAD-units) (A), plant height (B), stem width (C), and leaf area (D) in the leaves of tomato. T1: Ctrl_non_stressed. T2: Ctrl_stressed. T3: GABA_non_stressed. T4: GABA_stressed.
The GABA priming treatment also had a positive effect on the development of plant height. Still, among the plant populations exposed to drought stress later, the difference was only significant between the height of the GABA non-stressed and GABA-stressed plants (Figure 8B). The most negligible effect of GABA priming was on the stem width data, where no significant difference was measured in either of the populations (Figure 8C). The leaf area in the non-drought-stressed but GABA-primed plants increased significantly compared to the control stand, and the leaf area of the GABA-pretreated but drought-stressed combined-treated plants (Figure 8D).
Overall, it can be said that GABA has a beneficial effect on the development of some plant physiological parameters, which is more pronounced under optimal (stress-free) weather conditions than under cumulative stress factors. However, likely, its most significant advantage is not in increasing biomass, but instead in its stress-relieving effects.
4. Discussion
A pot experiment was performed on tomato plants under controlled conditions. The plant’s immunity was strengthened by GABA priming, and then the primed plants were exposed to drought stress. The control, untreated plants; plants exposed to drought stress; GABA-primed plants; and GABA-primed, drought-stressed, and combined-treated plants were compared. Based on the results, it can be stated that the same nine biochemical pathways were activated to the greatest extent by drought stress treatment, GABA priming, and prior GABA priming followed by drought stress. Of these, the starch and sucrose metabolism pathway was the most active.
Based on numerous preliminary literature references, it can be stated that plants begin to synthesize sugar and starch at an increased level under abiotic stress conditions [39,40,41,42]. When exposed to drought stress, it can be stated that their sucrose synthase enzyme activity is higher compared to plants with a regular water supply [43]. In our work, we made further contributions to the topic and, using our genome-wide studies, revealed the entire biochemical pathway, defined and identified all related stress-inducible enzymes. Thomas and Beena (2024) also suggested in their review that drought stress increased the activity of sucrose metabolism enzymes [44].
Li et al. (2023) also found, based on KEGG pathway analyses, that the activity of starch and sugar metabolism in grains exposed to drought stress changed significantly [45], which caused the plant to focus on energy and material accumulation, thereby enhancing the drought tolerance of the organism. In our experiments, the inducibility of starch and sucrose metabolism was successfully demonstrated, as well as its repeated inducibility in long-term stress memory, not only during drought stress but also during GABA-stimulatory pretreatment.
The second most active upregulated biochemical pathway was aminosugar and nucleotide sugar metabolism. Such sugars are activated forms of simple sugars and serve as intermediates in the conversion of sugar-containing compounds into one another. These biochemical processes synthesize, among others, trehalose, cellulose, hemicelluloses (xyloglucans, xylans, mannans), pectins, and starch. Nucleotide sugars are also used in the synthesis of galactolipids, sulfolipids, and ascorbic acid. They are also donor compounds in the synthesis of phytohormones and some secondary metabolites [46]. Trehalose is a disaccharide molecule that beneficially influences vital physiological processes, such as osmotic regulation, oxidative stress reduction, and cellular stability, which collectively enhance drought tolerance [47].
Cellulose synthesis, which provides stability to the plant cell wall, requires the activity of cellulose synthases, which are located along the microtubules of the cortex. Stress alters the structure of these microtubule networks, leading to increased cellulose synthesis. It has also been observed that stress-induced changes in the cell wall are closely linked to and activate plant hormone pathways [48]. In our experiments, both drought stress and GABA priming also activated the latter pathway, supporting the assumption that GABA can be considered a weak eustressor and has a stimulatory effect.
Xyloglucan endoglycosylases/hydrolases are involved in the structure of cell walls and play a crucial role in regulating cell wall elasticity and stress response [49]. In our experiments, these types of enzymes were also activated, which may strengthen the cell walls of stressed plants, thereby helping the plant to tolerate subsequent stress effects. Mannans are polysaccharide compounds that contain large amounts of mannose. In our experiments, enzymes involved in mannose synthesis were also activated, both under drought stress and in the presence of GABA, predicting an increase in mannan synthesis. All the polysaccharides that comprise the cell wall play a crucial role in the water storage capacity of plants, so their quantitative increase during drought stress is not surprising, as previous literature data also support this [50].
An increase in the amount of cellulose and pectins was also observed during drought stress, which together resulted in thickening of the cell walls and changes in their elasticity [51]. The increase in the amount of these compounds related to amino and nucleotide sugar metabolism is expected as a result of the increase in enzyme activity obtained from our experiments, which in all respects points towards the activation of adaptation mechanisms to stress effects in both the short and long term, supporting the inducing effect of GABA priming and stress memory.
Chen et al. (2018) observed an increase in galactolipid synthesis in maize leaves during drought stress, which they linked to leaf senescence [52]. The biosynthesis of galactolipids also requires a nucleotide sugar donor, so the enzymes activated in this pathway in our experiments may also indirectly activate stress-induced senescence processes. The biosynthesis of sulfolipids also starts from nucleotide sugars and is linked to the biosynthesis of other plant glycerolipids [53]; the latter biosynthetic pathway also showed strong activation during our experiments. Our treatments, therefore, affect the interconnected, joint functioning of two pathways that showed increased activity in our experiments.
Ascorbic acid is also a long-known antioxidant scavenger compound that can contribute to the success of plant defense processes. The formation of this compound also requires substrates synthesized in the amino sugar and nucleotide sugar metabolism pathways [54]. In connection with this, based on our experiments, the ascorbate and aldarate metabolism pathway is also among the nine most active pathways. Thus, it can be said that the cooperation and building on each other of two, in our experiments, very active pathways (nucleotide sugar metabolism and ascorbate metabolism pathways) result in the formation of one of the most powerful cellular scavenger compounds, ascorbic acid, which has a beneficial effect against oxidative stress resulting from primary stress effects.
Finally, the biosynthesis of plant hormones also begins with amino acids and nucleotide donors, whose activity affects the activation of the plant hormone signal transduction pathway. This latter pathway is also among the nine pathways that respond most actively to our treatments. Our experiments activated receptor molecules related to the synthesis of jasmonic acid, auxin, ethylene, and gibberellin. These hormones are all known to be involved in plant stress response processes [55,56,57,58]. Among the most active biochemical pathways in the experiments were also the pantothenate and CoA biosynthesis pathways. Pantothenate, or vitamin B5, is a precursor for the formation of the essential coenzyme A (CoA). Vitamin B5 is also known as the stress-relieving vitamin [59]. CoA acts as a transport compound in many metabolic pathways, so its increased production during periods of stress is not surprising [60].
In addition to the above, the porphyrin metabolism pathway was also strongly activated in all experimental settings. Literature data support that stress-tolerant plants are able to bind intermediate compounds in porphyrin metabolism (e.g., protoporphyrin, protochlorophyllin, etc.) in their leaves under drought stress. Such plants show significantly better drought tolerance, as indicated by higher shoot water potential, less oxidative damage and more favorable redox balance [61]. We observed an increased intensity in the function of the Protochlorophyllide Reductase enzyme under drought stress, which was reinforced by GABA priming, since in the latter case, in addition to protochlorophyllide Reductase, the activity of protoporphyrin ferrochelatase together with several other enzymes also increased. This latter trend was also evident in the case of combined, long-term stress treatments, confirming the activation of stress memory.
The phenylalanine, tyrosine and tryptophan biosynthesis pathway were also strongly activated by our treatments. While the activation of tryptophan synthase is noteworthy in drought stress, in addition to GABA priming, additional enzymes (e.g., shikimate dehydrogenase, etc.) also became active, which activity was also observed in the combined treatment. It is well known that stress effects activate secondary metabolic pathways—especially the shikimic acid pathway- in which numerous secondary metabolites and protective compounds are synthesized. Aromatic amino acids, such as phenylalanine and tyrosine, significantly stimulate this process, as both compounds are involved in the work of the shikimic acid pathway, catalyzed by the key enzyme phenylalanine ammonia-lyase (PAL) [62].
Among the nine most active metabolic pathways, the phenylalanine metabolism pathway, which functions in connection with the previous pathway, also showed outstanding activity in our treatments. The enzyme phenylalanine ammonia-lyase was activated, among others, even under drought stress, and subsequently, its activity increased in both other treatments. Additionally, other enzymes were activated in response to phenylalanine ammonia-lyase. PAL is an essential regulatory enzyme that allows the “switching” from the primary metabolism of the plant to the secondary metabolism and leads to the formation of several secondary metabolites based on the phenylpropanoid skeleton [63]. PAL can be activated under various environmental influences, which makes it an excellent inducer and triggers reactions aimed at increasing the resistance of plants [64].
Returning to the biosynthetic pathway of phenylalanine, tyrosine, and tryptophan, the metabolism of tryptophan in plants produces essential compounds such as auxin, melatonin, serotonin, immunoglobulins, and camalexin. These metabolites are also necessary for the development of plant stress responses. Additionally, tryptophan plays a crucial role in plant defense mechanisms as a precursor to indoleamines [65].
In addition to the upregulated and overexpressed pathways, biochemical pathways that were downregulated as a result of the treatments should also be mentioned. We highlighted the five most active biochemical pathways, which had a standard feature that the number of genes downregulated as a result of drought stress was significantly reduced by GABA and complex treatments, i.e., we can speak of a quasi-increase in activity in the case of these pathways.
Our treatments induced some genes of the downregulated mitogen-activated protein kinase (MAPK) signaling cascade, which can be considered the main center of plant signal transduction. They play a key role in the transmission of various extracellular stimuli, such as biotic and abiotic stresses [66]. Their primary task is to transmit external environmental stimuli and then ensure the earliest feedback of cellular responses; therefore, they are understandably of particular importance in stress responses [67].
The transcription of information encoded in DNA does not always proceed smoothly, and errors can occasionally slip into mRNA synthesis. This is especially true when stress causes the sudden expression of a multitude of genes in response to plant defense. In such cases, mechanisms responsible for repairing errors, such as mRNA surveillance, play a significant role [68]. The mRNA surveillance pathway, which becomes downregulated as a result of drought stress, also showed activation under the influence of GABA priming and combined treatment, highlighting the importance of this repair mechanism in the development of plant cellular immune responses.
Of the five pathways mentioned above, the most actively changing were genes involved in spliceosome function. The splicing mechanism is a process linked to the function of the spliceosome, which regulates pre-mRNA processing and the repair of potential errors. Literature data support the notion that the splicing mechanism is induced by the presence of several stressors, confirming the key role of splicing in stress adaptation [69].
Glycerophospholipid metabolism was also among the major downregulated biochemical pathways following the treatments. It is well known that phospholipids are the primary components of cell membranes; however, the literature data support the crucial role of this biochemical pathway during stress [70]. Abiotic stressors can activate specific lipid-dependent signaling pathways—as in our case—that regulate the expression of stress-responsive genes and contribute to plant stress adaptation [71].
Among the pathways downregulated during drought stress treatments but activated by GABA and combination treatments is the steroid biosynthesis pathway. In plant life, steroids are considered to be primary stress-induced compounds [72,73]. Several publications have demonstrated that steroid biosynthesis is increased during drought stress [52,67]. This depressed pathway also appeared to be activated by GABA priming and combination treatments.
To support the genome-wide transcriptomic analysis, we also examined the behavior of individual members of the plant antioxidant enzyme system, both in silico and in vitro. Our enzyme activity studies support the increase in antioxidant activation during drought stress, and then show a further increase in the case of GABA priming, confirming that the external application of GABA has an immunostimulatory effect. Both in silico gene expression studies and laboratory tests also showed that the previously applied GABA priming treatment, when combined with subsequent drought stress, results in stronger antioxidant enzyme activity, thus confirming the hypothesis that GABA priming treatment plays a role in the reactivation of subsequent stress memory.
5. Conclusions
Climate change presents multiple challenges to agricultural producers globally. The harm inflicted by environmental stresses is challenging to remediate thereafter, and the reduction in production can be substantial. Prevention is progressively becoming the focal point, enabling us to fully utilize the potential of healthy plants. A preventive strategy may involve the external delivery of GABA to healthy plants, which can stimulate plant cellular immune responses, thus equipping the plants to better withstand subsequent stresses. GABA priming was implemented into tomato plants and subsequently analyzed the cellular immune responses by genome-wide transcriptome analysis.
Our research indicates that GABA successfully stimulated certain metabolic pathways associated with plant stress responses, collectively triggering defensive systems in healthy plants. Furthermore, the study encompassed the examination of plants that had undergone prior priming and were subsequently subjected to drought stress, during which it was determined that the advantageous effects of earlier priming can be reinstated later. The identical molecular processes involved in plant stress responses might be restarted. In the latter scenario, these pathways elicit a far more vigorous immune response due to the actual stress effect compared to non-primed plants. In the future, it will be necessary to set up further experiments, also under field and uncontrolled conditions, to determine how effective the preventive application of GABA is in inducing stress memory.
In summary, GABA is a naturally occurring bioactive non-proteinogenic amino acid synthesized in plants that, when externally supplied, enhances the activation of cellular immune responses and consequently induces stress memory. Prevention can assist agriculture in addressing emerging difficulties in a cost-efficient and ecologically sustainable manner. The application of GABA can serve as an exceptional, innovative, entirely natural, preventive protection solution for farmers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15192012/s1, Table S1: Count table of the de novo transcript; Table S2: Differentially expressed genes between control and drought-stressed data; Table S3: Differentially expressed genes between control and GABA-treated (primed) data; Table S4: Differentially expressed genes between control, drought stressed and GABA-treated, drought-stressed data; Table S5: The most activated enzymes, based on a comparison of the control and drought-stressed datasets; Table S6: The most activated enzymes, based on a comparison of the control and GABA-primed datasets; Table S7: The most activated enzymes, based on a comparison of the control, drought-stressed, and GABA-drought-stressed datasets; Table S8: The activity of the starch and sucrose metabolism biochemical pathways in the drought stressed plants; Table S9: The activity of the starch and sucrose metabolism biochemical pathways in the GABA primed plants; Table S10: The activity of the amino sugar and nucleotide sugar metabolism biochemical pathways in the GABA-primed and drought-stressed plants; Table S11: The activity of the porphyrin metabolism biochemical pathways in the GABA-primed and drought-stressed plants; Table S12: The activity of the glycerolipid metabolism biochemical pathways in the GABA-primed and drought-stressed plants; Table S13: The activity of the phenylalanine, tyrosine, and tryptophan biosynthesis biochemical pathways in the GABA primed and drought stressed plants; Table S14: The activity of the phenylalanine metabolism biochemical pathways in the GABA-primed and drought-stressed plants; Table S15: The activity of the ascorbate and aldarate metabolism biochemical pathways in the GABA-primed and drought-stressed plants; Table S16: The activity of the pantothenate and CoA biosynthesis biochemical pathways in the GABA-primed and drought-stressed plants; Table S17: The activity of the plant hormone signal transduction pathways in the GABA-primed and drought-stressed plants; Table S18: The activity of glycerophospholipid metabolism biochemical pathways in drought-stressed plants; Table S19: The activity of glycerophospholipid metabolism biochemical pathways in GABA-primed plants.
Author Contributions
Conceptualization, K.D. and M.A.; data curation, K.D., M.A. and Z.T.; formal analysis, K.D. and M.A.; funding acquisition, Z.T.; investigation, K.D. and M.A.; methodology, K.D. and M.A.; project ad-ministration, Z.T.; resources, Z.T.; software, K.D. and M.A.; supervision, Z.T. and K.D.; validation, K.D. and M.A.; visualization, K.D.; writing—original draft, K.D. and M.A.; writing—review and editing, K.D., M.A. and Z.T. All authors have read and agreed to the published version of the manuscript.
Funding
The Hungarian University of Agriculture and Life Sciences Research Excellence Programme and Flagship Research Groups Programme supported this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw reads (SRA’s) were deposited in the National Center for Biotechnology Information (NCBI) database under the following accession numbers: Raw reads were deposited in the National Center for Biotechnology Information (NCBI) database under the following accessions: SubmissionID: SUB15530359; BioProject ID: PRJNA1304149, Tomato treated with biostimulants against drought stress. (1) Repository name: Tomato treated with biostimulants against drought stress; Data identification number: PRJNA1304149; Direct URL to data: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1304149 (accessed on 11 August 2025). (2) Repository name: Ctrl_non_stressed_R1; Data identification number: SRR34939869; Direct URL to data: https://www.ncbi.nlm.nih.gov/sra/?term SRR34939869 (accessed on 11 August 2025). (3) Repository name: Ctrl_stressed_R1; Data identification number: SRR34939868; Direct URL to data: https://www.ncbi.nlm.nih.gov/sra/?term SRR34939868 (accessed on 11 August 2025). (4) Repository name: GABA_non_stressed_R1; Data identification number: SRR34939867; Direct URL to data: https://www.ncbi.nlm.nih.gov/sra/?term SRR34939867 (accessed on 11 August 2025). (5) Repository name: GABA_stressed_R1; Data identification number: SRR34939866; Direct URL to data: https://www.ncbi.nlm.nih.gov/sra/?term SRR34939866 (accessed on 11 August 2025).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Decsi, K.; Ahmed, M.; Rizk, R.; Abdul-Hamid, D.; Kovács, G.P.; Tóth, Z. Emerging Trends in Non-Protein Amino Acids as Potential Priming Agents: Implications for Stress Management Strategies and Unveiling Their Regulatory Functions. Int. J. Mol. Sci. 2024, 25, 6203. [Google Scholar] [CrossRef]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. Chapter 13-γ-Aminobutyric Acid (GABA): Biosynthesis, Role, Commercial Production, and Applications. Stud. Nat. Prod. Chem. 2018, 57, 413–452. [Google Scholar]
- Kinnersley, A.M.; Turano, F.J. Gamma Aminobutyric Acid (GABA) and Plant Responses to Stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Yamniuk, A.P.; Rainaldi, M.; Vogel, H.J. Calmodulin Has the Potential to Function as a Ca2+-Dependent Adaptor Protein. Plant Signal. Behav. 2007, 2, 354–357. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Kaur, R.; Bindumadhava, H.; Nair, R.M.; Siddique, K.H.M.; Nayyar, H. GABA (γ-Aminobutyric Acid), as a Thermo-Protectant, to Improve the Reproductive Function of Heat-Stressed Mungbean Plants. Sci. Rep. 2019, 9, 7788. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous Application of Gamma-Aminobutyric Acid (GABA) Alleviates the Effect of Water Deficit Stress in Black Cumin (Nigella sativa L.). Ind. Crops Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]
- Sheng, Y.; Xiao, H.; Guo, C.; Wu, H.; Wang, X. Effects of Exogenous Gamma-Aminobutyric Acid on α-Amylase Activity in the Aleurone of Barley Seeds. Plant Physiol. Biochem. 2018, 127, 39–46. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-Aminobutyric Acid (GABA) Alleviates Salt Damage in Tomato by Modulating Na+ Uptake, the GAD Gene, Amino Acid Synthesis and Reactive Oxygen Species Metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse Role of γ-Aminobutyric Acid in Dynamic Plant Cell Responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. γ-Aminobutyric Acid and Related Amino Acids in Plant Immune Responses: Emerging Mechanisms of Action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Role of the γ-Aminobutyric Acid (GABA) in Plant Salt Stress Tolerance. Horticulturae 2023, 9, 230. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA Signalling Modulates Stomatal Opening to Enhance Plant Water Use Efficiency and Drought Resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, M.; Zhou, M.; Hassan, M.J.; Lin, L.; Lin, J.; Zhang, Y.; Li, Z. Drought Priming-Induced Stress Memory Improves Subsequent Drought or Heat Tolerance via Activation of γ-Aminobutyric Acid-Regulated Pathways in Creeping Bentgrass. Plant Biol. 2024. early view. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Gilliham, M. GABA Signalling in Guard Cells Acts as a ‘Stress Memory’to Optimise Plant Water Loss. bioRxiv 2019. [Google Scholar] [CrossRef]
- Lukić, N.; Kukavica, B.; Davidović-Plavšić, B.; Hasanagić, D.; Walter, J. Plant Stress Memory Is Linked to High Levels of Anti-Oxidative Enzymes over Several Weeks. Environ. Exp. Bot. 2020, 178, 104166. [Google Scholar] [CrossRef]
- Imakumbili, M.L.E.; Semu, E.; Semoka, J.M.R.; Abass, A.; Mkamilo, G. Managing Cassava Growth on Nutrient Poor Soils under Different Water Stress Conditions. Heliyon 2021, 7, e07331. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- BioBam Bioinformatics. OmicsBox; Bioinformatics Made Easy; BioBam Bioinformatics: Valencia, Spain, 2019. [Google Scholar]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-Throughput Functional Annotation and Data Mining with the Blast2GO Suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.C.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M.; et al. eggNOG 4.5: A Hierarchical Orthology Framework with Improved Functional Annotations for Eukaryotic, Prokaryotic and Viral Sequences. Nucleic Acids Res. 2016, 44, D286–D293. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Tarazona, S.; Furió-Tarí, P.; Turrà, D.; Pietro, A.D.; Nueda, M.J.; Ferrer, A.; Conesa, A. Data Quality Aware Analysis of Differential Expression in RNA-Seq with NOISeq R/Bioc Package. Nucleic Acids Res. 2015, 43, e140. [Google Scholar] [CrossRef]
- Tarazona, S.; García-Alcalde, F.; Dopazo, J.; Ferrer, A.; Conesa, A. Differential Expression in RNA-Seq: A Matter of Depth. Genome Res. 2011, 21, 2213–2223. [Google Scholar] [CrossRef]
- Ahmed, M.; Tóth, Z.; Marrez, D.A.; Rizk, R.; Abdul-Hamid, D.; Decsi, K. Transcriptome Datasets of Salt-Stressed Tomato Plants Treated with Zinc Oxide Nanoparticles. Data Brief 2025, 58, 111282. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Al-Shahrour, F.; Díaz-Uriarte, R.; Dopazo, J. FatiGO: A Web Tool for Finding Significant Associations of Gene Ontology Terms with Groups of Genes. Bioinformatics 2004, 20, 578–580. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Venisse, J.-S.; Gullner, G.; Brisset, M.-N. Evidence for the Involvement of an Oxidative Stress in the Initiation of Infection of Pear by Erwinia amylovora. Plant Physiol. 2001, 125, 2164–2172. [Google Scholar] [CrossRef]
- Ahmed, M.; Tóth, Z.; Rizk, R.; Abdul-Hamid, D.; Decsi, K. Investigation of Antioxidative Enzymes and Transcriptomic Analysis in Response to Foliar Application of Zinc Oxide Nanoparticles and Salinity Stress in Solanum Lycopersicum. Agronomy 2025, 15, 1715. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. [136] Assay of Catalases and Peroxidases. In Methods in Enzymology; Academic Press: New York, NY, USA, 1955; Volume 2, pp. 764–775. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bonnichsen, R.K.; Chance, B.; Theorell, H. Catalase Activity. Acta Chem. Scand. 1947, 1, 685–709. [Google Scholar] [CrossRef]
- Maehly, A.C. The Assay of Catalases and Peroxidases. In Methods of Biochemical Analysis; Wiley: Hoboken, NJ, USA, 1954; pp. 357–424. [Google Scholar]
- Ahmed, M.; Marrez, D.A.; Rizk, R.; Zedan, M.; Abdul-Hamid, D.; Decsi, K.; Kovács, G.P.; Tóth, Z. The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum lycopersicum L. Plants. Plants 2024, 13, 1418. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Bai, Y.; Yu, H.; Qian, Y.; Zhang, D.; Zhou, Y. Starch and Sucrose Metabolism and Plant Hormone Signaling Pathways Play Crucial Roles in Aquilegia Salt Stress Adaption. Int. J. Mol. Sci. 2023, 24, 3948. [Google Scholar] [CrossRef]
- Legay, S.; Lefèvre, I.; Lamoureux, D.; Barreda, C.; Luz, R.T.; Gutierrez, R.; Quiroz, R.; Hoffmann, L.; Hausman, J.-F.; Bonierbale, M.; et al. Carbohydrate Metabolism and Cell Protection Mechanisms Differentiate Drought Tolerance and Sensitivity in Advanced Potato Clones (Solanum tuberosum L.). Funct. Integr. Genomics 2011, 11, 275–291. [Google Scholar] [CrossRef]
- Valluru, R.; Link, J.; Claupein, W. Natural Variation and Morpho-Physiological Traits Associated with Water-Soluble Carbohydrate Concentration in Wheat under Different Nitrogen Levels. Field Crops Res. 2011, 124, 104–113. [Google Scholar] [CrossRef]
- Zahoor, R.; Dong, H.; Abid, M.; Zhao, W.; Wang, Y.; Zhou, Z. Potassium Fertilizer Improves Drought Stress Alleviation Potential in Cotton by Enhancing Photosynthesis and Carbohydrate Metabolism. Environ. Exp. Bot. 2017, 137, 73–83. [Google Scholar] [CrossRef]
- Nemati, F.; Ghanati, F.; Ahmadi Gavlighi, H.; Sharifi, M. Comparison of Sucrose Metabolism in Wheat Seedlings during Drought Stress and Subsequent Recovery. Biol. Plant. 2018, 62, 595–599. [Google Scholar] [CrossRef]
- Thomas, A.; Beena, R. Sucrose Metabolism in Plants under Drought Stress Condition: A Review. Indian J. Agric. Res. 2024, 58, 943. [Google Scholar] [CrossRef]
- Li, C.; Fu, K.; Guo, W.; Zhang, X.; Li, C.; Li, C. Starch and Sugar Metabolism Response to Post-Anthesis Drought Stress During Critical Periods of Elite Wheat (Triticum aestivum L.) Endosperm Development. J. Plant Growth Regul. 2023, 42, 5476–5494. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E.; Iglesias, A.A. Nucleotide-Sugar Metabolism in Plants: The Legacy of Luis F. Leloir. J. Exp. Bot. 2021, 72, 4053–4067. [Google Scholar] [CrossRef]
- Al Hinai, M.S.; Rehman, A.; Siddique, K.H.M.; Farooq, M. The Role of Trehalose in Improving Drought Tolerance in Wheat. J. Agron. Crop Sci. 2025, 211, e70053. [Google Scholar] [CrossRef]
- Kesten, C.; Menna, A.; Sánchez-Rodríguez, C. Regulation of Cellulose Synthesis in Response to Stress. Curr. Opin. Plant Biol. 2017, 40, 106–113. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Shen, Y.; Li, W. A Surprising Diversity of Xyloglucan Endotransglucosylase/Hydrolase in Wheat: New in Sight to the Roles in Drought Tolerance. Int. J. Mol. Sci. 2023, 24, 9886. [Google Scholar] [CrossRef]
- Ahl, L.I.; Mravec, J.; Jørgensen, B.; Rudall, P.J.; Rønsted, N.; Grace, O.M. Dynamics of Intracellular Mannan and Cell Wall Folding in the Drought Responses of Succulent Aloe Species. Plant Cell Environ. 2019, 42, 2458–2471. [Google Scholar] [CrossRef]
- Sun, D.; Lei, Z.; Carriquí, M.; Zhang, Y.; Liu, T.; Wang, S.; Song, K.; Zhu, L.; Zhang, W.; Zhang, Y. Reductions in Mesophyll Conductance under Drought Stress Are Influenced by Increases in Cell Wall Chelator-Soluble Pectin Content and Denser Microfibril Alignment in Cotton. J. Exp. Bot. 2025, 76, 1116–1130. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Qi, L.; Yin, L.; Deng, X. Galactolipid Remodeling Is Involved in Drought-Induced Leaf Senescence in Maize. Environ. Exp. Bot. 2018, 150, 57–68. [Google Scholar] [CrossRef]
- Shimojima, M. Biosynthesis and Functions of the Plant Sulfolipid. Prog. Lipid Res. 2011, 50, 234–239. [Google Scholar] [CrossRef]
- Khazaei, Z.; Esmaielpour, B.; Estaji, A. Ameliorative Effects of Ascorbic Acid on Tolerance to Drought Stress on Pepper (Capsicum annuum L.) Plants. Physiol. Mol. Biol. Plants 2020, 26, 1649–1662. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The Role of Gibberellin Signalling in Plant Responses to Abiotic Stress. J. Exp. Biol. 2014, 217 Pt 1, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Peter, P.; Gupta, R.; Kumari, S.; Nawaz, K.; Khan, M.I.R. Plant Hormone Ethylene: A Leading Edge in Conferring Drought Stress Tolerance. Physiol. Plant. 2024, 176, e14151. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, A.; Ramakrishnan, M.; Ha, C.V.; Zheng, B.; Bhardwaj, M.; Tran, L.-S.P. Roles of Abscisic Acid and Auxin in Plants during Drought: A Molecular Point of View. Plant Physiol. Biochem. 2023, 204, 108129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Raman, S.B.; Rathinasabapathi, B. Pantothenate Synthesis in Plants. Plant Sci. 2004, 167, 961–968. [Google Scholar] [CrossRef]
- Zhao, H.; Kosma, D.K.; Lü, S. Functional Role of Long-Chain Acyl-CoA Synthetases in Plant Development and Stress Responses. Front. Plant Sci. 2021, 12, 640996. [Google Scholar] [CrossRef]
- Phung, T.-H.; Jung, H.; Park, J.-H.; Kim, J.-G.; Back, K.; Jung, S. Porphyrin Biosynthesis Control under Water Stress: Sustained Porphyrin Status Correlates with Drought Tolerance in Transgenic Rice. Plant Physiol. 2011, 157, 1746–1764. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and Tyrosine as Exogenous Precursors of Wheat (Triticum aestivum L.) Secondary Metabolism through PAL-Associated Pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef]
- Rohde, A.; Morreel, K.; Ralph, J.; Goeminne, G.; Hostyn, V.; De Rycke, R.; Kushnir, S.; Van Doorsselaere, J.; Joseleau, J.-P.; Vuylsteke, M.; et al. Molecular Phenotyping of the Pal1 and Pal2 Mutants of Arabidopsis Thaliana Reveals Far-Reaching Consequences on Phenylpropanoid, Amino Acid, and Carbohydrate Metabolism. Plant Cell 2004, 16, 2749–2771. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional Analysis of the Arabidopsis PAL Gene Family in Plant Growth, Development, and Response to Environmental Stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Ayyanath, M.-M.; Shukla, M.R.; Sriskantharajah, K.; Hezema, Y.S.; Saxena, P.K. Stable Indoleamines Attenuate Stress—A Novel Paradigm in Tryptophan Metabolism in Plants. J. Pineal Res. 2024, 76, e12938. [Google Scholar] [CrossRef]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK Machinery in Plants. Plant Signal. Behav. 2010, 5, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.S.; Mawlong, I.; Ali, K.; Tyagi, A. Regulation of Phytosterol Biosynthetic Pathway during Drought Stress in Rice. Plant Physiol. Biochem. 2018, 129, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kawa, D. Security Notice: This Plant Immunity Is under mRNA Surveillance. Plant Cell 2020, 32, 803–804. [Google Scholar] [CrossRef]
- Punzo, P.; Grillo, S.; Batelli, G. Alternative Splicing in Plant Abiotic Stress Responses. Biochem. Soc. Trans. 2020, 48, 2117–2126. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, Y.; Liu, C.; Chen, K.; Li, M. Functions and Interaction of Plant Lipid Signalling under Abiotic Stresses. Plant Biol. 2023, 25, 361–378. [Google Scholar] [CrossRef]
- Gutierrez, S.; Ibañez, S.G.; Agostini, E.; Sosa Alderete, L.G. Influence of Arsenic Exposure on the Daily Changes of Glycerophospholipid Turnover and Assessment of Defence Mechanisms in Tobacco Hairy Roots. Plant Physiol. Biochem. 2025, 223, 109880. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the Roles of Plant Sterols in Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. The Role of Sterols in Plant Response to Abiotic Stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).