Powdery Mildew Resistance Gene (Pm) Stability and Blumeria graminis f. sp. avenae Virulence Trends in Poland (2021–2023): Challenges to Durable Resistance in Oat
Abstract
1. Introduction
2. Materials and Methods
2.1. Differential Sets
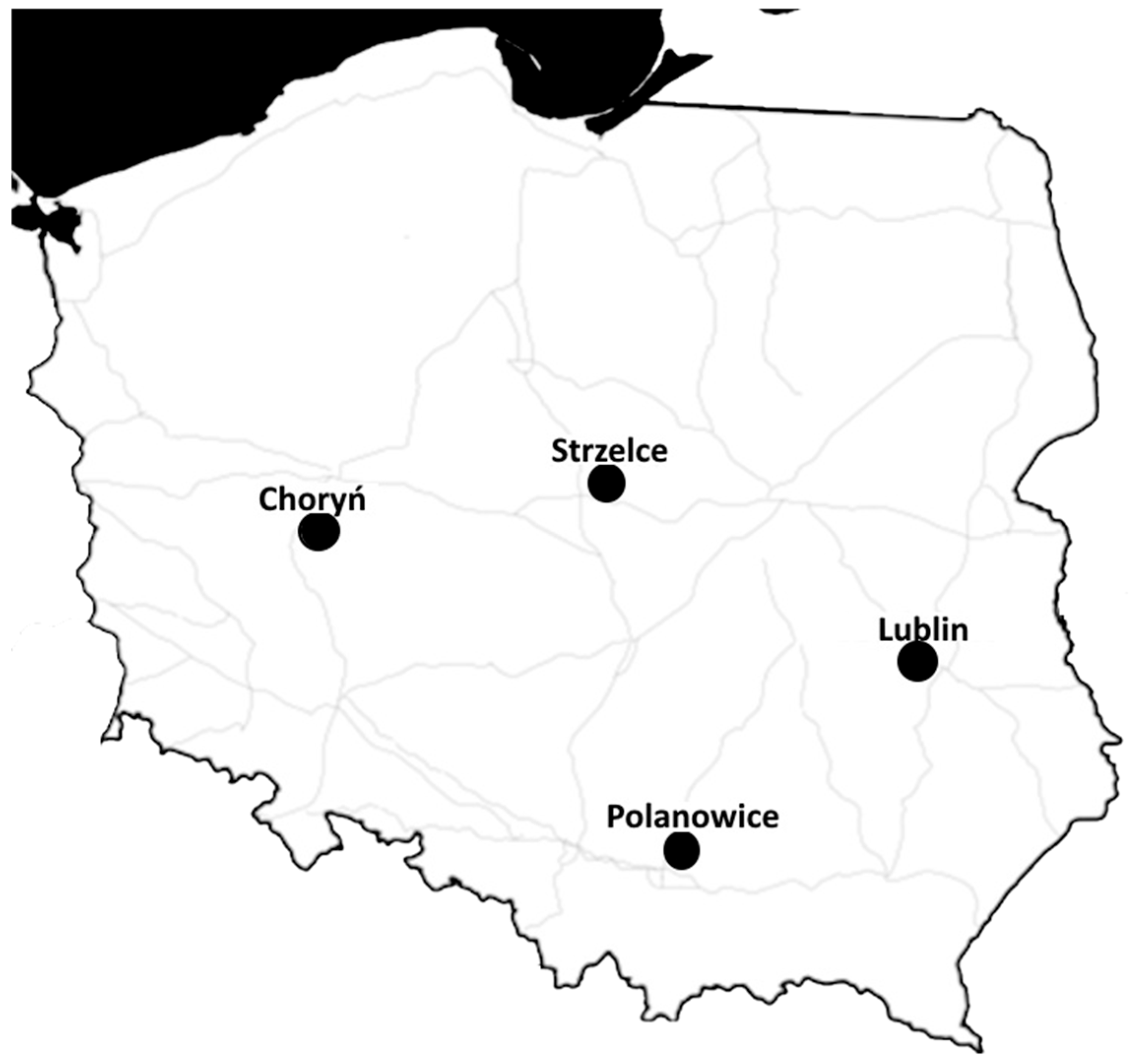
2.2. Pathogen Collection
2.3. Host–Pathogen Tests
2.4. Effectiveness of Pm Genes and Bga Population Diversity
3. Results
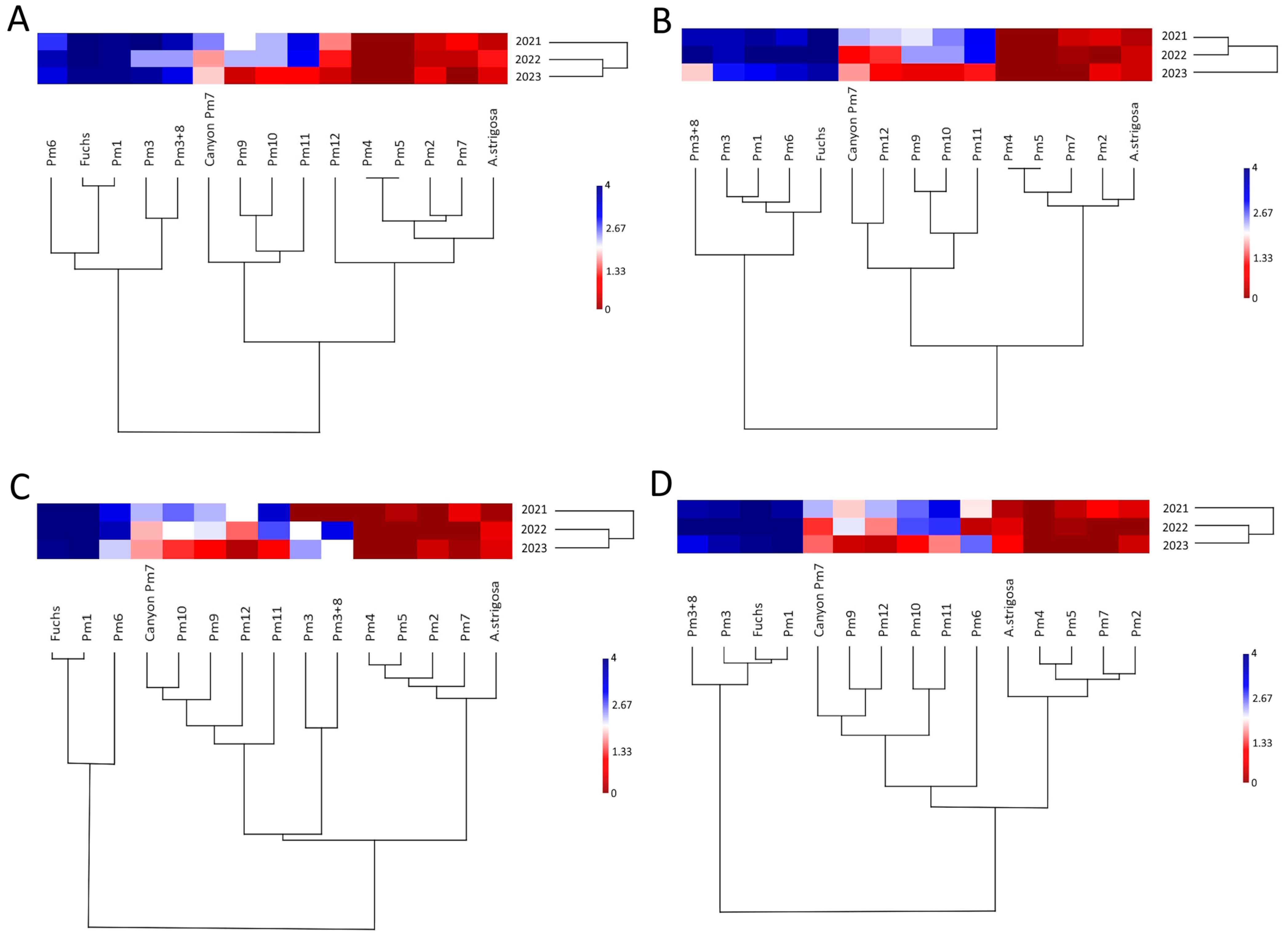
3.1. Pm Gene Effectiveness
3.2. Bga Virulence Frequency
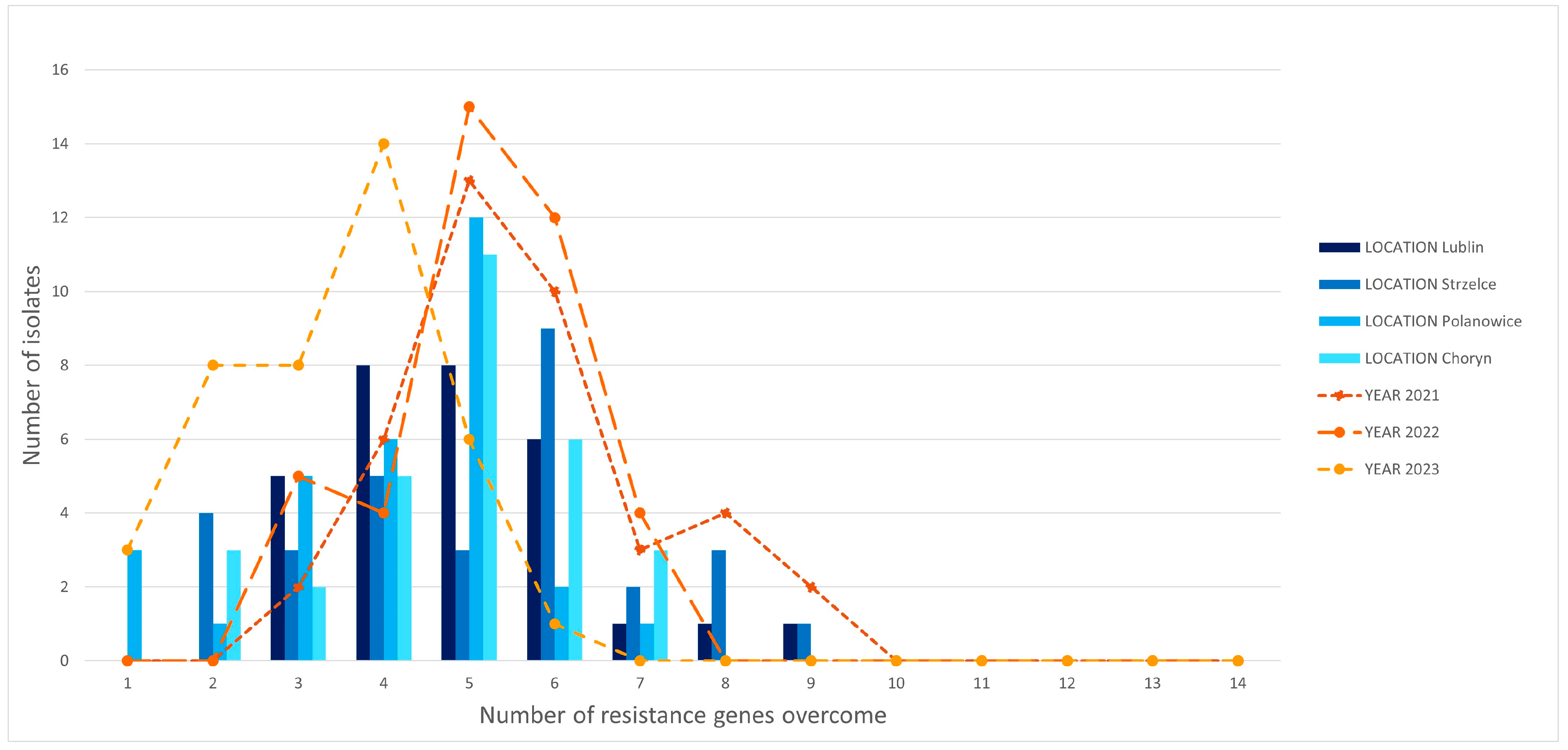
3.3. Complexity
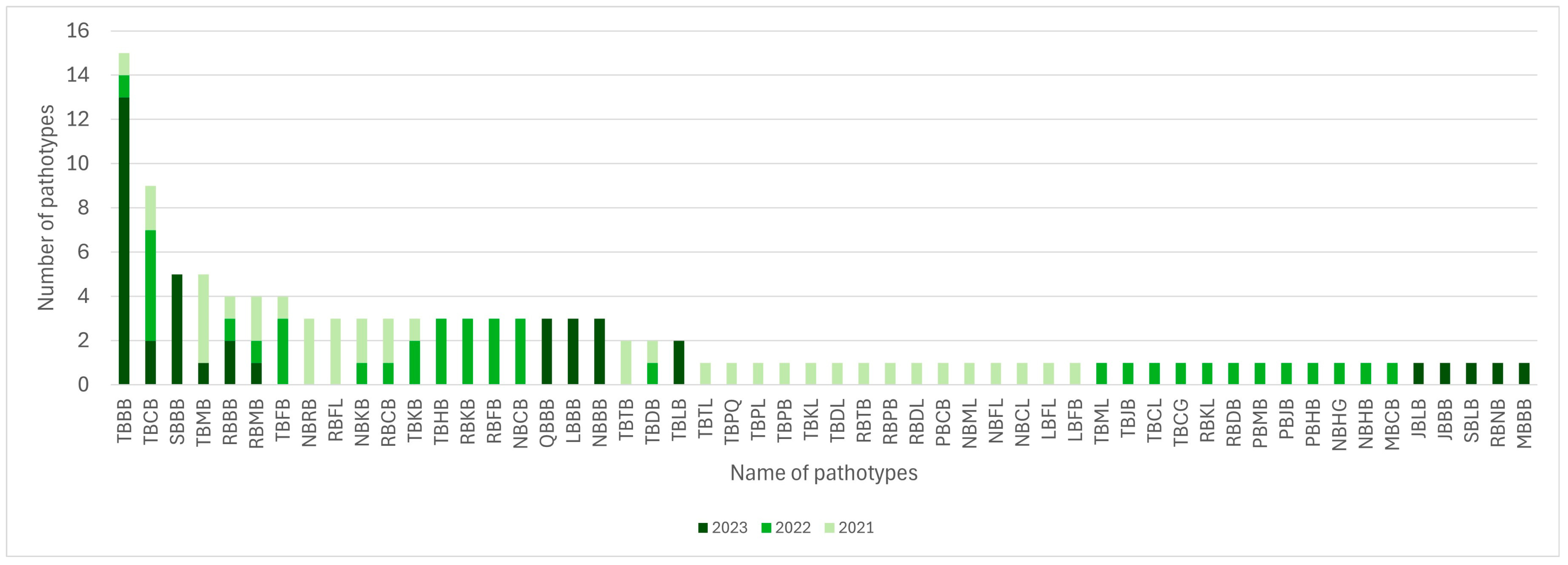
3.4. Pathotype Structure
3.5. Diversity Within Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stewart, D.; McDougall, G. Oat Agriculture, Cultivation and Breeding Targets: Implications for Human Nutrition and Health. Br. J. Nutr. 2014, 112, S50–S57. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-Based Milk Substitutes: Bioactive Compounds, Conventional and Novel Processes, Bioavailability Studies, and Health Effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-Dairy Plant-Based Milk Substitutes and Fermented Dairy-Type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.a.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Troch, V.; Audenaert, K.; Vanheule, A.; Bekaert, B.; Höfte, M.; Haesaert, G. Evaluation of Resistance to Powdery Mildew in Triticale Seedlings and Adult Plants. Plant Dis. 2013, 97, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Lawes, D.A.; Hayes, J.D. The Effect of Mildew (Erysiphe graminis f.sp. avenae) on Spring Oats. Plant Pathol. 1965, 14, 125–128. [Google Scholar] [CrossRef]
- Clifford, B.C. Diseases, Pests and Disorders of Oats. In The Oat Crop; Welch, R.W., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 252–278. ISBN 978-94-010-4010-5. [Google Scholar]
- Jones, I.T. The Effect on Grain Yield of Adult Plant Resistance to Mildew in Oats. Ann. Appl. Biol. 1977, 86, 267–277. [Google Scholar] [CrossRef]
- Golzar, H.; Shankar, M.; Sznajder, B.; Fox, R.; Reeves, K.; Mather, D.E. Genetic Mapping of Loci Affecting Seedling and Adult-Plant Resistance to Powdery Mildew Derived from Two CIMMYT Wheat Lines. Planta 2024, 260, 13. [Google Scholar] [CrossRef]
- Dracatos, P.M.; Lu, J.; Sánchez-Martín, J.; Wulff, B.B.H. Resistance That Stacks up: Engineering Rust and Mildew Disease Control in the Cereal Crops Wheat and Barley. Plant Biotechnol. J. 2023, 21, 1938–1951. [Google Scholar] [CrossRef]
- Dreiseitl, A. Powdery Mildew Resistance Genes in European Barley Cultivars Registered in the Czech Republic from 2016 to 2020. Genes 2022, 13, 1274. [Google Scholar] [CrossRef]
- European Parliament. Directive—2009/128-EN-EUR-Lex. Off. J. Eur. Union 2009, L 309/71, 71–86.
- Aktar-Uz-Zaman, M.; Tuhina-Khatun, M.; Hanafi, M.M.; Sahebi, M. Genetic Analysis of Rust Resistance Genes in Global Wheat Cultivars: An Overview. Biotechnol. Biotechnol. Equip. 2017, 31, 431–445. [Google Scholar] [CrossRef]
- Goutam, U.; Kukreja, S.; Yadav, R.; Salaria, N.; Thakur, K.; Goyal, A.K. Recent Trends and Perspectives of Molecular Markers against Fungal Diseases in Wheat. Front. Microbiol. 2015, 6, 861. [Google Scholar] [CrossRef]
- Stevens, D.E.J.; Armstrong, K.W.; Bezar, H.J.; Griffin, D.W.B.; Hampton, J.G. Fodder Oats: An Overview. In Fodder Oats: A World Overview, 33rd ed.; FAO: Rome, Italy, 2004; pp. 11–18. [Google Scholar]
- Duru, M.; Therond, O.; Martin, G.; Martin-Clouaire, R.; Magne, M.-A.; Justes, E.; Journet, E.-P.; Aubertot, J.-N.; Savary, S.; Bergez, J.-E.; et al. How to Implement Biodiversity-Based Agriculture to Enhance Ecosystem Services: A Review. Agron. Sustain. Dev. 2015, 35, 1259–1281. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Gu, B.; Ben Fekih, I.; Finger, R.; Kenis, M.; Lu, Y.; Subramanian, S.; Tang, F.H.M.; Weber, D.C.; Zhang, W.; et al. Restoring Functional Integrity of the Global Production Ecosystem through Biological Control. J. Environ. Manag. 2024, 370, 122446. [Google Scholar] [CrossRef]
- Médiène, S.; Valantin-Morison, M.; Sarthou, J.-P.; de Tourdonnet, S.; Gosme, M.; Bertrand, M.; Roger-Estrade, J.; Aubertot, J.-N.; Rusch, A.; Motisi, N.; et al. Agroecosystem Management and Biotic Interactions: A Review. Agron. Sustain. Dev. 2011, 31, 491–514. [Google Scholar] [CrossRef]
- Shea, K.; Possingham, H.P.; Murdoch, W.W.; Roush, R. Active Adaptive Management in Insect Pest and Weed Control: Intervention with a Plan for Learning. Ecol. Appl. 2002, 12, 927–936. [Google Scholar] [CrossRef]
- Kremen, C.; Miles, A. Ecosystem Services in Biologically Diversified versus Conventional Farming Systems: Benefits, Externalities, and Trade-Offs. Ecol. Soc. 2012, 17, 40. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, J.; Rubiales, D.; Prats, E. Resistance to Powdery Mildew (Blumeria graminis f.sp. avenae) in Oat Seedlings and Adult Plants. Plant Pathol. 2011, 60, 846–856. [Google Scholar] [CrossRef]
- Jensen, H.R.; Dreiseitl, A.; Sadiki, M.; Schoen, D.J. High Diversity, Low Spatial Structure and Rapid Pathotype Evolution in Moroccan Populations of Blumeria graminis f.sp. hordei. Eur. J. Plant Pathol. 2013, 136, 323–336. [Google Scholar] [CrossRef]
- Jigisha, J.; Ly, J.; Minadakis, N.; Freund, F.; Kunz, L.; Piechota, U.; Akin, B.; Balmas, V.; Ben-David, R.; Bencze, S.; et al. Population Genomics and Molecular Epidemiology of Wheat Powdery Mildew in Europe. PLoS Biol. 2025, 23, e3003097. [Google Scholar] [CrossRef]
- Mahaffee, W.F.; Thiessen, L.D.; King, K.M.; Choudhury, R.A. Detection and Monitoring of Plant Pathogens and Pests. Phyto Front. 2024, 4, 3–4. [Google Scholar] [CrossRef]
- Cowger, C.; Parks, R.; Kosman, E. Structure and Migration in U.S. Blumeria graminis f.sp. tritici Populations. Phytopathology 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Menardo, F.; Praz, C.R.; Wyder, S.; Ben-David, R.; Bourras, S.; Matsumae, H.; McNally, K.E.; Parlange, F.; Riba, A.; Roffler, S.; et al. Hybridization of Powdery Mildew Strains Gives Rise to Pathogens on Novel Agricultural Crop Species. Nat. Genet. 2016, 48, 201–205. [Google Scholar] [CrossRef]
- Herrmann, M.H.; Mohler, V. Locating Two Novel Genes for Resistance to Powdery Mildew from Avena byzantina in the Oat Genome. Plant Breed. 2018, 137, 832–838. [Google Scholar] [CrossRef]
- Hsam, S.L.K.; Mohler, V.; Zeller, F.J. The Genetics of Resistance to Powdery Mildew in Cultivated Oats (Avena sativa L.): Current Status of Major Genes. J. Appl. Genet. 2014, 55, 155–162. [Google Scholar] [CrossRef]
- Ociepa, T.; Okoń, S.; Nucia, A.; Leśniowska-Nowak, J.; Paczos-Grzęda, E.; Bisaga, M. Molecular Identification and Chromosomal Localization of New Powdery Mildew Resistance Gene Pm11 in Oat. Theor. Appl. Genet. 2020, 133, 179–185. [Google Scholar] [CrossRef]
- Ociepa, T.; Okoń, S. Chromosomal Location of Pm12—A Novel Powdery Mildew Resistance Gene from Avena sterilis. Genes 2022, 13, 2409. [Google Scholar] [CrossRef]
- Schurack, S.; Beuch, S.; Cowan, S.; Griffiths, I.; Lunzer, M.; Morales, L.; Tudor, S.; Buerstmayr, H.; Howarth, C.J.; Tinker, N.A. Genetic Mapping of the Powdery Mildew Resistance Gene Pm13 on Oat Chromosome 1D. bioRxiv 2024, 2024-01. [Google Scholar] [CrossRef]
- Yu, J.; Herrmann, M. Inheritance and Mapping of a Powdery Mildew Resistance Gene Introgressed from Avena macrostachya in Cultivated Oat. Theor. Appl. Genet. 2006, 113, 429–437. [Google Scholar] [CrossRef]
- Okoń, S.; Kowalczyk, K. Screening Oat Landraces for Resistance to Blumeria graminis f.sp. avenae. J. Plant Pathol. 2020, 102, 893–898. [Google Scholar] [CrossRef]
- Okoń, S.M. Effectiveness of Resistance Genes to Powdery Mildew in Oat. Crop Prot. 2015, 74, 48–50. [Google Scholar] [CrossRef]
- Cieplak, M.; Terlecka, K.; Ociepa, T.; Zimowska, B.; Okoń, S. Virulence Structure of Blumeria graminis f.sp. avena Populations in Poland across 2014–2015. Plant Pathol. J. 2021, 37, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, M.; Nucia, A.; Ociepa, T.; Okoń, S. Virulence Structure and Genetic Diversity of Blumeria graminis f.sp. avenae from Different Regions of Europe. Plants 2022, 11, 1358. [Google Scholar] [CrossRef] [PubMed]
- Hsam, S.L.K.; Paderina, E.V.; Gordei, S.; Zeller, F.J. Genetic Studies of Powdery Mildew Resistance in Cultivated Oat (Avena sativa L.) II. Cultivars and Breeding Lines Grown in Northern and Eastern Europe. Hereditas 1998, 230, 227–230. [Google Scholar] [CrossRef]
- Hsam, S.L.K.; Peters, N.; Paderina, E.V.; Felsenstein, F.; Oppitz, K.; Zeller, F.J. Genetic Studies of Powdery Mildew Resistance in Common Oat (Avena sativa L.) I. Cultivars and Breeding Lines Grown in Western Europe and North America. Euphytica 1997, 96, 421–427. [Google Scholar] [CrossRef]
- Mains, E.B. Inheritance of Resistance to Powdery Mildew, Erysiphe graminis tritici, in Wheat. Phytopathology 1934, 24, 1257–1261. [Google Scholar]
- Okoń, S.; Cieplak, M.; Kuzdraliński, A.; Ociepa, T. New Pathotype Nomenclature for Better Characterisation the Virulence and Diversity of Blumeria graminis f.sp. avenae Populations. Agronomy 2021, 11, 1852. [Google Scholar] [CrossRef]
- Dreiseitl, A.; Kosman, E. Virulence Phenotypes of Blumeria graminis f.sp. hordei in South Africa. Eur. J. Plant Pathol. 2015, 136, 113–121. [Google Scholar] [CrossRef]
- Kosman, E. Difference and Diversity of Plant Pathogen Populations: A New Approach for Measuring. Phytopathology 1996, 86, 1152–1155. [Google Scholar]
- Kosman, E.; Leonard, K.J. Conceptual Analysis of Methods Applied to Assessment of Diversity within and Distance Between Populations with Asexual or Mixed Mode of Reproduction. New Phytol. 2007, 174, 683–696. [Google Scholar] [CrossRef]
- Herrmann, A.; Löwer, C.F.; Schachtel, G.A. A New Tool for Entry and Analysis of Virulence Data for Plant Pathogens. Plant Pathol. 1999, 48, 154–158. [Google Scholar] [CrossRef]
- Schachtel, G.A.; Dinoor, A.; Herrmann, A.; Kosman, E. Comprehensive Evaluation of Virulence and Resistance Data: A New Analysis Tool. Plant Dis. 2012, 96, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Manoharachary, C. (Eds.) Future Challenges in Crop Protection Against Fungal Pathogens; Fungal Biology; Springer: New York, NY, USA, 2014; ISBN 978-1-4939-1187-5. [Google Scholar]
- Nürnberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate Immunity in Plants and Animals: Striking Similarities and Obvious Differences. Immunol. Rev. 2004, 198, 249–266. [Google Scholar] [CrossRef]
- Okoń, S.M.; Ociepa, T. Virulence Structure of the Blumeria graminis DC f.sp. avenae Populations Occurring in Poland Across 2010–2013. Eur. J. Plant Pathol. 2017, 149, 711–718. [Google Scholar] [CrossRef]
- Brodführer, S.; Mohler, V.; Stadlmeier, M.; Okoń, S.; Beuch, S.; Mascher, M.; Tinker, N.A.; Bekele, W.A.; Hackauf, B.; Herrmann, M.H. Genetic Mapping of the Powdery Mildew Resistance Gene Pm7 on Oat Chromosome 5D. Theor. Appl. Genet. 2023, 136, 53. [Google Scholar] [CrossRef]
- Cieplak, M.; Okoń, S. Resistant or Susceptible? How Central European Oat (A. sativa L.) Cultivars React to B. graminis f.sp. avenae Infection. Plants 2023, 12, 3825. [Google Scholar] [CrossRef] [PubMed]
- Okoń, S.; Ociepa, T.; Paczos-Grzęda, E.; Kowalczyk, K. Analysis of the resistance level of Polish common oat (Avena sativa L.) varieties to powdery mildew (Blumeria graminis DC. f. sp. avenae Em. Marchal.). Agron. Sci. 2016, 71, 51–60. [Google Scholar] [CrossRef]
- Reilly, A.; Okoń, S.; Cieplak, M.; Finnan, J.; Kildea, S.; Feechan, A. Breadth of Resistance to Powdery Mildew in Commercial Oat Cultivars Available in Ireland. Crop Prot. 2024, 176, 106517. [Google Scholar] [CrossRef]
- Babayants, O.V.; Babayants, L.T.; Traskovetskaya, V.A.; Gorash, A.F.; Saulyak, N.I.; Galaev, A.V. Race Composition of Blumeria graminis (DC) Speer f. sp. tritici in the South of Ukraine and Effectiveness of Pm-Genes in 2004–2013. Cereal Res. Commun. 2015, 43, 449–458. [Google Scholar] [CrossRef]
- Traskovetskaya, V.; Gorash, A.; Liatukas, Ž.; Saulyak, N.; Ternovyi, K.; Babayants, O.; Ruzgas, V.; Leistrumaitė, A. Virulence and Diversity of the Blumeria graminis f. sp. tritici Populations in Lithuania and Southern Ukraine. Zemdirb.-Agric. 2019, 106, 107–116. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. The Population Genetics of Plant Pathogens and Breeding Strategies for Durable Resistance. Euphytica 2002, 124, 163–180. [Google Scholar] [CrossRef]
- Bargués-Ribera, M.; Gokhale, C.S. Eco-Evolutionary Agriculture: Host-Pathogen Dynamics in Crop Rotations. PLoS Comput. Biol. 2020, 16, e1007546. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, S.; Okoń, S.; Rai, M.; Jadhav, P.; Prokisch, J. Recent Advances in CRISPR/Cas9 Technology for Engineering Disease Resistance in Plants. Acta Agrobot. 2025, 78, 1–31. [Google Scholar] [CrossRef]
- Manzoor, S.; Nabi, S.U.; Rather, T.R.; Gani, G.; Mir, Z.A.; Wani, A.W.; Ali, S.; Tyagi, A.; Manzar, N. Advancing Crop Disease Resistance through Genome Editing: A Promising Approach for Enhancing Agricultural Production. Front. Genome Ed. 2024, 6, 1399051. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ishiguro, K.; Nakajima, T.; Kim, H.Y.; Okada, M.; Kobayashi, K. Effects of Elevated Atmospheric CO2 Concentration on the Infection of Rice Blast and Sheath Blight. Phytopathology 2006, 96, 425–431. [Google Scholar] [CrossRef]
- Váry, Z.; Mullins, E.; McElwain, J.C.; Doohan, F.M. The Severity of Wheat Diseases Increases When Plants and Pathogens Are Acclimatized to Elevated Carbon Dioxide. Glob. Change Biol. 2015, 21, 2661–2669. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Zhang, C.; Xu, F.; Liu, W.; Fan, J.; Ma, Z.; Zhou, Y. Key Infection Stages Defending Heat Stress in High-Temperature-Resistant Blumeria graminis f. sp. tritici Isolates. Front. Microbiol. 2022, 13, 1045796. [Google Scholar] [CrossRef]
- Clarkson, J.P.; Fawcett, L.; Anthony, S.G.; Young, C. A Model for Sclerotinia sclerotiorum Infection and Disease Development in Lettuce, Based on the Effects of Temperature, Relative Humidity and Ascospore Density. PLoS ONE 2014, 9, e94049. [Google Scholar] [CrossRef]
- Granke, L.L.; Hausbeck, M.K. Effects of Temperature, Humidity, and Wounding on Development of Phytophthora Rot of Cucumber Fruit. Plant Dis. 2010, 94, 1417–1424. [Google Scholar] [CrossRef]
- Magarey, R.D.; Sutton, T.B.; Thayer, C.L. A Simple Generic Infection Model for Foliar Fungal Plant Pathogens. Phytopathology 2005, 95, 92–100. [Google Scholar] [CrossRef]
- Wyand, R.A.; Brown, J.K.M. Genetic and Forma Specialis Diversity in Blumeria graminis of Cereals and Its Implications for Host-pathogen Co-evolution. Mol. Plant Pathol. 2003, 4, 187–198. [Google Scholar] [CrossRef]
- Klocke, B.; Flath, K.; Miedaner, T. Virulence Phenotypes in Powdery Mildew (Blumeria graminis) Populations and Resistance Genes in Triticale (× Triticosecale). Eur. J. Plant Pathol. 2013, 137, 463–476. [Google Scholar] [CrossRef]
- Miedaner, T.; Klocke, B.; Flath, K.; Geiger, H.H.; Weber, W.E. Diversity, Spatial Variation, and Temporal Dynamics of Virulences in the German Leaf Rust (Puccinia recondita f. sp. secalis) Population in Winter Rye. Eur. J. Plant Pathol. 2012, 132, 23–35. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Hovmøller, M.S. Aerial Dispersal of Pathogens on the Global and Continental Scales and Its Impact on Plant Disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, J.E.; Torp, U.; Prahm, L.P. Studies of Transport of Live Spores of Cereal Mildew and Rust Fungi across the North Sea. Grana 1978, 17, 41–46. [Google Scholar] [CrossRef]
| Reference Set | Year | Location | |||||
|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2023 | Lublin | Strzelce | Polanowice | Choryń | |
| Pm1 | 1 | 1 | 0.95 | 1 | 0.933 | 1 | 1 |
| Pm2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pm3 | 0.725 | 0.725 | 0.825 | 0.867 | 0.867 | 0.367 | 0.967 |
| Pm4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pm5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pm6 | 0.675 | 0.7 | 0.725 | 0.833 | 0.9 | 0.767 | 0.3 |
| Pm7 in APR122 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pm7 (in Canyon) | 0.45 | 0.075 | 0.175 | 0.267 | 0.2 | 0.267 | 0.2 |
| Pm3+8 | 0.75 | 0.85 | 0.575 | 0.767 | 0.8 | 0.433 | 0.9 |
| Pm9 | 0.25 | 0.375 | 0 | 0.2 | 0.233 | 0.233 | 0.167 |
| Pm10 | 0.575 | 0.425 | 0.025 | 0.267 | 0.333 | 0.3 | 0.467 |
| Pm11 | 0.875 | 0.85 | 0.1 | 0.6 | 0.633 | 0.567 | 0.633 |
| Pm12 | 0.325 | 0.075 | 0 | 0.033 | 0.167 | 0.167 | 0.167 |
| A. strigosa | 0.025 | 0.05 | 0 | 0.067 | 0.033 | 0 | 0 |
| Fuchs | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Diversity Parameters | Year | Localization | |||||
|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2023 | Lublin | Strzelce | Polanowice | Choryń | |
| Gene diversity (Nei index Hs) equivalent to ADWm diversity | 0.219 | 0.176 | 0.119 | 0.176 | 0.186 | 0.217 | 0.167 |
| Simpson index Si | 0.956 | 0.943 | 0.85 | 0.927 | 0.944 | 0.94 | 0.922 |
| Shannon normalized index Sh | 0.884 | 0.824 | 0.624 | 0.829 | 0.881 | 0.873 | 0.789 |
| Kosman index KWm | 0.327 | 0.247 | 0.163 | 0.236 | 0.244 | 0.324 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzelak, W.; Nucia, A.; Okoń, S. Powdery Mildew Resistance Gene (Pm) Stability and Blumeria graminis f. sp. avenae Virulence Trends in Poland (2021–2023): Challenges to Durable Resistance in Oat. Agriculture 2025, 15, 1965. https://doi.org/10.3390/agriculture15181965
Grzelak W, Nucia A, Okoń S. Powdery Mildew Resistance Gene (Pm) Stability and Blumeria graminis f. sp. avenae Virulence Trends in Poland (2021–2023): Challenges to Durable Resistance in Oat. Agriculture. 2025; 15(18):1965. https://doi.org/10.3390/agriculture15181965
Chicago/Turabian StyleGrzelak, Weronika, Aleksandra Nucia, and Sylwia Okoń. 2025. "Powdery Mildew Resistance Gene (Pm) Stability and Blumeria graminis f. sp. avenae Virulence Trends in Poland (2021–2023): Challenges to Durable Resistance in Oat" Agriculture 15, no. 18: 1965. https://doi.org/10.3390/agriculture15181965
APA StyleGrzelak, W., Nucia, A., & Okoń, S. (2025). Powdery Mildew Resistance Gene (Pm) Stability and Blumeria graminis f. sp. avenae Virulence Trends in Poland (2021–2023): Challenges to Durable Resistance in Oat. Agriculture, 15(18), 1965. https://doi.org/10.3390/agriculture15181965








