Development and Properties of Starches in Vitreous and Floury Endosperm of Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Investigation of Starch Granules in Developing Endosperm
2.3. Investigation of Starch Structural Properties
2.4. Analysis of RNA-Seq
2.5. Analysis of qRT-PCR
2.6. Activity Analysis of Granule-Bound Starch Synthase I
2.7. Determination of Pyruvate Orthophosphate Dikinase Activity in Developing Endosperm
2.8. Statistical Analysis
3. Results
3.1. Morphology and Accumulation of Developing Starch in IE and OE
3.2. Structural Properties of Developing Starch
3.3. Statistical Analysis of Expressed Genes in IE and OE
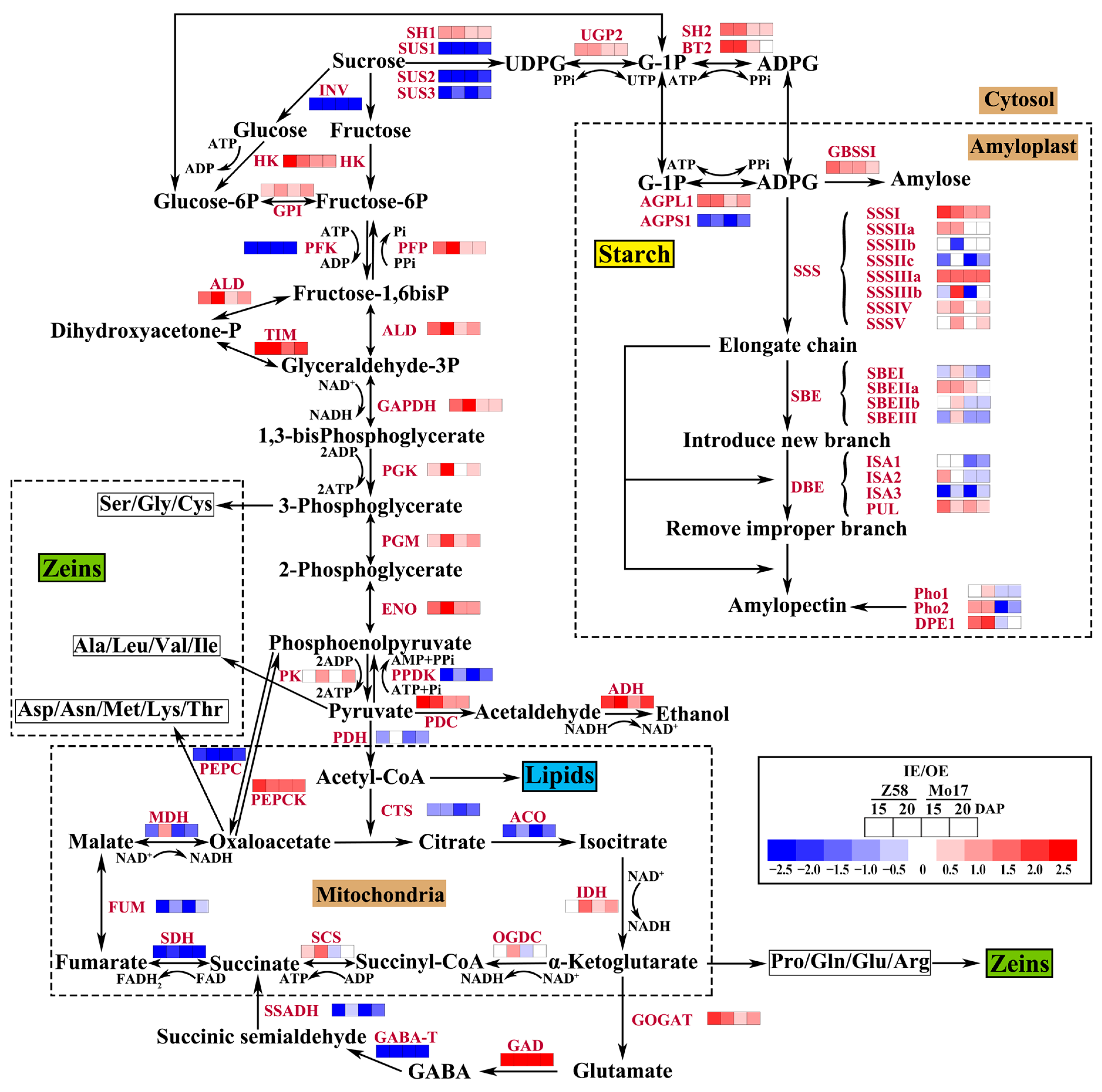
3.4. Differential Regulation of Energy Metabolism in IE and OE
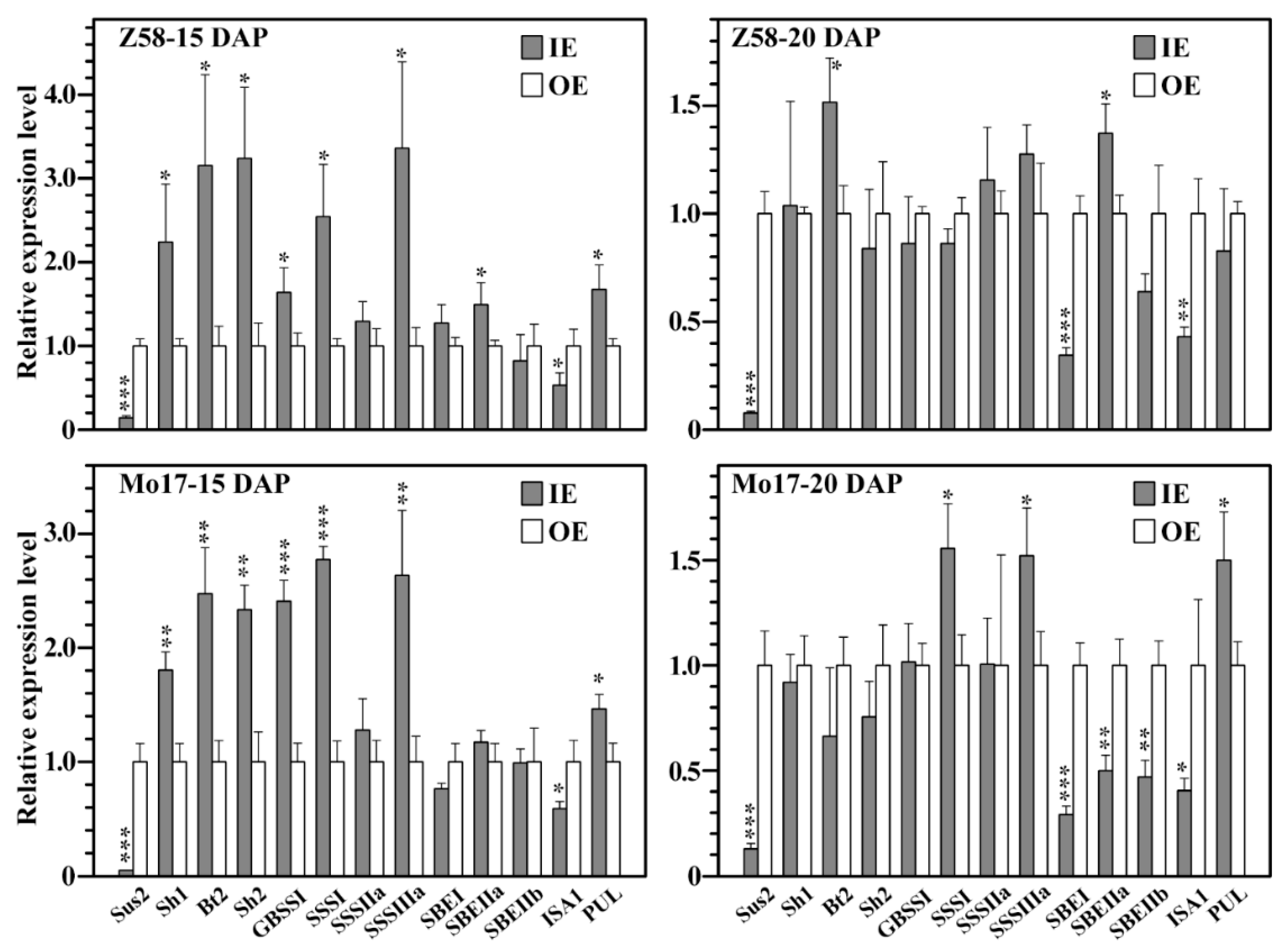
3.5. Relative Expression of Starch Synthesis Related Genes in IE and OE
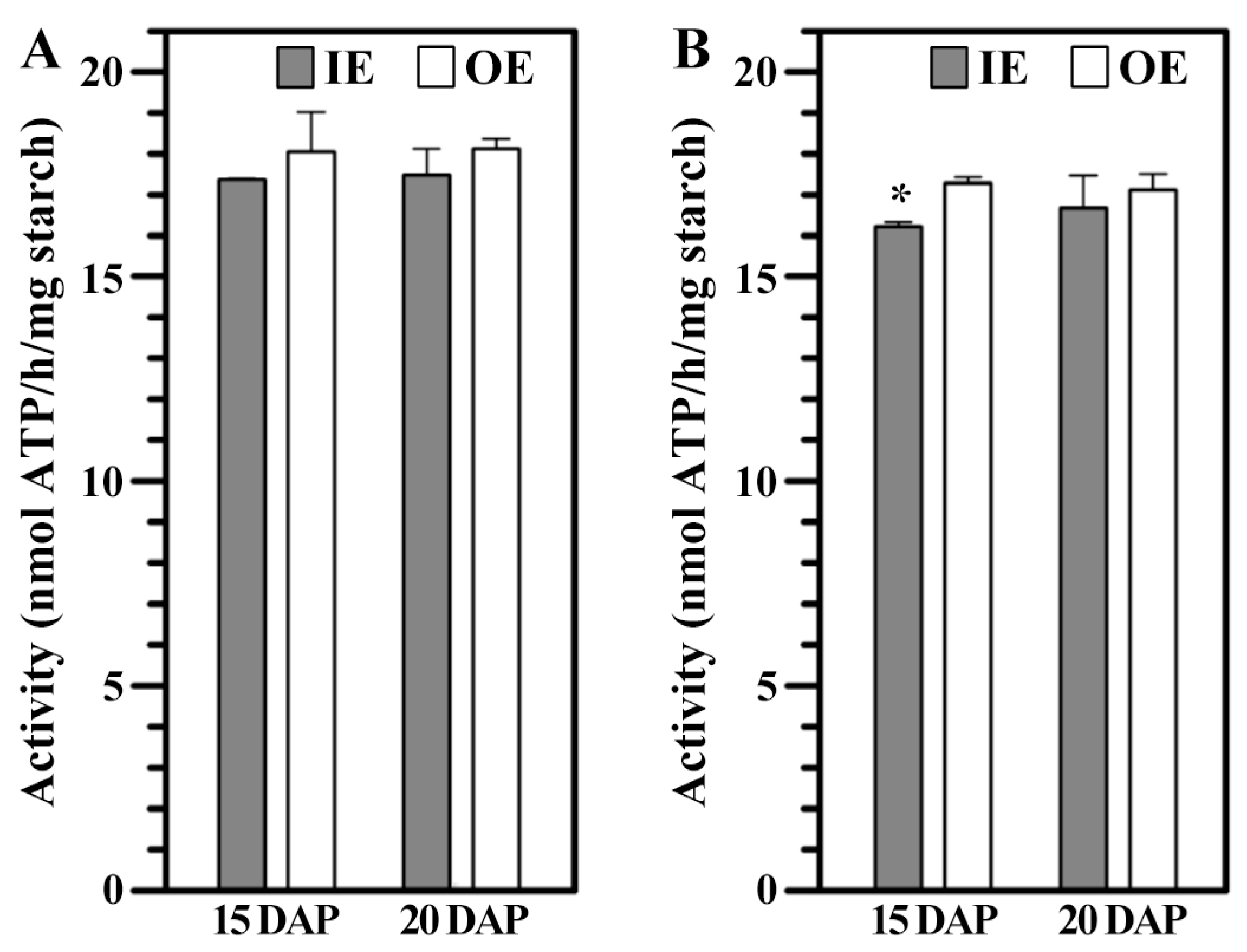
3.6. GBSSI Activity Within Developing Starch
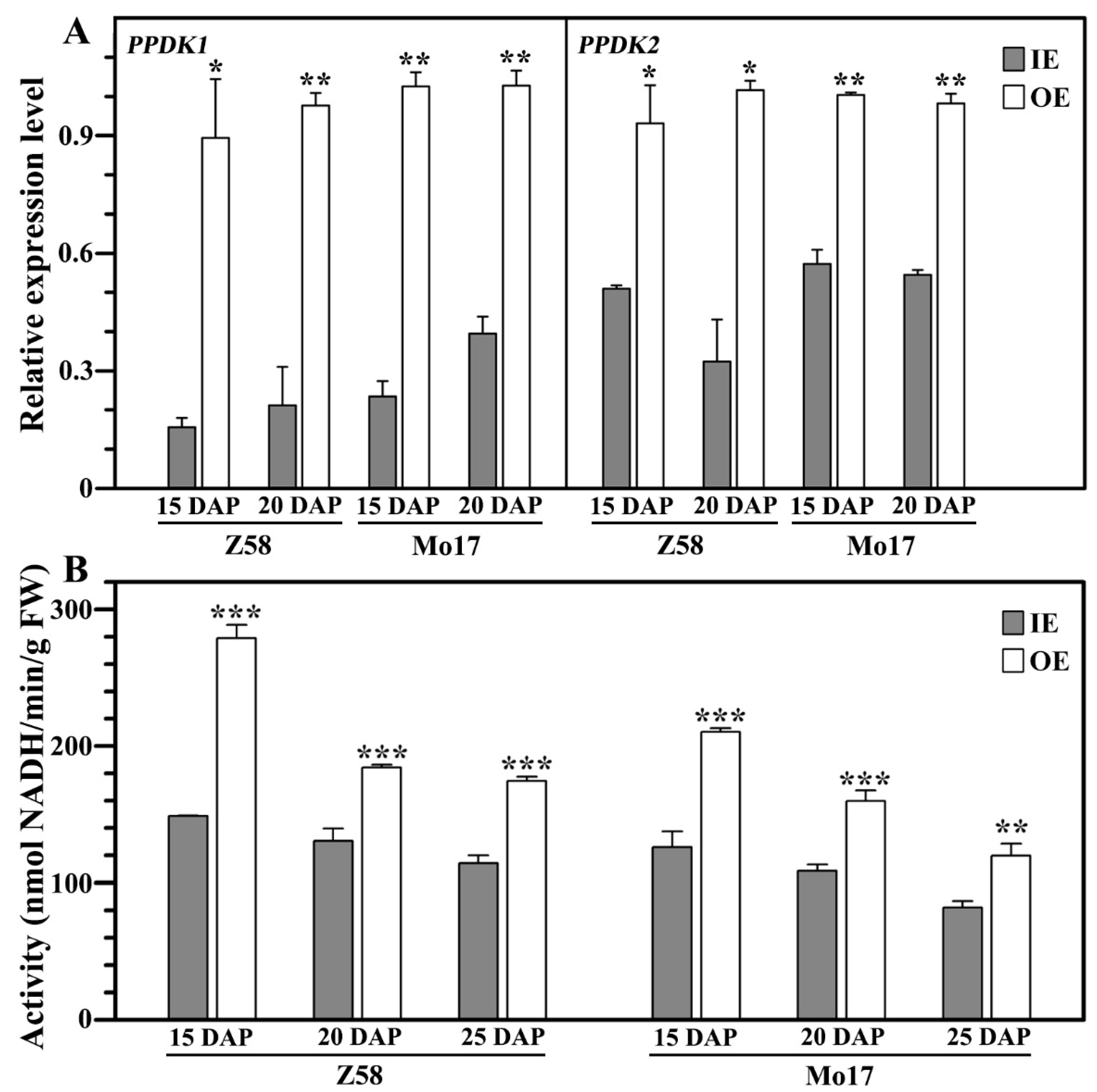
3.7. PPDK in IE and OE
4. Discussion
4.1. Nutrient Transportation and Oxygen Concentration Affect Starch Development and Accumulation in IE and OE
4.2. Large Starch Granule Size in OE Promotes Amylose Accumulation
4.3. Starch Granule Size and Amylose Content Influence Starch Properties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, R.C.; Davide, L.C.; Pedrozo, C.A.; Carneiro, N.P.; Souza, I.R.P.; Paiva, E. Relationship between structural and biochemical characteristics and texture of corn grains. Genet. Mol. Res. 2008, 7, 498–508. [Google Scholar] [CrossRef]
- Kljak, K.; Duvnjak, M.; Grbeša, D. Contribution of zein content and starch characteristics to vitreousness of commercial maize hybrids. J. Cereal Sci. 2018, 80, 57–62. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G. Physicochemical properties of vitreous and floury endosperm flours in maize. Food Sci. Nutr. 2019, 7, 2605–2612. [Google Scholar] [CrossRef]
- Gayral, M.; Bénédicte, B.; Dalgalarrondo, M.; Elmorjani, K.; Delluc, C.; Brunet, S.; Linossier, L.; Morel, M.H.; Marion, D. Lipid partitioning in maize (Zea mays L.) endosperm highlights relationships among starch lipids, amylose, and vitreousness. J. Agric. Food Chem. 2015, 63, 3551–3558. [Google Scholar] [CrossRef]
- Eckhoff, S.R.; Watson, S.A. Corn and sorghum starches: Production. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 373–440. [Google Scholar]
- Baasandorj, T.; Ohm, J.B.; Simsek, S. Effects of kernel vitreousness and protein level on protein molecular weight distribution, milling quality, and bread making quality in hard red spring wheat. Cereal Chem. 2016, 93, 426–434. [Google Scholar] [CrossRef]
- Gerde, J.A.; Spinozzi, J.I.; Borrás, L. Maize kernel hardness, endosperm zein profiles, and ethanol production. Bioenergy Res. 2017, 10, 760–771. [Google Scholar] [CrossRef]
- Juárez-García, E.; Agama-Acevedo, E.; Gómez-Montiel, N.O.; Pando-Robles, V.; Bello-Pérez, L.A. Proteomic analysis of the enzymes involved in the starch biosynthesis of maize with different endosperm type and characterization of the starch. J. Sci. Food Agric. 2013, 93, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Larkins, B.A. Proteins of the kernel. In Corn: Chemistry and Technology; Serna-Saldivar, S.O., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 319–336. [Google Scholar]
- Rolletschek, H.; Koch, K.; Wobus, U.; Borisjuk, L. Positional cues for the starch/lipid balance in maize kernels and resource partitioning to the embryo. Plant J. 2005, 42, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Gao, R.Q.; Dong, S.T. Anatomical and physiological characteristics associated with corn endosperm texture. Agron. J. 2011, 103, 1258–1264. [Google Scholar] [CrossRef]
- Dombrink-Kurtzman, M.A.; Bietz, J.A. Zein composition in hard and soft endosperm of maize. Cereal Chem. 1993, 70, 105–108. [Google Scholar]
- Fox, G.; Manley, M. Hardness methods for testing maize kernels. J. Agric. Food Chem. 2009, 57, 5647–5657. [Google Scholar] [CrossRef]
- Xu, A.; Lin, L.; Guo, K.; Liu, T.; Yin, Z.; Wei, C. Physicochemical properties of starches from vitreous and floury endosperms from the same maize kernels. Food Chem. 2019, 291, 149–156. [Google Scholar] [CrossRef]
- Gayral, M.; Elmorjani, K.; Dalgalarrondo, M.; Balzergue, S.M.; Pateyron, S.; Morel, M.H.; Brunet, S.; Linossier, L.; Delluc, C.; Bénédicte, B.; et al. Responses to hypoxia and endoplasmic reticulum stress discriminate the development of vitreous and floury endosperms of conventional maize (Zea mays) inbred lines. Front. Plant Sci. 2017, 8, 557. [Google Scholar] [CrossRef]
- Dombrink-Kurtzman, M.A.; Knutson, C.A. A study of maize endosperm hardness in relation to amylose content and susceptibility to damage. Cereal Chem. 1997, 74, 776–780. [Google Scholar] [CrossRef]
- Gayral, M.; Gaillard, C.; Bakan, B.; Dalgalarrondo, M.; Elmorjani, K.; Delluc, C.; Brunet, S.; Linossier, L.; Morel, M.H.; Marion, D. Transition from vitreous to floury endosperm in maize (Zea mays L.) kernels is related to protein and starch gradients. J. Cereal Sci. 2016, 68, 148–154. [Google Scholar] [CrossRef]
- Xu, A.; Qiu, J.; Yin, Z.; Wei, C. Morphological characteristics of endosperm in different regions of maize kernels with different vitreousness. J. Cereal Sci. 2019, 87, 273–279. [Google Scholar] [CrossRef]
- Cai, C.; Cai, J.; Man, J.; Yang, Y.; Wang, Z.; Wei, C. Allomorph distribution and granule structure of lotus rhizome C-type starch during gelatinization. Food Chem. 2014, 142, 408–415. [Google Scholar] [CrossRef]
- Prioul, J.L.; Méchin, V.; Lessard, P.; Thévenot, C.; Grimmer, M.; Chateau-Joubert, S.; Coates, S.; Hartings, H.; Kloiber-Maitz, M.; Murigneux, A.; et al. A joint transcriptomic, proteomic and metabolic analysis of maize endosperm development and starch filling. Plant Biotechnol. J. 2008, 6, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, X.; Yang, J.; Messing, J.; Wu, J. Maize endosperm-specific transcription factors O2 and PBF network the regulation of protein and starch synthesis. Proc. Natl Acad. Sci. USA 2016, 113, 10842–10847. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.V.; Denyer, K.; Packman, L.C.; Jones, M.K.; Howe, C.J. Molecular basis of the waxy endosperm starch phenotype in broomcorn millet (Panicum miliaceum L.). Mol. Biol. Evol. 2010, 27, 1478–1494. [Google Scholar] [CrossRef]
- Fujita, N.; Hasegawa, H.; Taira, T. The isolation and characterization of a waxy mutant of diploid wheat (Triticum monococcum L.). Plant Sci. 2001, 160, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Dong, X.; Luo, H.; Bi, W.; Chen, H.; Yu, S.; Zhang, X.; Dai, Y.; Cheng, X.; Xing, Y.; Fan, X.; et al. Transcriptome-wide identification and characterization of genes exhibit allele-specific imprinting in maize embryo and endosperm. BMC Plant Biol. 2023, 23, 470. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Li, K.; Liu, X.; Cao, X.; Zhou, Y.; Huang, C.; Peng, Y.; Hu, X. Characterization and transcriptome analysis of maize small-kernel mutant smk7a in different development stages. Plants 2023, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Tian, R.; Bean, S.R.; Yerka, M.; Jiao, Y. Transcriptome and metabolome analyses reveal regulatory networks associated with nutrition synthesis in sorghum seeds. Commun. Biol. 2024, 7, 841. [Google Scholar] [CrossRef]
- Hennen-Bierwagen, T.A.; Lin, Q.H.; Grimaud, F.; Planchot, V.; Keeling, P.L.; James, M.G.; Myers, A.M. Proteins from multiple metabolic pathways associate with starch biosynthetic enzymes in high molecular weight complexes: A model for regulation of carbon allocation in maize amyloplasts. Plant Physiol. 2009, 149, 1541–1559. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Park, S.; Matsuoka, M.; An, G. White-core endosperm flour endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J. 2005, 42, 901–911. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, A.; Zhang, L.; Yin, Z.; Wei, C. Spatiotemporal accumulation and characteristics of starch in developing maize caryopses. Plant Physiol. Biochem. 2018, 130, 493–500. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y.; Li, W.; Dong, M.; Bian, A. The development of endosperm in maize and its nutrients transporting way. J. Jiangsu Agric. Coll. 1997, 18, 1–7. (In Chinese) [Google Scholar]
- He, W.; Liu, X.; Lin, L.; Xu, A.; Wei, C. The defective effect of starch branching enzyme IIb from weak to strong induces the formation of biphasic starch granules in amylose-extender maize endosperm. Plant Mol. Biol. 2020, 103, 355–371. [Google Scholar] [CrossRef]
- Chastain, C.J.; Heck, J.W.; Colquhoun, T.A.; Voge, D.G.; Gu, X.Y. Posttranslational regulation of pyruvate, orthophosphate dikinase in developing rice (Oryza sativa) seeds. Planta 2006, 224, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Lappe, R.R.; Baier, J.W.; Boehlein, S.K.; Huffman, R.; Myers, A.M. Functions of maize genes encoding pyruvate phosphate dikinase in developing endosperm. Proc. Natl. Acad. Sci. USA 2018, 115, E24–E33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, L.; Lin, L.; Zhao, L.; Liu, Q.; Wei, C. A novel mutation of OsPPDKB, encoding pyruvate orthophosphate dikinase, affects metabolism and structure of starch in the rice endosperm. Int. J. Mol. Sci. 2018, 19, 2268. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Hasjim, J.; Gidley, M.J. Physicochemical and structural properties of maize and potato starches as a function of granule size. J. Agric. Food Chem. 2011, 59, 10151–10161. [Google Scholar] [CrossRef]
- Li, L.; Blanco, M.; Jane, J.L. Physicochemical properties of endosperm and pericarp starches during maize development. Carbohydr. Polym. 2007, 67, 630–639. [Google Scholar] [CrossRef]
- Naguleswaran, S.; Li, J.; Vasanthan, T.; Bressler, D.; Hoover, R. Amylolysis of large and small granules of native triticale, wheat and corn starches using a mixture of α-amylase and glucoamylase. Carbohydr. Polym. 2012, 88, 864–874. [Google Scholar] [CrossRef]
- Naguleswaran, S.; Vasanthan, T.; Hoover, R.; Bressler, D. The susceptibility of large and small granules of waxy, normal and high-amylose genotypes of barley and corn starches toward amylolysis at sub-gelatinization temperatures. Food Res. Int. 2013, 51, 771–782. [Google Scholar] [CrossRef]
- Takeda, Y.; Takeda, C.; Mizukami, H.; Hanashiro, I. Structures of large, medium and small starch granules of barley grain. Carbohydr. Polym. 1999, 38, 109–114. [Google Scholar] [CrossRef]
- Tang, H.; Ando, H.; Watanabe, K.; Takeda, Y.; Mitsunaga, T. Physicochemical properties and structure of large, medium and small granule starches in fractions of normal barley endosperm. Carbohydr. Res. 2001, 330, 241–248. [Google Scholar] [CrossRef]
- Utrilla-Coello, R.G.; Agama-Acevedo, E.; de la Rosa, A.P.B.; Rodríguez-Ambriz, S.L.; Bello-Pérez, L.A. Physicochemical and enzyme characterization of small and large starch granules isolated from two maize cultivars. Cereal Chem. 2010, 87, 50–56. [Google Scholar] [CrossRef]
- Pan, D.D.; Jane, J.L. Internal structure of normal maize starch granules revealed by chemical surface gelatinization. Biomacromolecules 2000, 1, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Lin, L.; Man, J.; Zhao, L.; Wei, C. Different structural properties of high-amylose maize starch fractions varying in granule size. J. Agric. Food Chem. 2014, 62, 11711–11721. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, J.; Zhao, L.; Wang, J.; Wei, C. Effect of granule size on the properties of lotus rhizome C-type starch. Carbohydr. Polym. 2015, 134, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Guo, D.; Huang, J.; Zhang, X.; Zhang, L.; Wei, C. Molecular structure and enzymatic hydrolysis properties of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocoll. 2016, 58, 246–254. [Google Scholar] [CrossRef]
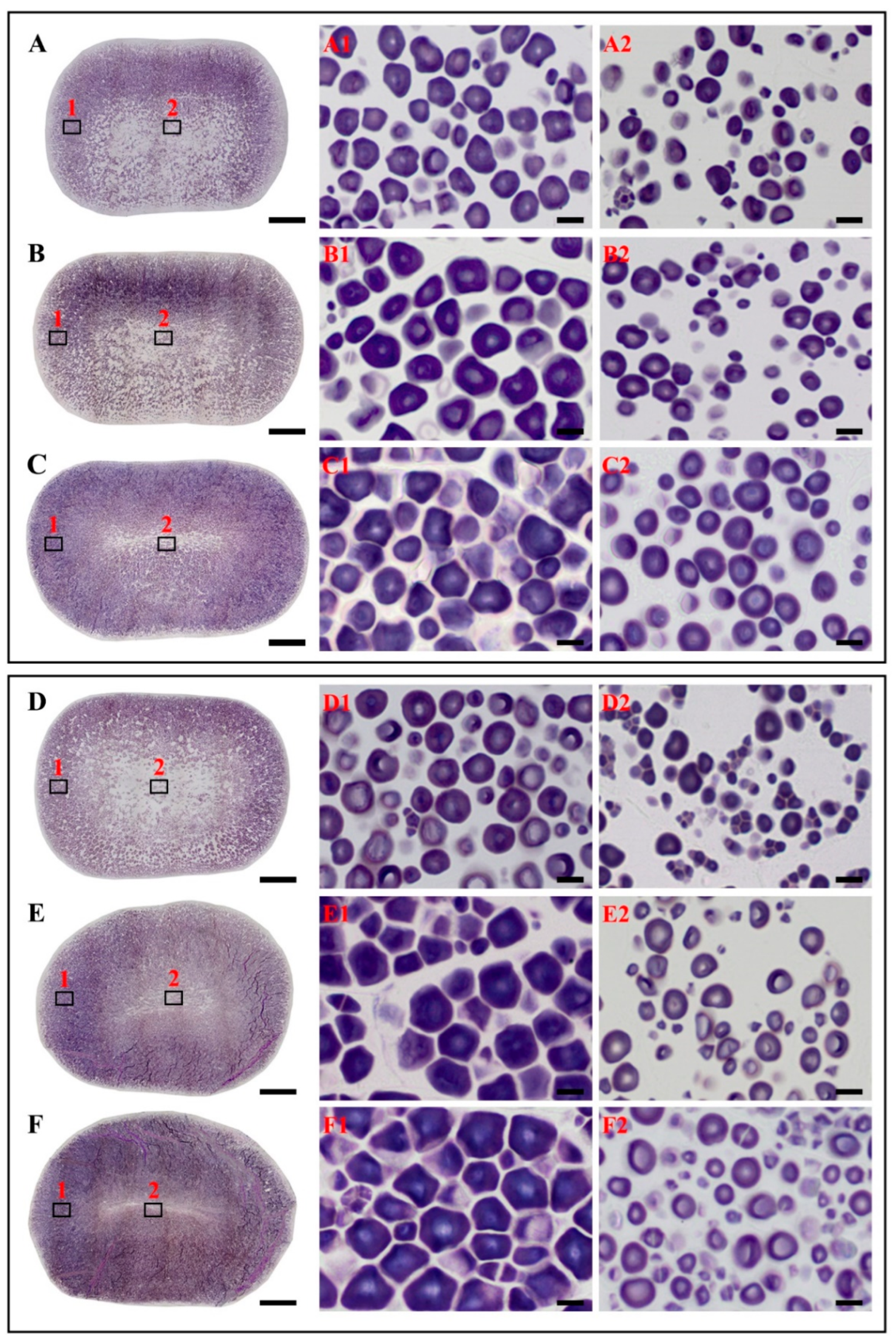

| Cultivar | Developing Stage | Region | Area (μm2) | Roundness | FD (%) |
|---|---|---|---|---|---|
| Z58 | 15 DAP | IE | 31.7 ± 17.1 | 1.16 ± 0.07 | UD |
| OE | 52.6 ± 26.9 *** | 1.18 ± 0.09 *** | 47.8 ± 2.2 | ||
| 20 DAP | IE | 46.5 ± 26.6 | 1.15 ± 0.04 | 21.7 ± 1.9 | |
| OE | 92.1 ± 46.9 *** | 1.28 ± 0.09 *** | 56.8 ± 1.9 *** | ||
| 25 DAP | IE | 45.7 ± 26.9 | 1.13 ± 0.03 | 34.2 ± 3.4 | |
| OE | 106.4 ± 60.3 *** | 1.21 ± 0.07 *** | 65.8 ± 2.3 *** | ||
| Mo17 | 15 DAP | IE | 23.6 ± 18.4 | 1.14 ± 0.06 | UD |
| OE | 54.9 ± 37.8 *** | 1.13 ± 0.04 * | 46.0 ± 2.5 | ||
| 20 DAP | IE | 38.2 ± 22.2 | 1.18 ± 0.07 | 28.5 ± 3.0 | |
| OE | 103.2 ± 73.5 *** | 1.27 ± 0.12 *** | 65.9 ± 5.8 *** | ||
| 25 DAP | IE | 35.2 ± 21.4 | 1.16 ± 0.05 | 36.4 ± 4.6 | |
| OE | 111.6 ± 70.7 *** | 1.32 ± 0.17 *** | 74.0 ± 4.4 *** |
| Cultivar | Developing Stage | Region | AC (%) | RC (%) | PI (counts) | Smax (Å−1) | D (nm) |
|---|---|---|---|---|---|---|---|
| Z58 | 15 DAP | IE | 17.6 ± 0.4 | 23.9 ± 1.3 | 432.6 ± 2.6 | 0.062 ± 0.000 | 10.1 ± 0.0 |
| OE | 18.8 ± 0.4 | 24.5 ± 0.0 | 408.6 ± 34.5 | 0.064 ± 0.001 | 9.9 ± 0.1 | ||
| 20 DAP | IE | 18.1 ± 0.1 | 23.9 ± 0.1 | 421.2 ± 26.7 | 0.062 ± 0.000 | 10.1 ± 0.0 | |
| OE | 20.0 ± 0.5 * | 23.7 ± 0.2 | 410.5 ± 3.5 | 0.062 ± 0.000 | 10.1 ± 0.0 | ||
| 25 DAP | IE | 17.8 ± 0.5 | 24.7 ± 0.4 | 425.2 ± 2.6 | 0.062 ± 0.000 | 10.1 ± 0.0 | |
| OE | 20.1 ± 0.4 * | 22.0 ± 0.2 * | 353.7 ± 3.0 ** | 0.062 ± 0.001 | 10.2 ± 0.1 | ||
| Mature | IE | 18.3 ± 0.2 | 21.4 ± 0.1 | 282.1 ± 6.2 | 0.061 ± 0.001 | 10.4 ± 0.1 | |
| OE | 20.6 ± 0.7 * | 19.9 ± 0.2 * | 148.4 ± 2.6 ** | 0.060 ± 0.000 | 10.5 ± 0.0 | ||
| Mo17 | 15 DAP | IE | 16.5 ± 0.6 | 24.2 ± 0.4 | 434.0 ± 46.0 | 0.063 ± 0.001 | 10.1 ± 0.1 |
| OE | 17.0 ± 0.0 | 24.7 ± 0.5 | 440.3 ± 6.3 | 0.063 ± 0.000 | 10.0 ± 0.0 | ||
| 20 DAP | IE | 17.5 ± 0.2 | 24.9 ± 0.1 | 443.3 ± 0.6 | 0.062 ± 0.000 | 10.1 ± 0.0 | |
| OE | 19.5 ± 0.3 * | 24.8 ± 1.1 | 409.6 ± 5.6 * | 0.062 ± 0.000 | 10.1 ± 0.0 | ||
| 25 DAP | IE | 18.1 ± 0.5 | 23.2 ± 0.3 | 381.2 ± 5.6 | 0.064 ± 0.001 | 9.9 ± 0.1 | |
| OE | 20.0 ± 0.3 * | 21.0 ± 0.2 * | 260.5 ± 2.6 ** | 0.063 ± 0.001 | 10.1 ± 0.1 | ||
| Mature | IE | 18.4 ± 0.1 | 22.8 ± 0.0 | 417.6 ± 7.1 | 0.062 ± 0.000 | 10.1 ± 0.0 | |
| OE | 20.8 ± 0.5 * | 21.8 ± 0.3 * | 234.5 ± 39.8 * | 0.061 ± 0.001 | 10.4 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wei, S.; Xu, A.; Wei, C. Development and Properties of Starches in Vitreous and Floury Endosperm of Maize. Agriculture 2025, 15, 1978. https://doi.org/10.3390/agriculture15181978
Han Y, Wei S, Xu A, Wei C. Development and Properties of Starches in Vitreous and Floury Endosperm of Maize. Agriculture. 2025; 15(18):1978. https://doi.org/10.3390/agriculture15181978
Chicago/Turabian StyleHan, Yuzhi, Shuchang Wei, Ahui Xu, and Cunxu Wei. 2025. "Development and Properties of Starches in Vitreous and Floury Endosperm of Maize" Agriculture 15, no. 18: 1978. https://doi.org/10.3390/agriculture15181978
APA StyleHan, Y., Wei, S., Xu, A., & Wei, C. (2025). Development and Properties of Starches in Vitreous and Floury Endosperm of Maize. Agriculture, 15(18), 1978. https://doi.org/10.3390/agriculture15181978





